Abstract
Objective
To describe patterns of diagnostic testing and antibiotic management of uncomplicated pneumonia in general community hospitals and children's hospitals within hospitals, and to determine the association between diagnostic testing and length of hospital stay.
Study design
We conducted a retrospective cohort study of children 1-17 years of age hospitalized with the diagnosis of pneumonia from 2007-2010 to hospitals contributing data to Perspective Database Warehouse, assessing patterns of diagnostic testing and antibiotic management. We constructed logistic regression models of log-transformed length of stay and grouped treatment models to ascertain whether performance of blood cultures and viral respiratory testing were associated with length of stay.
Results
17299 pneumonia cases occurred at 125 hospitals, with considerable variability in pneumonia management. Only 40 (0.2%) received ampicillin/penicillin G alone or in combination with other antibiotics, and 1318 (7.4%) received macrolide monotherapy as initial antibiotic management. Performance of blood culture and testing for respiratory viruses was associated with a statistically significant longer length of stay, but these differences did not persist in grouped treatment models.
Conclusions
We observed higher rates of diagnostic testing in this cohort of structurally diverse hospitals than previously reported at freestanding children's hospitals, with extremely low rates of narrow-spectrum antibiotic use. Tailored antibiotic stewardship initiatives at these hospitals are needed to achieve adherence to national guideline recommendations.
Keywords: community-acquired pneumonia, variation, antibiotics, antibiotic stewardship
Pneumonia is the leading cause of hospitalization for children in the US.1 The costs of pediatric pneumonia management in the inpatient setting are significant, with total healthcare costs exceeding one billion dollars annually.2 Studies conducted among pediatric specialists and at freestanding children's hospitals suggest that management of community-acquired pneumonia (CAP) varies across practitioners and hospitals, with widespread variability in diagnostic testing and antibiotic choices.3–5 However, little is known about the clinical practice patterns at general community hospitals and children's hospitals within larger hospitals, where approximately three-quarters of children receive their hospital care.6
Characterizing practice variation at structurally diverse hospitals that provide pediatric care is particularly important in light of recently published national recommendations for diagnosis and management of CAP.7 Successful implementation of clinical practice guidelines (CPGs) has been shown to decrease resource utilization and improve quality of care.8–10 However, CPGs may have limited influence on disease management for several reasons, including inertia related to previous practice patterns and perceived ineffectiveness of the guidelines.8 Authors of the national CAP CPG acknowledge limited evidence to support several guideline recommendations,7 and few studies have explored whether performance of CPG-recommended diagnostic tests may have unintended consequences, such as escalation of diagnostic testing or prolonged hospital stay. Characterizing patterns of care and associated outcomes may provide valuable data upon which institutional CAP CPGs could be developed at both children's hospitals and general community hospitals.
The objectives of this study were twofold. First, we aimed to describe patterns of diagnostic testing and antibiotic management of uncomplicated pneumonia among previously well children admitted to a large sample of structurally diverse hospitals, with particular focus on diagnostic tests and antibiotics discussed in the Infectious Diseases Society of American/Pediatric Infectious Diseases Society (IDSA/PIDS) CPG. Second, to test the hypothesis that diagnostic testing may be associated with increased length of stay (LOS), we sought to determine associations between performance of blood cultures and tests for respiratory viruses on LOS.
Methods
We conducted a retrospective cohort study of previously well children 1-17 years of age admitted to hospital with CAP from July 1, 2007 to June 30, 2010, preceding publication of the IDSA/PIDS CPG. Children were included in the cohort if they had a principal diagnosis of pneumonia (ICD-9-CM 480.0-2, 480.8-9, 481, 482.0, 482.30-2, 482.41-2, 482.83, 482.89-90, 483, 484.3, 485, 486, 487.0), defined using a previously validated algorithm,11 and if antibiotics were initiated on the first day of hospitalization. Children with complex chronic conditions were excluded using an established algorithm.12,13 Infants less than one year of age were excluded given their high prevalence of bronchiolitis and increased likelihood of misclassifyied respiratory illness.3,14,15 Patients transferred to or from outside facilities were excluded as we were unable to accurately assess LOS and full course of hospital treatments. Subjects who left hospital against medical advice, and those with diagnosis-related-group codes inconsistent with a hospitalization primarily for pneumonia were excluded. We excluded subjects with ICD-9-CM codes for complicated pneumonia using a previously published classification system.7,16 General community hospitals and children's hospitals within larger hospitals (hereafter referred to as children's hospitals) were included; freestanding children's hospitals were excluded. Lastly, we limited our sample to hospitals in which at least 50 patients with uncomplicated pneumonia were admitted during the 3-year study period to allow a sufficient sample size to calculate stable hospital-level statistics. Permission to conduct the study was obtained from the Baystate Medical Center IRB.
Data for this research was accessed from the Perspective Data Warehouse (PDW) (Premier Healthcare Informatics, Charlotte, NC), a highly detailed administrative database that measures healthcare utilization. PDW contains similar data elements to the Pediatric Health Information System (PHIS), including fully de-identified information including patient demographic characteristics, length of stay, all ICD-9-CM discharge diagnoses, as well as a date-specific record of all billed items, including diagnostic tests, medications and associated costs. Although PHIS is used by freestanding, tertiary care children's hospitals affiliated with the Child Health Corporation of America (Shawnee Mission, Kansas) only, PDW is used by a geographically and structurally diverse group of hospitals and represents approximately 15% of all hospitalizations nationally.17 Previous research has shown PDW to be sufficiently similar to a national probability-based sample of pediatric hospitalizations to develop national estimates of pediatric medication use.18
Subjects were characterized by age, sex, race/ethnicity (as recorded by the staff of participating hospitals using hospital-defined options), insurance status, and comorbid conditions. The admitting healthcare provider type was classified as: (1) general pediatrician (including hospitalists); (2) subspecialty pediatrician; or (3) other (including family practice providers and adult-medicine specialties). Characteristics of the admitting hospitals were recorded, including geographic region, bed size, urban/rural location, children's hospital versus general community hospital, and teaching status. Similar to previous research, children's hospitals were defined according to (i) American Hospital Association (AHA) children's hospital designation; or (ii) institutions that had 10 or more pediatric subspecialties recorded in the database.19 Hospitals that did not meet this criterion were categorized as general community hospitals.
We examined billing codes to identify the use of investigations and therapies performed during the hospitalization, with a focus on those discussed in the IDSA/PIDS CPG including: (1) blood culture; (2) chest radiograph; (3) tests for viral respiratory pathogens; (4) erythrocyte sedimentation rate (ESR); (5) C-reactive protein (CRP); and (6) complete blood counts (CBC). Initial antibiotics choice was defined as antibiotics initiated in the emergency department (ED) or on the first day of hospitalization.
Statistical analyses
We calculated patient-level summary statistics and assessed for differences between children admitted to children's hospitals and general community hospitals using chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. We then assessed the proportion of patients receiving each test/therapy at the hospital level, assessing for differences between hospital types using Kruskal-Wallis tests. To describe the degree of variation in treatment rates across hospitals, we calculated the interquartile range (absolute difference between the 75th and the 25th percentiles) for each variable.
We then developed multivariable regression models to ascertain whether performance of two diagnostic tests recommended by the CAP CPG—blood cultures and tests for viral respiratory pathogens, performed on the first day of hospitalization—was associated with LOS. To account for the potential lack of independence among patients treated within the same hospital, we constructed hierarchical mixed effects logistic regression models of log-transformed LOS, controlling for all patient and hospital characteristics shown in Table I. Second, to address potential of confounding by indication, in which sicker patients were more likely to have diagnostic testing, we developed models combining a grouped treatment variable with individual-level covariates to predict LOS, an adaptation of the instrumental variable approach.20 Each patient was assigned a probability of having a diagnostic test performed (blood culture or test for viral respiratory pathogens) that was equal to the diagnostic testing rate at the hospital where they were admitted. This approach attempts to answer the question, “Was treatment at an institution where blood cultures or tests of viral respiratory pathogens were used more frequently associated with a different length of stay, regardless of whether a particular patient received the diagnostic test?” We then constructed multivariable models, substituting the hospital rate of diagnostic testing for the performance of the diagnostic test for each patient. All analyses were carried out using SAS 9.2 (Cary, NC: SAS Institute Inc). Permission to conduct the study was obtained from the Baystate Medical Center IRB.
Table 1.
Patient and hospital characteristics among children admitted with pneumonia.
| Characteristic | n (%) | Admitted to general community hospitals n=12013 n (%) | Admitted to children's hospitals n=5286 n (%) | p-value |
|---|---|---|---|---|
| Patient level (n=17299) | ||||
| Age, years (median [IQR] | 3 [1-6] | 3 [1-6] | 3 [1-6] | 0.01 |
| Sex (female) | 8036 (46.5) | 5541 (46.1) | 2495 (47.2) | 0.19 |
| Race/ethnicity : | ||||
| White | 8249 (47.7) | 5605 (46.7) | 2644 (50.0) | <0.001 |
| Black | 3008 (17.4) | 1800 (15.0) | 1208 (22.9) | |
| Hispanic | 2691 (15.6) | 1746 (14.5) | 945 (17.9) | |
| Other/unknown | 3351 (19.4) | 2862 (23.8) | 489 (9.3) | |
| Insurance status: | ||||
| Public | 8721 (50.4) | 6289 (52.4) | 2432 (46.0) | <0.001 |
| Private | 7742 (44.8) | 5229 (43.5) | 2513 (47.5) | |
| Uninsured | 582 (3.4) | 139 (1.2) | 115 (2.2) | |
| Unknown | 254 (1.5) | 356 (3) | 226 (4.3) | |
| Other conditions: | ||||
| Asthma | 7318 (42.3) | 4772 (39.7) | 2546 (48.2) | <0.001 |
| Influenza | 875 (5.1) | 549 (4.6) | 326 (6.2) | <0.001 |
| Disorders of fluids and electrolytes | 4428 (25.6) | 3245 (27) | 1183 (22.4) | <0.001 |
| Provider specialty:* | ||||
| General pediatrician | 13090 (75.7) | 9621 (80.1) | 3469 (65.6) | <0.001 |
| Subspecialty pediatrician | 1671 (9.7) | 307 (2.6) | 1364 (25.8) | |
| Other | 2538 (14.7) | 2085 (17.4) | 453 (8.6) | |
| Admission source: | ||||
| Emergency department | 10513 (60.8) | 6596 (54.9) | 3917 (74.1) | <0.001 |
| Other | 6786 (39.2) | 5417 (45.1) | 1369 (25.9) | |
| Hospital level (n=125) | ||||
| Geographic region: | ||||
| South | 9633 (55.7) | 6051 (50.4) | 3582 (67.8) | <0.001 |
| Northeast | 2188 (12.6) | 1265 (10.5) | 923 (17.5) | |
| Midwest | 2883 (16.7) | 2722 (22.7) | 161 (3.0) | |
| West | 2595 (15.0) | 1975 (16.4) | 620 (11.7) | |
| Number of beds: | ||||
| ≤ 200 | 2711 (15.7) | 2711 (22.6) | 0 (0) | <0.001 |
| 201-400 | 6593 (38.1) | 5639 (46.9) | 954 (18.0) | |
| ≥ 400 | 7995 (46.2) | 3663 (30.5) | 4332 (82.0) | |
| Urban | 14459 (83.6) | 9173 (76.4) | 5286 (100) | <0.001 |
| Teaching center | 6431 (37.2) | 3518 (29.3) | 2913 (55.1) | <0.001 |
Results
A total of 32558 subjects ages 1-17 years with a principal diagnosis of pneumonia were admitted to 293 hospitals contributing data to the PDW during the study period. We excluded: 4284 subjects with complex chronic conditions, 1905 subjects with complicated pneumonia, 4501 subjects in whom antibiotics were not initiated on the first day of hospitalization, and 1493 subjects who were transferred to or from outside facilities, left hospital against medical advice, had no overnight stay, or had a diagnosis-related-group code inconsistent with pneumonia hospitalization. Of the remaining 20375 patients, 2547 were admitted to 152 hospitals with less than 50 eligible hospitalizations during the study period and 529 were admitted to two freestanding children's hospitals, leaving a final sample of 17299 cases across 125 hospitals. A total of 12013 (69.4%) were admitted to general community hospitals, and 5286 (30.6%) were admitted to children's hospitals within larger hospitals.
The 125 hospitals included in our analysis represented all geographic regions of the United States and included 104 general community hospitals and 21 children's hospitals. There were several differences between children admitted to general community hospitals and children's hospitals (Table I). Children admitted to general community hospitals were more likely to have public health insurance and were more likely to be cared for by general pediatricians than children admitted to children's hospitals.
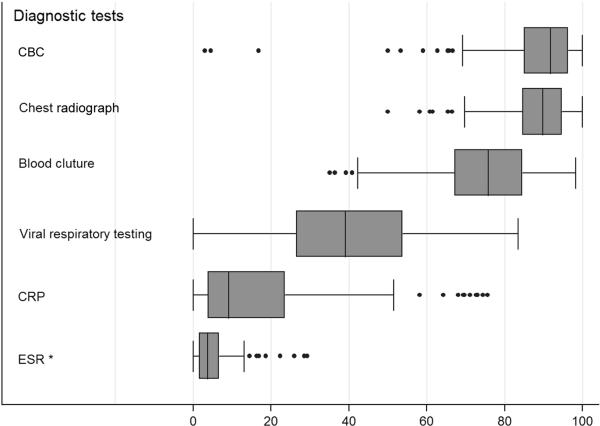
Approximately three-quarters of children had a blood culture performed and more than 80% had a CBC and chest radiograph (Table II). Less than one-half received tests for viral respiratory pathogens, and approximately one-quarter had acute phase reactants measured. We observed similar rates of chest radiograph, blood culture and viral respiratory testing among children admitted to general community hospitals and children's hospitals, but children admitted to children's hospitals were significantly more likely to have acute phase reactants assessed than children admitted to general community hospitals. A considerable proportion of children received the same investigations multiple times during their hospitalization (Table II). Among children receiving a given diagnostic test, more than 10% received more than one blood culture, and more than one-quarter received multiple chest radiographs, CBCs, and CRP assessments.
Table 2.
Performance of diagnostic tests during hospitalization, illustrating proportion of children receiving repeat diagnostic testing during their hospital stay.
| Diagnostic test | Children receiving diagnostic test (n = 17299) n (%) | Admitted to general community hospitals (n=12013) n (%) | Admitted to children's hospitals (n=5286) n (%) | p-value | Number (%) of children receiving repeat diagnostic tests during hospitalization | ||
|---|---|---|---|---|---|---|---|
| 2 tests n (%) | ≥3 tests n (%) | % receiving repeat testinga | |||||
| Blood culture | 12849 (74.3) | 8897 (74.1) | 3952 (74.8) | 0.33 | 1241 (7.2) | 95 (0.6) | 10.4% |
| Chest radiograph | 15334 (88.6) | 10617 (88.4) | 4717 (89.2) | 0.10 | 3364 (19.5) | 978 (5.7) | 28.3% |
| Viral respiratory testing | 7199 (41.6) | 4994 (41.6) | 2205 (41.7) | 0.86 | 291 (1.7) | 6 (0.03) | 4.1% |
| Complete blood count | 15117 (87.4) | 10634 (88.5) | 4483 (84.8) | <0.001 | 3754 (21.7) | 1799 (10.4) | 36.7% |
| ESR | 1261 (7.3) | 549 (4.6) | 712 (13.5) | <0.001 | 143 (0.8) | 51 (0.3) | 15.4% |
| CRP | 3627 (21.0) | 2257 (18.8) | 1370 (25.9) | <0.001 | 784 (4.5) | 398 (2.3) | 32.6% |
among children receiving the test
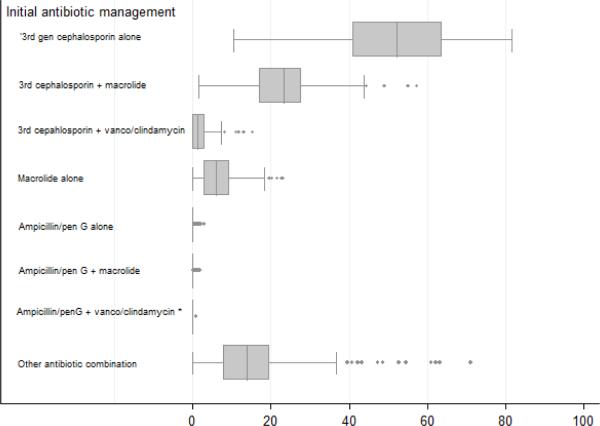
A third-generation cephalosporin, used alone or in combination with a macrolide or anti-staphylococcal antibiotic, was the most commonly used antibiotics for initial disease management. Use of parenteral ampicillin or penicillin G was extremely rare, either alone or in combination with other antibiotics. Only 29 (0.17%) received ampicillin or penicillin G alone, and 11 (0.06%) received ampicillin or pencillin G in combination with a macrolide, vancomycin or clindamycin as initial management.
A minority of children had antibiotics modified on the second day of hospitalization. Six children (0.03%) had therapy changed from ceftriaxone to ampicillin or penicillin G on the second day of hospitalization, and 1 (0.01%) was changed from macrolide monotherapy to ampicillin. A total of 100 children (0.58%) initially treated with ceftriaxone/macrolide combination therapy had coverage narrowed to ceftriaxone alone on the second day of hospitalization.
Variation in Resource Use
We observed considerable variation in resource utilization at the hospital level, with the greatest variation observed in use of viral respiratory testing, CRP and blood culture (Figure 1). At some hospitals more than one in four children received at least 1 CRP; at the other end of the testing spectrum, fewer than 1 in 20 children underwent CRP testing. We observed substantial variation at the hospital level in the use of antibiotics for pneumonia management, particularly regarding third-generation cephalosporin and third-generation cephalosporin-macrolide combination therapy (Figure 2). Antibiotic prescribing was similar at children's hospitals and general community hospitals; the only significant difference was observed in cephalosporin and anti-staphylococcal combination therapy, with children's hospitals having a higher median prescribing rate for this combination than general community hospitals.
Figure 1.
Box plot illustrating hospital-level variation in rates of diagnostic testing.**
Figure 1 footnote: * significantly different between children's hospitals and general community hospitals (p<0.01)
Figure 1 footnote: ** center box illustrates median and interquartile range; whiskers illustrate range excluding outliers; dots illustrate outliers.
Figure 2.
Boxplot illustrating hospital-level variation in initial antibiotic management**
Figure 2 footnote: * significantly different between children's hospitals and general community hospitals (p<0.01)
Figure 2 footnote: ** center box illustrates median and interquartile range; whiskers illustrate range excluding outliers; dots illustrate outliers.
Total costs of pneumonia hospitalization also varied across hospitals. The median cost of hospitalization at the patient level was $3264 (IQR $2224-4939), and the median cost at the hospital level was $3165 (IQR $2591-3734). LOS was less varied, with a median of 2 days (IQR 2-3) at the patient level and a median of 2 days (IQR 2-2) at the hospital level.
Association between diagnostic tests and length of stay
Accounting for clustering within hospitals and patient and hospital characteristics, performance of blood culture was associated with a statistically significant increased LOS (RR1.06, 95%CI 1.04-1.07). Given the mean length of stay of 2.5 days in our cohort, this is equivalent to one child staying in hospital for one additional day for every seven children receiving a blood culture. In our grouped treatment analysis, the direction of the effect was similar but no longer statistically significant (RR 1.10, 95%CI 0.98-1.23). Similarly, diagnostic testing for viral respiratory pathogens on the first day of hospitalization was associated with a small but statistically significant increased LOS in our hierarchical mixed effects logistic regression model (RR1.03, 95%CI 1.01-1.05). However, this difference was not seen in our grouped treatment analysis intended to control for unmeasured confounders at the patient level (RR1.06, 95%CI 0.92-1.23).
Discussion
This study characterizes the management of uncomplicated pneumonia among children at 125 structurally diverse hospitals across the United States, illustrating substantial variation in the performance of diagnostic tests and antibiotic management. A considerable proportion of children received repeated investigations during hospitals stays averaging 2 days in duration. Ampicillin was administered very infrequently, with only 40 of 17299 children receiving ampicillin alone or in combination with a macrolide or clindamycin/vancomycin as the initial antibiotic. We also observed considerable variation across hospitals with respect to macrolide use, either alone or in combination with other antibiotics.
Our results, reflecting patterns of care at a large cohort of structurally diverse acute care hospitals, align with a previous study illustrating significant variation in diagnostic testing and antimicrobial management at 27 freestanding children's hospitals.3 In our cohort, we observed higher rates of diagnostic testing than that observed at freestanding children's hospitals, despite the fact that the studies examined temporally similar cohorts of children. These differences may be influenced, in part, by the 2007 American Thoracic Society/IDSA guidelines which recommend chest radiographs and blood culture for adults with CAP.21 Children cared for at general community hospitals and children's hospitals affiliated with adult centers may be more likely to receive their initial care at Emergency Departments in which care is provided to both adults and children, which may have resulted in application of the adult CAP guidelines to children. Similarly, we observed significantly lower rates of ampicillin use and higher rates of macrolide monotherapy than that observed at freestanding children's hospitals.
In 2007, the IDSA established recommendations that hospitals develop antimicrobial stewardship (AS) programs to monitor and improve the use of appropriate antimicrobials, with goals of optimizing clinical outcomes while minimizing drug toxicity, selection of pathogenic organisms and emergence of antibiotic resistance.22 Correspondingly, ampicillin or penicillin G is strongly recommended for first-line management of uncomplicated pediatric CAP in the inpatient setting barring substantial high level penicillin resistance of Streptococcus pneumonaie in the community.7 Successful initiatives to increase ampicillin use while decreasing ceftriaxone use have been established at freestanding children's hospitals using combinations of institutional guidelines, AS working groups, and prospective audit with feedback programs.23–25 The very low rates of ampicillin use and relatively high rates of macrolide monotherapy observed in our cohort highlight a need for effective pediatric AS initiatives beyond freestanding children's hospitals. In the absence of pediatric specific guidelines, general community hospitals may face a number of unique challenges to implementing pediatric AS programs, including a relative lack of pediatric resources such as pediatric ED physicians and infectious diseases subspecialists, who have traditionally led AS programs in children's hospitals. As a result, development of unique AS programs that align with community hospitals’ resources and clinical practice models is needed.
The substantial proportion of children in our cohort who received repeated diagnostic testing is a notable finding, particularly given that we limited our study to children with uncomplicated CAP and without complex chronic conditions. Among children receiving initial diagnostic testing, more than 10% had multiple blood cultures performed and more than a quarter of children had repeat chest radiograph, CBC or CRP assessments. The adverse effects of over-testing have been discussed extensively within the adult literature,26,27 prompting the American Board of Internal Medicine Foundation's Choosing Wisely campaign to aim to improve patient safety by reducing diagnostic tests with unproven benefits.28,29 Our results support the notion that diagnostic testing begets more diagnostic testing, and highlight the importance of having a robust justification for initial diagnostic testing among children with uncomplicated presentation of disease.
Given that false-positive blood cultures can lead to further diagnostic testing and that many institutions do not report final blood culture results until 48-72 hours following the blood testing, we hypothesized that high rates of blood culture testing would be associated with increased LOS. However, our analyses do not support this hypothesis – although we found small but statistically significant increased LOS associated with diagnostic testing in our initial regression models, these association were no longer significant in grouped treatment analysis, a rigorous method to account for potential confounding by indication. It is noteworthy that blood culture and viral respiratory pathogen testing, both strongly recommended in the IDSA/PIDS CPG, do not appear to have considerable impacts on LOS.
Our results should be interpreted in light of several limitations. First, we used ICD-9-CM codes to retrospectively identify patients with pneumonia, which may have resulted in misclassification. We attempted to minimize misclassification by using a validated pneumonia definition,11 excluding complications using a previously established classification scheme,16 and limiting our sample to patients for whom antibiotics were initiated on the first day of hospitalization. Second, because our analysis used administrative data, we did not have clinical data such as oxygen saturation and other vital signs. As a result, we had limited ability to assess pneumonia severity or other factors that may have influenced decisions regarding diagnostic testing and antibiotic management. However, we limited our cohort to a relatively homogenous population, excluding children with complex chronic conditions and those with complications of pneumonia. Third, we are unable to discern whether antibiotics were initiated in the emergency department or upon admission for inpatient care. Finally, our study reflects practice patterns prior to publication of the PIDS/IDSA CAP CPG; follow-up studies are needed to assess practice patterns following CPG publication.
Our results provide valuable baseline data to incentivize pediatric AS programs beyond freestanding children's hospitals. In addition, this study provides an example of how highly detailed administrative data may be applied to explore patterns of resource utilization at structurally diverse hospitals similar to previous studies conducted at freestanding children's hospitals using PHIS.3,30–32 By linking PDW with AHA survey data, as done in this study, investigators have the opportunity to understand disease management and outcomes in a variety of hospital environments, thereby increasing our health services research knowledge base.
Acknowledgments
J.L. was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences and the National Institutes of Health (NIH: UL1 RR025752). T.L. is supported by the National Heart, Lung, and Blood Institute of the NIH (K01HL114745). The content is solely the responsibility of the authors and does not necessarily represent the official news of the NIH.
Abbreviations
- CAP
Community-acquired pneumonia
- CBC
complete blood count
- CPG
clinical practice guideline
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- ICD-9-CM
International Classification of Disease, 9th Edition, Clinical Modification
- LOS
Length of stay
- PDW
Perspective Data Warehouse
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Yu H, Wier LM, Elixhauser A. Hospital Stays for Children, 2009. Healthc cost Utilization Project Statistical Brief 118. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb1. [PubMed]
- 2.Agency for Healthcare Research and Quality HCUPnet. doi: 10.1080/15360280802537332. http://hcupnet.ahrq.gov/HCUPnet.jsp. [DOI] [PubMed]
- 3.Hersh AL, Shapiro DJ, Newland JG, Polgreen PM, Beekmann SE, Shah SS. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31:1036–41. doi: 10.1097/INF.0b013e31825f2b10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambroggio L, Tabb LP, O'Meara T, Sheffler-Collins S, McGowan KL, Shah SS. Influence of antibiotic susceptibility patterns on empiric antibiotic prescribing for children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31:331–6. doi: 10.1097/INF.0b013e3182489cc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hersh AL, Shapiro DJ, Newland JG, Polgreen PM, Beekmann SE, Shah SS. Variability in pediatric infectious disease consultants’ recommendations for management of community-acquired pneumonia. PLoS One. 2011;6:e20325. doi: 10.1371/journal.pone.0020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry JG, Hall M, Hall DE, Kuo DZ, Cohen E, Agrawal R, et al. Inpatient Growth and Resource Use in 28 Children's Hospitals: A Longitudinal, Multi-institutional Study. JAMA Pediatr. 2013;167:170–7. doi: 10.1001/jamapediatrics.2013.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–76. doi: 10.1093/cid/cir531. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud P-AC, et al. Why Don't Physicians Follow Clinical Practice Guidelines? A Framework for Improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 9.Cabana MD, Flores G. The role of clinical practice guidelines in enhancing quality and reducing racial / ethnic disparities in paediatrics. Paediatr Respir Rev. 2002;3:52–58. doi: 10.1053/prrv.2002.0182. [DOI] [PubMed] [Google Scholar]
- 10.Davis DA, Taylor-Vaisey A. Translating guidelines into practice. Can Med Assoc J. 1997;157:408–16. [PMC free article] [PubMed] [Google Scholar]
- 11.Williams DJ, Shah SS, Myers A, Hall M, Auger K, Queen MA, et al. Identifying Pediatric Community-Acquired Pneumonia Hospitalizations: Accuracy of Administrative Billing Codes. JAMA Pediatr. 2013;37232:1–8. doi: 10.1001/jamapediatrics.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feudtner C, Christakis DA, Connell FA. Pediatric Deaths Attributable to Complex Chronic Conditions: A population based study of Washington State: 1980-1997. Pediatrics. 2000;106:205–9. [PubMed] [Google Scholar]
- 13.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths Attributed to Pediatric Complex Chronic Conditions: National Trends and Implications for Supportive Care Services. Pediatrics. 2001;107:e99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 14.Weiss AK, Hall M, Lee GE, Kronman MP, Sheffler-Collins S, Shah SS. Adjunct Corticosteroids in Children Hospitalized With Community-Acquired Pneumonia. Pediatrics. 2011;127:e255–e263. doi: 10.1542/peds.2010-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambroggio L, Taylor JA, Tabb LP, Newschaffer CJ, Evans AA, Shah SS. Comparative effectiveness of empiric β-lactam monotherapy and β-lactammacrolide combination therapy in children hospitalized with community-acquired pneumonia. J Pediatr. 2012;161:1097–103. doi: 10.1016/j.jpeds.2012.06.067. [DOI] [PubMed] [Google Scholar]
- 16.Lee GE, Lorch S a, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126:204–13. doi: 10.1542/peds.2009-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher BT, Lindenauer PK, Feudtner C. In-hospital databases. In: Strom BL, Kimmel SE, Hennessy S, editors. Pharmacoepidemiology. 5th ed. John Wiley and Sons; 2012. pp. 244–249. [Google Scholar]
- 18.Lasky T, Ernst FR, Greenspan J, Wang S, Gonzalez L. Estimating pediatric inpatient medication use in the United States. Pharmacoepidemiol drug safety. 2011;20:76–82. doi: 10.1002/pds.2063. [DOI] [PubMed] [Google Scholar]
- 19.Jan S, Slap G, Dai D, Rubin DM. Variation in surgical outcomes for adolescents and young adults with inflammatory bowel disease. Pediatrics. 2013;131(Suppl):S81–9. doi: 10.1542/peds.2012-1427j. [DOI] [PubMed] [Google Scholar]
- 20.Johnston SC, Henneman T, McCulloch C, van der Lann M. Modeling Treatment Effects on Binary Outcomes with Grouped-Treatment Variables and Individual Covariates. Am J Epidemiol. 2002;156(8):753–760. doi: 10.1093/aje/kwf095. [DOI] [PubMed] [Google Scholar]
- 21.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 23.Smith MJ, Kong M, Cambon A, Woods CR. Effectiveness of antimicrobial guidelines for community-acquired pneumonia in children. Pediatrics. 2012;129:e1326–33. doi: 10.1542/peds.2011-2412. [DOI] [PubMed] [Google Scholar]
- 24.Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129:e597–604. doi: 10.1542/peds.2011-1533. [DOI] [PubMed] [Google Scholar]
- 25.Ambroggio L, Thomson J, Murtagh Kurowski E, Courter J, Statile A, Graham C, et al. Quality improvement methods increase appropriate antibiotic prescribing for childhood pneumonia. Pediatrics. 2013;131:e1623–31. doi: 10.1542/peds.2012-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grady D, Redberg RF. Less is more: how less health care can result in better health. Arch Intern Med. 2010;170:749–750. doi: 10.1001/archinternmed.2010.90. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–7. doi: 10.1542/peds.2011-2726. [DOI] [PubMed] [Google Scholar]
- 28.Quinonez RA, Garber MD, Schroeder AR, Alverson BK, Nickel W, Goldstein J, et al. Choosing wisely in pediatric hospital medicine: Five opportunities for improved healthcare value. J Hosp Med. 2013;8:479–85. doi: 10.1002/jhm.2064. [DOI] [PubMed] [Google Scholar]
- 29.ABIM Foundation U.S. physician groups identify commonly used tests or procedures they say are often not necessary. Available at: http://www.abimfoundation.org/News/ABIM-Foundation-News/2012/Choosing-Wisely.aspx.
- 30.Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster M a, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164:300–5. doi: 10.1016/j.jpeds.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Mahant S, Keren R, Localio R, Luan X, Song L, Shah SS, et al. Variation in Quality of Tonsillectomy Perioperative Care and Revisit Rates in Children's Hospitals. Pediatrics. 2014;133:280–8. doi: 10.1542/peds.2013-1884. [DOI] [PubMed] [Google Scholar]
- 32.Jain S, Cheng J, Alpern ER, Thurm C, Schroeder L, Black K, et al. Management of Febrile Neonates in US Pediatric Emergency Departments. Pediatrics. 2014;133:187–95. doi: 10.1542/peds.2013-1820. [DOI] [PubMed] [Google Scholar]