Abstract
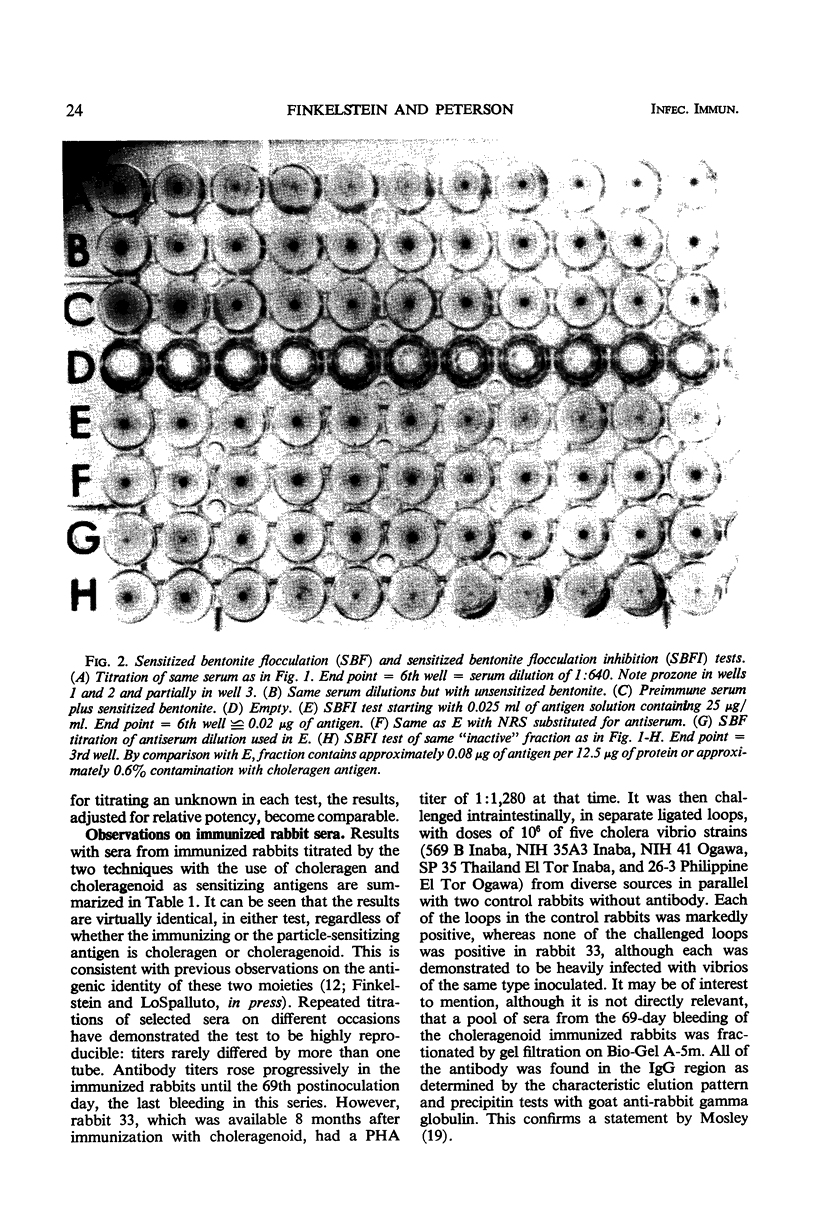
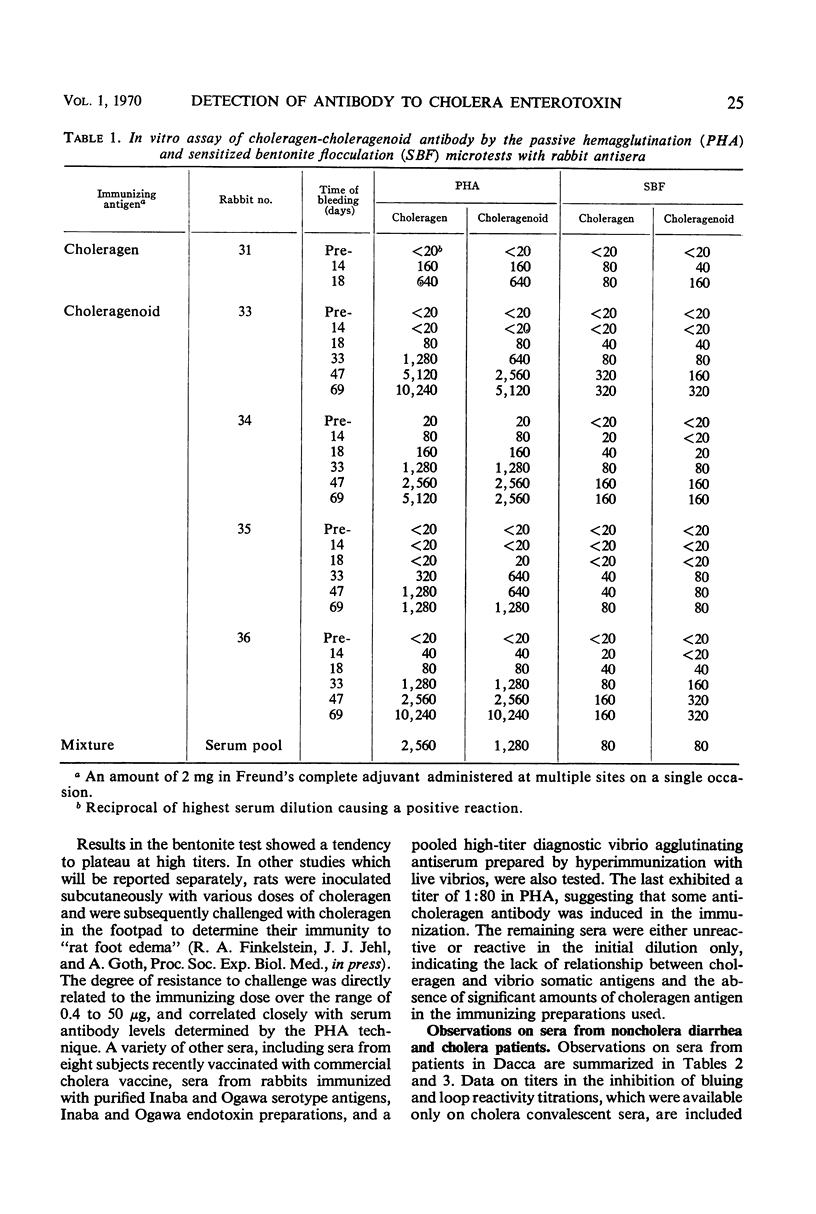
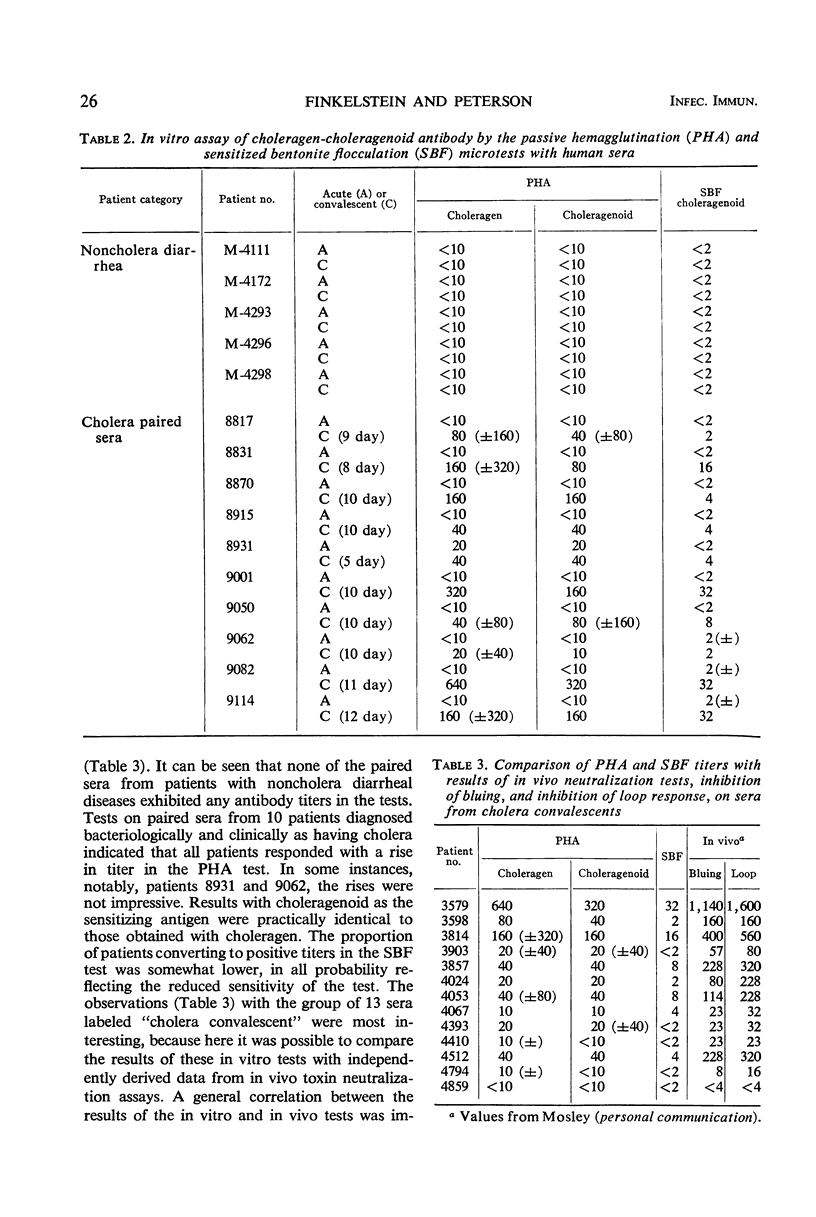
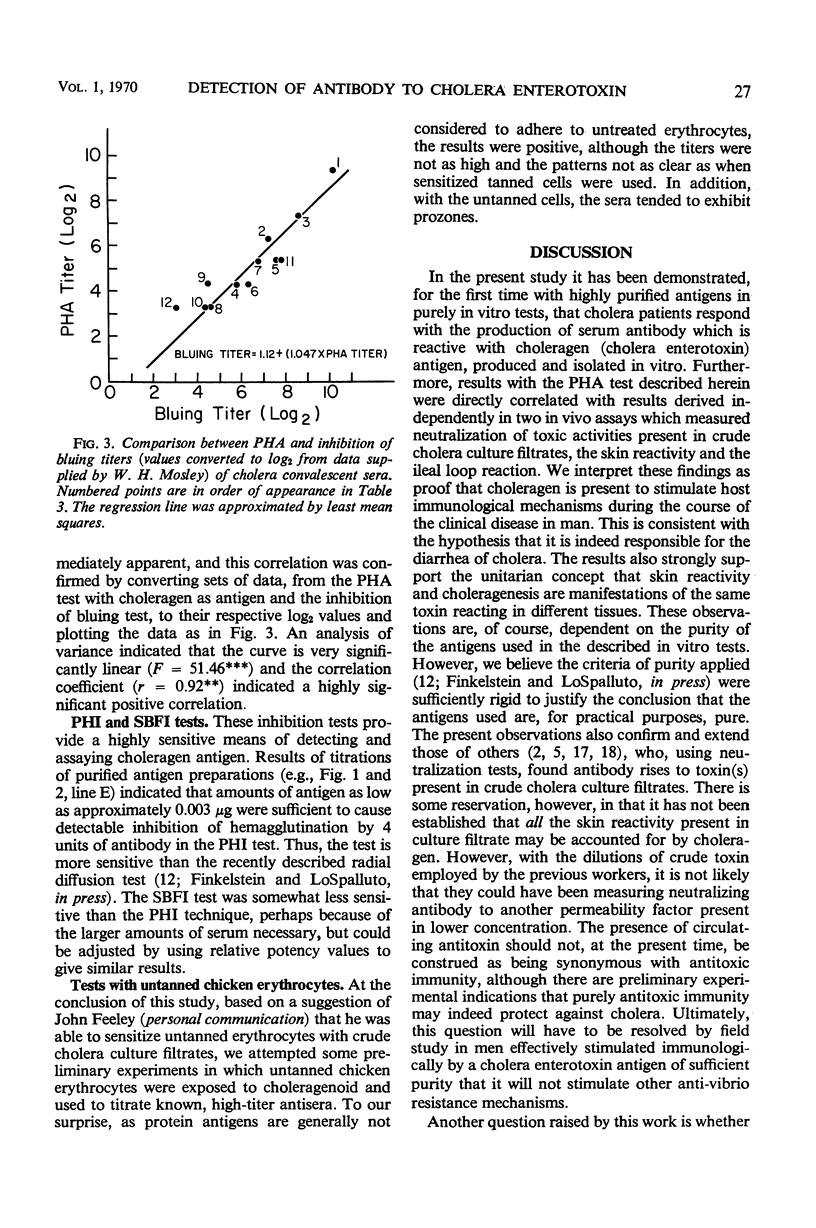
Two new in vitro microtests for anticholeragen (cholera exo-enterotoxin) antibodies are described and compared. In both tests, choleragen and choleragenoid, antigenically identical purified moieties which differ in size, charge, and toxicity, may be used as sensitizing antigens with apparently equal facility. The passive hemagglutination (PHA) test in which sensitized tanned chicken erythrocytes are used was found to be more sensitive than the sensitized bentonite flocculation test. Tests with sera from cholera patients almost invariably demonstrated a rise in titer on convalescence. Results with the PHA test were directly correlated with results derived from in vivo toxin neutralization assays involving inhibition of skin reactivity and the ileal loop reaction. These observations strengthen the hypothesis that choleragen is involved in the pathogenesis of cholera in man and support the unitarian concept that skin reactivity and choleragenesis are manifestations of the same toxin acting in different tissues. Both of the tests described have been modified and used as inhibition tests in the detection and assay of choleragen antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. V. The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951 Feb;93(2):107–120. doi: 10.1084/jem.93.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benenson A. S., Saad A., Mosley W. H., Ahmed A. Serological studies in cholera. 3. Serum toxin neutralization--rise in titre in response to infection with Vibrio cholerae, and the level in the "normal" population of East Pakistan. Bull World Health Organ. 1968;38(2):287–295. [PMC free article] [PubMed] [Google Scholar]
- Benenson A. S., Saad A., Mosley W. H. Serological studies in cholera. 2. The vibriocidal antibody response of cholera patients determined by a microtechnique. Bull World Health Organ. 1968;38(2):277–285. [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Lincoln R. E., Lamanna C. Status of bacterial toxins and their nomenclature: need for discipline and clarity of expression. Bacteriol Rev. 1967 Jun;31(2):95–109. doi: 10.1128/br.31.2.95-109.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- DUTTA N. K., HABBU M. K. Experimental cholera in infant rabbits: a method for chemotherapeutic investigation. Br J Pharmacol Chemother. 1955 Jun;10(2):153–159. doi: 10.1111/j.1476-5381.1955.tb00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Richardson S. H. In vitro production of choleragen and vascular permeability factor by Vibrio cholerae. J Bacteriol. 1968 Jul;96(1):126–130. doi: 10.1128/jb.96.1.126-130.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN R. A., NORRIS H. T., DUTTA N. K. PATHOGENESIS EXPERIMENTAL CHOLERA IN INFANT RABBITS. I. OBSERVATIONS ON THE INTRAINTESTINAL INFECTION AND EXPERIMENTAL CHOLERA PRODUCED WITH CELL-FREE PRODUCTS. J Infect Dis. 1964 Jun;114:203–216. doi: 10.1093/infdis/114.3.203. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P. Immunity against experimental cholera. Proc Soc Exp Biol Med. 1967 Jun;125(2):465–469. doi: 10.3181/00379727-125-32121. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Nye S. W., Atthasampunna P., Charunmethee P. Pathogenesis of experimental cholera. Effect of choleragen on vascular permeability. Lab Invest. 1966 Oct;15(10):1601–1609. [PubMed] [Google Scholar]
- Finkelstein R. A., Sobocinski P. Z., Atthasampunna P., Charunmethee P. Pathogenesis of experimental cholera: identification of choleragen (procholeragen A) by disc immunoelectrophoresis and its differentiation from cholera mucinase. J Immunol. 1966 Jul;97(1):25–33. [PubMed] [Google Scholar]
- Kasai G. J., Burrows W. The titration of cholera toxin and antitoxin in the rabbit ileal loop. J Infect Dis. 1966 Dec;116(5):606–614. doi: 10.1093/infdis/116.5.606. [DOI] [PubMed] [Google Scholar]
- Martin A. R., Vernon T. M., Mosley W. H. Neutralization of the vascular permeability factor of Vibrio Cholerae in man. Am J Trop Med Hyg. 1969 Mar;18(2):253–257. [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):360–367. [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. II. Specific applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):368–375. [PubMed] [Google Scholar]




