Abstract
Ixodes scapularis can be infected with Borrelia burgdorferi, Anaplasma phagocytophilum, Bartonella spp., Babesia microti, and Rickettsia spp., including spotted-fever group Rickettsia. As all of these microorganisms have been reported in Maryland, the potential for these ticks to have concurrent infections exists in this region. To assess the frequency of these complex infections, 348 I. scapularis nymphs collected in 2003 were screened for these microorganisms by PCR with positives being confirmed by DNA sequencing. Borrelia burgdorferi was detected in 14.7% of nymphs. Anaplasma phagocytophilum (0.3%), Rickettsia spp. (19.5%), and an uncategorized agent (0.9%) was also detected. Dual infections were detected with B. burgdorferi and Rickettsia spp. as well as a triple infection with B. burgdorferi, Rickettsia spp., and an uncategorized agent. Infections with B. burgdorferi and Rickettsia spp. were statistically independent of one another. However, infection with B. burgdorferi and any one of these other microorganisms appears to occur more frequently than by chance alone, probably as a result of shared enzootic cycles. This study confirms that multiple microorganisms co-circulate with B. burgdorferi in I. scapularis in Maryland and demonstrates that Rickettsia spp. and B. burgdorferi circulate independently and at nearly equal frequencies, while A. phagocytophilum and other unrecognized organisms are less common.
Keyword Index: Ixodes scapularis, Borrelia burgdorferi, Anaplasma phagocytophilum, Rickettsia spp., co-circulating
Introduction
The blacklegged tick Ixodes scapularis is an established vector of several human pathogens including bacteria, protozoa, and viruses (Burgdorfer and Gage 1986, Ebel et al. 2000, Adelson et al. 2004). Borrelia burgdorferi, the causative spirochete of Lyme disease, has historically been the most notorious pathogen associated with this tick due to the number of human cases. However, other microorganisms can also be present in I. scapularis. Although the frequency of transmission and rate of infection with other pathogens is considerably lower than that of B. burgdorferi in most geographic areas, these organisms are often maintained in an enzootic cycle involving I. scapularis and rodent hosts such as Peromyscus leucopus. Due to the similarities in the maintenance cycles of these organisms, concurrent infections with B. burgdorferi have been reported in ticks, but the effects of these complex infections on the transmission and pathogen maintenance of these agents are not completely understood (Halos et al. 2005). Other microorganisms with similar maintenance cycles are Anaplasma phagocytophilum (causing human granulocytic anaplasmosis [HGA]), Babesia microti (causing babesiosis), Bartonella spp., and Rickettsia spp. (Spielman 1976, Pancholi et al. 1995, Richter et al. 1996, Magnarelli et al. 1997, Raoult and Roux 1997, Schouls et al. 1999, Dumler et al. 2001, Sanogo et al. 2003). Study of these complex infections in I. scapularis will assist in determining whether infection with one agent affects the acquisition of other organisms and aid in understanding the effect that concurrent infection has on the transmission of individual agents.
Due to the expansive endemic regions for Lyme disease in the northern hemisphere, many of these other tick-borne organisms have a high potential to co-circulate with B. burgdorferi in vector populations. A recent review of co-infection studies determined that dual infection in Ixodes ticks is highest in areas where Lyme disease is endemic (Swanson et al. 2006). In the United States, studies have reported between 1% and 28% of Ixodes ticks are dually infected with any two of B. burgdorferi, B. microti, or A. phagocytophilum (Swanson et al. 2006). In I. ricinus collected in France, two pathogens were detected in 7.6% of ticks (Halos et al. 2005). In that study, any of three co-circulating pathogens (B. burgdorferi, Babesia spp., Bartonella spp.) were detected in adult ticks at a significantly higher rate than nymphs. In northern New Jersey, more than one pathogen (B. burgdorferi, B. microti, A. phagocytophilum, or Bartonella spp.) was detected by PCR in 14% of I. scapularis; 8.4% of these were concurrently infected with B. burgdorferi and Bartonella spp. (Adelson et al. 2004). Although these previous two studies, as well as others, have detected Bartonella spp., including B. henselae, in Ixodes spp., transmission of Bartonella spp. has not been demonstrated (Chang et al. 2001, Sanogo et al. 2003, Adelson et al. 2004, Morozova et al. 2004, Halos et al. 2005, Holden et al. 2006). In Delaware, where the prevalence of A. phagocytophilum in ticks is 4%, the density of I. scapularis has increased by 34% in a 10-year time span (Curran et al. 2000), dramatically increasing chances for humans to have contact with ticks and potentially be exposed to these tick-borne pathogens. Concurrently infected ticks also pose a potential increased risk to humans. Ticks collected from mice that are concurrently infected with B. burgdorferi and A. phagocytophilum have a higher infection prevalence with A. phagocytophilum than those from A. phagocytophilum-only infected mice (Thomas et al. 2001). Interestingly, ticks can efficiently co-transmit these pathogens (Levin and Fish 2000).
Studies of concurrent infection in ticks, mice, and humans have primarily focused on B. burgdorferi, A. phagocytophilum, and B. microti. Given the biology of Bartonella transmission and the apparent increase in Rickettsia spp. and unidentified endosymbionts and other microorganisms detected in ticks, concurrent infection in I. scapularis with these agents and B. burgdorferi is likely to occur in Lyme disease endemic regions. Understanding the effect that concurrent infections have on the transmission and maintenance of B. burgdorferi is important in understanding the role that these complex infections play in the epidemiology of Lyme disease and other potential human pathogens. The prevalence of these concurrent infections in the mid-Atlantic region of North America is unknown. However, B. microti is known to be endemic in regions immediately north of Maryland and human cases of HGA have been reported in Maryland. Therefore, a preliminary survey of I. scapularis nymphs collected on the Eastern Shore of Maryland was conducted to determine the prevalence and geographic distributions of B. burgdorferi, A. phagocytophilum, B. microti, Bartonella spp., and Rickettsia spp. in the region. We hypothesized that the presence of other pathogens (A. phagocytophilum, Rickettsia spp., Bartonella spp, B. microti), either as a competing infection or as a co-circulating infection, would affect the prevalence rate of B. burgdorferi in I. scapularis nymphs.
Materials and Methods
Tick collection
Questing nymphs were collected between June and August 2003 by flagging a 50 m × 200 m grid at 27 forested (deciduous, pine, or mixed) locations on the Eastern Shore of Maryland (Figure 1). At each site, flagging for questing ticks was conducted for approximately 45 min within the grid. Flags were examined for ticks every 15 m, and ticks were placed in collection vials. In the lab, ticks were morphologically identified and stored in 1.5 ml centrifuge tubes at -20º C until further processing.
Figure 1.
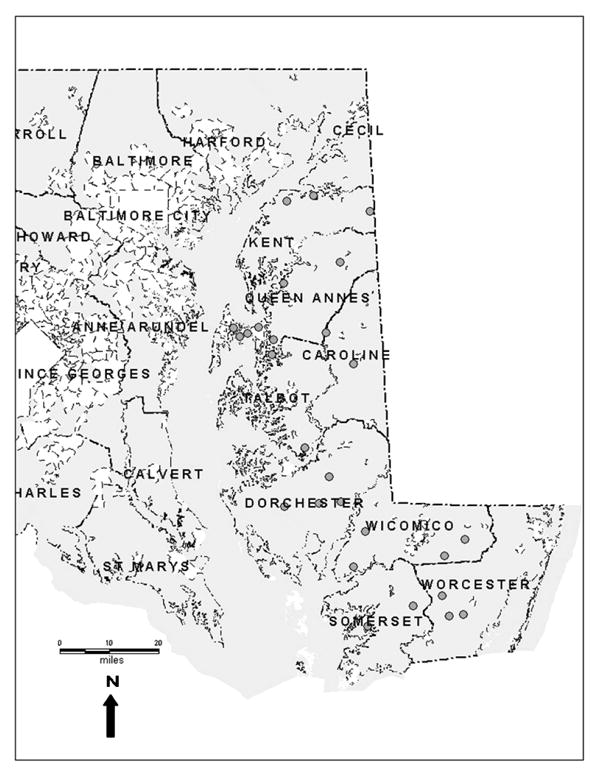
Tick collection sites on the Eastern Shore of Maryland.
DNA extraction
DNA was extracted from each I. scapularis nymph using either a modified hexadecyltrimethylammoniumbromide (CTAB) extraction (Black et al. 1997, Anderson et al. 2004) or a QIAamp DNA MiniKit (Qiagen, Inc., Valencia, CA). Due to concerns about the long-term DNA stability of the extractions, the QIAamp DNA MiniKit was utilized to produce more stable extractions. The manufacturer's protocol for tissue extraction was followed for the QIAamp kit with the addition of an extended proteinase K digestion of approximately 15 h (overnight) and a fnal elution volume of 30 µl.
Microorganism detection by PCR
The presence of B. burgdorferi was determined by PCR for two target gene fragments: flagellin (flaB) and outer surface protein C (ospC). The nested PCR for flaB followed the protocol of Johnson et al. (1992). The exterior reaction was conducted in a 25 µl volume. The interior reaction was conducted in 50 µl, using 1 µl of the exterior product as template for the second reaction. The ospC PCR amplified the 3′ end of the ospC fragment (Wang et al. 1999, Alghaferi et al. 2005, Anderson and Norris 2006). This semi-nested protocol used both exterior primers for the first round PCR and the interior forward and exterior reverse primers for the second round PCR. Only 1.5 µl (˜ 22 ng) of template DNA was used for the first round and 0.5 µl of exterior product was used as the template for the second round. Both the first and second rounds of PCR were conducted in 50 µl reactions. The positive control used was a pure B. burgdorferi culture provided by J.S. Dumler, Johns Hopkins University School of Medicine.
Anaplasma phagocytophilum 16S rRNA was detected using primers ge9f and ge10r described by Chen et al. (1994). Each 50 µl reaction contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% w/v gelatin, 400 µM each dNTP, 100 pmol each primer, 1 U Taq polymerase, and 1.0 µl (˜ 15 ng) template DNA. Cycling conditions involved an initial denaturation for 3 min at 95º C followed by 35 cycles of denaturation for 30 s at 94º C, annealing for 30 s at 55º C, and extension for 1 min at 72º C. The expected product size was 919 bp. The positive control was extracted genomic DNA provided by J.S. Dumler, The Johns Hopkins University School of Medicine.
Detection of a 238-bp fragment of the small subunit rRNA of Babesia microti was performed following the protocol of Persing et al. (1992) using primers Bab1 and Bab4. Modifications to the reaction mixture were 2.0 µl (˜ 30 ng) template DNA and 1 U Taq polymerase in each 20 µl reaction. Positive control material was extracted genomic DNA provided by E. Hofmeister, United States Geological Survey.
Bartonella spp. were identified through PCR amplification of a fragment of the citrate synthase (gltA) gene using primers BhCS.781p and BhCS.1137n as described by Norman et al. (1995). Each 50 µl reaction mixture was modified to contain 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% w/v gelatin, 400 µM each dNTP, 50 pmol each primer, 1 U Taq polymerase, and 1 µl (˜ 15 ng) template DNA. Cycling conditions were an initial denaturation for 5 min at 95º C followed by one cycle of denaturation for 20 s at 95º C, annealing for 30 s at 37º C, and extension for 2 min at 72º C followed by 35 cycles of denaturation for 20 s at 95º C, annealing for 30 s at 42º C, and extension for 2 min at 72º C. Bartonella elizabethae culture (ATCC 49927) obtained from ATCC (Manassas, VA) was used as the positive control.
Detection of a citrate synthase gene (gltA) fragment from Rickettsia spp. was used to determine the presence of Rickettsia spp. The primers (RpCS.877p, RpCS.1258n) and cycling conditions were previously described by Regnery et al. (1991). Each 50 µl reaction mixture contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% w/v gelatin, 400 µM each dNTP, 50 pmol each primer, ˜ 2.5 U Taq polymerase, and 1.5 µl (˜ 22 ng) template DNA. Samples which were positive for Rickettsia spp. were further tested for the presence of the rOmpA gene of spotted fever group (SFG) rickettsiae as described by Regnery et al. (1991), using primers Rr190.70p and Rr190.602n in a 50 µl reaction. Extracted genomic DNA for positive controls for both Rickettsia spp. and SFG- rickettsiae were provided by W. Nicholson, Centers for Disease Control and Prevention.
All PCR amplifications were completed on a PTC-200 thermal cycler (MJ Research, Inc., Watertown, MA). Positive and negative controls (no template) were performed for all PCR reactions. Products were visualized on 2% agarose gels using ethidium bromide. A sample was considered positive if a band of the expected size was visualized on agarose. Positive PCR results for A. phagocytophilum, Rickettsia spp., and Bartonella spp. were confirmed through direct sequencing and comparison to sequences available on GenBank. For samples positive for the Rickettsia spp. and Bartonella spp. gltA, sample sequences and sequences available on GenBank were aligned using BioEdit (Hall 1999) and ClustalX (Thompson et al. 1997). Phylogenetic trees were constructed in ClustalX using the Neighbor Joining method and 1000 bootstrap replicates to evaluate the strength of the clustering analysis.
Data analysis
To determine whether the infections with B. burgdorferi and the other pathogens were statistically independent, the odds ratio and 95% confidence intervals were calculated using Epi Info™ Version 3.3.2 (Dean et al. 2002).
Results
A total of 348 questing I. scapularis nymphs were collected from the Eastern Shore of Maryland during the summer months of 2003. Nymphal I. scapularis were collected at only 66.7% (18/27) of the sites. The majority of the ticks (n=333, 95.7%) were collected from 14 sites located in counties of the Upper Eastern Shore (Talbot, Caroline, Queen Anne's, and Kent counties) (Table 1). Although substantial numbers were collected, none of the adult, nymphal, or larval metastriate ticks was included in this analysis. The 27 forested sites were composed of deciduous trees (n=10, 37%), pine trees (n=2, 7.4%), or a mixture of deciduous and pine trees (n=15, 55.6%) (Table 1). Ticks, including nymphal I. scapularis, were collected at all three habitat types.
Table 1.
Summary of tick collections and PCR status of I. scapularis nymphs on the Eastern Shore of Maryland.
| County | Site Number |
Habitat Type |
Ixodes scapularis nymphs |
Ixodes scapularis adults |
Dermacentor variabilis adults |
Amblyomma americanum adults |
Metastriate nymphs |
Larvae (Y/N) |
B.b. |
Rickettsia spp. (SFG) |
A.p. |
Bartonella spp. |
B.m. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Sites Total | 348 | 7 | 109 | 276 | 1973 | 51 | 68 (43) | 1 | 3 | 0 | |||
| Caroline | 108 | Deciduous | 40 | 2 | 9 | 7 | 79 | N | 6 | 4 (2) | 0 | 0 | 0 |
| 109 | Mixed forest | 4 | 0 | 1 | 4 | 72 | N | 0 | 0 | 0 | 0 | 0 | |
| Total | 44 | 2 | 10 | 11 | 151 | 6 | 4 (2) | 0 | 0 | 0 | |||
| Dorchester | 103 | Deciduous | 2 | 0 | 2 | 8 | 27 | N | 0 | 0 | 0 | 0 | 0 |
| 104 | Mixed forest | 2 | 1 | 6 | 3 | 38 | N | 0 | 0 | 0 | 0 | 0 | |
| 126 | Mixed forest | 0 | 0 | 3 | 0 | 41 | Y | 0 | 0 | 0 | 0 | 0 | |
| 127 | Mixed forest | 0 | 0 | 0 | 0 | 139 | Y | 0 | 0 | 0 | 0 | 0 | |
| Total | 4 | 1 | 11 | 11 | 245 | 0 | 0 | 0 | 0 | 0 | |||
| Kent | 110 | Pine | 60 | 2 | 8 | 4 | 86 | N | 8 | 4 (4) | 1 | 0 | 0 |
| 111 | Deciduous | 0 | 0 | 2 | 0 | 0 | N | 0 | 0 | 0 | 0 | 0 | |
| 112 | Deciduous | 0 | 1 | 0 | 1 | 0 | N | 0 | 0 | 0 | 0 | 0 | |
| Total | 60 | 3 | 10 | 5 | 86 | 8 | 4 (4) | 1 | 0 | 0 | |||
| Queen Anne's | 101 | Mixed forest | 69 | 1 | 48 | 156 | 394 | N | 17 | 29 (24) | 0 | 2 | 0 |
| 102 | Mixed forest | 62 | 0 | 3 | 9 | 33 | N | 5 | 3 (2) | 0 | 0 | 0 | |
| 105 | Mixed forest | 15 | 0 | 1 | 3 | 4 | N | 1 | 0 | 0 | 0 | 0 | |
| 106 | Mixed forest | 33 | 0 | 13 | 13 | 125 | N | 9 | 3 (2) | 0 | 0 | 0 | |
| 107 | Deciduous | 28 | 0 | 1 | 45 | 351 | N | 1 | 1 (0) | 0 | 1 | 0 | |
| 114 | Deciduous | 17 | 0 | 3 | 0 | 19 | Y | 1 | 17 (5) | 0 | 0 | 0 | |
| 124 | Deciduous | 0 | 0 | 0 | 0 | 3 | N | 0 | 0 | 0 | 0 | 0 | |
| 125 | Deciduous | 0 | 0 | 0 | 0 | 8 | N | 0 | 0 | 0 | 0 | 0 | |
| Total | 224 | 1 | 69 | 226 | 937 | 34 | 53 (33) | 0 | 3 | 0 | |||
| Somerset | 120 | Deciduous | 1 | 0 | 0 | 1 | 280 | Y | 0 | 1 (0) | 0 | 0 | 0 |
| 121 | Deciduous | 3 | 0 | 0 | 2 | 223 | Y | 1 | 1 (0) | 0 | 0 | 0 | |
| Total | 4 | 0 | 0 | 3 | 503 | 1 | 2 (0) | 0 | 0 | o | |||
| Talbot | 113 | Mixed forest | 5 | 0 | 1 | 8 | 39 | Y | 0 | 1 (0) | 0 | 0 | 0 |
| Total | 5 | 0 | 1 | 8 | 39 | 0 | 1 (0) | 0 | 0 | o | |||
| Wicomico | 115 | Mixed forest | 2 | 0 | 2 | 1 | 28 | N | 1 | 2 (2) | 0 | 0 | 0 |
| 116 | Mixed forest | 2 | 0 | 1 | 0 | 33 | N | 0 | 1 (1) | 0 | 0 | 0 | |
| 117 | Mixed forest | 1 | 0 | 2 | 9 | 91 | N | 1 | 0 | 0 | 0 | 0 | |
| 118 | Pine | 0 | 0 | 0 | 0 | 10 | Y | 0 | 0 | 0 | 0 | 0 | |
| Total | 5 | 0 | 5 | 10 | 162 | 2 | 3 (3) | 0 | 0 | 0 | |||
| Worcester | 119 | Mixed forest | 0 | 0 | 1 | 0 | 36 | Y | 0 | 0 | 0 | 0 | 0 |
| 122 | Mixed forest | 2 | 0 | 1 | 0 | 17 | Y | 0 | 1 (1) | 0 | 0 | 0 | |
| 123 | Mixed forest | 0 | 0 | 1 | 2 | 20 | Y | 0 | 0 | 0 | 0 | 0 | |
| Total | 2 | 0 | 3 | 2 | 73 | 0 | 1 (1) | 0 | 0 | 0 |
Abbreviations: B.b. - Borrelia burgdorferi; A.p. - Anaplasma phagocytophilum ; B.m. - Babesia microti
Extractions of I. scapularis nymphs were considered to be positive for B. burgdorferi if the PCR results were positive for either flaB, ospC, or both. The B. burgdorferi infection prevalence was 14.7% (51/348) in I. scapularis. Of the 51 positive samples, 10 (19.6%) were positive for flaB only, 18 (35.3%) were positive for ospC only, and 23 (45.1%) were positive for both. The infection prevalence was not significantly associated with the county of collection (χ2=4.97, p=0.55), even when the counties were clustered as Upper or Lower Eastern Shore (Fisher's exact=0.21). Infected I. scapularis were present in all three forest types. Infection prevalence with each organism is shown in Table 1.
Anaplasma phagocytophilum 16S rRNA genes were detected in only 0.3% (1/348) of the nymphs. The presence of A. phagocytophilum was confirmed through sequencing of the amplicon and comparison to sequences available on GenBank using BLAST (Altschul et al. 1990) and revealed 98% identity with the 16S rRNA gene of A. phagocytophilum.
Using the Bartonella spp. primers, a PCR product of the expected size (356 bp) was obtained from three (0.9%) of the nymphs. These samples were sequenced directly using the primers described by Norman et al. (1995), but the recovered sequences did not cluster with those of Bartonella species available on GenBank (Figure 2). The sequences have been deposited into GenBank (Accession numbers EF662053-EF662055). Of these three nymphs, one was positive for both Borrelia burgdorferi and a non-SFG Rickettsia spp. DNA. Another tested positive for both Rickettsia spp. gltA and ompA.
Figure 2.
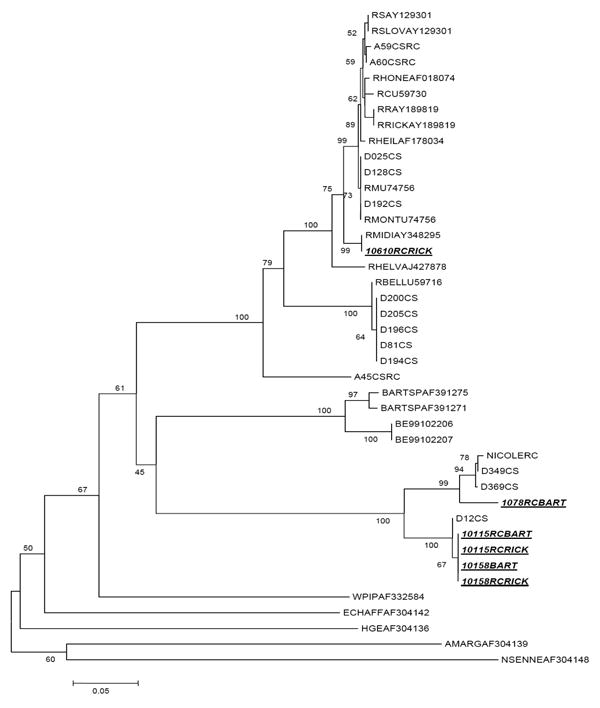
Neighbor-joining phylogenetic tree based on alignment of citrate synthase gene fragments from Rickettsia spp. and Bartonella spp. Samples from this study are underlined and italicized. (GenBank Accession Numbers EF662053-EF662058).
Amplicons were obtained by PCR for Rickettsia spp. gltA in 19.5% (68/348) of the nymphs. These positive samples were subsequently analyzed for SFG-rickettsiae. The infection prevalence of SFG-rickettsiae was 63.2% (43/68) of the rickettsia-positive nymphs. Of the 68 nymphs that were positive for Rickettsia spp., 22.1% (15/68) were also positive for B. burgdorferi. The odds ratio for concurrent infection with B. burgdorferi and Rickettsia spp. was 1.92 (95% CI: 0.93, 3.94) and not statistically significant, indicating that the infections are independent of each other. Simultaneous infection with SFG-rickettsiae and B. burgdorferi was detected in 11/15 (73.3%) of the nymphs, a relationship that was also not statistically significant (OR: 1.80; 95% CI: 0.51, 6.43). Representatives of Rickettsia spp. gltA-positive samples were sequenced to confirm the PCR results. Two had no full-length GenBank matches, with all matches being fragments less than 65 nucleotides. The sequences were similar to two derived using the Bartonella-specific primers. The sequence from the third sample had 99% identity with Rickettsia spp. gltA available on GenBank. This third sequence clustered with Rickettsia “midichlorii” (AY348295) and R. helvetica (AJ427878) (Figure 2). These sequences have been deposited into GenBank (Accession numbers EF662056-EF662058). None of the 348 nymphs were positive for Babesia microti.
The association between Borrelia burgdorferi infection and detection of any other agent (A. phagocytophilum, Rickettsia spp., or Bartonella-like) produced an odds ratio of 2.94 (95% CI: 1.41, 6.09). This is statistically significant, indicating a trend towards concurrent infection.
Discussion
Several microorganisms are known to infect I. scapularis. This study confirms that at least three found in I. scapularis on the Eastern Shore in Maryland can co-circulate with B. burgdorferi. The most commonly detected microorganisms in I. scapularis nymphs were B. burgdorferi and Rickettsia spp., both individually and as co-circulating microorganisms. An unidentified agent or agents detected with Bartonella primers and A. phagocytophilum were rarely observed, and Babesia microti was not detected in any nymphs.
Our study relied on data from nymphal I. scapularis since we collected ticks during the summer months (June, July, and August), the times when nymphal ticks are most active (Wilson and Spielman 1985). Adults become active in the early spring and late summer and were therefore not collected at a high frequency. In addition, nymphs are considered to be more important epidemiologically in terms of transmission than are larvae or adults (Orloski et al. 2000).
The overall prevalence of B. burgdorferi in nymphal I. scapularis was 14.7%. In the northern counties of Queen Anne's, Kent, and Caroline, where 94% of the nymphs were collected, the prevalence was geographically stable, ranging from 13.3% to 15%. In the other fve counties of the Eastern Shore (Talbot, Wicomico, Worcester, Somerset, and Dorchester), the sample sizes were quite small, with fewer than ten nymphs collected per county. The reasons for the low density of I. scapularis in the southern Eastern Shore need to be determined to fully understand B. burgdorferi ecology in this region. Potential explanations include the lack of adequate tick habitat, the lack of suitable tick hosts or pathogen reservoirs, soil composition, or other abiotic factors.
The regional infection rate of B. burgdorferi in nymphal I. scapularis (14.7%) is low compared to nymphal infection rates from the western coastal plain of Maryland (40% to 98%) (Anderson 2004). These infection rates seem further distorted considering that the human incidence of Lyme disease in the northern part of this collection region can be as high as 80 cases per 100,000 persons while the incidence in the western coastal plains is usually less than 50 cases per 100,000 persons (Frank et al. 2002). This may be due to the small sample size; however, the prevalence of B. burgdorferi infection is consistent with the previously reported prevalence rate of 15% in I. scapularis collected from deer on the Eastern Shore (Amerasinghe et al. 1992, Amerasinghe et al. 1993).
Other researchers have examined the presence of Rickettsia spp. in Dermacentor variabilis in Maryland and found the prevalence of SFG-rickettsia to range from 3.8% to 8.6% (Schriefer and Azad 1994, Ammerman et al. 2004). Rickettsia spp. have been reported in I. scapularis from both laboratory colonies and from field specimens collected in Connecticut (Magnarelli et al. 1991, Magnarelli and Swihart 1991, Magnarelli et al. 1995, Noda et al. 1997). Our results not only establish the presence of Rickettsia spp. in field specimens of I. scapularis in Maryland, but also establish that the prevalence of SFG-rickettsiae in I. scapularis is higher than reported in D. variabilis (Schriefer and Azad 1994, Ammerman et al. 2004). The high prevalence of Rickettsia spp.-positive ticks among our samples (19.5%) and lack of epidemiologically associated human disease on the Eastern Shore of Maryland supports the hypothesis put forth by Noda et al. (1997) and Weller et al. (1998) that the rickettsial organisms detected are endosymbionts of I. scapularis rather than human pathogens.
Human granulocytic anaplasmosis was not a reportable disease in Maryland until 2002, so long term data regarding human cases is not available. However, the prevalence of infection in Maryland is low (0.4%-1.8%), based on samples from either blood donors or from patients with concurrent Lyme disease (personal communication, J.S. Dumler). Based on serological data from samples collected between 1987 and 1997, the estimated prevalence of HGA in Maryland was 1.6% (Comer et al. 1999). In 2001-2002, the incidence of HGA in Maryland was reported to be 0.46 to 1.62 cases per million people (Demma et al. 2005). Serological data supports the presence of A. phagocytophilum antibodies in the reservoir P. leucopus in Maryland with a prevalence of 6.7% (Nicholson et al. 1998). In mid-Atlantic and northeastern states, the prevalence of antibodies in P. leucopus ranges from 4.3% in New Jersey to 23.0% in New York (Nicholson et al. 1998). In the eastern United States, the prevalence of A. phagocytophilum in I. scapularis ranges from 1.6% in the southeast to between 4% and 90.9% in the northeast (Magnarelli et al. 1995, Telford et al. 1996, Schwartz et al. 1997, Varde et al. 1998, Curran et al. 2000, Fang et al. 2002). The A. phagocytophilum prevalence of 0.3% observed in I. scapularis nymphs in this study is much lower than reported from surrounding regions, but is consistent with the low frequency of clinical HGA observed in Maryland. It is also consistent with results from other surveys conducted in our laboratory, where A. phagocytophilum has not been detected in approximately 1,000 ticks screened (unpublished data). Our use of a single-step PCR protocol rather than a nested protocol may have resulted in a conservative prevalence of A. phagocytophilum since nested PCR has a greater sensitivity (Massung and Slater 2003). However, in I. scapularis collected in 1997 and 1998 throughout Maryland, Delaware, Pennsylvania, and New Jersey, only three of 979 (0.3%) ticks were positive for A. phagocytophilum (Bunnell 1999). The consistency between our data and that of Bunnell indicates that infection with A. phagocytophilum in I. scapularis is not common but appears to be regionally stable.
Bartonella spp., including human pathogens, have been detected in I. pacificus (Chang et al. 2001); however, maintenance in the ticks and transmission to mammals has not been demonstrated. In our study, 0.9% of I. scapularis nymphs contained DNA for an organism using a PCR primer set designed for amplification of a gltA gene fragment from Bartonella spp. (Norman et al. 1995). However, sequencing results indicated that the amplicons did not originate from any known Bartonella spp. In fact, the sequences did not cluster with any citrate synthase sequences available from GenBank. These sequences did cluster with other unidentified citrate synthase amplicons recovered from D. variabilis by our laboratory. The sequence alignments and phylogenetic tree both confirm the presence of an unknown agent or agents in Maryland I. scapularis. These entities appear to be most closely related to Bartonella spp. The definitive identification of these organisms is pending in expanded studies in our laboratory.
Although B. burgdorferi and Rickettsia spp. were co-circulating in 4.3% of the nymphs, the presence of these organisms appears to be independent of each other. Different enzootic maintenance mechanisms could afect the rate at which each organism occurs in ticks. Unlike B. burgdorferi, SFG-rickettsiae can be maintained through transovarial transmission.
Concurrent infections with Borrelia burgdorferi and any of the microorganisms studied (Rickettsia spp., Bartonella spp., or A. phagocytophilum), when considered together, do not occur independently. However, concurrent infections may not necessarily occur as a result of concurrent B. burgdorferi infection. The chances of concurrent infections with B. burgdorferi and other tick-borne pathogens may increase due to their common transmission cycles.
Concurrently infected nymphs have the potential to transmit more than one pathogen to a host during feeding which is important because altered immune responses can occur in mice and in humans with multiple infections (Belongia 2002). In human concurrent infections with B. burgdorferi and B. microti, the increased severity of clinical Lyme disease seen with the dual infection is thought to be the result of an increased B. burgdorferi spirochete load and the immune response elicited by B. microti (Persing and Conrad 1995, Krause et al. 1996). In mice infected with both B. burgdorferi and A. phagocytophilum, the immunological response elicited by each of the pathogens results in increased densities of spirochetes (Holden et al. 2005). However, recent in vitro data demonstrated that a greater proportion of B. burgdorferi were able to cross an endothelial cell barrier when A. phagocytophilum-infected cells were present, thereby possibly explaining the increased spirochete load in the both the blood and tissue (Nyarko et al. 2006).
Borrelia burgdorferi remains the primary microorganism of concern with I. scapularis on the Eastern Shore of Maryland; however, other microorganisms, some pathogenic for humans, can co-circulate with B. burgdorferi in these ticks. While concurrent infections with B. burgdorferi and any one of the other microorganisms in this study appears related, probably due to the overlap between the common enzootic cycles, the sample size is relatively small. Therefore, the expanded examination with a larger sample size of the prevalence of these microorganisms and the association between concurrently infecting microorganisms in both adult I. scapularis and in P. leucopus would help to understand these enzootic cycles. The potential epidemiological risk associated with concurrent infections in the vector population and subsequent transmission of complex bacterial infections to humans is not yet fully understood. This is complicated by a paucity of epidemiological data for illness associated with many of these potential or confirmed pathogens. Further study is warranted to better delineate the distribution, identity, and risks associated with transmission of these microorganisms to humans.
Acknowledgments
We thank J. Stephen Dumler from The Johns Hopkins University School of Medicine, Eric Hofmeister from the United States Geological Survey, and William Nicholson from Centers for Disease Control and Prevention for providing positive control samples. This research was supported by a National Institute of Environmental Health Sciences Training Fellowship (T32ES07141) awarded to KIS and a Centers for Disease Control and Prevention Cooperative Agreement (U50/CCU319554) awarded to DEN.
References Cited
- Adelson ME, Rao RVS, Tilton RC, Cabets K, Eskow E, Fein L, Occi JL, Mordechai E. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in northern New Jersey. J Clin Microbiol. 2004;42:2799–2801. doi: 10.1128/JCM.42.6.2799-2801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghaferi MY, Anderson JM, Park J, Auwaerter PG, Aucott JN, Norris DE, Dumler JS. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of Northern Maryland and Southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J Clin Microbiol. 2005;43:1879–1884. doi: 10.1128/JCM.43.4.1879-1884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tools. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amerasinghe FP, Breisch NL, Neidhardt K, Pagac B, Scott TW. Increasing density and Borrelia burgdorferi infection of deer-infesting Ixodes dammini(Acari: Ixodidae)in Maryland. J Med Entomol. 1993;30:858–864. doi: 10.1093/jmedent/30.5.858. [DOI] [PubMed] [Google Scholar]
- Amerasinghe FP, Breisch NL, Azad AF, Gimpel WF, Greco M, Neidhardt K, Pagac B, Piesman J, Sandt J, Scott TW, Sweeney K. Distribution, density, and Lyme disease spirochete infection in Ixodes dammini (Acari: Ixodidae) on white-tailed deer in Maryland. J Med Entomol. 1992;29:54–61. doi: 10.1093/jmedent/29.1.54. [DOI] [PubMed] [Google Scholar]
- Ammerman NC, Swanson KI, Anderson JA, Schwartz TR, Seaberg EC, Glass GE, Norris DE. Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg Infect Dis. 2004;10:1478–1481. doi: 10.3201/eid1008.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM. Molecular Microbiology and Immunology. Johns Hopkins Bloomberg School of Public Health; Baltimore, MD.: 2004. Ecological and genetic factors contributing to enzootic Lyme disease in southern Maryland; p. 320. [Google Scholar]
- Anderson JM, Ammerman NC, Norris DE. Molecular differentiation of metastriate tick immatures. Vector Borne Zoonotic Dis. 2006;4:334–342. doi: 10.1089/vbz.2004.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Norris DE. Genetic diversity of Borrelia burgdorferi sensu stricto in Peromyscus leucopus, the primary reservoir of Lyme disease in a region of endemicity in Southern Maryland. Appl Environ Microbiol. 2006;72:5331–5341. doi: 10.1128/AEM.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belongia EA. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2002;2:265–273. doi: 10.1089/153036602321653851. [DOI] [PubMed] [Google Scholar]
- Black WC, IV, Klompen Js, Keirans JE. Phylogenetic relationships among tick subfamilies (Ixodida: Ixodidae: Argasidae) based on the 18S nuclear rDNA gene. Mol Phylogenet Evol. 1997;7:129–144. doi: 10.1006/mpev.1996.0382. [DOI] [PubMed] [Google Scholar]
- Bunnell JE. Pathobiology of granulocytic ehrlichiosis, Lyme borreliosis, and their tick vector in the middle Atlantic region, USA. Johns Hopkins University School of Hygiene and Public Health; Baltimore, MD: 1999. p. 143. [Google Scholar]
- Burgdorfer W, Gage KL. Susceptibility of the black-legged tick, Ixodes scapularis, to the Lyme disease spirochete, Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg [A] 1986;263:15–20. doi: 10.1016/s0176-6724(86)80096-7. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chomel BB, Kasten RW, Romano V, Tietze N. Molecular evidence of Bartonella spp in questing adult Ixodes pacificus ticks in California. J Clin Microbiol. 2001;39:1221–1226. doi: 10.1128/JCM.39.4.1221-1226.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer JA, Nicholson WL, Olson JG, Childs JE. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J Clin Microbiol. 1999;37:558–564. doi: 10.1128/jcm.37.3.558-564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran KL, Kidd JB, Vassallo J, van Meter VL. Borrelia burgdorferi and the causative agent of human granulocytic ehrlichiosis in deer ticks, Delaware. Emerg Infect Dis. 2000;6:408–411. doi: 10.3201/eid0604.000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AG, Arner TG, Sunki GG, Friedman R, Lantinga M, Sangam S, Zubieta JC, Sullivan KM, Brendel KA, Gao Z, Fontaine N, Shu M, Fuller G. Epi Info™, a database and statistics program for public health professionals. Centers for Disease Control and Prevention; Atlanta, GA: 2002. [Google Scholar]
- Demma LJ, Holman RC, McQuiston JH, Krebs JW, Swerdlow DL. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001-2002. Am J Trop Med Hyg. 2005;73:400–409. [PubMed] [Google Scholar]
- Dumler J, Barbet A, Bekker C, Dasch G, Palmer G, Ray S, Rikihisa Y, Rurangirwa F. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- Ebel GD, Campbell E, Goethert HK, Spielman A, Telford SR. Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am J Trop Med Hyg. 2000;63:36–42. doi: 10.4269/ajtmh.2000.63.36. [DOI] [PubMed] [Google Scholar]
- Fang QQ, Mixson TR, Hughes M, Dunham B, Sapp J. Prevalence of the agent of human granulocytic ehrlichiosis in Ixodes scapularis (Acari: Ixodidae) in the coastal southeastern United States. J Med Entomol. 2002;39:251–255. doi: 10.1603/0022-2585-39.2.251. [DOI] [PubMed] [Google Scholar]
- Frank C, Fix AD, Pena CA, Strickland GT. Mapping Lyme disease incidence for diagnostic and preventive decisions, Maryland. Emerg Infect Dis. 2002;8:427–429. doi: 10.3201/eid0804.000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Halos L, Jamal T, Maillard R, Beugnet F, Le Menach A, Boulouis HJ, Vayssier-Taussat M. Evidence of Bartonella sp in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet Res. 2005;36:79–87. doi: 10.1051/vetres:2004052. [DOI] [PubMed] [Google Scholar]
- Holden K, Boothby JT, Kasten RW, Chomel BB. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus ticks from California, USA. Vector Borne Zoonotic Dis. 2006;6:99–102. doi: 10.1089/vbz.2006.6.99. [DOI] [PubMed] [Google Scholar]
- Holden K, Hodzic E, Feng S, Freet KJ, Lefebvre RB, Barthold SW. Coinfection with Anaplasma phagocytophilum alters Borrelia burgdorferi population distribution in C3H/HeN mice. Infect Immun. 2005;73:3440–3444. doi: 10.1128/IAI.73.6.3440-3444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJB, Happ CM, Mayer LW, Piesman J. Detection of Borrelia burgdorferi in ticks by species-specific amplification of the flagellin gene. Am J Trop Med Hyg. 1992;47:730–741. doi: 10.4269/ajtmh.1992.47.730. [DOI] [PubMed] [Google Scholar]
- Krause PJ, Telford SRI, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing DH. Concurrent Lyme disease and babesiosis Evidence for increased severity and duration of illness. J Am Med Assoc. 1996;275:1657–1660. [PubMed] [Google Scholar]
- Levin ML, Fish D. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect Immun. 2000;68:2183–2186. doi: 10.1128/iai.68.4.2183-2186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L, Anderson J, Staford KC, 3rd, Dumler J. Antibodies to multiple tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in whitefooted mice. J Wildl Dis. 1997;33:466–473. doi: 10.7589/0090-3558-33.3.466. [DOI] [PubMed] [Google Scholar]
- Magnarelli L, Staford K, 3rd, Mather T, Yeh M, Horn K, Dumler J. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710–2714. doi: 10.1128/jcm.33.10.2710-2714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli LA, Swihart RK. Spotted fever group rickettsiae or Borrelia burgdorferi in Ixodes cookei (Ixodidae) in Connecticut. J Clin Microbiol. 1991;29:1520–1522. doi: 10.1128/jcm.29.7.1520-1522.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli LA, Andreadis TG, Staford KCI, Holland CJ. Rickettsiae and Borrelia burgdorferi in ixodid ticks. J Clin Microbiol. 1991;29:2798–2804. doi: 10.1128/jcm.29.12.2798-2804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massung RF, Slater KG. Comparison of PCR assays for detection of the agent of human granulocytic ehrlichiosis, Anaplasma phagocytophilum. J Clin Microbiol. 2003;41:717–722. doi: 10.1128/JCM.41.2.717-722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova OV, Cabello FC, Dobrotvorsky AK. Semi-nested PCR detection of Bartonella henselae in Ixodes persulcatus ticks from Western Siberia, Russia. Vector Borne Zoonotic Dis. 2004;4:306–309. doi: 10.1089/vbz.2004.4.306. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Muir S, Sumner JW, Childs JE. Serologic evidence of infection with Ehrlichia spp. in wild rodents (Muridae: Sigmodontinae) in the United States. J Clin Microbiol. 1998;36:695–700. doi: 10.1128/jcm.36.3.695-700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Munderloh U, Kurtti T. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Environ Microbiol. 1997;63:3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AF, Regnery R, Jameson P, Greene C, Krause DC. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyarko E, Grab DJ, Dumler JS. Anaplasma phagocytophilum-infected neutrophils enhance transmigration of Borrelia burgdorferi across the human blood brain barrier in vitro. Int J Parasitol. 2006;36:601–605. doi: 10.1016/j.ijpara.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Orloski KA, Hayes EB, Campbell GL, Dennis DT. Surveillance for Lyme disease – United States. MMWR. 2000;49:1–11. [PubMed] [Google Scholar]
- Pancholi P, Kolbert C, Mitchell P, Reed KJ, Dumler J, Bakken J, Telford SR, Persing D. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- Persing DH, Conrad PA. Babesiosis: new insights from phylogenetic analysis. Infect Agents Dis. 1995;4:182–195. [PubMed] [Google Scholar]
- Persing DH, Mathiesen D, Marshall WF, Telford SR, Spielman A, Thomford JW, Conrad PA. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719. doi: 10.1128/cmr.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P, Kimsey R, Madigan J, Barlough J, Dumler J, Brooks D. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae) J Med Entomol. 1996;33:1–5. doi: 10.1093/jmedent/33.1.1. [DOI] [PubMed] [Google Scholar]
- Sanogo YO, Zeaiter Z, Caruso G, Merola F, Shpynov S, Brouqui P, Raoult D. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno Province, Italy. Emerg Infect Dis. 2003;9:329–332. doi: 10.3201/eid0903.020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouls LM, Van de Pol I, Rijpkema SGT, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–2222. doi: 10.1128/jcm.37.7.2215-2222.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriefer ME, Azad AF. Changing ecology of Rocky Mountain spotted fever. In: Sonenshine DE, Mather TN, editors. Ecological Dynamics of Tick-borne Zoonoses. Oxford Univ. Press; New York: 1994. pp. 314–326. [Google Scholar]
- Schwartz I, Fish D, Daniels TJ. Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. New Engl J Med. 1997;337:49–50. doi: 10.1056/NEJM199707033370111. [DOI] [PubMed] [Google Scholar]
- Spielman A. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am J Trop Med Hyg. 1976;25:784–787. doi: 10.4269/ajtmh.1976.25.784. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708–727. doi: 10.1128/CMR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SRI, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tickrodent cycle. Proc Natl Acad Sci U S A. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V, Anguita J, Barthold SW, Fikrig E. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect Immun. 2001;69:3359–3371. doi: 10.1128/IAI.69.5.3359-3371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varde S, Beckley J, Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey county. Emerg Infect Dis. 1998;4:97–99. doi: 10.3201/eid0401.980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller SJ, Baldridge GD, Munderloh UG, Noda H, Simser J, Kurtti TJ. Phylogenetic placement of Rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J Clin Microbiol. 1998;36:1305–1317. doi: 10.1128/jcm.36.5.1305-1317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Spielman A. Seasonal activity of immature Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1985;22:408–414. doi: 10.1093/jmedent/22.4.408. [DOI] [PubMed] [Google Scholar]


