Abstract
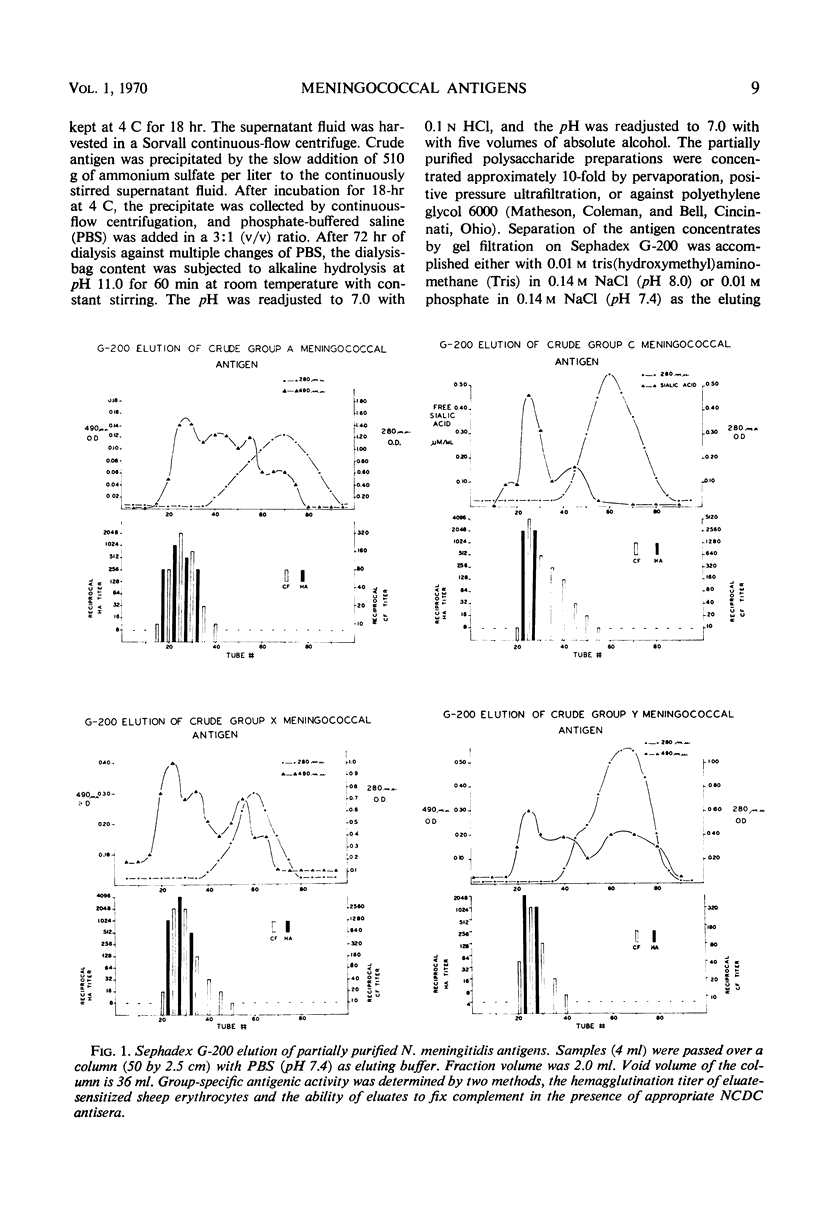
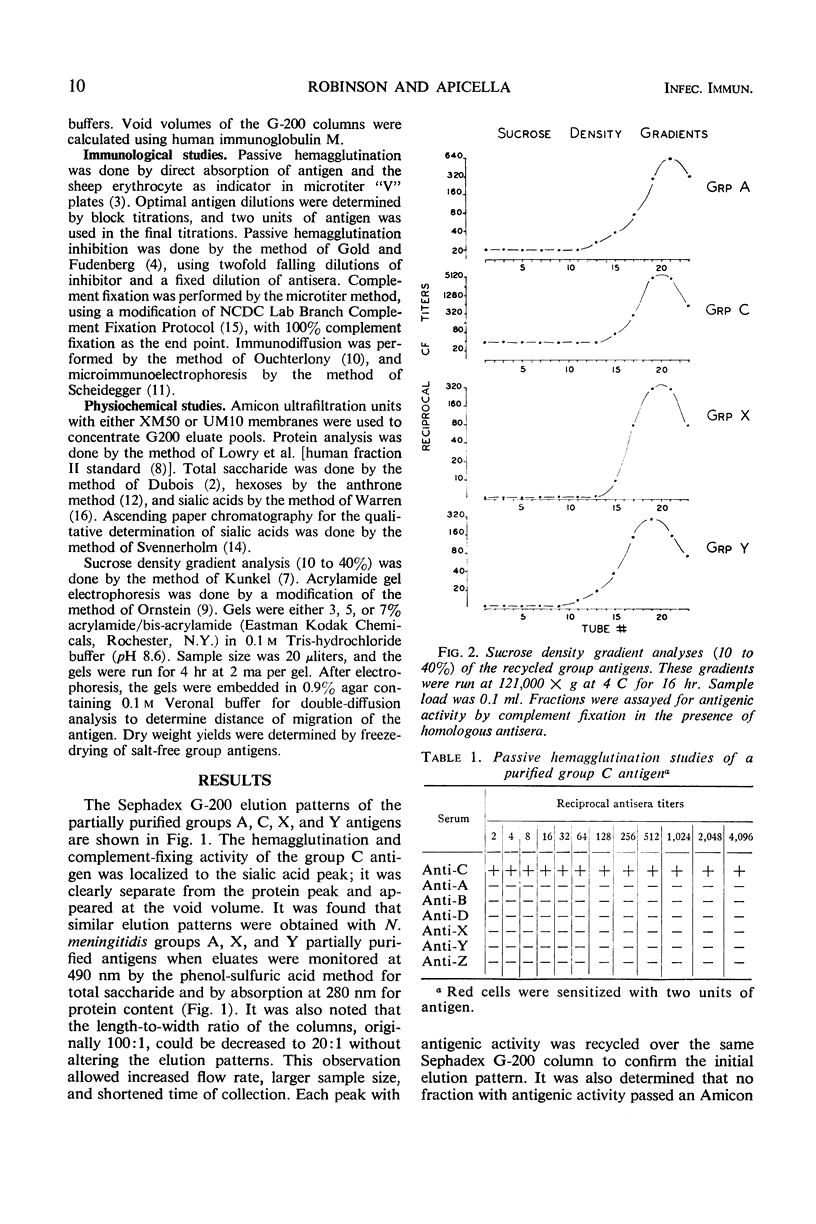
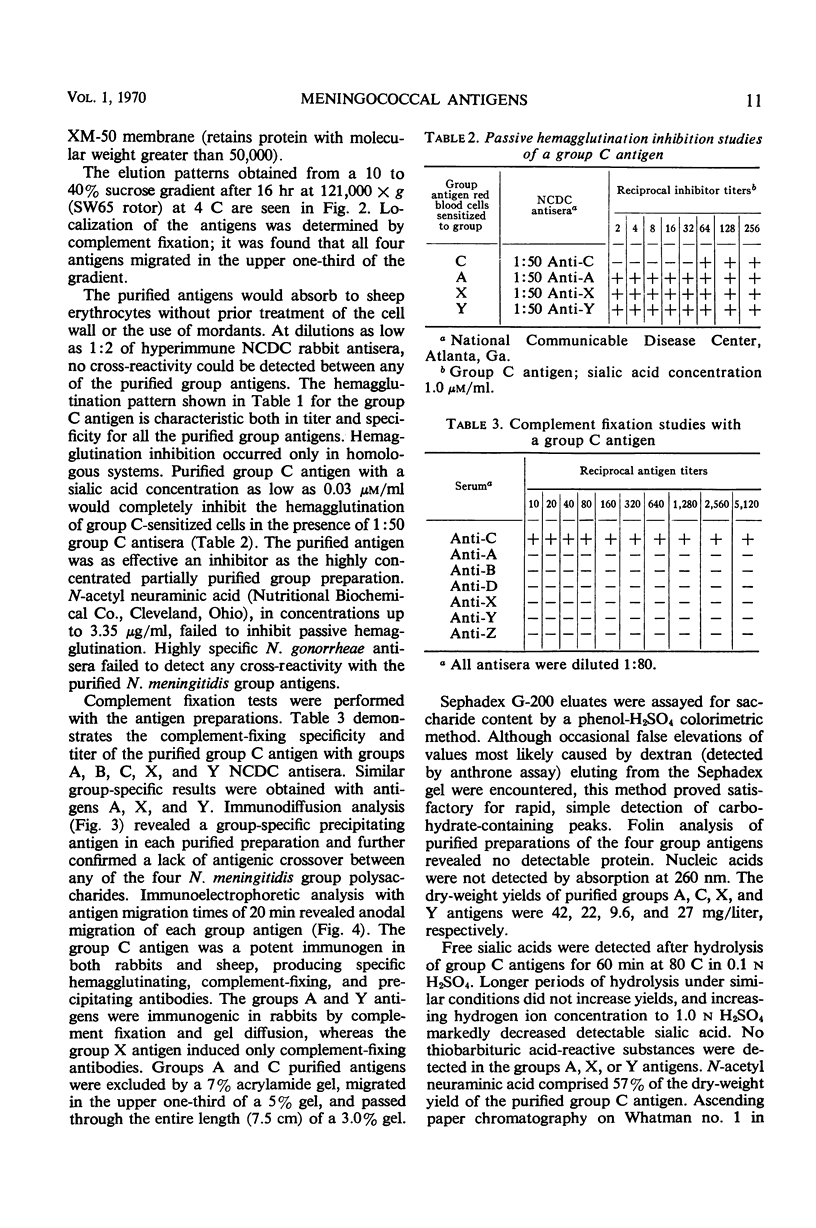
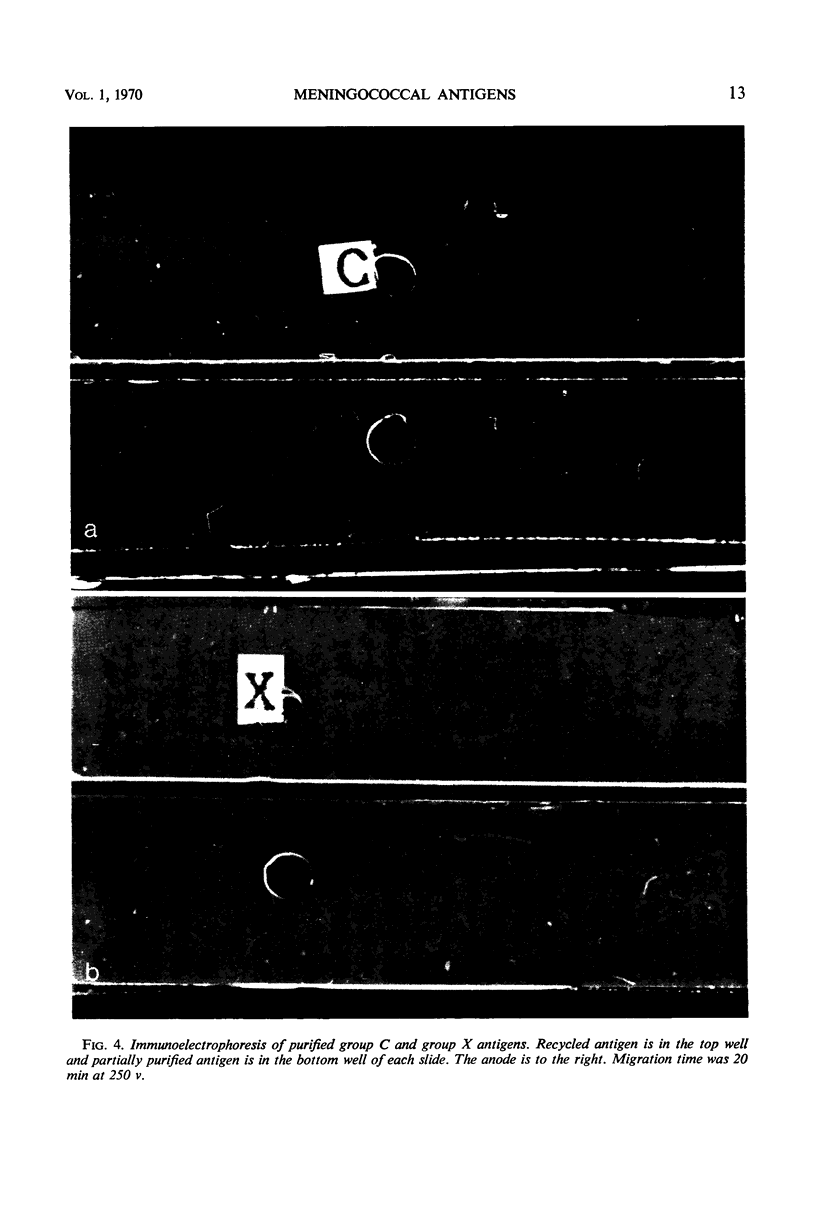
To prepare Neisseria meningitidis groups A, C, X, and Y polysaccharide antigens, culture supernatant fluids were subjected to serial processes of salt precipitation, alkaline hydrolysis, ethyl alcohol precipitation, and Sephadex G-200 chromatography. This method resulted in the isolation of large quantities of group antigens. All are acidic polysaccharides, the group C antigen being a polymer of n-acetyl neuraminic acid. Thiobarbituric acid assay failed to reveal sialic acids in the other group antigens. Protein was undetectable by absorption at 280 nm or by Folin analysis. These antigens are of similar molecular size, the majority of which are excluded by Sephadex G-200. They migrate in the upper one-third of sucrose density gradients and are retained by 5% acrylamide gel. All are highly group-specific and react only with homologous hyperimmune antisera in hemagglutination, complement fixation, and immunodiffusion systems. As little as 0.03 μmoles of n-acetyl neuraminic acid in group C antigen inhibits the hemagglutination of group C-sensitized red cells. All antigens are immunogenic in rabbits. These techniques afford a simplified method for the production of relatively large yields of highly specific group antigens which participate in multiple immunologic systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond B. W., Kingsbury D. T., Weiss E. Modification of meningococcal polysaccharide antigens for use in passive hemagglutination tests. J Immunol. 1968 Oct;101(4):808–809. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SVENNERHOLM E., SVENNERHOLM L. Quantitative paper partition chromatography of sialic acids. Nature. 1958 Apr 19;181(4616):1154–1155. doi: 10.1038/1811154a0. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WATSON R. G., SCHERP H. W. The specific hapten of group C (group II alpha) meningococcus. I. Preparation and immunological behavior. J Immunol. 1958 Oct;81(4):331–336. [PubMed] [Google Scholar]





