Abstract
Background
Between the 1970s and 1990s, the World Health Organization promoted traditional birth attendant (TBA) training as one strategy to reduce maternal and neonatal mortality. To date, evidence in support of TBA training is limited but promising for some mortality outcomes.
Objectives
To assess the effects of TBA training on health behaviours and pregnancy outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (18 June 2012), citation alerts from our work and reference lists of studies identified in the search.
Selection criteria
Published and unpublished randomised controlled trials (RCT), comparing trained versus untrained TBAs, additionally trained versus trained TBAs, or women cared for/living in areas served by TBAs.
Data collection and analysis
Three authors independently assessed study quality and extracted data in the original and first update review. Three authors and one external reviewer independently assessed study quality and two extracted data in this second update.
Main results
Six studies involving over 1345 TBAs, more than 32,000 women and approximately 57,000 births that examined the effects of TBA training for trained versus untrained TBAs (one study) and additionally trained TBA training versus trained TBAs (five studies) are included in this review. These studies consist of individual randomised trials (two studies) and cluster‐randomised trials (four studies). The primary outcomes across the sample of studies were perinatal deaths, stillbirths and neonatal deaths (early, late and overall).
Trained TBAs versus untrained TBAs: one cluster‐randomised trial found a significantly lower perinatal death rate in the trained versus untrained TBA clusters (adjusted odds ratio (OR) 0.70, 95% confidence interval (CI) 0.59 to 0.83), lower stillbirth rate (adjusted OR 0.69, 95% CI 0.57 to 0.83) and lower neonatal death rate (adjusted OR 0.71, 95% CI 0.61 to 0.82). This study also found the maternal death rate was lower but not significant (adjusted OR 0.74, 95% CI 0.45 to 1.22).
Additionally trained TBAs versus trained TBAs: three large cluster‐randomised trials compared TBAs who received additional training in initial steps of resuscitation, including bag‐valve‐mask ventilation, with TBAs who had received basic training in safe, clean delivery and immediate newborn care. Basic training included mouth‐to‐mouth resuscitation (two studies) or bag‐valve‐mask resuscitation (one study). There was no significant difference in the perinatal death rate between the intervention and control clusters (one study, adjusted OR 0.79, 95% CI 0.61 to 1.02) and no significant difference in late neonatal death rate between intervention and control clusters (one study, adjusted risk ratio (RR) 0.47, 95% CI 0.20 to 1.11). The neonatal death rate, however, was 45% lower in intervention compared with the control clusters (one study, 22.8% versus 40.2%, adjusted RR 0.54, 95% CI 0.32 to 0.92).
We conducted a meta‐analysis on two outcomes: stillbirths and early neonatal death. There was no significant difference between the additionally trained TBAs versus trained TBAs for stillbirths (two studies, mean weighted adjusted RR 0.99, 95% CI 0.76 to 1.28) or early neonatal death rate (three studies, mean weighted adjusted RR 0.83, 95% CI 0.68 to 1.01).
Authors' conclusions
The results are promising for some outcomes (perinatal death, stillbirth and neonatal death). However, most outcomes are reported in only one study. A lack of contrast in training in the intervention and control clusters may have contributed to the null result for stillbirths and an insufficient number of studies may have contributed to the failure to achieve significance for early neonatal deaths. Despite the additional studies included in this updated systematic review, there remains insufficient evidence to establish the potential of TBA training to improve peri‐neonatal mortality.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Health Behavior; Infant Mortality; Maternal Mortality; Midwifery; Midwifery/education; Midwifery/standards; Perinatal Mortality; Pregnancy Outcome; Pregnancy Outcome/epidemiology; Randomized Controlled Trials as Topic; Stillbirth; Stillbirth/epidemiology
Plain language summary
Traditional birth attendant training for improving health behaviours and pregnancy outcomes
Traditional birth attendants are important providers of maternity care in developing countries. Many women in those countries give birth at home, assisted by family members or traditional birth attendants (TBAs). TBAs lack formal training and their skills are initially acquired by delivering babies and apprenticeships with other TBAs. Governments and other organisations have conducted training programmes to improve their skills and to link TBAs to health services. There is disagreement about whether these training programmes are effective. This review included six studies involving over 1345 TBAs, more than 32,000 women and approximately 57,000 births and examined the effect of TBA training, or additional training, on TBA behaviour and on pregnancy outcomes. We conclude that while there are a few more studies meeting the inclusion criteria and the results are promising for some outcomes, more evidence is needed to establish the potential of TBA training to improve peri‐neonatal mortality. A lack of contrast in training in the intervention and control clusters and an insufficient number of studies may have contributed to the lack of observed differences in maternal deaths and deaths of their babies (early neonatal deaths).
Summary of findings
for the main comparison.
| Additionally trained TBAs compared with trained TBAs for stillbirths and early neonatal mortality | |||||
|
Patient or population: pregnant women giving birth Settings: home Intervention: additional training Comparison: trained | |||||
| Studies | Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Carlo2010; Gill 2011 |
Stillbirths | RR 0.99 (0.76 to 1.28) | 27,594 births (2 studies) |
⊕⊕⊕⊝ moderate | The quality of evidence is moderate. GRADE criteria rates randomised trials as high, but factors such as limitations in blinding, as well as the high likelihood of recruitment and contamination bias in the Gill 2011 study downgrades the evidence to more moderate quality. |
|
Azad 2010; Carlo2010; Gill 2011. |
Early neonatal death (0 to 6 days) | RR 0.83 (0.68 to 1.01) | 37,494 births (3 studies) |
⊕⊕⊕⊝ moderate | Azad 2010 accounts for two‐thirds of the weight of the evidence. The quality of evidence is moderate. GRADE criteria rates randomised trials as high, but factors such as limitations in blinding, as well as the high likelihood of recruitment bias in the Azad 2010 and Gill 2011 studies, as well as contamination bias in the Gill 2011 study downgrades the evidence to moderate quality. |
| CI: confidence interval; RR: risk ratio; TBAs: traditional birth attendants | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Background
The World Health Organization (WHO) (WHO 1992) defines a traditional birth attendant (TBA) as a person who assists the mother during childbirth and who initially acquires skills by delivering babies herself or through an apprenticeship to other TBAs. Individual TBAs and their roles vary. However, certain characteristics are commonly seen across continents and regions (Fortney 1997a). TBAs tend to be older women, respected in the community for their knowledge and experience. They are often non‐literate and have learned their skills through older, more experienced TBAs. They may work independently, in collaboration with an individual provider or facility or they may be integrated into the health system. Their role may include, in addition to birth attendance, bathing and massage, domestic chores and provision of care during the later postpartum or postnatal period. TBAs may perform other roles depending on local custom, their own interests and expertise. The number of births TBAs attend each year ranges from a few births to as many as 120 births per year. Typically, TBAs attract clients by reputation and word‐of‐mouth. Usually they receive some remuneration for their services.
TBAs are important providers of maternity care in developing countries. Analysis of the 1995 to 1999 Demographic Health Surveys (Measure DHS 2002) using STATCompiler found that TBAs (trained and untrained) assisted at 24% of 200,633 live births (ranging from less than 1% to 66%) in 44 developing countries representing five regions of the world. Reporting on attendance at birth does not clearly distinguish between a TBA, a family TBA (one who has been designated by an extended family to attend births only in that family) or a relative who occasionally attends birth. When these categories are combined, TBAs, relatives and others assisted at 43% of all live births, ranging from less than 1% to 89% of live births. Up to 12% of births are unassisted in some settings (Sibley 2004a). Our re‐analysis of the 2005 to 2009 Demographic and Health Surveys (Measure DHS 2011) using STATCompiler for 20 sub‐Saharan African countries shows that 42% of births from 2002 to 2006 were attended by semi‐ or unskilled attendants (TBAs and relatives/others attend 26% and 17% of births, respectively, while 6% of births were unattended). These statistics are similar for five South Asian countries, where 53% of births are attended by unskilled or semi‐skilled attendants (TBAs attend 23% of births, relatives/others 28% and 2% are unattended).
Maternal mortality estimates for 2005 (WHO 2007) indicated that approximately 536,000 women die each year from causes related to childbearing; 99% in developing countries, mostly due to direct obstetric causes including severe bleeding (haemorrhage), infection, complications of unsafe abortion, eclampsia and obstructed labour (Khan 2006). Although more recent estimates show that a number of countries have made progress and are on track to achieve Millennium Development Goal (MDG) 5 to reduce the maternal mortality ratio by three‐quarters, sub‐Saharan Africa and South Asia together still carry the greatest burden of death, with 87% of maternal deaths (WHO 2010b).
An estimated 4 million babies die within four weeks of birth, mostly (75%) within the first week and on the first day of life; a similar number of babies are stillborn. Like maternal mortality, 99% of neonatal deaths take place in developing countries, mostly due to preterm birth, severe infections and asphyxia (Lawn 2005; WHO 2006). Although progress has been made towards MDG 4 to reduce the under‐five mortality rate by two‐thirds between 1990 and 2015, this is primarily due to reductions in child mortality. To achieve MDG 4, reductions in neonatal mortality are also necessary. The highest numbers of neonatal deaths are in south‐central Asian countries and the highest rates are generally in sub‐Saharan Africa. Most countries in these regions have made little progress in reducing these deaths (Lawn 2005; WHO 2006). Stillbirths and early neonatal deaths have similar obstetric origins and could be largely prevented, highlighting the importance of skilled care during labour, delivery and the early postnatal period for both mothers and babies (WHO 2006).
During the 1970s through to the 1990s, the World Health Organization promoted training of TBAs as a major public health strategy to reduce this tragic and preventable loss of life. According to the WHO (WHO 1992), a trained TBA is any TBA who has received a short course of training through the modern health sector to upgrade skills. The broad goals of TBA training were to reduce maternal and child morbidity and mortality and to improve the reproductive health of women. Objectives included enhancing linkages between modern healthcare services and the community, increasing the number of TBA‐attended births, and improving TBA skills and stature. Training programmes differed considerably in the way in which they address these objectives (Fortney 1997a). TBAs were trained by individuals, non‐governmental organisations and missions, as well as by local, state and national governments. The training programmes ranged from very basic to quite elaborate and may last from several days to several months. They may, but often did not, include clinical practice at a referral facility. They may, but sometimes did not, include continued contact with trained TBAs through supervision and further education. The content of TBA training also varied but usually includes performance of hygienic deliveries and cord care and use of appropriate techniques for delivery of the placenta to prevent immediate postpartum haemorrhage. Consistent with the emphasis on extending the reach of primary health care, however, many TBAs were also trained to take on expanded functions focusing on prevention, screening and referral. Usually TBAs were not trained to provide initial management for major maternal and neonatal complications such as postpartum haemorrhage or birth asphyxia (Sibley 2004c). Moreover, TBAs typically practised in resource‐poor environments where access to and availability of quality emergency obstetric care are severely constrained. Thus, their ability to impact maternal and neonatal mortality was limited. After more than three decades of experience, the evidence to support TBA training was limited and conflicting. By the late 1990s the uncertain impact of TBAs on maternal and neonatal mortality stimulated a passionate policy debate over the cost‐effectiveness of TBA training (Bang 1994; Bang 1999; Bergstrom 2001; Fortney 1997a; Kumar 1998; Levitt 1997; Maine 1992, Maine 1993; Rahman 1982; Sibley 2004a; Starrs 1998; Tinker 1993; UNICEF 1997; WHO 1992). Since the mid‐1990s, international policy has promoted skilled birth attendance. At the same time, countries with the highest maternal and neonatal mortality are often challenged by maternal and neonatal health workforce shortages, poor performance, inequitable distribution and high costs. These countries have, perhaps by default, a greater proportion of births attended by TBAs, relatives and others. While recognising the critical importance of skilled birth attendance in reducing maternal and neonatal mortality, concern persists among policy makers and planners about what to do about TBAs (and other less skilled health workers) during the transition to skilled birth attendance.
Rigorous evaluation of TBA training is methodologically and logistically challenging. The distinction between a trained TBA and an untrained TBA may be blurred because untrained TBAs are exposed frequently to biomedical concepts and practices. Moreover, TBA training is often one component of comprehensive interventions, for example, community mobilisation and upgrading of referral facilities. Both behavioural and health outcomes such as maternal and neonatal morbidity are typically based on self report and suffer the limitations of this method (Filippi 2000; Fortney 1997b; Ronsmans 1997). Finally, measuring the magnitude of the impact of TBA training on maternal mortality requires special studies with large bio‐statistical denominators. As a result, while donors, governments and non‐governmental organisations have invested heavily in TBA training programmes over the years, they have not invested equally in the systematic evaluation of training effectiveness (Fortney 1997b; Miller 2003).
Two of the review authors (LM Sibley, TA Sipe) conducted a meta‐analysis for the period 1970 to 1999 on the effect of TBA training and a subsequent update through 2002 (Sibley 2002; Sibley 2004c). The original systematic review included 60 eligible studies measuring 1695 unique outcomes reflecting attributes of knowledge, attitude, behaviour and advice (a subset of behaviour) related to maternal and child health care (MCH), as well as maternal and perinatal mortality. The studies span 30 years and three world regions. Main findings include moderate to large, positive effects for MCH knowledge, attitudes, behaviour and advice associated with training and small effects associated with perinatal health outcomes, such as cause‐specific neonatal mortality due to birth asphyxia, which demonstrated an 11% decrease over the untrained TBA baseline). The data were not sufficient to document an association between training and maternal mortality (Sibley 2002; Sibley 2004c). The 2004 updated systematic review (n = 16) included a stratified analysis to examine the influence of methodological variables and outcomes pertaining to antenatal care service (Sibley 2004a) and emergency obstetric care (Sibley 2004b). Main findings from the systematic review include a medium, positive, but non‐significant association between training and knowledge of risk factors and health conditions requiring referral (danger signs); with small, positive, significant associations between training and TBA referral behaviour (a 36% increase over the untrained TBA baseline), as well as maternal service use (a 22% increase over the untrained TBA baseline). These findings suggest that the real effects of TBA training on TBA and maternal referral behaviour are likely to be small, and emphasise the complexity of the referral process (Sibley 2004b). Unfortunately, the overall quality of studies included in this systematic review did not permit causal attribution to training. Even in the more rigorous studies, TBA training was part of an integrated package of interventions in an integrated package of interventions (Sibley 2002; Sibley 2004c). Thus, countries that are considering current and future TBA training do not have adequate evidence needed to guide sound policy and programme decisions. As others have persuasively argued (Buekens 2003; Miller 2003), rigorous evaluation is both necessary and feasible. Our original and first update of this review (2007, 2009) included four studies. We concluded that the potential of TBA training to reduce peri‐neonatal mortality is promising when combined with improved health services. However, the number of studies meeting the inclusion criteria was insufficient to provide the evidence needed to establish training effectiveness.
Responsive to the reality that lay health workers and TBAs continue to provide maternal and neonatal health in high mortality countries, the WHO has initiated consultative steps towards developing guidance for Member States on optimising the roles and capacity of frontline workers (including lay health workers and TBAs) as a possible strategy for scaling‐up implementation of evidence‐based care practices to achieve Millennium Development Goals 4 and 5 (WHO 2010a). With the addition of three new large cluster‐randomised trials, we hope that this 2011 update review on TBA training effectiveness will contribute evidence that is needed for guidance on TBA training.
Objectives
The objective of this review is to assess the effects of traditional birth attendant (TBA) training on TBA and maternal behaviours thought to mediate positive pregnancy outcomes, as well as on maternal, perinatal and newborn mortality and morbidity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials (including cluster‐randomised trials).
In previous versions of the review we also considered studies with other types of design (interrupted time series and controlled before and after studies). In view of the increasing evidence from randomised trials, in this updated review inclusion criteria for types of studies has been restricted to randomised and quasi‐randomised controlled trials.
Types of participants
This review uses the World Health Organization (WHO 1992) definition of a traditional birth attendant (TBA), which defines a TBA as a person who assists the mother during childbirth and who initially acquired her skills by delivering babies herself or through an apprenticeship to other TBAs. Eligible participants include:
trained and untrained TBAs as well as additionally trained and trained TBAs (reference to target intervention);
mothers and neonates cared for by trained and untrained TBAs as well as additionally trained and trained TBAs (or those who are living in areas where such TBAs attend a majority of births ‐ a proxy for exposure of women to TBAs); or
areas (or communities) having #1 and #2 (in the case of cluster‐randomised trials).
Only studies where the participants were TBAs and/or mothers and neonates in the care of TBAs were considered for inclusion. Studies with interventions that did not include TBAs or those in their care were excluded.
Where available, we provide information on the following characteristics of participants: TBA age, socioeconomic status, educational attainment, number of deliveries per year and number of years of practice, as well as maternal age, parity, socioeconomic status and educational attainment.
Types of interventions
TBA training is the intervention of interest.
Where available, we provide information on the following characteristics of the intervention: training method, content, duration, contact hours, trainer/trainee ratio, supervision and continuing education after training; whether training is a single intervention or a component of a complex intervention; as well as whether training was implemented in the context of an enabling environment that include elements such as advocacy, community mobilisation, emergency transportation or referral sites capable of emergency obstetric and newborn care.
Only studies with interventions that included TBA training were considered. Studies with interventions that did not include TBA training were excluded.
Types of outcome measures
Primary outcomes
1. Measure of maternal mortality:
maternal death (number of maternal deaths per 100,000 live births).
2. Measures of peri‐neonatal mortality:
stillbirth (number per 1000 live births);
early neonatal death (number 0 to 7 days per 1000 live births);
late neonatal death (number 8 to 28 days per 1000 live births);
neonatal death (number 0 to 28 days per 1000 live births); and
perinatal death (number stillbirths + live births 0 to 7 days per 1000 live births).
Secondary outcomes
1. Measures of maternal morbidity. We included studies containing outcomes based on self report of conditions in which a TBA's or woman's behaviour could affect the occurrence or severity of the condition, as long as the studies specify accepted case definitions or diagnostic criteria; for example, studies using or modifying the WHO verbal autopsy method (WHO 1995). The following conditions include:
prolonged or obstructed labour (> 24 hours duration of labour);
postpartum haemorrhage (> 500 ml blood loss following vaginal birth); and
postpartum infection.
2. Measures of peri‐neonatal morbidity. As with maternal morbidity, we included studies containing outcomes based on self report of conditions in which a TBA's or woman's behaviour could affect the occurrence or severity of the condition, as long as the studies specify accepted case definitions or diagnostic criteria (Marsh 2003; WHO 1999). The following conditions include:
low birthweight (< 2500 grams at birth);
birth asphyxia (failure to breathe within one minute of birth); and
infection (systemic, acute respiratory infection, tetanus).
3. TBA or maternal behaviours, or both, thought to mediate positive pregnancy outcomes include:
TBA advice to use or distribution of antenatal iron folic acid, or maternal use of antenatal iron folic acid supplementation;
TBA advice to use or distribution of vitamin A, or maternal use of antenatal and postnatal vitamin A supplementation;
TBA advice to use or distribution of malaria prophylaxis, or maternal use of malaria prophylaxis;
TBA advice to be immunised against tetanus, or maternal acceptance of tetanus immunisation;
TBA use of hygienic delivery practices, advice to use hygienic delivery practices, or maternal use of hygienic delivery practices;
TBA advice regarding or maternal initiation of early and exclusive breastfeeding, or both;
TBA home‐based management of selected life‐threatening conditions (listed below);
completed referral to health facility for emergency obstetric or neonatal care (where facilities exist and such care is available), or both;
TBA advice to use or maternal use of family planning; and
TBA advice to use or maternal use of antenatal and postnatal care.
We provide information for characteristics of outcome measures such as the timing of observation relative to the intervention, as well as data collection method and data source, where available.
Search methods for identification of studies
Electronic searches
For this update, we searched the Cochrane Pregnancy and Childbirth Group's Trials Register (18 June 2012). For details of supplemental searches we have conducted for previous versions of the review, seeAppendix 1.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched citation alerts from our work and reference lists of studies identified in the search.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 3.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion. We did not require third person consultation.
Data extraction and management
We used the form designed for the original review to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion and did not require third‐person consultation. We entered data into Review Manager software (RevMan 2011) and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Three review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion. In addition we used items specific for cluster‐randomised trials when appropriate (see Handbook sections 16.3.2 and 16.4.3). In the following section, we describe the criteria used to assess risk of bias for the different types of study designs.
A. Individually randomised controlled trials
1. Random sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
low risk (any truly random process, e.g., random number table; computer random number generator);
high risk (any non‐random process, e.g., odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk (e.g., telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
3. Blinding (checking for possible detection and performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes. We assessed the methods as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel;
low risk, high risk or unclear risk for outcome assessors.
4. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed data as low risk if there was no more than 20% missing data. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
low risk;
high risk;
unclear risk.
5. Selective reporting bias (checking for reporting bias)
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk.
6. Other sources of bias
We describe for each included study any important concerns we have about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk;
high risk;
unclear risk.
B. Cluster‐randomised trials
1. Recruitment bias
Recruitment bias can occur when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an ‘intervention’ or ‘control’ cluster could affect the types of participants recruited. We assessed the methods as:
low risk;
high risk;
unclear risk.
2. Baseline imbalance
Cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters or statistical adjustment. We assessed the methods as:
low risk (comparable groups in terms of clusters individuals or statistical adjustment in the analysis);
high risk;
unclear risk.
3. Loss of clusters
Occasionally complete clusters are lost from a trial, and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. Missing outcomes for individuals within clusters may also lead to a risk of bias in cluster‐randomised trials. We assessed the methods as:
low risk (no loss of clusters, < 20% loss of individuals, < 20% missing data on primary outcomes);
high risk;
unclear risk.
4. Incorrect statistical methods (unit of analysis error)
Cluster‐randomised trials are sometimes analysed by incorrect statistical methods, not taking the clustering into account. Such analyses create a ‘unit of analysis error’ and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis. We assessed the methods as:
low risk;
high risk;
unclear risk.
C. Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) for individually randomised trials and (1) to (4) for cluster‐randomised controlled trials, above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We planned to explore the impact of the level of bias through sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
We calculated effect sizes for each outcome reported in individual studies. We calculated summary effect estimates for similar outcomes reported by two or more studies.
Dichotomous data
For individual studies reporting dichotomous data, we used the odds ratios or risk ratios provided by the authors and entered the data into RevMan using the inverse variance method. When necessary we calculated effect estimates from raw data provided by the authors and we present data that the authors report as well (e.g., percentages). Because most of the studies reported cluster‐adjusted outcomes, we were unable to transform all of the effect sizes into a common metric.
Continuous data
For individual studies reporting continuous data, we calculated the mean difference and 95% confidence intervals. We provide the data that the authors report as well (e.g., group means, standard deviations (SDs)).
Unit of analysis issues
We stratified on the type of comparison group in our analysis. We analysed studies comparing trained versus untrained TBAs separately from studies comparing additionally trained versus trained TBAs. We included four cluster‐randomised trials in the analyses along with two individually randomised trials. All of the cluster trials used an appropriate method of analysis to handle the cluster level by either calculating an intra‐class correlation coefficient (ICC) (Azad 2010; Carlo 2010) or by using multilevel modelling to adjust for cluster randomisation (Gill 2011; Jokhio 2005). We calculated effect sizes using the inverse variance method described in the Handbook (Section 16.3.3). For the two outcomes we were able to combine, measures of heterogeneity did not exceed the threshold (I² < 50%).
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect with a sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e., we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if T² was greater than zero and if either I² was greater than 50% or there was a low P value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
There were six included studies in this update of the review. As such, we did not investigate reporting bias beyond the level of the individual study. In future updates of this review, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we will use the test proposed by Egger 1997, and for dichotomous outcomes we will use the test proposed by Harbord 2006. If asymmetry is detected in any of these tests or is suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e., where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. We were able to combine studies for two outcomes: stillbirths and early neonatal deaths. We combined results only from studies having similar comparison groups and similar designs.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analysis for primary outcomes, stratifying on participant and intervention characteristics.
Sensitivity analysis
We planned to conduct sensitivity analysis for the primary outcomes included in meta‐analyses when there were sufficient numbers of studies. Specifically, we planned a one‐study‐removed analysis to determine if any one study has an undue influence on results. Other sensitivity analyses were planned as needed once the data were examined, e.g., impact of level of bias on treatment effect.
Results
Description of studies
Results of the search
The original review electronic searches yielded a total of 147 citations, including three citations from the Cochrane Pregnancy and Childbirth Group's Trials Register, 113 from the Cochrane Effective Practice and Organisation of Care (EPOC) Group's Trials Register and 43 citations from the supplemental search. We screened the studies in two stages. First, two members of the review team independently screened the titles or abstracts from these citations, reaching 99% agreement. They discussed and resolved differences of opinion, where possible, and eliminated all studies which they agreed did not meet preset criteria for types of participants, interventions and outcome measures. We obtained the full text documents of 18 studies. Second, two different members of the team independently read the full text and screened all 18 studies according to the preset criteria for types of studies, reaching 94% agreement. Differences of opinion were discussed and resolved in favour of including four and excluding 14 studies from the review (seeCharacteristics of excluded studies table). In addition, a staff member from the Cochrane EPOC Group reviewed the studies under disagreement and provided a third opinion regarding eligibility.
For this 2012 update, we followed the same search strategy used in the original review, but did not update the supplemental search. We also screened citation alerts from our publications on this topic. The updated electronic searches yielded a total of 28 citations. We reached consensus in favour of including three studies and excluding nine studies from the review. Four trial reports are awaiting classification (Costello 2011; Matendo 2011; Morrison 2011; Pasha 2010).
In this update we changed the inclusion criteria for types of studies and now include only randomised or quasi‐randomised trials; one controlled before and after study that was previously included has now been excluded (O'Rourke 1994).
This review is now comprised of six included studies and 24 excluded studies.
Included studies
The six included studies covered a period of 22 years and included four large cluster‐randomised controlled trials from Bangladesh (Azad 2010); the Democratic Republic of Congo, Guatemala, India, Pakistan, Zambia (Carlo 2010); Zambia (Gill 2011); and Pakistan (Jokhio 2005), along with smaller individual randomised controlled trials from Malawi (Bullough 1989) and Bangladesh (Hossain 2000). The six studies include five papers published in peer‐reviewed journals and an unpublished technical report of operations research (seeCharacteristics of included studies table).
Participants
Participants included traditional birth attendants (TBAs) and lactating mothers living in the intervention and control areas (Hossain 2000), pregnant women living in the intervention and control clusters identified, recruited and followed through the postpartum and/or postnatal periods (Azad 2010; Carlo 2010; Jokhio 2005), and women recently delivered by and/or referred to a health facility by TBAs (Bullough 1989; Gill 2011). See 'Characteristics of TBAs' (Table 2) and 'Characteristics of women' (Table 3) for available social and demographic information about the study populations.
1. Characteristics of traditional birth attendants (participants).
| Study ID (year) | Sample | Age (years) % | Educational level % | Marital status % | Other sources | Experience (years) % | Prior training % | Place of training |
| Azad 2010 | Intervention and control: n = 482 |
Not reported | Not reported | Not reported | Not reported | Not reported | Intervention: Yes = 100 Control: Yes = 100 |
Not reported |
| Bullough 1989 | Intervention: n = 2104 Control: n = 2123 |
Not reported | Not reported | Not reported | Not reported | Not reported | Intervention: Yes = 100 Control: Yes = 100 | Not reported |
| Carlo 2010 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Intervention: Yes = 100 Control: Yes = 100 | Not reported |
| Gill 2011 | Intervention: n = 60 Control: n = 67 |
Intervention: mean = 49.2, SE = 0.79 Control: mean = 49.6, SE = 1.32 |
Intervention: None = 5 Some primary = 78 Some secondary = 17 Mean = 6.3 Control: None = 29 Some primary = 62 Some secondary = 9 Mean = 4.3 |
Intervention: Married = 70 Single = 2 Divorced = 13 Widowed = 15 Control: Married = 81 Single = 2 Divorced = 2 Widowed = 16 |
Not reported | Intervention: mean = 6.3, SE = 0.48 Control: mean = 4.0, SE = 0.55 |
Intervention: Yes = 100 Control: Yes = 100 | Not reported |
| Hossain 2000 | Intervention n = 85 Control n = 86 |
Intervention = < 30 = 3.3 30 to 39 = 24.4 40 to 49 = 38.9 50 to 59 = 23.3 60+ = 10.0 Control: < 30 = 1.2 30 to 39 = 19.8 40 to 49 = 45.7 50 to 59 = 21.0 60+ = 12.3 | Intervention: < Primary = 72.2 Class 5 to 9 = 26.7 SSC = 1.1 HSC = 0.0 Control: < Primary = 71.6 Class 5 to 9 = 24.7 SSC = 2.5 HSC = 1.2 | Intervention: Married = 67.8 Unmarried = 0.0 Widow = 32.2 Control: Married = 63.0 Unmarried = 1.2 Widow = 35.8 | Intervention: Nothing = 36.7 Work in = Other house = 5.6 Small business = 2.2 Poultry rearing = 35.6 Cow rearing = 3.3 Other = 16.7 Control: Nothing = 46.9 Work in = Other house = 1.2 Small business = 3.7 Poultry rearing = 28.4 Cow rearing = 0.0 Other = 19.8 | Intervention: < 10 = 45.6 10 to 19 = 31.0 20 to 29 = 16.7 30+ = 6.7 Control: < 10 = 33.3 10 to 19 = 46.9 20 to 29 = 17.3 30+ = 2.5 | Intervention: Yes = 96.7 Control: Yes = 70.4 | Not reported |
| Jokhio 2005 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
HSC: Higher School Certificate SE: standard error SSC: Secondary School Certificate
2. Characteristics of women (participants) served by traditional birth attendants.
| Study ID (year) | Sample | Age in years (%) | Parity (%) | Education (%) | Marital status (%) | SES (%) | Other (%) |
| Azad 2010 | Intervention n = 12,519 home births (8618 attended by any TBA and 2792 attended by TBA trained in bag‐valve‐mask resuscitation) Control n = 13,195 home births (9171 attended by any TBA and 2536 attended by TBA trained in bag‐valve‐mask resuscitation) Intervention n = 3213 women Control = 3176 women |
Intervention: < 20 = 16 20 to 29 = 65 > 30 = 19 Control: < 20 = 13 20 to 29 = 60 > 30 = 26 |
Not reported | Intervention: None = 59 Primary = 32 Secondary or more = 18 Control: None = 48 Primary = 28 Secondary or more = 24 |
Not reported | Intervention: Own agricultural land = 49 Own house = 98 Own appliances (wardrobe, radio, sewing machine, bicycle) = 30 Own none of above appliances = 10 Use of sanitary latrine = 32 Access to tube well = 97 Control: Own agricultural land = 49 Own house = 96 Own appliances (wardrobe, radio, sewing machine, bicycle) = 44 Own none of above appliances = 8 Use of sanitary latrine = 46 Access to tube well = 95 |
NGO membership Intervention: 27 Control: 32 Religion Intervention: Islam = 88 Hindu = 12 Control: Islam = 81 Hindu = 19 |
| Bullough 1989 | Intervention n = 2104 Control n = 2123 |
Not reported | Intervention: primigravida = 12.0 1 to 3 = 40.3 4 to 6 = 29.4 > 7 = 12.4 Unknown = 6.0 Control: primigravida = 13.6 1 to 3 = 41.5 4 to 6 = 1.0 > 7 = 12.2 Unknown = 1.7 | Not reported | Not reported | Not reported | Not reported |
| Carlo 2010 | Intervention: 10,770 women delivered by TBA Control: 13,327 women delivered by TBA |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Gill 2011 | Intervention: 1920 women Control: 1517 women |
Intervention: 25.3 Control: 25.3 |
Not reported | Intervention: No formal = 16.9 Some primary = 68.9 Some secondary = 13.9 Some higher = 0.3 Control: No formal = 18.5 Some primary = 69.3 Some secondary = 11.8 Some higher = 0.4 |
Intervention: Married = 89.0 Widowed = 0.9 Sep/div = 2.5 Never married = 7.6 Control: Married = 90.1 Widowed = 0.6 Sep/div = 2.7 Never married = 6.6 |
Not reported | Mean # people living in household Intervention: 5.2 Control: 5.3 Mean # ANC visits Intervention: 3.3 Control: 3.2 Content of ANC received Intervention: Malaria tx = 88.7 Deworm = 65.2 Folic acid = 85.0 Iron = 91.6 TT = 72.0 Control: Malaria tx = 87.4 Deworm = 64.0 Folic acid = 83.8 Iron = 91.8 TT = 72.4 |
| Hossain 2000 | Intervention n = 354 Control n = 360 | Intervention: < 20 = 15.9 20 to 29 = 62.9 30 to 39 = 19.8 40 to 49 = 1.5 Control: < 20 = 20.7 20 to 29 = 57.7 30 to 39 = 19.9 40 to 49 = 1.6 | Total children. Intervention 1 to 2 = 61.7 3 to 4 = 25.1 5 to 6 = 10.5 7+ = 2.7 Control: 1 to 2 = 57.5 3 to 4 = 31.5 5 to 6 = 8.7 7+ = 2.4 | Intervention: No school = 46.7 Class 1 to 4 = 21.0 Class 5 to 6 = 27.2 SSC = 4.2 HSC = 0.9 Control: No school = 45.9 Class 1 to 4 = 15.0 Class 5 to 6 = 31.0 SSC = 6.8 HSC = 1.3 | Not reported | Housing structure. Intervention: Jhupri = 3.6 One room of clay/bamboo/straw = 26.0 More than one room of clay/bamboo/straw = 10.2 Tin‐roofed house = 55.4 Concrete = 3.9 Shelter = 0.9 Control: Jhupri = 3.7 One room of clay/bamboo/straw = 27.8 More than one room of clay/bamboo/straw = 9.7 Tin‐roofed house = 52.2 Concrete = 5.0 Shelter = 1.6 | Not reported |
| Jokhio 2005 | Intervention n = 10,114 Control n = 9443 | Intervention:
mean = 26.7
SD = 5.9 Control: mean = 26.0 SD = 6.2 |
Intervention: mean = 3.5 SD = 2.8 Control: mean (no.) = 3.7 SD = 2.9 | Intervention:
mean (yrs.) = 1.1
SD = 2.8 Control: mean (yrs.) = 1.4; SD = 3.2 |
Not reported | Not reported | Distance to nearest health facility (km)
Intervention:
mean (km) = 2.7
SD = 3.0 Control: mean (km) = 2.5 SD = 3.0 |
ANC: antenatal care HSC: Higher School Certificate NGO: non‐governmental organisation SD: standard deviation SES: socioeconomic status SSC: Secondary School Certificate TBA: traditional birth attendant TT: tetanus toxoid tx: treatment
Interventions
In two studies, the authors categorised TBAs targeted by interventions as 'untrained' TBAs (Hossain 2000; Jokhio 2005). However, in one of these studies, the large majority of TBAs in both the intervention and control groups had received some prior biomedical training through government or non‐governmental organisations, or both; for example, 97% of the intervention group TBAs and 70% of the control group TBAs in the Bangladesh study (Hossain 2000) had received prior training. Thus we categorised this study into the additionally trained TBAs versus trained TBAs. In the remaining study (Bullough 1989), all TBAs received some prior biomedical training (seeCharacteristics of included studies table).
In four studies, the interventions consisted of educational instruction in management of normal delivery, timely detection and referral of women and/or newborns with complications, as well as importance of linking women to essential obstetric care services (Azad 2010; Carlo 2010; Gill 2011; Jokhio 2005).
In some of the studies, TBA training was part of a package of interventions including community and improved facility‐based care components. For example, in one study (Jokhio 2005), the improvements involved staff training in essential and emergency obstetric care and clinical outreach by team physicians. Two study interventions focused on breastfeeding: one highlighted initiation of early exclusive breastfeeding and the introduction of weaning foods (Hossain 2000), while the other intervention emphasised initiation of early suckling before placental delivery to reduce postpartum blood loss (Bullough 1989). Finally, the interventions in the three new studies included in this 2011 review (Azad 2010; Carlo 2010; Gill 2011) focused on essential newborn care and resuscitation.
Duration of training among studies ranged from two to three days (Azad 2010; Carlo 2010) to up to one week (Gill 2011). Apart from content and duration, few authors reported other characteristics of the TBA training interventions (see 'Intervention characteristics' (Table 4)).
3. Intervention characteristics of traditional birth attendant training.
| Author (year) | Characteristics | Trainer information | Training modality | Curriculum info | Training focus | Duration/intensity | Trainee information | Post‐training |
| Azad 2010 | Evidence base: bag‐valve‐mask ventilation reduces birth asphyxia mortality Based on clinical practice guidelines: not clear Purpose: improved management, initiation of new management Nature of desired change: improved neonatal resuscitation Source of funding: Women and Children First, UK Big Lottery Fund, Bill & Melinda Gates Foundation, UK Department for International Development Ethical approval: not clear |
Deliverer: not clear Trainer qualification: not clear Trainers training: not clear Experience with low or non‐literate trainees: not clear |
Theoretical: done Practical: done Format: interpersonal, simulation |
Curriculum source: not clear | Content: all TBAs, clean safe delivery, danger signs emergency preparedness, mouth‐to‐mouth ventilation; intervention TBAs, bag‐valve‐mask resuscitation |
Duration: not clear Total contact hours: not clear |
Trainees per cohort: not clear Total trained in programme year: 482 TBAs, number in intervention and control clusters not clear |
Supervision: not clear Follow‐up: not clear (only for data collection) Continuing education: not clear |
| Bullough 1989 | Evidence base: not done (no evidence that early suckling reduces postpartum blood loss) Based on clinical practice guidelines: not done Purpose: appropriate management Nature of the desired change: initiation of new management Source of funding: charitable trust (Bert Trust, Wellcome Trust) Ethical approval: done (Health Sciences Research Committee/ Malawi Ministry of Health) | Deliverer: not clear
Trainer qualification: not clear Trainers training: not clear Experience with low or non‐literate trainees: not clear |
Theoretical: not clear Practical (clinical): not clear Format: interpersonal, audio/visual |
Curriculum source: developed by/for project, local expert body Curriculum pre‐tested or pilot tested: not clear | Content: record keeping, physiology of 3rd stage labour, management of 3rd stage labour, causes of haemorrhage, measurement of blood loss, reason for referral of 3rd stage labour complication plus early sucking for the intervention group | Duration: 2 days Total contact hours: not clear | Trainees per cohort: 6 to 7 TBAs Total trained in programme year: 69 TBAs | Supervision: community midwives Follow‐up: monthly Continuing education: not clear |
|
Carlo 2010 |
Evidence base: done Based on clinical guidelines: done Purpose: improved management and initiation of new management Nature of desired change: basic care and neonatal resuscitation Funding source: Eunice Kennedy Shriver National Institute of Child Health and Human Development Global Network for Women’s and Children’s Health Research, and the Bill and Melinda Gates Foundation Ethical approval: not clear (but informed consent, data safety and monitoring board) |
Deliverer: local expert Trainer qualification: not clear (experienced) Trainers training: cascade‐trainers trained master trainers, who trained community co‐ordinators Experience with low or non‐literate trainees: not clear |
Theoretical: done Practical: done Format: interpersonal, clinical practice, demonstration, materials adapted for low/non‐literate participants |
Curriculum source: WHO 2004 Essential Newborn Care Program, modified American Academy of Pediatrics Neonatal Resuscitation Program Curriculum pre‐tested or pilot tested: done |
Content: all birth attendants ‐ routine neonatal care, initiation of breathing and resuscitation (bag‐valve‐mask ventilation), thermoregulation, early exclusive breastfeeding, kangaroo care, danger signs. Intervention birth attendants ‐ in‐depth theoretical and practical training in initial steps in resuscitation and bag‐valve‐mask ventilation | Duration: 3 days Essential Newborn Care Program, 3 days Newborn Resuscitation Program Total contact hours: not clear |
Trainees per cohort: not clear Total trained in program year: not clear |
Supervision: not clear (only for data collection) Follow‐up: not clear (only for data collection) Continuing education: 6 mo. refresher for intervention birth attendants |
| Gill 2011 | Evidence base: done Based on clinical guidelines: done Purpose: improved management and initiation of new management Nature of desired change: basic care, resuscitation, sepsis treatment, referral Funding source: Co‐operative agreement, Boston University and USAID, with support from American Academy of Pediatrics and UNICEF Ethical approval: Boston University Medical Center and the Tropical Diseases Research Centre, Ndola, Zambia, informed consent in local languages, data safety monitoring board |
Deliverer: not clear Trainer qualification: not clear (members of the study team) Trainers training: not clear Experience with low or non‐literate trainees: not clear |
Theoretical: done Practical: done Format: lectures, demonstrations, small group sessions and skills practice using infant manikins |
Curriculum source: American Pediatric Association and American Heart Association neonatal resuscitation protocol Curriculum pre‐tested or pilot tested: not clear |
Content: all TBAs trained in prevention of neonatal hypothermia, mouth‐to‐mouth ventilation, record keeping, following up mothers‐newborns Intervention TBAs trained in bag‐valve‐mask ventilation, sepsis management, facilitated referral | Duration: 2 1‐week sessions Total contact hours: not clear |
Trainees per cohort: 60 intervention and 60 control TBAs Total trained in program year: 120 TBAs |
Supervision: not clear (only for data collection) Follow‐up: not clear (only for data collection) Continuing education: not clear |
| Hossain 2000 | Evidence base: done Purpose: appropriate management Nature of the desired change: increase established management Source of funding: voluntary body Ethical approval: not clear | Deliverer: local expert Trainer qualification: not clear Trainers had trainer training: not clear Experience with low or non‐literate trainees: not clear | Theoretical: not clear Practical (clinical): not clear Format: not clear | Curriculum source: developed by/for project, national expert body (Bangladesh Breast Feeding Program) Curriculum pre‐ tested or pilot tested: not clear | Breastfeeding advice including benefits, early, exclusive feeding, introduction/types of complementary weaning foods, disadvantages of bottle feeding | Duration: 2 days Total contact hours: not clear | Trainees per cohort: 15 Total trained in program year: 85 | Supervision: not clear Follow‐up: not clear |
| Jokhio 2005 | Evidence base: not clear (generally accepted best practices) Purpose: appropriate management Nature of the desired change: initiation of new management (new components) Source of funding: government organisation for capital costs (Family Health Project of Sindh Government Health Department) and other for data entry (University of Birmingham, UK) Ethical approval: not clear (protocol discussed and approved after meeting with provincial leaders) | Deliverer: local experts Trainer qualification: paramedics (LHW) and obstetricians Trainers had trainer training: not clear Experience with low or non‐literate trainees: not clear | Theoretical: not clear Practical (clinical): not clear Format: interpersonal, audio/visual | Curriculum source: developed by/for project, local expert body Curriculum pre‐tested or pilot tested: not clear | Advice on antepartum, intrapartum postpartum care, how to conduct clean delivery, how to use disposable delivery kit, referral for obstetric emergencies, newborn care | Duration: 3 days Total contact hours: not clear | Trainees per cohort: not clear Total trained in program year: 565 | Supervision: LHW support and data collection Follow‐up: not clear |
LHW: lady health workers mo.: month TBA: traditional birth attendant
Outcomes
The six studies contained outcomes of maternal deaths, maternal morbidity, perinatal deaths, stillbirths, and early and/or late neonatal deaths, as well as TBA or maternal behaviours thought to mediate positive pregnancy outcomes. Perinatal deaths, stillbirths, early and late neonatal deaths and referral were reported in more than one study. We were able to pool the results for studies containing the outcomes stillbirths and early neonatal deaths in the analysis.
Excluded studies
We excluded 24 studies. The two major reasons for study exclusion were ineligible study design and type of participant (seeCharacteristics of excluded studies table).
Risk of bias in included studies
Of the six randomised controlled trials four were cluster‐randomised. No study was low risk of bias for all criteria (seeCharacteristics of included studies) thus all studies were either high risk or unclear risk, meaning that there is some level of plausible bias in each of the studies reviewed or it is unknown if there is bias. Assessment of key criteria and justification for risk level assignation is described in Characteristics of included studies and summarised below. Figure 1 and Figure 2 provide graphical summaries.
1.
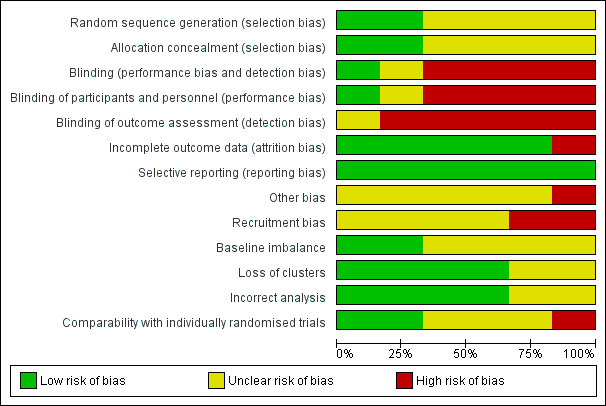
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
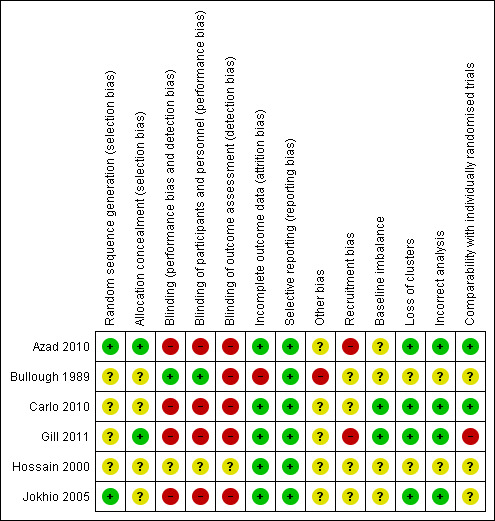
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Individually randomised and cluster‐randomised controlled trials
1. Random sequence generation
Bullough 1989, Hossain 2000 and Carlo 2010 did not report details on sequence generation for randomisation so the risk of selection bias is unclear for both studies. Azad 2010 and Gill 2011 randomly allocated unions and TBAs, respectively, by drawing slips of paper from a container. However, the Gill 2011 study later added seven TBAs to the control group through a non‐random procedure, and thus the risk of bias is unclear. Jokhio 2005 randomised sub‐districts (talukas) using a computer‐generated randomisation procedure. The allocation sequence in these three studies was decided on before randomisation and before collection and analysis of baseline data.
2. Allocation concealment
Information about allocation concealment procedures used in Bullough 1989, Hossain 2000, Carlo 2010 and Jokhio 2005 was not reported. Risk of selection bias is thus unclear for these studies. The procedure of drawing slips from a container in Azad 2010 and Gill 2011 carried a low risk of bias.
3. Blinding
Bullough 1989 reported that project data collectors were not blinded to the intervention status of the TBAs, but that the TBAs, who measured blood loss, were not informed that they were participating in a trial. Hossain 2000 did not report on blinding of providers, recipients of care or outcome assessors. The risk of performance and detection bias is therefore high for Bullough 1989 and unclear for Hossain 2000.
Blinding of participants and personnel is often not possible for cluster‐randomised trials. Performance bias may be an issue as increased training of TBAs may have instilled greater confidence and adherence from community members for all aspects of care. Carlo 2010, Azad 2010, Gill 2011 and Jokhio 2005 all used TBAs as the primary outcome assessors, with verification of data provided by other unblinded government health workers or interviewers.
Azad 2010 provided a 60 Taka incentive to TBAs for accurate birth/death identification, regardless of whether the TBA was the delivery attendant. However TBAs may have still underreported deaths for deliveries they attended if greater social or reputation disincentives prevailed. Gill 2011 and Jokhio 2005 both used prospective monitoring of pregnant women, which likely reduced detection bias of deaths. Gill 2011 also utilised a panel of independent neonatologists, blinded to group allocation, which reviewed delivery and verbal autopsy reports for stillbirths and neonatal deaths. Jokhio 2005 also notes that the Lady Health Workers and TBAs were not made aware of the purpose or comparative nature of the study. We consider the risk of bias due to lack of blinding for all four cluster‐randomised trials to be high.
4. Incomplete outcome data
Bullough 1989 reported that about 30% of TBAs in each group, intervention and control, were excluded from the analysis because they were "untrainable", "failed quality control check", or were "strongly suspected of fabricating results" (page 524). Among the remaining TBAs, outcomes were reported for more than 90% of women. Hossain 2000 reported outcomes for more than 90% of participants. We consider risk of attrition bias to be high for Bullough 1989 and low for Hossain 2000.
None of the four cluster‐randomised control trials had clusters lost to follow‐up. Additionally, all studies reported results for all primary outcomes listed, with a high and comparable percentage for control and intervention clusters (above 80% in all studies). In Azad 2010 only home deliveries were included in the analysis, which is appropriate due to the nature of the intervention. Intention‐to‐treat analysis was performed for all primary and secondary mortality outcomes, yet analysis for behavioural secondary outcomes excluded temporary and tea garden residents. As the only specified outcome for the TBA intervention was early neonatal mortality, there is a low risk of incomplete outcome data. Carlo 2010 excluded births for which the birth weight was unmeasured from the analysis (5.1%), and reported that results were “materially unchanged” with this exclusion.
5. Selective reporting
Reporting bias was low in all studies.
6. Other biases
Bullough 1989 noted that "The accuracy of blood loss measurements by TBAs who were mostly illiterate and/or innumerate may be doubted" (page 524).
Azad 2010 stated that randomisation may be compromised due to the purposive selection of districts, sampling stratification and the restricted number of clusters. Confounding with a women’s group intervention and potentially differential interventions for health services inputs may have also occurred. Underlying secular trends in declining mortality also appeared to be present in control areas. Carlo 2010 reported that participation appeared to be non‐differential between intervention and control. Gill 2011 described various methods for combating misclassification of failed resuscitation as stillbirths. Jokhio 2005 was not sufficiently powered to detect a difference for the maternal mortality outcome. Furthermore, as the outcomes of referrals were not followed, it is possible that the intervention could have altered the composition of referred women (i.e., a greater proportion of high‐risk women electing to be referred), which could have biased results. Finally, the validity of self report of maternal complications may lead to bias.
B. Cluster‐randomised trials only
1. Recruitment bias
Azad 2010 used a factorial design in which clusters were first randomised for a women’s group intervention, then secondarily for the TBA intervention. Women were recruited after the clusters were randomised, placing it at high risk of recruitment bias. Gill 2011 was also at a high risk of bias as seven TBAs (“clusters”) were added to the control group after randomisation. Carlo 2010 and Jokhio 2005 all had an unclear risk of recruitment bias. The method of recruitment was not clearly stated, and the proximity of intervention and control clusters to each other was of concern.
2. Baseline imbalance
All four studies performed a baseline survey and all but Jokhio 2005 controlled for baseline differences in the analysis. In Azad 2010, chance inclusion of three tea garden estates in control clusters, which “had substantially worse health and socioeconomic indicators than did the rest of the study area” occurred, and delayed surveillance activities. The baseline survey found that intervention group mothers were more likely to be younger, have no education and no household assets than control mothers. While baseline differences were adjusted for in the women’s group component of the study, it was unclear whether these differences persisted, or were controlled for, through the second‐level of randomisation for the TBA component of the study. Furthermore, the Jokhio 2005 baseline survey collected data on a limited number of covariates and the comparability of clusters remained unclear. Carlo 2010 and Gill 2011 had comprehensive baseline surveys and large numbers of clusters, thus the risk of baseline imbalance for both was low.
3. Loss of clusters
None of the four studies had loss of clusters.
4. Incorrect analysis
All four studies adjusted for clustering using appropriate methods and are thus at low risk of bias.
5. Comparability with individual trials
Of the four cluster trials, Gill 2011 had a high risk of bias for comparability with individual trials due to apparent contamination of participants from control to intervention clusters. Gill 2011 uniquely defined a cluster by TBA, versus by administrative units like districts. Intervention group TBA catchment areas often overlapped with control group TBA areas, and intervention TBAs attended significantly larger number of deliveries, a figure which increased over the course of the study. Contamination did not appear to be the case for the other studies. However the use of clusters may facilitate natural diffusion of TBA training messages and foster an environment for changes in community‐level knowledge, attitudes and practices. The use of governmental administration units (e.g., sub‐districts) as clusters could also promote cohesion across health system actors and strengthen referral systems. The multiple components of the TBA intervention in Jokhio 2005 seemed particularly aimed at these goals. The implications of these cluster‐level activities made comparability with individual trials unclear.
C. Overall risk of bias
Allocation concealment is generally not a problem for cluster‐randomised control trials as all clusters are randomised at once. However, some authors did not clearly state details for allocation or randomisation sequence, prohibiting judgement on the expected direction and magnitude of the bias.
Lack of blinding of personnel, participants and outcome assessors was problematic across all studies, largely due to the nature of the intervention. This may have resulted in performance bias and detection bias. However, as most of the performance bias expected is due to TBA and communities’ increased confidence in care provided due to the training intervention, this could arguably be considered a valid component of the intervention effect intended. Additionally, it is unclear that detection bias would have been differential between intervention and control clusters. Furthermore the direction of bias could have favoured the control groups, as better‐trained TBAs in intervention clusters may have been able to more accurately detect maternal deaths. Conversely, knowledge of the intervention may have favoured the intervention group, especially for more subjective secondary outcomes such as maternal morbidity or behaviours. Prospective monitoring of pregnant women to minimise issues of disclosure of deaths by both women and TBAs is recommended, as is supervision of TBAs for data collection and follow‐up.
Overall, incomplete outcome data and selective reporting did not appear to be problematic, neither did loss of clusters or incorrect analysis. The largest threat to comparability with individually randomised trials was concerns of a “herd effect” due to natural diffusion of education amongst mothers within intervention clusters and contamination of control cluster participants to intervention clusters due to knowledge of the intervention (also an issue for recruitment bias). The former would overestimate the effect, while the latter may underestimate the effect.
Baseline imbalance was addressed in some way by all four cluster‐RCTs. However, a small number of clusters or incomplete baseline data proved problematic for two studies. In Jokhio 2005 the direction and magnitude of bias due to baseline differences is unclear, as insufficient data on these differences were collected. In Azad 2010 it is unclear in which direction baseline differences may have influenced the effect size, as it was not clear if the authors measured or controlled for these differences during the second level of randomisation for the TBA intervention.
Misclassification bias for stillbirths versus failed resuscitations was another potential source of bias in studies, which would likely underestimate the effect, as better trained TBAs would be more likely to attempt resuscitation. The magnitude of this bias is not clear.
Effects of interventions
See: Table 1
Trained traditional birth attendants (TBAs) versus untrained TBAs
Primary outcomes: Mortality
Maternal mortality
Jokhio 2005 measured maternal mortality and found 27 deaths in the intervention and 34 deaths in the control clusters, corresponding to a 268 and 360 deaths per 100,000 pregnancies, respectively. This 26% difference in favour of women living in the intervention clusters was non‐significant (0.27% versus 0.36%, adjusted odds ratio (OR) 0.74, 95% confidence interval (CI) 0.45 to 1.22, N = 19,525) (Analysis 1.1).
1.1. Analysis.
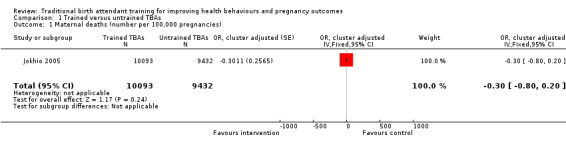
Comparison 1 Trained versus untrained TBAs, Outcome 1 Maternal deaths (number per 100,000 pregnancies).
Stillbirths
Jokhio 2005 identified 483 and 638 stillbirths in intervention and control clusters, respectively. The stillbirth rate difference was significant, 31% lower in intervention compared with control clusters (5.0% versus 7.1%, adjusted OR 0.69, 95% CI 0.57 to 0.83, N = 18,699) (Analysis 1.2).
1.2. Analysis.

Comparison 1 Trained versus untrained TBAs, Outcome 2 Stillbirths (number per 1000 live births and stillbirths).
Neonatal death
Jokhio 2005 identified 340 and 349 neonatal deaths in the intervention and control clusters, respectively. The neonatal death rate difference was significant, 29% lower in the intervention compared with the control clusters (3.5% versus 4.88, adjusted OR 0.71, 95% CI 0.61 to 0.82, P < 0.001, N = 18,699) (Analysis 1.3).
1.3. Analysis.

Comparison 1 Trained versus untrained TBAs, Outcome 3 Neonatal deaths (number per 1000 live births).
Perinatal death
Jokhio 2005 also measured perinatal death among singleton births and found 823 and 1077 deaths in the intervention and control clusters, respectively, corresponding to 85 and 120 deaths per 1000 live births and stillbirths, respectively. The death rate difference was significant, 30% lower in the intervention compared with the control clusters (8.5% versus 12%, adjusted OR 0.70, 95% CI 0.59 to 0.83, N = 18,699) (Analysis 1.4).
1.4. Analysis.

Comparison 1 Trained versus untrained TBAs, Outcome 4 Perinatal deaths (number per 1000 live births and stillbirths).
Secondary outcomes: Morbidity
Obstructed labour
Jokhio 2005 found that the frequency of obstructed labour was significantly higher among women living in intervention clusters compared with women living in control clusters (6% versus 5%, adjusted OR 1.26, 95% CI 1.03 to 1.54, N = 19,525) (Analysis 1.5).
1.5. Analysis.

Comparison 1 Trained versus untrained TBAs, Outcome 5 Prolonged or obstructed labour.
Haemorrhage
Jokhio 2005 found that frequency of haemorrhage (antepartum, intrapartum and postpartum combined) was significantly lower among women living in the intervention clusters, compared with women living in the control clusters (1.7% versus 2.8%, adjusted OR 0.61, 95% CI 0.47 to 0.79, N = 19,525) (Analysis 1.6).
1.6. Analysis.

Comparison 1 Trained versus untrained TBAs, Outcome 6 Postpartum haemorrhage.
Puerperal sepsis
Jokhio 2005 found the frequency of puerperal sepsis was significantly lower among women living in intervention clusters compared with control clusters (0.8% versus 4.2%, adjusted OR 0.17, 95% CI 0.13 to 0.23, N = 19,525) (Analysis 1.7).
1.7. Analysis.

Comparison 1 Trained versus untrained TBAs, Outcome 7 Puerperal sepsis.
Secondary outcomes: Measures of behaviours
Referral
Although referral rates were low in both intervention and control clusters, Jokhio 2005 found that women living in intervention clusters were significantly more likely to have been referred to any health facility for a complication of pregnancy, delivery or postpartum period, than women living in control clusters; 10% versus 7% respectively (adjusted odds ratio (OR) 1.50, 95% CI 1.18 to 1.90, N = 19,525) (Analysis 1.8).
1.8. Analysis.

Comparison 1 Trained versus untrained TBAs, Outcome 8 Referral to emergency obstetrical care.
Additionally trained TBAs versus trained TBAs
Primary outcomes: Mortality
Maternal deaths
Gill 2011 reported two maternal deaths, one in both the intervention and control groups (unadjusted risk ratio (RR) 0.79, 95% CI 0.05 to 12.62, N = 3437 (Analysis 2.1)).
2.1. Analysis.
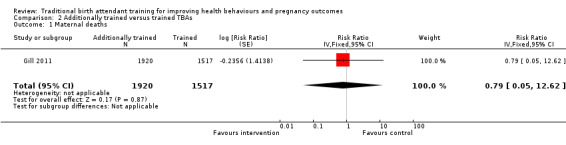
Comparison 2 Additionally trained versus trained TBAs, Outcome 1 Maternal deaths.
Perinatal death
Carlo 2010 identified 306 and 476 perinatal deaths (defined as stillbirths plus neonatal deaths in first seven days) in the intervention and control clusters, respectively. The perinatal death rate difference was not significant as reported by the author (28.5 versus 35.8 deaths per 1000 births, adjusted RR 0.79, 95% CI 0.61 to 1.02, N = 24,097 (Analysis 2.2)).
2.2. Analysis.
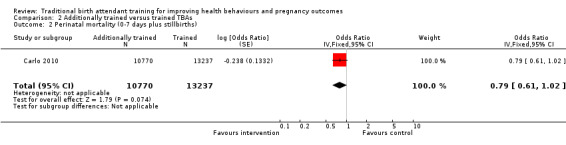
Comparison 2 Additionally trained versus trained TBAs, Outcome 2 Perinatal mortality (0‐7 days plus stillbirths).
Stillbirth
Carlo 2010 also identified 147 and 189 stillbirths in the intervention and control clusters, respectively (13.6 versus 14.2 per 1000 births, adjusted RR 0.96, 95% CI 0.71 to 1.30). Similarly, Gill 2011 identified 38 and 28 stillbirths in the intervention and control clusters, respectively (19.4 versus 18.9 deaths per 1000 births, adjusted RR 1.07, 95% CI 0.64 to 1.77). The overall weighted mean effect estimate was close to 1.0 and not statistically significant (adjusted RR 0.99, 95% CI 0.76 to 1.28, N = 27,594) (Analysis 2.3; Figure 3).
2.3. Analysis.
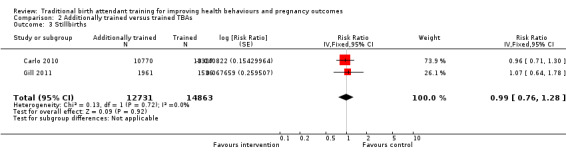
Comparison 2 Additionally trained versus trained TBAs, Outcome 3 Stillbirths.
3.
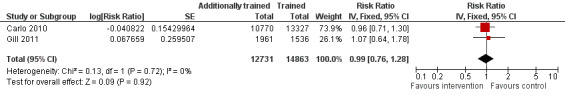
Forest plot of comparison: 2 Additional training versus basic training, outcome: 2.4 Stillbirths.
Early neonatal death
Three of the new studies included in this update measured early neonatal deaths (defined as deaths within seven days of birth, excluding stillbirths). Azad 2010 found 25.4 versus 26.5 deaths per 1000 live births in the intervention and control clusters (adjusted RR 0.95, 95% CI 0.75 to 1.21). Carlo 2010 identified 15.0 versus 21.9 deaths per 1000 births in the intervention and control clusters, respectively (adjusted RR 0.68, 95% CI 0.45 to 1.03). Gill 2011 identified 18.2 versus 30.5 deaths per 1000 births in the intervention and control clusters, respectively (adjusted RR 0.56, 95% CI 0.31 to 1.01). The overall weighted mean effect estimate for the three studies approached but was not statistically significant (adjusted RR 0.83, 95% CI 0.68 to 1.01, N = 37,494 (Analysis 2.4; Figure 4)).
2.4. Analysis.
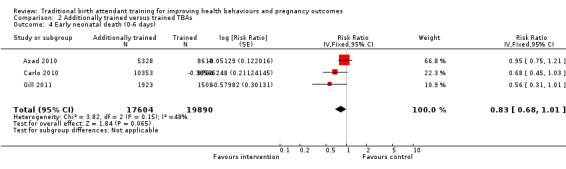
Comparison 2 Additionally trained versus trained TBAs, Outcome 4 Early neonatal death (0‐6 days).
4.
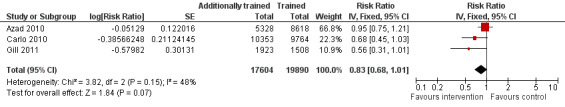
Forest plot of comparison: 2 Additional training versus basic training, outcome: 2.5 Early neonatal death (0‐6 days).
Neonatal death
Gill 2011 measured neonatal death (defined as death during the first 28 days, excluding stillbirths), identifying 43 and 59 deaths in the intervention and control clusters, respectively. The neonatal death rate difference was significant, 45% lower in the intervention compared with the control clusters (adjusted RR 0.54, 95% CI 0.32 to 0.92, N = 3355 (Analysis 2.5)). When stillbirths were included in the analysis, the rate difference decreased but was no longer significant (adjusted RR 0.72, 95% CI 0.51 to 1.01, N = 3421 (Analysis 2.6)).
2.5. Analysis.
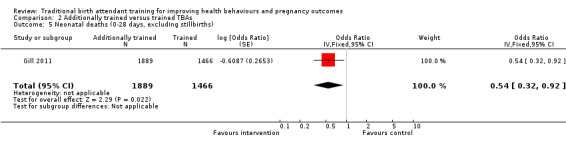
Comparison 2 Additionally trained versus trained TBAs, Outcome 5 Neonatal deaths (0‐28 days, excluding stillbirths).
2.6. Analysis.
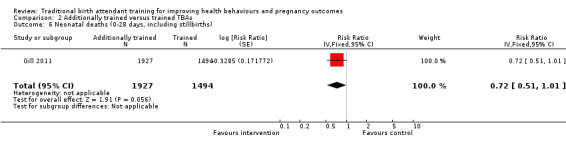
Comparison 2 Additionally trained versus trained TBAs, Outcome 6 Neonatal deaths (0‐28 days, including stillbirths).
Late neonatal death
Gill 2011 measured late neonatal death (defined as death from two to four weeks after birth) identifying eight and 13 deaths in the intervention and control clusters, respectively. The late neonatal death rate difference was not significant (adjusted OR 0.47, 95% CI 0.20 to 1.11, N = 3274 (Analysis 2.7)). This study also reported cause‐specific neonatal death; this was not one of our prespecified outcomes and we have set out findings as reported by the trialists in the Notes section of the Characteristics of included studies table.
2.7. Analysis.
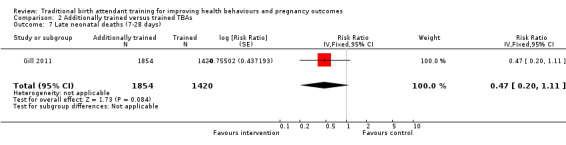
Comparison 2 Additionally trained versus trained TBAs, Outcome 7 Late neonatal deaths (7‐28 days).
Neonatal death within 24 hours of life
Carlo 2010 also measured neonatal death within 24 hours of birth and found 5.5 versus 7.3 deaths per 1000 live births in the intervention and control clusters respectively (adjusted RR 0.75, 95% CI 0.44 to 1.27, N = 24,097 (Analysis 2.8)).
2.8. Analysis.
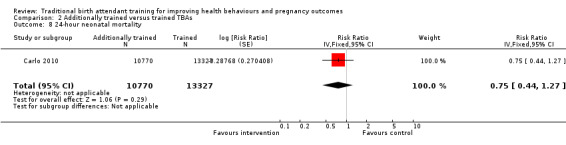
Comparison 2 Additionally trained versus trained TBAs, Outcome 8 24‐hour neonatal mortality.
Secondary outcomes: Morbidity
Postpartum haemorrhage
Bullough 1989 demonstrated a non‐significant difference in the frequency of postpartum haemorrhage among women cared for by additionally trained TBAs versus trained TBAs (7.9% versus 8.4%, OR 0.94, 95% CI 0.76 to 1.17, N = 4227 (Analysis 2.9)). (In this study postpartum haemorrhage was defined as blood loss greater than 500 ml during the third stage of labour and within the first 24 hours after delivery.)
2.9. Analysis.
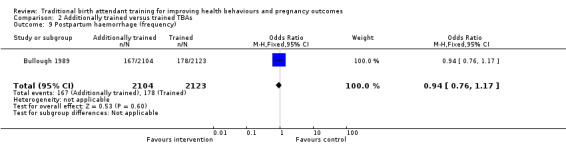
Comparison 2 Additionally trained versus trained TBAs, Outcome 9 Postpartum haemorrhage (frequency).
Blood loss
Bullough 1989 demonstrated a non‐significant difference in mean blood loss among women cared for by additionally trained TBAs versus trained TBAs (weighted mean difference 2.00 ml, 95% CI ‐7.39 to 11.39, N = 4227 (Analysis 2.10)).
2.10. Analysis.
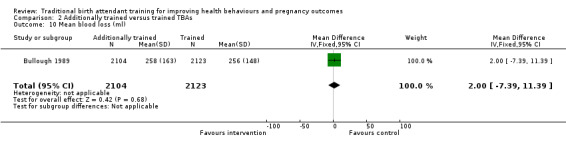
Comparison 2 Additionally trained versus trained TBAs, Outcome 10 Mean blood loss (ml).
Secondary outcomes: Measures of behaviours
Advice about immediate feeding of colostrum
Hossain 2000. Our analysis shows that the effect size estimates of the difference in additionally trained versus trained TBAs at post‐test for three months (OR 1.21, 95% CI 0.39 to 3.79, N = 162) and at seven months follow‐up (OR 1.37, 95% CI 0.62 to 3.03, N = 165) were not significant (Analysis 2.11).
2.11. Analysis.
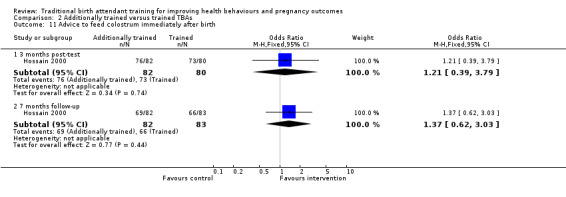
Comparison 2 Additionally trained versus trained TBAs, Outcome 11 Advice to feed colostrum immediately after birth.
Advice about introduction of complementary foods
Hossain 2000. Our analysis of these data demonstrates that the effect size estimates of the post‐test difference for additionally trained TBAs versus trained TBAs at both three months (OR 3.11, 95% CI 1.63 to 5.92, N = 162) and seven months follow‐up (OR 2.07, 95% CI 1.10 to 3.90, N = 165) favour the intervention group (Analysis 2.12).
2.12. Analysis.
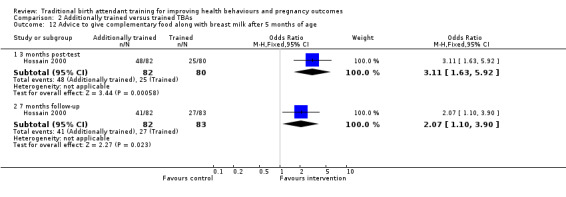
Comparison 2 Additionally trained versus trained TBAs, Outcome 12 Advice to give complementary food along with breast milk after 5 months of age.
Exclusively breastfeeding post delivery
Gill 2011 found similar rates of breastfeeding between women delivered by additionally trained TBAs (96.8%) versus trained TBAs (97.1%) (unadjusted RR 1.01, 95% CI 1.00 to 1.01, N = 3437) (Analysis 2.13).
2.13. Analysis.
Comparison 2 Additionally trained versus trained TBAs, Outcome 13 Exclusively breastfeeding at 1‐4 weeks.
Subgroup analyses
The number of studies was too small to conduct subgroup analyses.
Sensitivity analyses
We performed a sensitivity analysis for early neonatal death by removing each study and recalculating the effect size. With Azad 2010 removed, the effect estimate for early neonatal death is larger and significant (adjusted RR 0.64, 95% CI 0.45 to 0.90). Removal of either of the other two studies (Carlo 2010 or Gill 2011) did not change the original results.
Discussion
Summary of main results
Six studies involving over 1345 traditional birth attendants (TBAs), more than 32,000 women and approximately 57,000 births that examined the effects of TBA training for trained versus untrained TBAs (one study) and additionally trained TBA training versus trained TBAs (five studies) are included in this review. These studies consist of individual randomised trials (two studies) and cluster‐randomised trials (four studies). The quality of the evidence in the included studies was mixed, several studies had problems with attrition and none of the studies was blinded; for outcomes that were self reported by women and for clinical outcomes recorded by TBAs the potential impact of bias is difficult to determine but results should be interpreted with caution. The primary outcomes across the sample of studies were perinatal deaths, stillbirths and neonatal deaths (early, late and overall). The strengths of the three new studies included in this update are their design (community cluster‐randomised trials), size (large scale) and common focus (newborn care and peri‐neonatal outcomes). This allows us to begin to build the evidence needed to evaluate TBA training effectiveness in relation to peri‐neonatal mortality, which we were unable to do in previous versions of this review (2007 and 2009). The main results are summarised below and focus on primary outcomes.
Trained TBAs versus untrained TBAs
One cluster‐randomised trial found a significantly 30% lower perinatal death rate, 39% lower stillbirth rate and 29% lower neonatal death rate in the trained versus untrained TBA cluster.
Additionally trained TBAs versus trained TBAs
Three large cluster‐randomised trials compared TBAs who received additional training in initial steps of resuscitation, including bag‐valve‐mask ventilation, with TBAs who had received basic training in safe, clean delivery and immediate newborn care. Basic training included mouth‐to‐mouth resuscitation (two studies) or bag‐valve‐mask resuscitation (one study). There was no significant perinatal death rate difference in the intervention and control clusters and no significant late neonatal death rate difference in intervention and control clusters. The neonatal death rate, however, was 45% lower in the intervention compared with the control clusters.
A meta‐analysis conducted on two outcomes showed no significant differences between additionally trained TBAs versus trained TBAs for stillbirths (two studies) or early neonatal death rate (three studies). In the studies that examined stillbirths, all intervention and control cluster TBAs were trained in neonatal resuscitation, which may have resulted in apparent stillborns being resuscitated (and a reduction in births classified as stillbirths in both groups). Inadequate contrast between intervention and control TBAs’ training may also explain the null result. In the three studies that reported early neonatal death rates (Azad 2010; Carlo 2010; Gill 2011), reduction in the early neonatal death rate appears to favour additional TBA training in bag‐valve‐mask ventilation over basic TBA training, including mouth‐to‐mouth ventilation, but the differences were not significant. Note that Azad 2010 is heavily influencing the overall mean effect for early neonatal death rate and accounts for two‐thirds of the weight in the weighted effect estimate, primarily due to a small standard error. As previously noted, a sensitivity analysis with this study removed reveals an effect estimate for early neonatal death that is larger and significant (adjusted risk ratio (RR) 0.64, 95% confidence interval (CI) 0.45 to 0.90). Removal of either of the other two studies does not change the original results.
Overall completeness and applicability of evidence
All studies included the appropriate types of participants, interventions and outcomes. However, most of the authors did not provide sufficient descriptions of the TBA training, TBA supervision and follow‐up support (apart from data collection follow‐up), or comparable information on the characteristics of the TBAs and women (seeTable 2; Table 3; Table 4). While the number of studies in this updated review was too small to conduct subgroup analyses, this information would be useful to better understand the relationship between the intervention and results going forward and may have relevance for policy and practice.
The evidence in the review addressed the broad question about the effect of TBA training on health behaviours and pregnancy outcomes. It is based on findings from six studies from rural populations in sub‐Saharan Africa (Republic of Congo, Zambia) and South Asia (Bangladesh, India, Pakistan). These are high mortality regions and/or countries where TBAs continue to assist women and families during labour, birth and the early postnatal period and access to skilled care is still limited. The findings are applicable to ongoing international policy discussions on how to best optimise training and deployment of different kinds of health workers (including lay health workers such as TBAs) for maternal and newborn health during the slow but gradual transition to skilled birth attendants.
There was very little information in the included studies on the identification of, and referral for, obstetric complications by TBAs. In earlier versions of this review, given the overall paucity of evidence from randomised trials we included findings from a controlled before and after study examining additional training for TBAs (O'Rourke 1994). This study reported findings relating to the identification and referral for malpresentation, prolonged labour and preterm birth before and after a training intervention for TBAs. Overall, the mean number of monthly referrals over the study period showed non‐significant differences between intervention and control communities both during and after the intervention and baseline to post intervention identification of and referral for obstetric complications of malpresentation, prolonged labour and preterm labour among women referred by TBAs showed no significant difference for timely referral between the intervention and control communities.
Quality of the evidence
Assessment of risk of bias in these studies raises concerns about the quality of evidence. Among individually randomised trials, risk of selection bias (sequence generation and allocation concealment) was high in Bullough 1989 and unclear in Hossain 2000; risks of performance and detection bias (blinding) and incomplete outcome data (attrition) were high in Bullough 1989 and unclear in Hossain 2000; risk of incomplete outcome data was high in Bullough 1989. Both studies reported all relevant outcomes.
Among cluster‐randomised trials, risk of recruitment bias was high in Azad 2010, and unclear in Gill 2011 and Jokhio 2005. Risk of baseline imbalance was unclear in Azad 2010 and Jokhio 2005. All authors reported minimal loss of clusters and individuals and conducted the appropriate statistical analyses, adjusting for design effect and baseline covariates. Moreover, all reported relevant outcome measures. None of the studies used blinding and thus were at risk for performance and detection bias. Although many of the primary and secondary outcomes were objective (mortality), the likely direction and magnitude of performance and detection biases on outcomes are uncertain (Figure 1; Figure 2).
For the studies included in the meta‐analysis of stillbirths (Carlo 2010; Gill 2011) and early neonatal deaths (Azad 2010; Carlo 2010; Gill 2011), most of the information was of low or unclear risk of bias.
A major limitation of this review is that most of the outcomes were reported in only one study. This could lead to a bias in interpretation of the results for these outcomes.
Potential biases in the review process
We used a number of methods to mitigate potential bias in the review process. We searched both the Cochrane Pregnancy and Childbirth Group's Trials Register and Cochrane Effective Practice and Organisation of Care Group (EPOC) Trials Register as well as citation alert, and conducted handsearches of the references of included studies. Two of the review authors independently extracted the data and four review authors assessed the included studies for risk of bias and reached consensus through virtual discussion (one external assessor). The analysis was conducted according to Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Among the studies in the review, one study included TBA training as a second intervention (Azad 2010) and the other included TBAs as a subgroup of birth attendants (Carlo 2010). The amount of information about TBAs in these studies was limited to outcomes. We sought, but were unable to obtain additional information about TBA training intervention and results from Azad 2010.
Agreements and disagreements with other studies or reviews
In this review, the results of the meta‐analysis revealed no substantial impact of TBA training on stillbirths and a promising 17% decrease in early neonatal mortality in the intervention clusters. A 2009 systematic review by Darmstadt and colleagues (Darmstadt 2009) of the evidence of the ability of community‐level providers to reduce intrapartum‐related deaths found low to moderate quality evidence that TBA training may improve linkages with facilities and perinatal outcomes. Their review was based on the Jokhio 2005 study that is included in this update review, and our own earlier meta‐analysis of TBA training effectiveness which demonstrated an 11% reduction in perinatal mortality (Sibley 2004c). More recently, Lassi 2010 reviewed 18 cluster‐randomised and quasi‐randomised trials and found significant reductions maternal morbidity (25%), stillbirths (16%), perinatal mortality (20%) and neonatal mortality (24%), along with increases in referral for complications (40%) and early breastfeeding (94%) as a consequence of implementing a broad range of community‐level maternal and newborn care packages, many of which included a relationship with TBAs. As Osrin and Prost (Osrin 2010) note: “TBAs have been largely abandoned as a cadre who could take useful health actions to reduce PMR. This abandonment has been at the level of international policy rather than ground reality.” This ground reality and the two systematic reviews (Darmstadt 2009; Lassi 2010) summarise a growing body of evidence for scaling‐up community‐based maternal and newborn care through packages that can be implemented by a range of community‐level health workers.
Authors' conclusions
Implications for practice.
Rural settings in the developing world vary in terms of the role of traditional birth attendants (TBAs), the proportion of TBA‐attended births, cultural norms and values regarding childbirth and childcare practices, general health of young girls and women, local causes of maternal and perinatal mortality and morbidity, and the social standing, functional status and resources of the health services. The relationships among poverty, women's health status and access to quality health care are especially important. Each is relevant to any discussion and practical decision regarding the feasibility and potential of TBA training to contribute to improved pregnancy outcomes in a given setting. Thus, in settings where there is an insufficient number of skilled birth attendants or limited access to health facilities, and women prefer TBAs, TBA training may be the only means to optimise the use of community‐level heath workers for maternal and newborn health. Where skilled birth attendants and health facilities exist and are accessible, and women prefer TBAs, TBA training coupled with strategies to effectively engage them with the health system may be considered. In this update review, the only study of trained TBAs versus untrained TBAs suggests that biomedical training of TBAs who have not been exposed to such training may be beneficial. The remaining five studies comparing additionally trained TBAs and trained TBAs show no clear advantage of additional or advanced training. However, this finding may be due to an insufficient number of studies and the methodological issues in the included studies (risk of bias, lack of contrast in the intervention between the comparison groups).
Implications for research.
This updated review contains four large cluster‐randomised trials. The small number of studies continues to reflect, in part, the challenges associated with conducting large community trials on the outcomes of complex public health interventions.
This review, nonetheless, provides the foundation upon which to incorporate future studies of the effect of TBA training for improved health behaviours and pregnancy outcomes that is necessary for building the evidence base. The design and methods of future studies must include adequate sample size, protection against common sources of bias, sufficient contrast between intervention and control groups to adequately assess the effect of TBA training, and, most importantly, report similar perinatal and neonatal mortality definitions and outcomes. A focus on TBA training in relation to reducing perinatal and neonatal death, the potential mechanisms involved in reducing deaths, strategies that link TBAs to health systems, and costs continue to hold the most promise. Future studies should include also at least the following information on participants, the intervention and outcomes, to permit subgroup analyses:
TBA age, socioeconomic status, educational attainment, experience (number of deliveries per year and number of years of practice), and proportion of all births attended in the study area;
maternal age, parity, socioeconomic status and educational attainment;
training method, content, duration, contact hours, trainer/trainee ratio, supportive supervision and education after training, boosters and context, e.g., whether training is a single invention or part of complex intervention, whether it is situated within an enabling environment that includes elements such as advocacy, community mobilisation, emergency transportation or adequate accessible referral sites; and
timing of measurement (observations) relative to the intervention, as well as data collection method and source.
There is also need for research that seeks to improve family and community‐based interventions, in relation to TBA training, and the links to skilled facility‐based clinical care to reduce maternal and neonatal mortality and morbidity. Key knowledge gaps continue to exist in these regards. Examples include:
more effective approaches for promotion of care seeking for life‐threatening complications by improving problem recognition, increasing demand for quality care, and overcoming cultural barriers to referral;
methods to improve prevention or initial home‐based management (first aid stabilisation), or both, and safe referral care;
best combinations of frontline health workers for family and community service delivery and the phasing of their roles or skills during the transition to skilled birth attendance;
strategies to optimise the deployment of frontline health workers and to improve collaboration and partnership among frontline health workers and the health system; and
better ways to monitor and evaluate health outcomes of community‐based interventions, including vital registration, community‐based surveillance, improved verbal autopsy methods to assess specific medical causes and contributing factors to maternal and neonatal mortality and morbidity.
This updated review suggests that trained TBAs may be viable health workers in community‐level intervention packages, particularly given their role during the vulnerable period in and around the time of birth. However, the evidence in the updated review remains inconclusive with respect to peri‐neonatal mortality reduction due to an insufficient number of studies and the methodological issues of the included studies.
Feedback
Abba, 14 April 2008
Summary
This review was selected for presentation at one of our regular journal clubs. A number of comments arose from our discussions, and we thought we would like to share them with you.
Title and inclusion criteria
The title of the review and inclusion criteria do not best reflect the scope of this review, which is not simply training for traditional birth attendants, but also more complex intervention packages. The more complex interventions include improvements to facility based care and community interventions, supply of materials, liaison with the formal health sector, and supervision. We suggest the title refers to ‘traditional birth attendants programmes’ or ‘traditional birth attendant training and support programmes’.
Background
It would be useful to include a short general description of the varied social contexts in which traditional birth attendants work, how their relationships with formal health services were initiated and developed, and whose agenda the relationships were fulfilling. Also, it would be helpful to mention the strategy of including traditional birth attendants in biomedically orientated training and service improvement programmes.
Description of included studies
The interventions are complex and contextually bound, so it is important to describe the context and intervention used in each study as fully as possible. Also, when evaluating such a complex intervention, it is important to take account of the content and delivery of the training provided, if these are available. The authors describe the review as a ‘systematic narrative review’. In which case more detail is required, in the included studies table and the additional tables, on the components of the training programmes/ interventions, including any improvements to the health system. In the case of the training for example, where did the training take place? How long was the training? What was the mix of theory and practice? What pedagogic approach was used? Were the trainers trained themselves to teach?
Conclusions
One large study showed significant reductions in stillbirths, perinatal and neonatal deaths, and serious maternal complications, plus a reduction in maternal mortality that, while not significant, at 26% is certainly encouraging. This suggests that traditional birth attendant training programmes, in conjunction with improved health systems, have the potential to significantly improve outcome for mothers and babies. It is worth looking more closely into how this programme achieved such remarkable results. It suggests that some further research should concentrate on evaluating whether this model of provision might be effective in other contexts, and even how it might be improved on.
(Summary of feedback from Katharine Abba, on behalf of the International Health Group Journal Club at the Liverpool School of Tropical Medicine, April 2008)
Reply
In response, to each section of the feedback:
Title and inclusion criteria
The inclusion criteria state that the intervention is traditional birth attendant training. Like most interventions, traditional birth attendant training is nested within health systems and settings with a variety of interrelated components. Only two of the four included studies described traditional birth attendant training as a component of a more complex program or health service (Jokhio 2005 and O'Rourke 1994a). For this reason, we do not agree that changing the title of the review would improve the fit between title and scope of review; in fact it might be misleading. In the review we do discuss the challenge of evaluating complex program interventions. Training is a key activity in any traditional birth attendant program. Adding the term ‘program’, and changing the title to read ‘Traditional birth attendant training programs for improving health behaviours and pregnancy outcomes’ might be appropriate, though we are not sure this is an improvement.
Background
The background does describe the typical work arrangements, payment agreements and settings for traditional birth attendants. We are not sure what additional information is being requested, and would welcome specific examples. We agree traditional birth attendant training reflects a biomedical agenda. We do not address the implications of this for traditional birth attendant training because we did not see the review as the appropriate place for such a discussion.
Description of included studies
We agree with the feedback. In the Criteria for considering studies for this review we state that where available we provide information on characteristics of participants and of the intervention. Information in the main body of the review, in the included studies table, and in the additional tables reflects what was reported for each included studies. Where information is missing it is because it was not reported. The lack of sufficient descriptive information about participants and the intervention is a major limitation of most reports. In the conclusions to the review we urge future trials to provide complete descriptions. For future updates we will contact trial authors to ask for this additional information.
We used the term ‘systematic narrative review’ because we were unable to pool the study findings in a quantitative synthesis due to heterogeneity in the outcomes reported.
Conclusions
We agree with the feedback, and state this in the conclusion. We note that potential mechanisms underlying these promising effects were not addressed in this trial, and that another trial suggests appropriate referral to improved health services is a key factor. In implications for research, we will add the phrase ‘further research to examine the mechanisms underlying the effects of this promising program is warranted’.
(Response from Lynn Sibley, July 2008)
Contributors
Lynn Sibley
What's new
| Date | Event | Description |
|---|---|---|
| 18 June 2012 | New citation required but conclusions have not changed | Review updated. |
| 18 June 2012 | New search has been performed | Search updated. Three cluster‐randomised trials have been included (Azad 2010; Carlo 2010; Gill 2011). Meta‐analysis performed for two outcomes: stillbirths and early neonatal deaths. Nine new trials have been excluded (Ajenifuja 2010; Bhandari 2003; Bhutta 2011; Ellis 2011; Falle 2009; Olusanya 2011; Rowen 2009; Saravanan 2011; Satishchandra 2009). In addition we have excluded a non‐randomised study which was included in previous versions of the review (O'Rourke 1994). This review is now comprised of six included studies and 24 excluded studies. There are four trial reports in 'Studies awaiting classification' (Costello 2011; Matendo 2011; Morrison 2011; Pasha 2010). |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | No changes ‐ republished to fix technical problem. |
| 30 June 2008 | New search has been performed | Search updated. No new trial reports identified. |
| 24 June 2008 | Feedback has been incorporated | Feedback and reply from author added. |
| 19 June 2008 | Amended | Converted to new review format. Added sentence to 'Implications for research' in response to feedback. |
Acknowledgements
We thank Sonja Henderson and Lynn Hampson (Cochrane Childbirth and Pregnancy Group), and Alain Mayhew, Jessie McGowan and Michelle Fiander (Cochrane Effective Practice and Organisation of Care Group) for their encouragement and helpful advice over the course of this review; and Therese Dowswell for her independent 'Risk of bias' assessments for the four cluster‐randomised trials included in this 2012 update review. We also thank Carolyn Brown, Melissa Diallo, Kathryn McNatt and Nancy Habarta for their substantial contribution as co‐authors of the original 2007 review.
Appendices
Appendix 1. Supplemental search
Supplemental search Cochrane Effective Practice and Organisation of Care (EPOC) Trial's Register
The EPOC register is based upon retrospective and prospective sensitive searches of key bibliographic databases (including MEDLINE and CINAHL), handsearching of key journals and reference lists of published literature reviews (see 'Search strategies for the identification of studies' section within the editorial information about EPOC (Cochrane Effective Practice and Organisation of Care). (Last searched May 2011)
The supplemental search also included the following electronic databases representing the fields of education, social and health sciences:
African Index Medicus;
Anthropological PLUS (Anthropological Literature and Anthropological Index);
BIOSIS Previews;
Cambridge Scientific Biological Sciences Set;
CINAHL;
Current Contents Connect;
Database of Abstracts of Reviews of Effectiveness (DARE);
Dissertation Abstracts;
EMBASE;
Education Abstracts;
ERIC;
History of Science and Technology;
LILACS;
MEDLINE;
OCLC First Search Contents First;
PAIS International (Public Affaires Information Service);
POPLINE;
RLIN Bibliographic File;
Sociological Abstracts; and
Web of Science.
These databases were searched from inception to June 2006 using the search strategy (modified for each database, as necessary) in Appendix 2.
We also contacted agencies involved in global reproductive health for unpublished literature. We retrieved and screened the references of published studies for additional potentially relevant documents, and retrieved copies of the published articles. We searched the Internet for reports of training projects implemented and evaluated by international organisations, United Nations organisations such as United Nations Fund for Population Activities and the World Health Organization, as well as other non‐governmental organisations engaged in community‐based work such as CARE, Save the Children Foundation and World Vision. Finally, we contacted experts in the field for information and collection of unpublished material.
Appendix 2. Search strategy
TRADITIONAL BIRTH ATTENDANT*
TBA*
TRADITIONAL MIDWIFE OR TRADITIONAL MIDWIVES
LAY MIDWIFE/
LAY MIDWIFE OR LAY MIDWIVES
TRADITIONAL FAMILY BIRTH ATTENDANT*
TRADITIONAL HOME BIRTH ATTENDANT*
FAMILY BIRTH ATTENDANT*
OR/1‐8
TRAIN* or TEACH or EDUCAT* or INSTRUCT*
EVALUAT*
COMPAR*
EFFECT*
IMPACT*
OUTCOME*
PERFORM*
KNOWLEDGE/ATTITUDES AND PRACTICE/
TEACHING/
OR/10‐18
9 AND 19
Note: * by the term denotes truncating the term and searching it as a text word / at the end of a search statement means subject heading Search statement with nothing at the end means text word search
Appendix 3. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Bullough 1989; Hossain 2000; Jokhio 2005 and O'Rourke 1994.
Selection of studies
We assessed potentially eligible studies for inclusion against the following criteria: (a) research design is a randomised controlled trial, a time series study or a controlled before/after study; (b) intervention is traditional birth attendant (TBA) training; (c) intervention and comparison group data are derived from trained and untrained TBAs (reference to target intervention), or mothers and neonates whose care is provided by trained and untrained TBAs, or who are living in areas where trained and untrained TBAs attend a majority of births; and (d) dependent measures are categorical or continuous and concern TBA and maternal behaviours listed above or life‐threatening conditions listed above and defined using standard case definitions, and pregnancy outcomes.
We calculated inter‐coder reliability for eligibility using percentage agreement, resolved any differences by discussion and noted the reasons for excluding a particular study. We entered all studies into the Review Manager software (RevMan 2003). Details of the search procedure are provided in the 'Description of studies' section.
Data extraction and management
We used the Effective Practice and Organisation of Care (EPOC) Data Collection Checklist and Data Collection Template, as well as developed and pretested supplementary data collection forms such that we abstracted additional information pertaining to study references and authors, verification of eligibility, as well as study methods, participants, interventions, outcome measures and results. Three co‐authors independently extracted and coded the data, discussed and resolved any differences. A fourth author independently checked a portion of the abstraction. We contacted an investigator for two studies having incomplete information. In some cases the authors provided enough information for us to calculate the missing outcome data using EXCEL software and entered data into the Review Manager software for analysis (RevMan 2003).
Assessment of methodological quality of included studies
We assessed studies for methodological quality examining different sources of bias following criteria established by the Cochrane EPOC Group (www.epoc.uottawa.ca) and summarising risk of bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). The Cochrane EPOC Group's standard criteria for appraising the quality of studies include baseline measurement of outcomes, follow‐up of professionals, follow‐up of patients/episodes of care (protection against exclusion bias), blinded assessment of primary outcomes (protection against detection bias), reliable outcome measures and protection against contamination. An additional criterion for randomised controlled trials and controlled clinical trials study includes concealment of allocation, while the analogous criterion for controlled before‐after studies includes characteristics for studies using second site as control (protection against selection bias). To derive an overall summary assessment of how valid the results of each study are, we used three categories in which a 'low risk' of bias exists when all criteria are met (plausible bias unlikely to seriously alter results); a 'moderate risk' of bias exists when one or more criteria are partially met (plausible bias raises some doubt about results); and a 'high risk' of bias exists when one or more criteria are not met (plausible bias seriously weakens confidence results) (Higgins 2005).
Measures of treatment effect and unit of analysis issues
We used RevMan 2003 to calculate individual effect sizes of odds ratio and 95% confidence intervals or mean difference for the individual outcomes when possible. We used descriptive statistics to report characteristics of the participants, interventions and outcome measures detailed above. Due to the small number of studies, varied study designs and heterogeneity of the outcomes and measures, we were not able to calculate pooled summary effect sizes.
Data and analyses
Comparison 1. Trained versus untrained TBAs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal deaths (number per 100,000 pregnancies) | 1 | 19525 | OR, cluster adjusted (Fixed, 95% CI) | ‐0.30 [‐0.80, 0.20] |
| 2 Stillbirths (number per 1000 live births and stillbirths) | 1 | 18699 | OR, cluster adjusted (Fixed, 95% CI) | 0.69 [0.57, 0.83] |
| 3 Neonatal deaths (number per 1000 live births) | 1 | 18699 | OR, cluster adjusted (Fixed, 95% CI) | 0.71 [0.61, 0.82] |
| 4 Perinatal deaths (number per 1000 live births and stillbirths) | 1 | 18699 | OR, cluster adjusted (Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 5 Prolonged or obstructed labour | 1 | 19525 | OR, cluster adjusted (Fixed, 95% CI) | 1.26 [1.03, 1.54] |
| 6 Postpartum haemorrhage | 1 | 19525 | OR, cluster adjusted (Fixed, 95% CI) | 0.61 [0.47, 0.79] |
| 7 Puerperal sepsis | 1 | 19525 | OR, cluster adjusted (Fixed, 95% CI) | 0.17 [0.13, 0.23] |
| 8 Referral to emergency obstetrical care | 1 | 19525 | OR, cluster adjusted (Fixed, 95% CI) | 1.50 [1.18, 1.90] |
Comparison 2. Additionally trained versus trained TBAs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal deaths | 1 | 3437 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.05, 12.62] |
| 2 Perinatal mortality (0‐7 days plus stillbirths) | 1 | 24007 | Odds Ratio (Fixed, 95% CI) | 0.79 [0.61, 1.02] |
| 3 Stillbirths | 2 | 27594 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.76, 1.28] |
| 4 Early neonatal death (0‐6 days) | 3 | 37494 | Risk Ratio (Fixed, 95% CI) | 0.83 [0.68, 1.01] |
| 5 Neonatal deaths (0‐28 days, excluding stillbirths) | 1 | 3355 | Odds Ratio (Fixed, 95% CI) | 0.54 [0.32, 0.92] |
| 6 Neonatal deaths (0‐28 days, including stillbirths) | 1 | 3421 | Risk Ratio (Fixed, 95% CI) | 0.72 [0.51, 1.01] |
| 7 Late neonatal deaths (7‐28 days) | 1 | 3274 | Risk Ratio (Fixed, 95% CI) | 0.47 [0.20, 1.11] |
| 8 24‐hour neonatal mortality | 1 | 24097 | Risk Ratio (Fixed, 95% CI) | 0.75 [0.44, 1.27] |
| 9 Postpartum haemorrhage (frequency) | 1 | 4227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.76, 1.17] |
| 10 Mean blood loss (ml) | 1 | 4227 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐7.39, 11.39] |
| 11 Advice to feed colostrum immediately after birth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 3 months post‐test | 1 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.39, 3.79] |
| 11.2 7 months follow‐up | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.62, 3.03] |
| 12 Advice to give complementary food along with breast milk after 5 months of age | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 3 months post‐test | 1 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.11 [1.63, 5.92] |
| 12.2 7 months follow‐up | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.10, 3.90] |
| 13 Exclusively breastfeeding at 1‐4 weeks | 1 | 3437 | Risk Ratio (Fixed, 95% CI) | 1.01 [1.00, 1.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azad 2010.
| Methods | Cluster‐randomised controlled trial, factorial design (2 interventions: women's groups and TBA training), 18 clusters total. | |
| Participants | Bangladesh, 3 rural districts, 6 unions per district, 18 unions (lowest‐level administrative unit in rural Bangladesh), pop. 15,441 to 35,110 each. Women’s groups and TBAs, and pregnant women living in the clusters served by them. Women’s groups: 18 groups (1 per cluster) comprised of women of reproductive age, mothers‐in‐law, adolescents, others. TBAs: 482 TBAs in the combined intervention and control clusters. TBAs in intervention clusters attended 12,519 home births (8618 attended by any TBA and 2792 attended by TBA trained in bag‐valve‐mask resuscitation). TBAs in control clusters attended 13,195 home births (9171 attended by any TBA and 2536 attended by TBA trained in bag‐valve‐mask resuscitation). |
|
| Interventions | For the TBA intervention only: All TBAs received basic training in clean safe delivery, danger sign recognition, emergency preparedness and mouth‐to‐mouth resuscitation. Intervention cluster TBAs additionally received training in bag‐valve‐mask resuscitation. Control cluster TBAs did not training in bag‐valve‐mask resuscitation. Intervention was based on implementation of clinical practice guidelines. |
|
| Outcomes | Unit of analysis: unions, births to women living in study unions. Primary outcome women’s group intervention: neonatal mortality rate (within 28 completed days of birth). Pre‐specified secondary outcomes: maternal deaths, stillbirths, up‐take of antenatal and delivery services, home care practices, care seeking, perinatal mortality and early neonatal mortality. Primary outcome TBA intervention: early neonatal mortality rate (within 6 completed days of birth). |
|
| Notes | Only outcomes for the TBA intervention are included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment done before data collection and analysis of baseline data. “Unions were randomly allocated to either intervention or control groups by district in the presence of four project staff (including the project director and project manager) and two external individuals… For each district, cluster names were written on pieces of paper, which were folded and placed in a bottle. The first three cluster names drawn from the bottle were allocated to the women’s group intervention and the remaining three to control. The project manager drew the papers from the bottle. The allocation sequence was decided upon by the project team before drawing the papers and was based on clusters rather than individuals.” This method was repeated for a second‐level of randomisation. |
| Allocation concealment (selection bias) | Low risk | None of the staff attending the randomisation process had any previous knowledge of demographic and health status of the chosen clusters. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participant and clinician blinding is not possible in this study design; “Neither the study investigators nor the participants were masked to group allocation.” It is plausible that TBAs trained in bag‐valve‐mask resuscitation had greater confidence in providing all other services they received training on, and/or that the presence of this equipment instilled greater confidence from the community in the TBA’s skills, and in their subsequent adherence to TBA recommendations. This may have resulted performance bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the study investigators nor the participants were masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was performed by the key informants, i.e., TBAs (death outcome identification for all births, regardless if the TBA was the attendant), and verified by interviewers within 6 weeks of delivery. It is not explicitly stated whether these interviewers were blinded. TBAs were incentivised (60 Taka) for accurate birth/death identification, which may have helped minimise risk of detection bias. It is unclear, however, if this would have been a sufficient incentive to counter perhaps greater disincentives such as jeopardised reputations for being associated with a newborn death. This disincentive may have been more acute since verbal autopsies were performed with mothers for each stillbirth or neonatal death, which could have further implicated the TBA or further jeopardised her reputation. Primary outcome measures (mortality) were objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although the authors removed data for some analyses, the data used for this review had little/no attrition. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcome results were reported. The authors were contacted for other papers that may report intervention interaction effects and the remaining TBA intervention outcomes. |
| Other bias | Unclear risk | This study was not stopped early. Secondary outcome data by randomisation group (for TBAs) were not reported in this study. Randomisation may have been compromised due to the purposive selection of districts and upazilas, the stratification of sampling, as well as the restricted number of clusters. While the women’s group intervention did not show statistical interaction with the TBA intervention, confounding by the women’s group intervention could be present as differences in climactic conditions, gender‐based barriers, presence of NGOs which offered financial incentives (and thus decreased participation) could have occurred, especially as all these factors were associated with the clusters in which the women’s group intervention was present. The authors reported that a greater proportion of women’s group intervention clusters had less education and household assets, and were younger, compared to control cluster women. Though not stated, it is plausible that the various “health service inputs,” provided at various health services levels (e.g., training provided to doctors, nurses and paramedical staff working at district upazila and union levels) differed between the intervention and control groups. The authors note that declines in mortality in control areas may be due to other underlying secular trends. |
| Recruitment bias | High risk | Randomisation was conducted separately for the women’s group and TBA interventions “using the same method.” Clusters may have received both interventions (women’s group intervention plus bag‐valve‐mask trained TBAs), one of the two interventions, or neither. Although method of allocation appears transparent and was conducted before baseline data collection and analysis, neither study investigators nor participants were masked to group allocation. Additionally, women were recruited after the clusters had been randomised (page 1195). The authors do not comment on whether they believe women from control clusters elected to deliver with intervention cluster TBAs because of knowledge of the cluster’s intervention status, though this is unlikely. About 10% of women in the study area were temporary residents who had come to the cluster areas to give birth. It is not clear if these women would have been more or less likely to receive care from trained or untrained TBAs, nor whether this would have differed between the two clusters. |
| Baseline imbalance | Unclear risk | The number of clusters was relatively small (n = 18). “We obtained background demographic and socioeconomic information to investigate cluster comparability.” A cross‐sectional baseline survey was completed for more than 6000 mothers who had delivered in the past year on household and demographic characteristics as well as pregnancy, delivery and neonatal outcomes. By chance, the control clusters included three tea garden estates “that had substantially worse health and socioeconomic indicators than did the rest of the study area.” Surveillance also started late in this area due to entry restrictions. Baseline survey revealed differences in maternal education, maternal age and household assets between clusters which received the women’s group intervention versus those which did not. It is unclear if these differences, or others, existed between the clusters further randomised to the TBA intervention versus those which were not. While these differences in baseline characteristics were adjusted for in the women’s group study analysis, it is not stated that this was done for the TBA study. While the authors’ use of a stratified cluster‐level analysis reduces the risk of bias from chance baseline differences due to the small number of clusters in each group, inadequate information on the areas listed above result in an unclear risk of bias. |
| Loss of clusters | Low risk | No clusters were lost to follow‐up. In interventions areas, 84% and 82% of interviews were completed for births registered by key informants in the intervention and control clusters. “The main reason for failure to interview was maternal migration,” as about 10% of mothers in the study area were temporary residents who had come into the cluster areas to give birth. While these figures were not reported within the strata of home births, it seems unlikely that bias due to loss of these participants would be different between intervention and control clusters. “In the assessment of the traditional birth attendant intervention, only home deliveries were included in the analysis.” This exclusion is not likely to carry a high risk of bias. It probably best captures those who were exposed to the TBAs’ bag‐valve‐mask resuscitation skills. It also likely creates a better comparison group (i.e., other home births) for assessing the effectiveness of this intervention. (Further stratified analysis comparing births in which a TBA who received the intervention was present to those in which the TBA was not present would have been useful for assessing the true effect of the intervention, as done in the Gill et al study). Of 12,519 home births in intervention clusters, 8618 (69%) were attended by any TBA, but only 2792 (22%) were attended by a TBA who received the bag‐valve‐mask resuscitation training. Similarly, of 13,195 home births in control clusters, 9171 (70%) were attended by any TBA and 2536 by a TBA trained in mouth‐to‐mouth resuscitation (19%). ITT for the primary mortality outcomes were reported. Temporary and tea garden residents were excluded from secondary outcome analysis, “since they were unlikely to have been exposed to the intervention.” We assess this study as low risk of bias for primary outcomes (including TBA outcomes), but high risk of bias for secondary outcomes. |
| Incorrect analysis | Low risk | Stratified cluster‐level analysis was performed. RRs for early neonatal mortality rate were calculated for each stratum, and then an overall weighted mean of these were used in a stratified t‐test. The authors note that no interactions between the TBA and women’s group intervention. |
| Comparability with individually randomised trials | Low risk | No evidence of contamination effect. The initial women’s group component may have contributed to a “herd effect” by creating a foundation for increased community mobilisation and knowledge‐sharing. However, as the authors were able to demonstrate the absence of statistical interaction between the women’s group and TBA interventions, the risk of bias for comparability with individual trials was determined to be low. |
Bullough 1989.
| Methods | Randomised controlled trial. Unit of analysis: individual women cared for by the study TBAs. |
|
| Participants | Malawi, 3 rural regions. TBAs and women living in study areas who were attended by TBAs (2184 and 2201 women were attended by intervention and control group TBAs, respectively). |
|
| Interventions | TBAs in both intervention and control groups attended a 2‐day refresher course. The content focused on patient education and advice around breastfeeding. Intervention group TBAs subsequently received additional instruction on immediate suckling before placental delivery, and were also trained to measure all blood loss after delivery using a transparent plastic jug. Control group TBAs did not receive additional instruction in immediate suckling before placental delivery but they were trained to measure all blood loss after delivery using the plastic jug. |
|
| Outcomes | Unit of analysis: individual women cared for by the TBAs (unit of analysis error). Primary outcome: postpartum haemorrhage (> 500 ml blood loss during third stage of labour or within 24 hours of delivery; mean blood loss; and transfer to the hospital for third stage labour complications. |
|
| Notes | Follow‐up: 3 to 4 weeks after training, then every 5 weeks over 6 to 9 months, not specified who conducted follow‐up although community midwives also attended 1 follow‐up visit. The authors questioned the accuracy of measurement of blood loss (plastic jug). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation procedure conducted by the Malawi Ministry of Health Department of Statistics was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Random sequence generation procedure conducted by the Malawi Ministry of Health Department of Statistics was not reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All TBAs measured blood loss and none were told they were participating in a study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Same as above. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The author states that 29% of TBAs were excluded from the follow‐up because they were considered un‐trainable, failed quality control check, or were strongly suspected of data fabrication (page 524). Of the remaining TBAs, outcomes were reported for more than 90% of women. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | See above. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported. |
| Other bias | High risk | Unit of analysis error. The analysis conducted in this study was based on methods appropriate for an individual randomised controlled trial. The analysis would have been more appropriate had cluster‐randomised controlled trial analytic methods been used (a cluster being defined as all women whose birth was attended by a given TBA). Reliability of outcome measure. The authors questioned the accuracy of the measurement of blood loss by TBAs (transparent plastic jug). |
| Recruitment bias | Unclear risk | N/A |
| Baseline imbalance | Unclear risk | N/A |
| Loss of clusters | Unclear risk | N/A |
| Incorrect analysis | Unclear risk | N/A |
| Comparability with individually randomised trials | Unclear risk | N/A |
Carlo 2010.
| Methods | Two‐part study design: (1) Essential Newborn Care Study (before‐and‐after) and (2) Neonatal Resuscitation Program (cluster RCT). This review reports results from the cluster‐RCT. Unit of randomisation: communities (with at least 300 births per year), 88 clusters. | |
| Participants | Five countries (Democratic Republic of Congo, Guatemala, India, Pakistan, Zambia), 88 rural communities (n = 43 and 45 intervention and control communities, respectively). Community birth attendants, including TBAs, nurses, midwives and physicians. TBAs attended 39.2% of all births in the study area after an Essential Newborn Care program and before implementation of the Neonatal Resuscitation Program study. Women attended by community birth attendants in the study area (TBAs attended 10,770 and 13,327 births in the intervention and control areas, respectively). |
|
| Interventions | All community birth attendants received a 3‐day WHO 2004 Essential Newborn Care course. Content included routine neonatal care, initiation of breathing and resuscitation (bag‐valve‐mask ventilation), thermoregulation, early exclusive breastfeeding, kangaroo care, danger sign recognition. Intervention birth attendants received an additional 3‐day Neonatal Resuscitation Program and 6 mo. refresher. Content consisted of in‐depth theoretical and practical training in initial steps in resuscitation and bag‐valve‐mask ventilation. Control birth attendants did not receive the additional Neonatal Resuscitation Program. | |
| Outcomes | Unit of analysis: communities (births to women attended by community birth attendants). Primary outcome: all‐cause early neonatal mortality rate (within 7 completed days after birth) among infants with birth weights at least 1500 g. Pre‐specified secondary outcomes: birth asphyxia specific early neonatal mortality rate, stillbirth rate, perinatal mortality rate, 24‐hour neonatal mortality rate, death rate stratified by sex, birth weight, location of birth and type of birth attendant, Apgar scores < 4 at 1 and 5 min, use of resuscitation techniques and neurological outcomes. | |
| Notes | A Supplementary Appendix provided by the authors provides outcomes disaggregated by type of community birth attendant: Table 1 (7‐day neonatal mortality rates according to characteristics of the infant or delivery), Table 2 (stillbirth rates according to characteristics of the infant or delivery), and Table 3 (perinatal mortality rates according to characteristics of the infant or delivery). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The procedure for sequence generation was not reported. The clusters underwent randomisation, followed by training in the Neonatal Resuscitation Program and data collection. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Community birth attendants who implemented the intervention were not masked. Government officials and community staff facilitated data collection by the birth attendants were not masked. It is plausible that the TBA intervention group had greater confidence in providing resuscitation, and women might become aware of this additional training. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Fetuses and neonates < 1500 g birth weight were excluded. Where weight was not measured, infants were included if the birth weight was estimated by the birth attendant to be > 1500 g. It is plausible that birth weights were reported as lower in instances of stillbirths or neonatal deaths, as birth attendants may not have wanted these to appear to contribute to their performance. Detection bias on this outcome may be an issue, but it is likely to be low as 94.9% of births did have a birth weight that was measured. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The authors state that the project’s community co‐ordinators closely supervised the birth attendants to promote reliable data collection. Government officials and community staff who facilitated data collection by the birth attendants were not masked. The primary outcome (mortality) was objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The discharge outcome and 7‐day outcome was collected on 97.7% and 96.2% of the infants whose mothers consented. |
| Selective reporting (reporting bias) | Low risk | The authors did not report results of the analysis for births in which a birth weight was measured (not estimated), though they did state that the results were “materially unchanged.” Additionally, as 94.9% of births had a measured birth weight, this is likely to not carry a high risk of bias. Results were reported for all outcomes, regardless of statistical significance. |
| Other bias | Unclear risk | Whether mothers declined participation in the study varied by intervention status is not likely to be a high risk for bias, as this was low in both Part 1 and Part 2. The authors note that lack of an effect of bag‐valve‐mask resuscitation training on perinatal death rate could be due to fact that Essential Newborn Care training (which includes basic resuscitation training), was already in place before the Neonatal Resuscitation Program began. |
| Recruitment bias | Unclear risk | The Neonatal Resuscitation Program study was initiated following the Essential Newborn Care study in rural communities in 5 of 7 original study countries. The authors state that communities were selected to represent rural areas, to be geographically distinct, and to have at least 300 births per year. The procedure of allocating communities to clusters was not described. Moreover, how the TBAs and women were recruited is not described. Whether individuals were recruited or self selected to intervention or control communities is unknown. Proximity of clusters to each other, which may effect recruitment, is not provided. Note: Birth attendants would ascertain whether a death was a stillbirth or a live birth, whether the babies were eligible (weight greater than 1500g), and cause of death. (There appeared to be more exclusions of stillbirths < 1500 g in the control clusters and fewer exclusions of live births < 1500 g). |
| Baseline imbalance | Low risk | Baseline data were collected before provision of the training outlined in Part 1 or Part 2. Results were adjusted for significant variables in the models among the following explanatory variables: trial site, maternal age at delivery, maternal education, gestational age, parity, birth weight, sex, birth location and category of birth attendant. Note that there were more multiple births in the intervention clusters. |
| Loss of clusters | Low risk | No loss of clusters was apparent. Follow‐up rate for the Newborn Resuscitation Program study was 98% (Of the 35,017 infants included, only 300 did not have an outcome recorded at discharge). Fetuses and neonates with a birth weight of less than 1500 g were excluded because, “advanced medical care for very‐low‐birth‐weight infants was not available in most of the study communities.” These numbers were comparable in the intervention and control clusters. For primary outcomes there appeared to be little loss of data. Data for some outcomes (e.g., birth weight for babies > 1500 g) were missing (5% data not available). |
| Incorrect analysis | Low risk | The intra‐cluster correlation coefficient was estimate via simulation and confirmed with baseline data. Variances were adjusted for the primary outcome variable to account for the intra‐cluster correlation (design effect). Multivariate logistic‐regression models with generalised estimating equations accounting for the cluster effect were used to test for differences in the rates of early neonatal mortality rates, and to assess interactions of death with category of birth attendant and location of birth. |
| Comparability with individually randomised trials | Low risk | No contamination effect noted. |
Gill 2011.
| Methods | Cluster‐randomised controlled trial. Unit of randomisation: TBA (a cluster was defined as all births delivered by a given TBA). |
|
| Participants | Zambia, all TBAs within one rural district (total = 127). TBAs, women and their newborns, regardless of vital status at birth. TBAs attended 1961 and 1536 births in the intervention and control groups, respectively. |
|
| Interventions | All TBAs were trained in the prevention of neonatal hypothermia, reduction of birth asphyxia using mouth‐to‐mouth ventilation, and were given birth kits and supplies. Intervention TBAs were additionally trained in a modified American Pediatric Association and American Heart Association neonatal resuscitation protocol (bag‐valve‐mask ventilation), as well as in sepsis management (single dose amoxicillin) and facilitated referral to a health facility. Control TBAs did not receive the additional training and continued to provide their existing standard of care (basic clean safe delivery). | |
| Outcomes | Unit of analysis: TBA (births to women attended by a given TBAs in the study area). Primary outcome: proportion of live‐born neonates who died from all causes within 28 completed days of birth. Secondary outcomes: stillbirths, neonatal mortality rate at 1 week, neonatal mortality rate between 2 to 4 weeks of birth and cause of death. | |
| Notes | Gill 2011 was the only study to include results for cause‐specific neonatal mortality based on verbal autopsy. The authors identified 16 and 17 deaths attributed to serious infection in the intervention and control clusters, respectively (unadjusted RR 0.73, 95% CI 0.37 to 1.43, N = 3365 ); 10 and 21 deaths attributed to birth asphyxia in the intervention and control clusters (unadjusted RR 0.37, 95% CI 0.17 to 0.78, N = 3365 ); 0 and 4 deaths from diarrhoea (unadjusted RR 0.09, 95% CI 0.00 to 1.59, N = 3365 ); 1 and 0 deaths from tetanus in the intervention and control clusters (unadjusted RR 2.32, 95% CI 0.09 to 56.82, N = 3365 ). Most neonatal deaths (64%) were attributed to birth asphyxia and serious infection. Deaths attributed to infection occurred at similar rates in the intervention and control clusters, while deaths from apparent birth asphyxia were significantly lower (63%) in the intervention compared with the control clusters. Two neonatal deaths, 1 and 1 in the intervention and control clusters, respectively, were not assigned a cause. Other neonatal mortality outcomes were reported but these are not a focus of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | TBAs were randomly allocated (1:1) to intervention and control groups. Random assignment was done before baseline data were collected. For women, data collectors carried out up to 2 follow‐up visits at 1 and 4 weeks after birth. At the first visit, they collected baseline data including maternal household demographic and economic data, data regarding payments made to the birth attendant and maternal reproductive history. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by generating 120 allocation “slips” (60 intervention and 60 control), which were placed in an opaque container. During a public ceremony, witnessed by all TBAs and study staff, participants individually drew a slip from the container and allocation was announced to the whole group. |
| Blinding (performance bias and detection bias) All outcomes | High risk | TBAs and data collectors were not blinded to TBA allocation status. TBAs interact in their communities and some exchange of knowledge may have occurred from intervention to control group TBAs. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Same as above. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | TBAs and the data collectors were not blinded. TBAs conducted case ascertainment. In rural communities without basic facilities the difference between a stillbirth and a baby that does not respond to resuscitation may not be easy to discern. TBAs completed and shared standardised birth records for every delivery (including infant’s vital status on the day of delivery) with their data collector within 48 hours of delivery. Data collectors followed activities of all TBAs in their specific geographic area via weekly face‐to‐face contact. Prospective monitoring of the TBAs’ pregnant woman and due dates likely reduced detection bias of deaths. The data collector reviewed and verified the report contents with the TBA and carried out follow‐up visits (at 1 and 4 weeks) with the mother and infant pair. A panel of 3 neonatologists, who were blinded to group allocation and had no contact with TBAs or data collectors, independently reviewed delivery reports and verbal autopsies for stillbirths and neonatal deaths recorded at any time in the first month after birth. They assigned a presumptive cause of death, selecting from a pre‐specified list of causes. Concordance of 2 reviewers was recorded. Their assessments were necessarily based on the TBA and data collectors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was very little attrition and a modified ITT analysis was used. |
| Selective reporting (reporting bias) | Low risk | The study was not stopped. All primary and secondary outcome results were reported. |
| Other bias | Unclear risk | The authors note that while it is plausible that intervention TBAs could have shared their additional skills with control TBAs, there was no evidence this occurred, and that the effect of this is likely to be minimal. They state that this is because the intervention TBAs received amoxicillin and equipment (e.g., resuscitation masks, suction bulbs) that weren't likely to be transferred to control TBAs. Additionally, they note “the effect of cross contamination of skills” would render the control birth attendants more like the intervention ones,” resulting in a bias towards the null. It is not clear whether intervention TBAs were more likely to identify pregnancies at high risk and refer women for birth at a health centre (there were more referrals from the intervention group in the postnatal period); it is possible that there was a disproportionate number of intervention TBA deliveries at health centres. Numbers of early labour and high‐risk pregnancy referrals were not reported. Much attention to quality data collection in this study. Misclassification of failed resuscitations as stillbirths was addressed by comparing stillbirth rates between the intervention and control groups and calculating overall mortality rates, including and excluding stillbirths. |
| Recruitment bias | High risk | TBAs were recruited to the study before random allocation. TBAs (n = 120) were recruited to the study before random allocation, but 7 TBAs were added to the control group after randomisation during the last 6 months of the study. |
| Baseline imbalance | Low risk | Baseline data were collected on the TBAs as well as on the mothers and babies at 1 week post delivery. Data included maternal household demographic and economic data, data regarding payments made to the TBA by the mother and maternal reproductive history. The authors used models that were cluster‐adjusted (no other covariates adjusted for), as well as cluster‐adjusted models additionally adjusted for baseline imbalances. These included years of education, birth attendant’s marital status and whether the TBA reported that being a birth attendant was her primary job. Maternal and infant characteristics were similar between intervention and control groups. |
| Loss of clusters | Low risk | No loss of clusters (TBAs) occurred. Before final vital status had been determined at 28 days, 76 infants (2.1%) were lost to follow‐up: 34 of 2007 (1.7%) intervention deliveries and 42 of 1552 (2.7%) of control deliveries. The loss of participants was low and not significantly different in both groups. Some reports from one data collector had been falsified. All data on deliveries reported that data collector (including reports for both intervention and control TBAs) were excluded from the final analysis. Still, 98.3% (3497 of 3559) babies delivered in the study contributed valid data for the analysis. The number of participants excluded was low, and included both intervention and control births. A modified ITT analysis was used for mortality endpoints where participants who were lost during follow‐up were treated as missing rather than as deaths. Sensitivity analysis was also performed or the primary endpoint (neonatal mortality rate). |
| Incorrect analysis | Low risk | The investigators adjusted for clustering on assumption of a “by TBA” effect associated with outcomes. The Wilcoxon rank sum test was used to contrast the mean number of babies delivered by intervention or control birth attendants. Rate ratios were cluster‐adjusted using binomial regression in a generalised estimating equation to regress the risk of death as a function of the assigned treatment group. Clustering of babies within a birth attendant was adjusted for by specifying an exchangeable correlation matrix. A modified ITT analysis was used, where participants lost to follow‐up were treated as missing rather than as deaths. |
| Comparability with individually randomised trials | High risk | It is notable that intervention group TBAs attended significantly more deliveries than control group TBAs (P = 0.03), a difference that increased over the study, suggesting that pregnant women may have become aware of the TBAs’ additional training. Thus contamination of participants may have occurred. While the clusters roughly corresponded to the catchment area in which each TBA operated, adjacent catchment areas often overlapped. Participants may have learned about the additional training and services the intervention TBAs provided and elected to use these TBAs. |
Hossain 2000.
| Methods | Randomised controlled trial. | |
| Participants | Bangladesh, 12 rural thanas (6 intervention and 6 control). TBAs and village doctors. We include only TBAs in this review. There were 85 and 86 TBAs in the intervention and control groups, respectively. Postpartum lactating women: There were 1065 and 1067 women in the intervention and control groups, respectively. |
|
| Interventions | Intervention group TBAs and village doctors received a 2‐day training on knowledge and advice related to breastfeeding. The module was based on guidelines from the International Conference on Population and Development. Control group TBAs and village doctors received no training. |
|
| Outcomes | Unit of analysis: individual TBAs and women living in study areas served by TBAs. Primary outcomes (for TBAs): whether and what types of advice on breastfeeding; knowledge and benefits of colostrum; knowledge about initiation of breastfeeding and weaning foods; knowledge and benefits of exclusive breastfeeding; and knowledge about breast‐milk substitutes. Primary outcomes (for women): feeding practices at birth; timing and benefits of breastfeeding; whether, what and who provided information about breastfeeding; knowledge of the disadvantages of bottle‐feeding. Pre‐specified secondary outcomes (for women): use of antenatal care and place of delivery. |
|
| Notes | Most (71%) TBAs in intervention and control thanas had received previous training, including on breastfeeding. The study follow‐up survey was conducted at 3, 5 and 7 to 8 mo. after TBA training. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | The authors randomly selected 12 sub‐districts (thanas), 6 intervention and 6 control. Procedure for concealment not reported. Fifteen TBAs from each thana were selected. How they were selected and whether those selected represented some or all TBAs in a given thana, was not reported. All lactating women were eligible for inclusion in the study. Baseline measures of outcomes were reported and the intervention and control groups were comparable on several known potentially confounding variables. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors report > 90% of TBAs and village doctors assessed at midterm and final (p. 4). |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported. |
| Other bias | Unclear risk | None noted. |
| Recruitment bias | Unclear risk | N/A |
| Baseline imbalance | Unclear risk | N/A |
| Loss of clusters | Unclear risk | N/A |
| Incorrect analysis | Unclear risk | N/A |
| Comparability with individually randomised trials | Unclear risk | N/A |
Jokhio 2005.
| Methods | Cluster‐randomised controlled trial. | |
| Participants | Pakistan, 7 rural sub‐districts (talukas), 3 intervention and 4 control talukas. TBAs (565 TBAs and an unknown number of TBAs in the intervention and control clusters, respectively). Lady Health Workers (LHWs) (811 and 819 LHWs in the intervention and control clusters, respectively). Women living in the study clusters served by the TBAs and LHWs (10,114 women with 9710 singleton births, and 9443 women with 8989 singleton births in the intervention and control clusters, respectively). |
|
| Interventions | Intervention cluster TBAs received a 3‐day training on clean delivery and detection of complications and referral. Delivery kits were provided. Control cluster TBAs did not receive any training and did not receive delivery kits. LHWs were trained to support the TBAs in both intervention and control clusters. Obstetric teams provided consultation and outreach in the intervention clusters only. |
|
| Outcomes | Unit of analysis: talukas, individual women living in study talukas served by TBAs. Primary outcomes: perinatal death rate; stillbirth rate; neonatal death rate; maternal death; major obstetric complications; referral by TBA for emergency obstetric care. |
|
| Notes | Follow‐up: pregnant women were recruited 1 mo. after the TBA training over a 6‐mo. period and followed by the LHWs until 42 days postpartum. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of clusters to intervention or control was accomplished “with a simple cluster‐randomisation sampling scheme, and with a computer‐generated procedure.” No other details on randomisation methods provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Seven clusters were allocated. All TBAs and pregnant women living in the 7 clusters were eligible to participate. It is not clear whether anyone knew the sequence before allocation or could change it. |
| Blinding (performance bias and detection bias) All outcomes | High risk | TBAs and pregnant women were not masked to cluster allocation status. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Follow‐up in both groups involved the collection of information by Lady Health Workers, who asked the women, their families, and traditional birth attendants for details of the progress and outcome of each pregnancy that was registered. In cases of maternal death, the cause was ascertained by Lady Health Workers on the basis of oral reports from relatives, neighbours, or traditional birth attendants.” The authors also state that they had no data to ascertain the accuracy of the reports of death or of the reported causes of maternal death. For maternal complications (e.g., haemorrhage) blinding may have had an effect (see other bias). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Follow‐up in both groups involved the collection of information by Lady Health Workers, who asked the women, their families, and traditional birth attendants for details of the progress and outcome of each pregnancy that was registered. In cases of maternal death, the cause was ascertained by Lady Health Workers on the basis of oral reports from relatives, neighbours, or traditional birth attendants.” The authors also state that they had no data to ascertain the accuracy of the reports of death or of the reported causes of maternal death. For maternal complications (e.g., haemorrhage) blinding may have had an effect (see other bias). Lady Health Workers (who collected data) were not blinded but authors felt they were not aware of the purpose of comparative nature of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the intervention clusters, 10,114 women were recruited, 21 (0.2%) were lost to follow‐up, and 2 had unknown birth outcomes. In the control clusters, 9443 women were recruited and 11 (0.1%) were lost to follow‐up. The number of women lost to follow‐up is small and comparable in both intervention and control clusters. Only singleton births were included in the analysis of perinatal outcome. In the intervention clusters, 126 women (1.2%) had multiple births and 130 (1.4%) had multiple births in the control clusters. This per cent is low in both, and comparable. The exclusion of multiple births is thus not likely to lead to differential bias with respect to intervention or control status. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported. |
| Other bias | Unclear risk | Difficult to assess without access to study protocol. The study did not have the power to identify a difference between groups for 1 of the 2 selected primary outcomes (maternal death). The authors state, however, “We do not have information on the acceptance or the outcomes of these referrals [to public or private facilities].” While they report that the intervention did not significantly increase the number of referrals in intervention clusters, it is not clear if the different types of women [perhaps those who learned they were at high risk because of the intervention] subsequently went to the facilities and lower risk women [who felt confident delivering at home because of the intervention] decided not to deliver at facilities. If this happened, then it could be a source for bias, in that high‐risk women were not counted in the birth outcomes results, showing an artificially beneficial result of the intervention on mortality. While maternal mortality is an objective outcome, maternal morbidity as measured was not in this study. LHWs were taught the signs of the major obstetric complications and used a list with definitions to ask postpartum women if they had each complication. It is impossible to assess the validity of self report of complications. |
| Recruitment bias | Unclear risk | Unclear if random assignment done before data collection and analysis of baseline data. |
| Baseline imbalance | Unclear risk | “The baseline maternal characteristics [age, parity, month of pregnancy when recruited, husband’s education, distance from nearest primary health care facility] were similar for the study groups and across clusters with respect to all measured variables except years of women’s education, which were slightly greater among women in the control group.” The control group also had significantly more spontaneous abortions. The authors did not state if they controlled for covariates in the analysis. It is not clear to what degree the difference in maternal education would have impacted the results. A small number of clusters was randomised (7) and the clusters were of quite different sizes (all relatively large). It was not clear whether the cluster areas were similar. Only a small number of maternal variables were selected to demonstrate the comparability of areas and there was no attempt to match areas in any way. There were considerable differences in perinatal deaths in areas within clusters; with such a small number of clusters it would have been helpful to have information about death rates in the different areas before the intervention. |
| Loss of clusters | Low risk | There appear to be no loss of clusters. Less than 1% of eligible women were lost to follow‐up at 42 days postpartum in the intervention group (21/10,114) and control group (11/9732). Pregnancy ended in spontaneous abortion for 2.5% and 3.3% of women in the intervention and control groups, respectively. Multiple births occurred for 1.2% and 1.4% of women in the intervention and control groups, respectively. |
| Incorrect analysis | Low risk | Evaluation by ITT. Multi‐level modelling used to account for cluster‐randomisation. Quasi‐likelihood binary regression model with random intercept to estimate the degree of over‐dispersion in cluster‐specific maternal and perinatal death rates. Sample size was restricted due to funding (7 clusters total, average size 4000 pregnant women each). |
| Comparability with individually randomised trials | Unclear risk | An obstetric team for antenatal care outreach clinics joined mothers together in care‐seeking activities while strengthening use of the talukas as clusters provided a cohesive way to connect TBAs with existing health resources. Cluster‐level interventions like the use of obstetric teams for antenatal care outreach clinics in intervention clusters may have resulted in a “herd effect” of the TBA by facilitating knowledge sharing amongst women and changes in care‐seeking attitudes for referrals. |
CBA: controlled before/after study CI: confidence interval ITT: intention‐to‐treat LHW: lady health workers mo.: month MOH: ministry of health NGO: non‐governmental organisation OB: obstetric PPH: postpartum haemorrhage RCT: randomised controlled trial RR: risk ratio TBA: traditional birth attendant
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ajenifuja 2010 | Study design is a hospital study of PPH and has nothing to do with TBAs. |
| Alisjahbana 1995 | Study design CBA but does not include randomisation or baseline assessment of outcomes. |
| Bailey 2002 | Relevant data are not presented nor obtainable from author. |
| Bang 1999 | Intervention targeted training village health workers. Trained TBAs assisted the trained village health workers. |
| Bhandari 2003 | Training targeted health and nutrition workers at multiple levels and included TBAs. The data reported were not disaggregated by category of worker and the results cannot be attributed to any one category of worker. |
| Bhutta 2011 | Training targeted to Lady Health Workers and TBAs. The data reported were not disaggregated by TBAs. |
| Ellis 2011 | Intervention targeted TBA to collect birth outcome data for cluster‐randomised trial. |
| Falle 2009 | Study design is a one‐group before after design (no control). |
| Gloyd 2001 | Study design is an observational cross‐sectional design. |
| Greenwood 1999 | Intervention and control groups are not comparable. |
| John 2002 | Study design is time series but does not contain at least 3 data points both before and after the intervention. |
| Menendez 1994a | Population is trained TBAs only. No comparison to untrained TBAs. Comparison is iron supplement versus placebo. |
| Menendez 1994b | Population is trained TBAs only. No comparison to untrained TBAs. Comparison is maloprim (chemoprophylaxis for malaria) versus placebo. |
| Nyamwaya 1993 | Intervention and control groups are not comparable. Control site is not untrained TBAs only. |
| O'Rourke 1994 | Study design is controlled before and after study on the effect of additional TBA training in 10 areas in Guatemala. |
| Olusanya 2011 | Study design is a descriptive study looking at site of delivery and outcomes. |
| Prata 2005 | Study design is an observational cross‐sectional design. |
| Rahman 1982 | Study design included random selection but not random assignment to groups. |
| Rowen 2009 | Study design is a one‐group before after design (no control). |
| Saravanan 2011 | Study design is a descriptive study comparing post TBA training KAP with women's KAP. |
| Satishchandra 2009 | Study design is a one‐group before after design (no control) on effect of 2‐day TBA training on KAP of safe clean delivery and newborn care. |
| Singhal 2001 | Population is trained TBAs only. Comparison is TBAs trained by lecture versus TBAs trained by interaction problem‐based method. |
| Theron 1999 | Population is professional midwives and not TBAs. Training is not TBA training. |
| Theron 2000 | Population is professional midwives and not TBAs. Training is not TBA training. |
CBA: controlled before/after study KAP: knowledge, attitude and practice PPH: postpartum haemorrhage TBA: traditional birth attendants
Differences between protocol and review
In this version of the review we have changed the inclusion criteria for types of studies. In view of the small number of studies in this topic area, in previous versions we included study designs other than randomised or quasi‐randomised trials and included a controlled before and after study. In this update, as the amount of evidence from randomised trials has increased, we have now excluded this non‐randomised study.
Contributions of authors
LM Sibley (contact author and guarantor for the review) conceived, designed and co‐ordinated the review; developed study screening and abstraction forms; conducted second stage screening of studies for inclusion into review; conducted data abstraction and 'Risk of bias' assessments; updated the summary tables for intervention, TBA and woman characteristics; interpreted results and conclusions; and drafted the final review.
TA Sipe (co‐author) provided feedback on the draft protocol; developed study screening and abstraction forms; conducted second stage of screening of the studies for inclusion into the review; co‐ordinated and conducted data extraction and 'Risk of bias' assessments; conducted data entry into Review Manager and data analysis, interpretation of results, and graphical displays of data; made all entries into RevMan; drafted parts of the review and provided feedback on the content of the draft review.
D Barry (co‐author) provided independent 'Risk of bias' assessments; updated the summary of risk of bias; and provided feedback on the content of the final draft review.
Sources of support
Internal sources
Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA, USA.
Woodruff Health Sciences Center Library, Emory University, Atlanta, GA, USA.
External sources
-
National Institute for Health Research, UK.
UK NIHR Programme of centrally‐managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS: 10/4001/02
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Azad 2010 {published data only (unpublished sought but not used)}
- Azad K, Barnett S, Banerjee B, Shaha S, Khan K, Rego AR, et al. Effect of scaling up women's groups on birth outcomes in three rural districts in Bangladesh: a cluster‐randomised controlled trial. Lancet 2010;375(9721):1193‐202. [DOI] [PubMed] [Google Scholar]
Bullough 1989 {published data only}
- Bullough CHW, Msuku RS, Karonde L. Early suckling and postpartum haemorrhage: controlled trial in deliveries by traditional birth attendants. Lancet 1989;2:522‐5. [0089] [DOI] [PubMed] [Google Scholar]
Carlo 2010 {published and unpublished data}
- Carlo WA, Goudar SS, Jehan I, Chomba E, Tshefu A, Garces A, et al. First Breath Study Group. Newborn‐care training and perinatal mortality in developing countries. New England Journal of Medicine 2010;362:614‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gill 2011 {published data only}
- Gill C, Mazala G, Guerina N, Kasimba J, Knapp A, Mulenga C, et al. Reducing neonatal mortality in rural Zambia with traditional birth attendants, a cluster randomized trial: the Lufwanyama neonatal survival project (LUNESP). Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Gill CJ. Lufwanyama neonatal survival project (LUNESP). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 19 July 2010).
- Gill CJ, Phiri‐Mazala G, Guerina NG, Kasimba J, Mulenga C, MacLeod WB, et al. Effect of training traditional birth attendants on neonatal mortality (Lufwanyama Neonatal Survival Project): randomised controlled study. BMJ 2011;342:d346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina NG, Mazala G, Kasimba J, Mulenga C, Knapp A, Mirochnick M, et al. Effectiveness of NRP adapted for use by traditional birth attendants (TBAs) in rural Zambia: the Lufwanyama Neonatal Survival Project (LUNESP). Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
Hossain 2000 {unpublished data only}
- Hossain Z, Ripon FH, Chowdury JH. Promotion of breastfeeding: an operations research on traditional birth attendants (TBAs) and village doctors. Unpublished report, Adabor, Shamoly, Phaka, Bangladesh: Development Support Services May 2000:1‐47. [200]
Jokhio 2005 {published data only}
- Jokhio AH, Winter HR, Cheng KK. An intervention involving traditional birth attendants and perinatal and maternal mortality in Pakistan. New England Journal of Medicine 2005;352(20):2091‐8. [0103] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ajenifuja 2010 {published data only}
- Ajenifuja KO, Adepiti CA, Ogunniyi SO. Post partum haemorrhage in a teaching hospital in Nigeria: a 5‐year experience. African Health Sciences 2010;10(1):71‐81. [PMC free article] [PubMed] [Google Scholar]
Alisjahbana 1995 {published data only}
- Alisjahbana A, Williams C, Dharmayanti R, Hermawan D, Kwast BE, Koblinsky M. An integrated village maternity service to improve referral patterns in a rural area in West‐Java. International Journal of Gynecology & Obstetrics 1995;48 Suppl:S83‐S94. [0083] [DOI] [PubMed] [Google Scholar]
Bailey 2002 {published and unpublished data}
- Bailey PE, Szaszdi JA, Glover L. Obstetric complications: does training traditional birth attendants make a difference?. Pan American Journal of Public Health 2002;11(1):15‐22. [0084] [DOI] [PubMed] [Google Scholar]
Bang 1999 {published data only}
- Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD. Effect of home‐based neonatal care and management of sepsis on neonatal mortality: field trials in rural India. Lancet 1999;354:1955‐61. [DOI] [PubMed] [Google Scholar]
Bhandari 2003 {published data only (unpublished sought but not used)}
- Bhandari N, Bahl R, Mazumdar S, Martines J, Black RE, Bhan MK, Infant Feeding Study Group. Effect of community‐based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet 2003;361(9367):1418‐23. [10.1016/S0140‐6736(03)13134‐0 10] [DOI] [PubMed] [Google Scholar]
- Bhandari N, Mazumder S, Bahl R, Martines J, Black RE, Bhan MK, et al. Use of multiple opportunities for improving feeding practices in under‐twos within child health programmes. Health Policy & Planning 2005;20(5):328‐36. [DOI] [PubMed] [Google Scholar]
Bhutta 2011 {published data only}
- Bhutta Z, Soofi S, Cousens S, Mohammad S, Memon ZA, Ali I, et al. Improvement of perinatal and newborn care in rural Pakistan through community‐based strategies: a cluster‐randomised effectiveness trial. Lancet 2011;377:403‐12. [DOI] [PubMed] [Google Scholar]
Ellis 2011 {published data only}
- Ellis M, Azad K, Banerjee B, Shaha SK, Prost A, Rego AR, et al. Intrapartum‐related stillbirths and neonatal deaths in rural Bangladesh: a prospective, community‐based cohort study. Pediatrics 2011;127(5):e1182‐90. [DOI: 10.1542/peds.2010-0842] [DOI] [PubMed] [Google Scholar]
Falle 2009 {published data only}
- Falle TY, Mullany LC, Thatte N, Khatry SK, LeClerq SC, Darmstadt GL, et al. Potential role of traditional birth attendants in neonatal healthcare in rural southern Nepal. Journal of Health, Population and Nutrition 2010;27(1):53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gloyd 2001 {published data only}
- Gloyd S, Floriano F, Seunda M, Chadreque MA, Nyangezi JM, Platas A. Impact of traditional birth attendant training in Mozambique: a controlled study. Journal of Midwifery & Women's Health 2001;46(4):210‐6. [0095] [DOI] [PubMed] [Google Scholar]
Greenwood 1999 {published data only}
- Greenwood AM, Bradley AK, Byass P, Greenwood BM, Snow RW, Bennet S, et al. Evaluation of primary health care programme in The Gambia. The impact of trained traditional birth attendants on the outcome of pregnancy. Journal of Tropical Medicine and Hygiene 1990;93:58‐66. [PubMed] [Google Scholar]
John 2002 {published data only}
- John ME, Udoma EJ, Udoh MO, Ndebbio TJ, Idiong MS. Knowledge and practice of traditional birth attendants concerning risk factors in pregnancy, labour and puerperium. Africa Journal of Nursing and Midwifery 2002;4(1):41‐5. [Google Scholar]
Menendez 1994a {published data only}
- Menendez C, Todd J, Alonso PL, Francis N, Lulat S, Ceesay S, et al. The effects of iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88:590‐3. [0112] [DOI] [PubMed] [Google Scholar]
Menendez 1994b {published data only}
- Menendez C, Todd J, Alonso PL, Lulat S, Francis N, Greenwood BM. Malaria chemoprophylaxis, infection of the placenta and birth weight in Gambian primigravidae. Journal of Tropical Medicine and Hygiene 1994;97:244‐8. [0111] [PubMed] [Google Scholar]
Nyamwaya 1993 {unpublished data only}
- Nyamwaya D, Morgan R, Lukhando M, Fisher A, Ndhlovu L. Expanding family planning delivery systems using traditional health practitioners: an operations research study in rural Kenya. Unpublished report, Population Council's Africa Operations Research and Technical Assistance Project, Nairobi, Kenya 1993.
O'Rourke 1994 {unpublished data only}
- O'Rourke KM. The effect of a traditional birth attendant training program on obstetrical practices and perinatal mortality in rural Guatemala [dissertation]. Amerherst: University of Massachusetts, 1994. [Google Scholar]
Olusanya 2011 {published data only}
- Olusanya BO, Inem VA, Abosede OA. Infants delivered in maternity homes run by traditional birth attendants in Urban Nigeria: a community‐based study. Health Care for Women International 2011;32(6):474‐91. [DOI: 10.1080/07399332.2011.565531] [DOI] [PubMed] [Google Scholar]
Prata 2005 {published data only}
- Prata N, Mbaruku G, Campbell M, Potts M, Vahidnia F. Controlling postpartum hemorrhage after home births in Tanzania. International Journal of Gynecology & Obstetrics 2005;90:51‐5. [0122] [DOI] [PubMed] [Google Scholar]
Rahman 1982 {published data only}
- Rahman S. The effect of traditional birth attendants and tetanus toxoid in reduction of neonatal mortality. Journal of Tropical Pediatrics 1982;28(4):163‐5. [DOI] [PubMed] [Google Scholar]
Rowen 2009 {published data only}
- Rowen T, Prata N, Passano P. Evaluation of a traditional birth attendant training programme in Bangladesh. Midwifery 2011;27(2):229‐36. [DOI: 10.1016/j.midw.2009.06.003] [DOI] [PubMed] [Google Scholar]
Saravanan 2011 {published data only}
- Saravanan S, Turrell G, Johnson H, Fraser J, Patterson C. Traditional birth attendant training and local birthing practices in India. Evaluation and Program Planning 2011;34(3):254‐65. [DOI] [PubMed] [Google Scholar]
Satishchandra 2009 {published data only}
- Satishchandra DM, Naik VA, Wantamutte AS, Mallapur MD. Impact of training of traditional birth attendants on the newborn care. Indian Journal of Pediatrics 2009;76(1):33‐6. [DOI] [PubMed] [Google Scholar]
Singhal 2001 {published data only}
- Singhal N, McMillan DD. Problem‐based teaching of birth attendants in the Philippines. Health Care for Women International 2001;22:569‐83. [0127] [DOI] [PubMed] [Google Scholar]
Theron 1999 {published data only}
- Theron GB. Effect of the maternal care manual of the perinatal education programme on the ability of midwives to interpret antenatal cards and partograms. Journal of Perinatology 1999;19(6):432‐5. [0008] [DOI] [PubMed] [Google Scholar]
Theron 2000 {published data only}
- Theron GB. Improved practical skills of midwives practicing in the Eastern Cape Province of the Republic of South Africa through the study of a self‐education manual. Journal of Perinatology 2000;3:184‐8. [0004] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Costello 2011 {published data only}
- Costello A. Improving newborn care and outcomes through training of traditional birth attendants in use of bag and mask resuscitation in three rural districts in Bangladesh. Current Controlled Trials (www.controlled‐trials.com) (accessed 27 June 2011).
Matendo 2011 {published data only}
- Matendo R, Engmann C, Ditekemena J, Gado J, Tshefu A, Kinoshita R, et al. Reduced perinatal mortality following enhanced training of birth attendants in the Democratic Republic of Congo: a time‐dependent effect. BMC Medicine 2011;9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2011 {published data only}
- Morrison J, Tumbahangphe KM, Budhathoki B, Neupane R, Sen A, Dahal K, et al. Community mobilisation and health management committee strengthening to increase birth attendance by trained health workers in rural Makwanpur, Nepal: study protocol for a cluster randomised controlled trial. Trials 2011;12:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasha 2010 {published data only}
- Pasha O, Goldenberg RL, McClure EM, Saleem S, Goudar SS, Althabe F, et al. Communities, birth attendants and health facilities: a continuum of emergency maternal and newborn care (the Global Network's EmONC trial). BMC Pregnancy and Childbirth 2010;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bang 1994
- Bang AT, Bang RA, Sontakk PG, SEARCH Team. Management of childhood pneumonia by traditional birth attendants. Bulletin of the World Health Organization 1994;72(6):897‐905. [PMC free article] [PubMed] [Google Scholar]
Bergstrom 2001
- Bergstrom S, Goodburn E. The role of traditional birth attendants in the reduction of maternal mortality. In: Brouwere V, Lerberghe VW editor(s). Safe Motherhood Strategies: a Review of the Evidence, Studies in Health Services Organization and Policy. Vol. 17, Antwerp: ITG Press, 2001. [Google Scholar]
Buekens 2003
- Buekens P. Review of: traditional birth attendant training effectiveness: a meta‐analysis, LM Sibley, TA Sipe. International Journal of Gynecology & Obstetrics 2003;83:121‐2. [DOI] [PubMed] [Google Scholar]
Darmstadt 2009
- Darmstadt GL, Lee AC, Cousens S, Sibley L, Bhutta ZA Donnay F, et al. 60 million non‐facility births: who can deliver in community settings to reduce intrapartum‐related deaths?. International Journal of Gynecology & Obstetrics 2009;107(Suppl 1):S89–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Filippi 2000
- Filippi V, Ronsmans C, Gandaho T, Graham W, Alihonou E, Santos P. Women's self‐report of severe (near‐miss) obstetric complications in Benin. Studies in Family Planning 2000;31(4):309‐24. [DOI] [PubMed] [Google Scholar]
Fortney 1997a
- Fortney JA, Smith JB. Training of Traditional Birth Attendants: Issues and Controversies. Durham, NC: Family Health International and United Nations Children's Fund, 1997. [Google Scholar]
Fortney 1997b
- Fortney JA, Smith JB. Methodological Issues in Research on Traditional Birth Attendants. Durham, NC: Family Health International and United Nations Children's Fund, 1997. [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. http://www.cochrane.org/resources/handbook/hbook.htm (accessed 31 May 2005).
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Khan 2006
- Khan KS, Say L, Gülmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066‐74. [DOI] [PubMed] [Google Scholar]
Kumar 1998
- Kumar R. Effectiveness of training traditional birth attendants for management of asphyxia neonatorum using resuscitation equipment. Prenatal and Neonatal Medicine 1998;3(2):225‐60. [Google Scholar]
Lassi 2010
- Lassi ZS, Haider BA, Bhutta ZA. Community‐based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD007754.pub2] [DOI] [PubMed] [Google Scholar]
Lawn 2005
- Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: who, when? where? why?. Lancet 2005;365(9462):891‐900. [DOI] [PubMed] [Google Scholar]
Levitt 1997
- Levitt MJ. When the training of TBAs is cost effective: trained TBAs and neonatal essential care in South Asia. Improving health of the neonate and infant in developing countries (conference draft). London: Kathmandu Mother and Infant Research Activities (MIRA) and Institute of Child Health, University College Medical School, 1997.
Maine 1992
- Maine D. Cost‐effectiveness of different safe motherhood options. Safe Motherhood Supplement. Geneva: World Health Organization, 1992. [Google Scholar]
Maine 1993
- Maine D. Safe Motherhood Programs: Options and Issues. New York: Columbia University School of Public Health, Prevention of Maternal Mortality, Center for Population and Family Health, 1993. [Google Scholar]
Marsh 2003
- Marsh D, Sadruddin S, Fickree FF, Krishnan C, Darmstadt G. Validation of verbal autopsy to determine cause of death in 137 neonatal deaths in Karachi, Pakistan. Pediatric and Perinatal Epidemiology 2003;17(2):132‐42. [DOI] [PubMed] [Google Scholar]
Measure DHS 2002
- Measure Demographic and Health Surveys. Macro International. http://www.measuredhs.com/ (accessed 2011) 2002.
Measure DHS 2011
- Measure Demographic and Health Surveys. Macro International. http://www.measuredhs.com/ (accessed 2011) 2011.
Miller 2003
- Miller S, Sloan N, Winikoff B, Langer A, Fikree F. Where is the "E" in MCH? The need for an evidence‐based approach in safe motherhood. Journal of Midwifery & Women's Health 2003;48(1):10‐8. [DOI] [PubMed] [Google Scholar]
Osrin 2010
- Osrin D, Prost A. Perinatal interventions and survival in resource‐poor settings: which work, which don't, which have the jury out?. Archives of Disease in Childhood 2010;95(12):1039‐46. [DOI: 10.1136/adc.2009.179366] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ronsmans 1997
- Ronsmans C, Achadi E, Cohen S, Zazri A. Women's recall of obstetric complications in South Kalimantan, Indonesia. Studies in Family Planning 1997;28:203‐14. [PubMed] [Google Scholar]
Sibley 2002
- Sibley LM, Sipe TA. Effectiveness of Traditional Birth Attendant Training: a Meta‐analysis. Final Technical Report. Washington, DC: Academy for Educational Development (SARA Project), 2002. [Google Scholar]
Sibley 2004a
- Sibley L, Sipe TA, Koblinsky M. Does TBA training increase use of professional antenatal care services: a review of the evidence. Journal of Midwifery & Women's Health 2004;49(4):298‐305. [DOI] [PubMed] [Google Scholar]
Sibley 2004b
- Sibley L, Sipe TA, Koblinsky M. Does TBA training improve referral of obstetric complications: a review of the evidence. Social Science and Medicine 2004;59(8):1757‐68. [DOI] [PubMed] [Google Scholar]
Sibley 2004c
- Sibley L, Sipe TA. Traditional birth attendant training and pregnancy outcomes: what can meta‐analysis tell us?. Midwifery 2004;20(1):51‐60. [DOI] [PubMed] [Google Scholar]
Starrs 1998
- Starrs A, Inter‐Agency Group for Safe Motherhood (IAGSM). The safe motherhood action agenda: priorities for the next decade. Report of the safe motherhood technical consultation; 1997 October 18‐23; Colombo, Sri Lanka. New York: Family Care, International and the Inter‐Agency Group for Safe Motherhood, 1998.
Tinker 1993
- Tinker A, Koblinsky M. Making Motherhood Safe. Washington, DC: World Bank, 1993. [Google Scholar]
UNICEF 1997
- United Nations International Children's Education Fund. Report on the consultation on attendance at birth: community birth attendants; 1997 June 9‐10. New York: Health Section, Programme Division, United Nations Children's Fund, 1997.
WHO 1992
- World Health Organization. Traditional birth attendants: a joint WHO/UNICEF/UNFPA statement. Geneva: World Health Organization, 1992. [Google Scholar]
WHO 1995
- World Health Organization. Verbal autopsies for maternal death. WHO/FHE/MSM/95.15. Geneva: World Health Organization, 1995. [Google Scholar]
WHO 1999
- World Health Organization. A standard verbal autopsy method for causes of death in infants and children. WHO/CDS/CSR/ISR/99/4. Geneva: World Health Organization, 1999. [Google Scholar]
WHO 2006
- World Health Organization. Neonatal and Perinatal Mortality: Country, Regional and Global Estimates. Geneva: WHO, 2006. [Google Scholar]
WHO 2007
- World Health Organization. Maternal Mortality in 2005: Estimates Developed by WHO, UNICEF, UNFPA, and the World Bank. Geneva: WHO, 2007. [Google Scholar]
WHO 2010a
- World Health Organization. Optimizing the delivery of key interventions to attain MDGs 4 and 5, Optimize4 MNH: background document for the first expert scoping meeting to develop WHO recommendations to optimise health workers’ roles to improve maternal and newborn health. Geneva: WHO, 6‐8 December 2010. [Google Scholar]
WHO 2010b
- World Health Organization. Trends in Maternal Mortality: 1990 to 2008: Estimates Developed by WHO, UNICEF, UNFPA and the World Bank. Geneva: WHO, 2010. [Google Scholar]
References to other published versions of this review
Sibley 2007
- Sibley LM, Sipe TA, Brown CM, Diallo MM, McNatt K, Habarta N. Traditional birth attendant training for improving health behaviours and pregnancy outcomes. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005460.pub2] [DOI] [PubMed] [Google Scholar]


