Abstract
Objective
To examine the effect of mode of birth on plasma purine and malondialdehyde levels in normal term infants.
Study Design
Umbilical arterial cord blood was obtained immediately after birth from a convenience sample of 119 normal term newborns born by vaginal delivery, with or without oxytocin augmentation or by elective cesarean delivery. Plasma was analyzed for purine and/or malondialdehyde levels. Numeric data were analyzed utilizing independent samples t-test and ordinal data were analyzed using Mann–Whitney test. Correlation coefficients were obtained using Spearman’s ρ.
Result
Uric acid levels were significantly elevated (P<0.001) in neonates undergoing vaginal birth, compared to neonates born by elective cesarean delivery. When the effect of oxytocin and length of labor was analyzed, neonates born to mothers on oxytocin had lower hypoxanthine, significantly lower xanthine (P = 0.05) and higher uric acid levels. In addition, malondialdehyde levels were significantly higher (P<0.006) in neonates born to mothers who received oxytocin compared to neonates born to mothers without oxytocin augmentation. We also found significant correlations between malondialdehyde (MDA) and hypoxanthine (r = −0.465, P< 0.039) and between MDA and xanthine (r = −0.753, P = 0.003) in neonates born via oxytocin-augmented birth. Mode of birth had no statistically significant effect on clinical outcomes, although infants born by elective cesarean had higher incidence of acute respiratory distress and transient tachypnea of the newborn compared to those born vaginally.
Conclusion
Neonates born by elective cesarean had the lowest purine levels in cord blood compared to neonates born vaginally. Oxytocin augmentation is associated with some degree of uterine hyperstimulation which may enhance the ATP degradation pathway resulting in the rapid conversion of hypoxanthine to xanthine and xanthine to uric acid. Significantly higher MDA levels in neonates whose mothers received oxytocin as well as significant correlation between MDA and the purines hypoxanthine and xanthine, suggest free-radical production, most likely due to xanthine oxidase activation. However, despite differences in plasma purine and malondialdehyde levels, no significant differences were seen in neonatal outcome. Further studies are required to fully characterize the effect of mode of birth on purine metabolism and free-radical production.
Keywords: mode of birth, purines, MDA
Introduction
In all tissues there is tight coupling between blood flow and metabolic demand. As the metabolic rate increases or as cellular oxygen or glucose concentrations decrease, local arterioles undergo compensatory vasodilation leading to reductions in vascular resistance and increases in flow. As blood flow and oxygenation improves, adenosine diphosphate (ADP) is phosphorylated to adenosine triphosphate (ATP) (Figure 1).1 However, if tissue metabolic needs continue to exceed oxygen and glucose supply, ATP is quickly degraded to ADP and adenosine monophosphate, then to adenosine, adenosine to inosine, inosine to hypoxanthine, hypoxanthine to xanthine and xanthine to uric acid (Figure 1).1 Xanthine oxidoreductase catalyzes the last two conversions of this degradative pathway. This enzyme exists in two forms, xanthine dehydrogenase and xanthine oxidase (XO). With sustained hypoxemia or ischemia and upon reperfusion, xanthine dehydrogenase is converted to its reactive oxygen species (ROS)-producing form, XO.1 ROS can change membranes, proteins and nucleic acids,2,3 resulting in lipid peroxidation or protein and nucleic acid modification. Malondialdehyde (MDA) is a product of such ROS-induced damage and reflects the degree of lipid peroxidation.4
Figure 1.
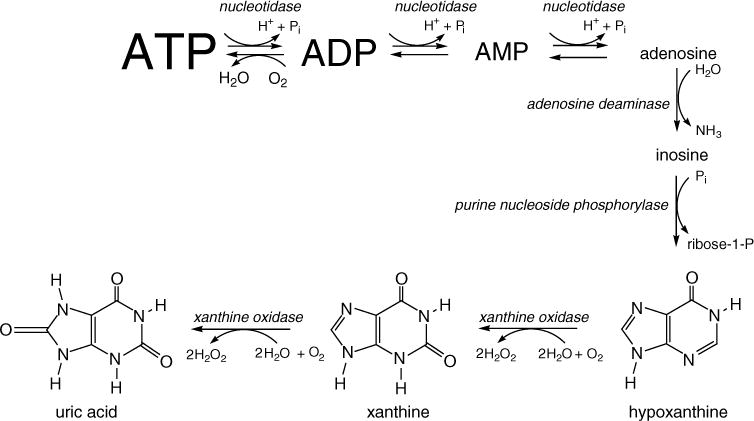
Pathway from adenosine triphosphate (ATP) breakdown to purine metabolism.
It is well documented that the fetus experiences significant reductions in oxygen saturation and short periods of moderate hypoxia-ischemia during the process of birth.5,6 What is unclear is whether this degree of intermittent physiologic fetal hypoxemia-ischemia during normal delivery is associated with quantifiable increases in hypoxanthine, xanthine, uric acid and malondialdehyde. More importantly, it is also unclear whether mode of birth or labor augmentation with oxytocin alters purine and malondialdehyde production in normal term deliveries.
To determine the specific effects of mode of delivery and labor augmentation on purine metabolism and lipid degradation in normal full-term infants, we measured levels of hypoxanthine, xanthine, uric acid and MDA in vaginal deliveries, with and without labor augmentation, and elective cesarean deliveries. Although umbilical cord uric acid levels7–9 and hypoxanthine levels10 have been reported in normal term newborns, there are currently no published baseline levels of all three breakdown products of ATP as well as MDA in the cord blood of normal term infants immediately after birth. In addition, the specific effects of oxytocin-augmentation on purine and MDA levels have not been measured. Given that purine metabolism can augment free-radical production and thus lipid peroxidation, and because these metabolites are often used as markers of hypoxia and oxidative stress, the effect of mode of birth, specifically oxytocin augmentation, on baseline levels of these biochemical markers are needed.
Methods
This study was reviewed and approved by Loma Linda University Institutional Review Board. Arterial blood from the umbilical artery (placental end) was obtained from a convenience sample of 119 normal full-term neonates. Inclusion criteria were as follows: Apgar score of at least 5 at 1 min and 6 at 5 min, estimated gestational age of 37 weeks or above and birth weight over 2500 g. Infants of mothers who smoke, had sickle-cell disease/trait or anemia were not included. In addition, infants born with severe congenital abnormalities were excluded from the study. Elective cesarean delivery refers to surgery planned at least 24 h in advance with no evidence of labor or uterine contractions at the time of surgery. All of the mothers delivering by elective cesarean received spinal anesthesia. Demographic and clinical characteristics such as birth weight, sex, estimated gestational age, Apgar score, maternal disease, labor augmentation and method of delivery were collected retrospectively. The condition of the neonate immediately after birth was collected retrospectively and stratified as (1) normal or (2) acute respiratory distress (grunting, flaring, retractions, cyanosis) requiring deep suctioning, stimulation and/or blow-by oxygen, which resolved in 4 h or less and (3) neonatal intensive care unit admission.
Purine assay
Blood was drawn from the umbilical artery (placental end) within 15 min of placental delivery, and transferred to an EDTA tube. The tube was transported on ice to a Fisher Scientific AccuSpin 1R (Fair Lawn, NJ, USA) centrifuge and was spun for 20 min at 1500 g, 4 °C. The plasma was removed, transferred to separate Eppendorf tubes and centrifuged in Beckman Microfuge 22R (Fullerton, CA, USA) centrifuge, for 30 min at 18000 g. The supernatant was protein depleted using Microcon centrifugal filter devices, 10K molecular weight cutoff (Millipore Corp.; Bedford, MA, USA), 500 μl per device, spun for 90 min at 14000 g, 4 °C. Filtrate was removed, and 200 μl were transferred to an Eppendorf tube containing 1 × 10−7 mol of 2-aminopurine as internal standard. HPLC (Waters 600E, Waters 996 PDA, 715 Ultra Wisp Sample Processor; Millipore Corp.) analysis was done on the same day, or the tubes were frozen at −78 °C until analysis. Previous HPLC analysis of plasma demonstrated that purine values remained stable despite freezing (data not shown).
Three 50 μl injections were done for each sample onto a Supelcosil LC-18-S 15 cm × 4.6 mm, 5 μm column (SGE; Austin, TX, USA), with the following isocratic conditions: 50 mM ammonium formate buffer, pH 5.5, flow rate 1.0 ml min−1. Hypoxanthine, xanthine, uric acid and 2-aminopurine were quantitated by obtaining peak areas at the appropriate retention times of approximately 6.4, 7.2, 3.2 and 10.8 min, and identified by absorbance at 248, 267, 288 and 305 nm, respectively. Once the peak area of 2-aminopurine was obtained, the area ratios of hypoxanthine, xanthine and uric acid to 2-aminopurine were determined and converted to concentrations using standard curves. Samples were analyzed in triplicates and values with a coefficient of variation of less than 10% were included in the final analyses.
MDA assay
Plasma MDA was determined using an adaptation of the selected ion-monitoring gas chromatography-mass spectrometry (GC-MS) analysis of phenylhydrazine-derivatized plasma, as described by Cighetti et al.11,12 Specifically, the sample was prepared by the mixture of 0.2 ml plasma, 0.26 nmol methyl malondialdehyde (MMDA), 5nmol butylated hydroxytoluene (10 μl of 0.5 mM), 0.2 ml citrate buffer (0.4 M, pH 4.0) and deionized water to a final volume of 480 μl. Then 20 μl 50 mM phenylhydrazine (1 μmol) was added as the derivatizing agent and incubated for 30 min at 25 °C. Extraction was started by the addition of 1 ml hexane. The samples were vortexed for 1 min and centrifuged (3000 r.p.m., 10 min) at 25 °C. The organic phase was removed, concentrated by nitrogen stream to 100 μl and analyzed by GC-MS, in selected ion-monitoring mode (injection volume of 2 μl). Ion 144.00 was monitored for MDA, and ion 158.00 for MMDA. The ion abundance ratios were converted to micromolar concentrations by use of a standard curve. All measurements were performed in triplicate. Values with a coefficient of variation of less than 10% were included in the final analyses.
The internal standard, MMDA, was synthesized using a modified method of Paroni et al.13 Briefly, 2-methyl-3-ethoxyprop-2-enal (Sigma; St Louis, MO, USA) was suspended in NaOH (7 M) and stirred for 150 min. This was diluted with 5 ml of water and extracted with three 5 ml volumes of CH2Cl2. Then the water was evaporated from the aqueous layer. The residue was crystallized once from 5 ml ethanol and 5 ml benzene and then three times from 5 ml ethanol and 5 ml diisopropyl ether. The resulting white powder was diluted in water, filtered and stored at −80 °C.
Statistics
All data were analyzed for normalcy. Continuous data were analyzed utilizing independent samples t-test and reported as the mean and standard deviation (Mean ± s.d.). Ordinal data were analyzed using Mann-Whitney or χ2-test. Correlation coefficients were obtained using Spearman’s ρ. All statistical analyses were performed using SPSS Statistics for Windows Version 10. Differences were considered significant at P<0.05.
Results
Umbilical arterial blood samples were obtained from 119 neonates. As described in Table 1, 40 babies were born by elective cesarean and 79 were born by vaginal delivery. For the infants born vaginally, 51 mothers received oxytocin augmentation while 28 did not. None of the infants born by elective cesarean went through labor, and none of the mothers experienced any cardiovascular or hematological complications. All of the mothers in the elective cesarean group received oxygen during the operative procedure. Only 18% of mothers delivering by non-oxytocin vaginal birth and 38% of mothers delivering by oxytocin-augmented birth received oxygen. There were no significant differences in gestational age, birth weight, gender, race or Apgar score between the groups. However, there were significantly more primigravidas in the vaginal birth group compared to the cesarean delivery group. Significant differences were also noted in parity. Of the measured purines, plasma levels of uric acid were highest while in many cases xanthine levels were less than 5 μM. Table 2 describes the number of samples for each purine and MDA after removing values with a coefficient of variation of more than 10% as a quality control standard.
Table 1.
Patient demographics
|
Elective cesarean (n = 40) |
Vaginal birth (n = 79) |
P-valuea | |
|---|---|---|---|
| EGA (week) | 39.1 ± 0.9 | 39.4 ± 1.0 | NS |
| Birth weight | 3550 ± 485 g | 3479 ± 498 g | NS |
| Gender | NS | ||
| Male | 16 (40%) | 41 (52%) | |
| Female | 24 (60%) | 38 (48%) | |
| Gravida | 0.011b | ||
| 1 | 2 (5%) | 25 (32%) | |
| 2–3 | 26 (65%) | 37 (47%) | |
| >4 | 12 (30%) | 17 (21%) | |
| Parity | 0.001b | ||
| 0–1 | 22 (55%) | 59 (75%) | |
| 2–3 | 13 (32.5%) | 17 (22%) | |
| >4 | 5 (12.5%) | 3 (3%) | |
| Race | NS | ||
| Caucasian | 16 (40%) | 25 (32%) | |
| Hispanic | 16 (40%) | 39 (49%) | |
| African-American | 3 (8) | 6 (8) | |
| Asian | 1 (2%) | 3 (4%) | |
| Not documented/other | 4 (10%) | 6 (6%) | |
| 1-min Apgar | NS | ||
| 5–6 | 0 (0%) | 5 (6%) | |
| 7–8 | 30 (75%) | 42 (53%) | |
| 9–10 | 10 (25%) | 32 (41%) | |
| 5-min Apgar | NS | ||
| 5–6 | 0 (0%) | 2 (2%) | |
| 7–8 | 9 (22.5%) | 9 (11%) | |
| 9–10 | 31 (77.5%) | 68 (86%) |
Abbreviations: EGA, estimated gestational age; NS, not significant.
Mann–Whitney for nominal data; independent samples t-test for numerical data.
χ2.
Table 2.
Effect of labor on umbilical cord plasma purines and MDA in vaginal vs elective cesarean births
| Elective cesarean | Vaginal birth | P-valuea | |
|---|---|---|---|
| Hypoxanthine (μM) | 11.6 ± 9.9 (n = 34) |
13.8 ± 12.5 (n = 69) |
0.358 |
| Xanthine (μM) | 3.1 ± 2.6 (n = 29) |
4.0 ± 3.8 (n = 47) |
0.518 |
| Uric Acid (μM) | 238.0 ± 47.8 (n = 40) |
285.9 ± 70.3 (n = 69) |
<0.001 |
| MDA (μM) | 3.7 ± 1.6 (n = 18) |
3.1±1.2 (n = 34) |
0.342 |
Abbreviation: MDA, malondialdehyde.
Mean ± s.d.
Independent samples t-test.
Bold values are statistically significant.
Purine and MDA levels in neonates born by elective cesarean delivery or vaginal birth
As described in Table 2, we found that neonates delivered vaginally had higher hypoxanthine and xanthine levels as well as significantly higher plasma uric acid levels (P<0.001). MDA levels tend to be higher in the elective cesarean group but the data were not statistically significant. Although we found a highly significant positive relationship between hypoxanthine and xanthine (r = 0.696, P<0.001) in the elective cesarean group, we found no significant relationship between MDA and purine metabolites (Table 3). This data suggest that in neonates born by elective cesarean delivery, elevations in MDA levels may not be related to purine breakdown or metabolism.
Table 3.
Correlation between purine metabolites and MDA in neonates born by elective cesarean delivery
| Hypoxanthine | Xanthine | Uric acid | |
|---|---|---|---|
| Hypoxanthine | |||
| Correlation coefficient | NS | 0.696 | NS |
| P-value | <0.001a | 34 | |
| N | 34 | 26 | |
| Uric acid | |||
| Correlation coefficient | NS | NS | NS |
| P-value | NS | NS | NS |
| N | 34 | 29 | 40 |
| MDA | |||
| Correlation coefficient | NS | NS | NS |
| P-value | NS | NS | NS |
| N | 15 | 13 | 18 |
Abbreviations: MDA, malondialdehyde; NS, not significant.
Spearman’s ρ.
Bold values are statistically significant.
Purine and MDA levels in neonates born by vaginal birth with and without oxytocin augmentation
As described in Table 4, neonates whose mothers received oxytocin experienced longer labor, tend to have lower hypoxanthine, significantly lower xanthine (P = 0.023), higher uric acid levels and significantly higher MDA levels (P< 0.002). In addition, there is a significant negative correlation between hypoxanthine and MDA (Spearman’s ρ, −0.465; P = 0.039) as well as xanthine and MDA (Spearman’s ρ, −0.753; P = 0.003) in those born with oxytocin-augmented births (Table 5), suggesting a significant relationship between purine breakdown and lipid peroxidation. Although mothers are usually placed on oxytocin due to ineffective contractions, we found that mothers on oxytocin have more frequent uterine contractions occurring within 1 min of each other in the last 2 h before birth (Table 4). Interestingly, we found a small but significant correlation between hypoxanthine and the number of times that uterine contractions occurred five times or more per 10 min in the last 2 h before birth (Spearman’s ρ, 0.557; P< 0.001) and between hypoxanthine and the number of times that contractions occurred within 1 min of each other in the last 2 h before birth (Spearman’s ρ, 0.417; P = 0.008) (Table 5). This suggests that as the frequency of uterine contractions increase, hypoxanthine levels also increase.
Table 4.
Effect of oxytocin augmentation on umbilical cord plasma purines and MDA
| Vaginal birth, no oxytocin | Vaginal birth with oxytocin | P-valuea | |
|---|---|---|---|
| Hypoxanthine (μM) | 14.8 ± 13.7 (n = 25) |
13.2 ± 11.7 (n = 44) |
0.736 |
| Xanthine (μM) | 5.3 ± 3.9 (n = 18) |
3.2 ± 3.5 (n = 29) |
0.023 |
| Uric acid (μM) | 277.9 ± 61.5 (n = 41) |
290.6 ± 75.3 (n = 75) |
0.566 |
| MDA (μM) | 2.4 ± 0.6 (n = 11) |
3.5 ± 1.2 (n = 21) |
0.002 |
| Clinical variables | |||
| Frequency of uterine contractions that were longer than 2 min in the last 2 h of labor | 0 (median) (n = 20) |
1 (median) (n = 44) |
0.882 |
| Frequency of uterine contractions that occurred more than five times in 10 min in the last 2 h of labor | 0 (median) (n = 20) |
0 (median) (n = 44) |
0.550 |
| Frequency of uterine contractions that occurred within 1 min of each other in the last 2 h of labor | 11 (median) (n = 25) |
18 (median) (n = 44) |
0.048 |
| Length of labor (hours) | 18.6 ± 10.6 (n = 22) |
24.1±15.5 (n = 47) |
0.088 |
Abbreviation: MDA, Malondialdehyde.
Mean ± s.d.
Mann – Whitney.
Bold values are statistically significant.
Table 5.
Correlation between purine metabolites and MDA in neonates born by oxytocin-augmented vaginal birth
| Hypoxanthine | Xanthine | Uric acid | |
|---|---|---|---|
| Xanthine: correlation coefficient | 0.833 | 0.408 | |
| P-valuea | <0.001 | 0.035 | |
| N | 27 | 27 | |
| MDA: correlation coefficient | −0.465 | −0.753 | |
| P-valuea | 0.039 | 0.003 | |
| N | 20 | 13 | |
| Frequency of uterine contractions that occurred more than five times in 10 min: correlation coefficient | 0.557 | ||
| P-valuea | <0.001 | ||
| N | 39 | ||
| Frequency of uterine contractions that occurred less than 1 min apart: correlation coefficient | 0.417 | ||
| P-valuea | 0.008 | ||
| N | 39 |
Abbreviation: MDA, Malondialdehyde.
Spearman’s ρ.
Bold values are statistically significant.
Effect of mode of birth on neonatal outcome
As shown in Table 6, mode of birth had no significant effect on neonatal outcome. Although neonates born by elective cesarean tended to have a higher incidence of acute respiratory distress, the data were not statistically significant.
Table 6.
Effect of mode of birth on the immediate post-partum outcome of neonates
| Elective cesarean (n = 40) | Vaginal birth, no oxytocin (n = 28) | Vaginal birth, with oxytocin (n = 51) | P-valuea | |
|---|---|---|---|---|
| Normal | 24 (60%) | 21 (75%) | 37 (73%) | NS |
| Acute respiratory distress which resolved in <4 h | 14 (35%) | 4 (14%) | 11 (21%) | NS |
| NICU admission | 2 (5%) | 3 (11%) | 3 (6%) | NS |
| Hypoglycemia—infant of diabetic mother | Poor feeding, possible sepsis | Possible sepsis | ||
| Respiratory distress | Possible patent ductus arteriosus | Meconium aspiration, hypoxemia, respiratory distress | ||
| Hyperbilirubinemia | Respiratory distress |
Abbreviations: NICU, neonatal intensive care unit; NS, not significant.
χ2 P-value = 0.352.
Discussion
While a plethora of animal studies have documented elevated purine levels in neonates born vaginally, only a few studies have measured purine levels in normal term human neonates. Monkus et al.7 measured uric acid levels in 45 infants born vaginally, Manzke et al.8 measured uric acid levels in a group of infants at birth and 12 h later and Wallenburg and van Kreel quantified uric acid levels in infants born by oxytocin-augmented birth.9 None of these studies examined the effect of mode of birth on ATP and purine metabolism. In addition, there are currently no published studies documenting baseline purine and MDA levels in normal term newborns immediately after birth. Because these substances are often used as markers of hypoxia and oxidative stress, baseline levels in normal neonates are needed. More importantly, we found no studies that examined the effect of mode of birth on all three purine moieties as well as MDA. Only Irestedt et al.10 investigated the effect of mode of birth on hypoxanthine. In this study, hypoxanthine levels were shown to be significantly higher in infants born vaginally, but no measures of xanthine, uric acid or MDA levels were performed. In addition, the authors did not specify if those who delivered vaginally had labors that were augmented by oxytocin. Considering that oxytocin is documented to produce a significant increase in frequency, duration and strength in uterine contractions14 which can result in fetal stress, we specifically investigated the effect of this mode of birth on purine and MDA levels and compared it to normal vaginal birth with no oxytocin augmentation and elective cesarean birth.
We found that neonates born by vaginal birth had higher hypoxanthine and xanthine levels as well as significantly higher plasma uric acid levels compared to neonates born by elective cesarean (Table 2). These data suggest that there is a significant relationship between labor and neonatal ATP metabolism. Myometrial contractions during labor can lead to a reduction in umbilicoplacental blood flow from compression of arcuate and spiral arteries15 decreasing blood flow to the fetus. Although these episodes of ischemia may be nonpathologic, they may also lead to decreased ATP synthesis in the fetus due to a reduction in the phosphorylation of ADP to ATP. When ADP phosphorylation decreases, it is degraded to its purine metabolites (Figure 1) leading to higher purine levels in umbilical arterial cord plasma.
As shown in Table 2, we found that MDA levels tend to be higher in those neonates born by elective cesarean delivery. However, we found no significant correlation between MDA and purine metabolites (Table 3), suggesting that MDA production in this cohort may be unrelated to purine degradation but may be due to maternal oxygen administration. All of the mothers in this cohort who delivered by elective cesarean received oxygen. Our data agree with the findings of Khaw et al.,16 who found higher MDA levels in those mothers who received oxygen vs air during cesarean section. These data suggest that although oxygen administration may improve fetal oxygenation status, it may also affect lipid peroxidation in the fetus and the neonate.
When we analyzed the effect of labor and oxytocin administration on purine and MDA levels, we found that neonates born via oxytocin-augmented vaginal birth had lower hypoxanthine, significantly lower xanthine and higher uric acid levels (Table 4). These values show a pattern of enhanced ATP breakdown in oxytocin-augmented births resulting in the rapid conversion of hypoxanthine to xanthine and xanthine to uric acid. Hypoxanthine is lower because it has been converted to xanthine and xanthine is significantly lower because it has been converted to uric acid.1 Because uric acid is the final breakdown product of purine degradation, its levels accumulate in plasma until it is excreted in the urine.1 More importantly, we also found that MDA levels were significantly higher in oxytocin-augmented births (Table 4) and MDA significantly correlates with xanthine and hypoxanthine (Table 5) suggesting that this pattern of enhanced purine degradation and MDA production may be mediated by the free-radical producing enzyme XO.1 Although patients are placed on oxytocin due to ineffective contractions, we found that mothers on oxytocin tend to have more frequent uterine contractions in the last 2 h before birth (Table 4), which may increase the number of times the fetus is exposed to some degree of hypoxia, increasing the number of hypoxia–ischemia–reperfusion events and XO activation. The significant correlation between hypoxanthine and frequency of uterine contractions in the last 2 h before birth (Table 5) suggest enhanced ATP utilization or degradation in this neonatal cohort. However, since our cohort was of normal neonates, the degree of ATP degradation we identified was, by definition, within normal range for a birth. In future studies, we intend to characterize these metabolites in the setting of neonates who have potentially undergone greater hypoxic–ischemic stress to determine whether such tests would be useful clinically.
Although babies born by elective cesarean delivery had the lowest purine levels, this group had the highest number of babies with signs and symptoms of acute respiratory distress or transient tachypnea of the newborn. Although the difference was not significant, our data agrees with Hack et al.17 and Jain and Eaton18 who showed that while the occurrence of birth asphyxia, trauma and meconium aspiration is reduced by elective cesarean delivery, the risk of respiratory distress secondary to transient tachypnea of the newborn, surfactant deficiency and pulmonary hypertension is increased. These data suggest that although plasma purine and MDA levels may predict the level of oxidative stress before and during birth, it may not determine neonatal outcome immediately after birth.
Limitations
Because the present sample size is relatively small and study inclusion and exclusion criteria are limited to normal full-term neonates, further studies of direct effects of the mode of delivery on purine and MDA levels are warranted. In addition, the effect of oxytocin is difficult to evaluate as subjects were not randomized to oxytocin. Our observation that neonates born by elective cesarean delivery appear to have more respiratory problems immediately after birth suggests that elevated purine metabolites, specifically uric acid, measured in neonates born by vaginal birth may provide the newborn with some level of protection. Several studies suggest that higher uric acid formation may provide a significant antioxidant defense against peroxynitrite and nitric oxide-derived oxidants.19,20 However, the role of elevated uric acid levels measured in term and preterm neonates with perinatal asphyxia21,22 remains unclear. Further characterization of the ATP degradation pathway and free-radical production in neonates is required and should include both normoxic and hypoxic newborns.
Acknowledgments
This study is partially funded by NIH Grants R21NR010407 (DA) and NIH IMSD 2R25GM060501 (TC). We thank Laura Gouveia, Katherine Angeles and LLUMC obstetric nurses and physicians for helping us with data collection.
References
- 1.Wakatsuki A, Okatani Y, Izumiya C, Ikenoue N. Effect of ischemia-reperfusion on xanthine oxidase activity in fetal rat brain capillaries. Am J Obstet Gynecol. 1999;181(3):731–735. doi: 10.1016/s0002-9378(99)70520-x. [DOI] [PubMed] [Google Scholar]
- 2.Dorrepaal CA, Berger HM, Benders MJ, Van Zoeren-Grobben D, Van de Bor M, Van Bel F. Nonprotein-bound iron in post asphyxial reperfusion injury of the newborn. Pediatrics. 1996;98:883–889. [PubMed] [Google Scholar]
- 3.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt H, Grune T, Muller R, Siems WG, Wauer RR. Increased levels of lipid peroxidation products malondialdehyde and 4-hydroxynonenal after perinatal hypoxia. Pediatr Res. 1996;40:15–20. doi: 10.1203/00006450-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Junge HD, Kunzel W, Klock FK. Acute reduction of uterine blood flow and fetal heart rate changes in pregnant sheep near term. J Perinat Med. 1977;5:39–55. doi: 10.1515/jpme.1977.5.1.39. [DOI] [PubMed] [Google Scholar]
- 6.Siristatidis C, Salamalekis E, Kassanos D, Loghis C, Creatsas G. Evaluation of fetal intrapartum hypoxia by middle cerebral and umbilical artery doppler velocimetry with simultaneous cardiotocography and pulse oximetry. Arch Gynecol Obstet. 2004;270:265–270. doi: 10.1007/s00404-003-0556-z. [DOI] [PubMed] [Google Scholar]
- 7.Monkus ES, Nyhan WL, Fogel BJ, Yankow S. Concentrations of uric acid in the serum of neonatal infants and their mothers. Am J Obstet Gynecol. 1970;108:91–97. doi: 10.1016/0002-9378(70)90211-5. [DOI] [PubMed] [Google Scholar]
- 8.Manzke H, Spreter von Kreudenstein P, Dorner K, Kruse K. Quantitative measurements of the urinary excretion of creatinine, uric acid, hypoxanthine and xanthine, uracil, cyclic AMP, and cyclic GMP in healthy newborn infants. Eur J Pediatr. 1980;133:157–161. doi: 10.1007/BF00441585. [DOI] [PubMed] [Google Scholar]
- 9.Wallenburg HCS, van Kreel BK. Fetal and maternal concentrations of uric acid and oxypurines during labor and post partum. Am J Obstet Gynecol. 1980;136:513–517. doi: 10.1016/0002-9378(80)90681-x. [DOI] [PubMed] [Google Scholar]
- 10.Irestedt L, Dahlin I, Hertzberg T, Sollevi A, Lagercrantz H. Adenosine concentration in umbilical cord blood of newborn infants after vaginal delivery and cesarean section. Pediatr Res. 1989;26:106–108. doi: 10.1203/00006450-198908000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Cighetti G, Debiasi S, Paroni R, Allevi P. Free and total malondialdehyde assessment in biological matrices by gas chromatography-mass spectrometry: what is needed for an accurate detection. Anal Biochem. 1999;266:222–229. doi: 10.1006/abio.1998.2952. [DOI] [PubMed] [Google Scholar]
- 12.Cighetti G, Allevi P, Anastasia L, Bortone L, Paroni R. Use of methyl malondialdehyde as an internal standard for malondialdehyde detection: validation by isotope-dilution gas chromatography-mass spectrometry. Clin Chem. 2002;48:2266–2269. [PubMed] [Google Scholar]
- 13.Paroni R, Fermo I, Cighetti G. Validation of methyl malondialdehyde as internal standard for malondialdehyde detection by capillary electrophoresis. Anal Biochem. 2002;307:92–98. doi: 10.1016/s0003-2697(02)00002-7. [DOI] [PubMed] [Google Scholar]
- 14.Pacheco LD, Rosen MP, Gei AF, Saade GR, Hankins GD. Management of uterine hyperstimulation with concomitant use of oxytocin and terbutaline. Am J Perinatol. 2006;23:377–380. doi: 10.1055/s-2006-948223. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Gudmundsson S, Olofsson P. Acute centralization of blood flow in compromised human fetuses evoked by uterine contractions. Early Hum Dev. 2006;82(11):747–752. doi: 10.1016/j.earlhumdev.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Khaw KS, Wang CC, Ngan Kee WD, Pang CP, Rogers MS. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88(1):18–23. doi: 10.1093/bja/88.1.18. [DOI] [PubMed] [Google Scholar]
- 17.Hack M, Fanaroff AA, Klaus MH, Mendelawitz BD, Merkatz IR. Neonatal respiratory distress following elective delivery: a preventable disease? Am J Obstet Gynecol. 1976;126:43–47. doi: 10.1016/0002-9378(76)90462-2. [DOI] [PubMed] [Google Scholar]
- 18.Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Semin Perinatol. 2006;30(1):34–43. doi: 10.1053/j.semperi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Teng RJ, Ye YZ, Parks DA, Beckman JS. Urate produced during hypoxia protects heart proteins from peroxynitrite-mediated protein nitration. Free Radic Biol Med. 2002;33(9):1243–1249. doi: 10.1016/s0891-5849(02)01020-1. [DOI] [PubMed] [Google Scholar]
- 20.Hooper D, Scott G, Zborek A, Mikheeva T, Kean R, Koprowski R, et al. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000;14:691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- 21.Perlman JM, Risser R. Relationship of uric acid concentrations and severe intraventricular hemorrhage/leukomalacia in the premature infant. J Pediatr Res. 1998;132:436–439. doi: 10.1016/s0022-3476(98)70016-2. [DOI] [PubMed] [Google Scholar]
- 22.Streitman K, Tóth A, Horváth I, Tálosi G. Renal injury in perinatal hypoxia: ultrasonography and changes in renal function. Eur J Pediatr. 2001;160(8):473–477. doi: 10.1007/s004310100769. [DOI] [PubMed] [Google Scholar]


