Abstract
Aims and Objectives:
The present study was designed to estimate and compare the superoxide dismutase and total antioxidant capacity levels in the serum of diabetes mellitus - Type 2 patients and healthy individuals with and without periodontal disease.
Materials and Methods:
The study was designed as a case-control study comprising of 150 subjects, inclusive of both genders in the age group of 35-65 years. They were divided into three groups of 50 patients each. Patients were categorized into diabetic with chronic periodontitis (DM-CP) and systemically healthy groups with and without periodontitis. Serum samples were collected and sent for biochemical analysis to estimate the total antioxidant capacity (TAOC) and superoxide dismutase (SOD) levels. Results obtained were then statistically analysed using ANOVA test.
Results:
The results showed a higher level of serum TAOC in the systemically healthy group without periodontitis (PH) compared to the other groups. The P value was found to be <0.05. The SOD levels were found to be highest in the DM-CP group.
Conclusion:
The results of this study indicate that serum TAOC were found to be highest in the PH group and lowest in the systemically healthy with chronic periodontitis (CP). The serum SOD levels were found to be highest in the DM-CP group. The increased levels of SOD seen in DM patients may be a result of a protective and adaptive mechanism against the oxidative stress developing in the tissue.
Keywords: Oxidative stress, superoxide dismutase, total antioxidant capacity
INTRODUCTION
The majority of periodontal tissue destruction is caused by an inappropriate host response to microorganisms and their products;[1] specifically, a loss of homeostatic balance between reactive oxygen species (ROS) and the antioxidant defense systems that protect and repair vital tissue cells and molecular components are believed to be responsible.[2]
One of the host defense mechanism involves the production of powerful oxidizing agents, which is characterized by a rapid increase in the uptake of oxygen. This phenomenon is known as the respiratory burst and results in the production of reactive oxidants such as superoxide, hydrogen peroxide and hydroxyl radicals. Chronic exposure to ROS can initiate a broad spectrum of pathologic reactions. These include lysing of cell membranes, DNA fragmentation, activation of proteolytic enzymes and the degradation of specific extracellular matrix components.[3] The body has evolved certain defense and repair systems inherently to prevent the accumulation of oxidatively damaged molecules that are toxic. Antioxidants are defined as those substances that when present at low concentrations, compared with those of an oxidizable substrate, will significantly delay or inhibit oxidation of that substrate. Oxidative stress occurs when the oxidant–antioxidant balance is disturbed in favor of ROS, which then causes tissue damage.
Superoxide dismutase (SOD) is one of the antioxidant enzymes that protects the cell against the deleterious effects of ROS. It specifically scavenges superoxide by catalyzing its dismutation to H2O2 and O2.[4]
Total antioxidant capacity (TAOC) assays provide an overview of the biological interactions between individual antioxidant species and also a measure of the capacity of biological systems to withstand oxidative attack.[5]
The pathways of vascular tissue complications in diabetes are mediated through the formation of advanced glycation end products (AGE) and increased production of ROS. Formation of AGE are among the factors that can lead to periodontal disease development and increased oxidative stress in the gingiva of diabetes patients. Oxidative stress is also thought to be increased by a decrease in the antioxidant mechanism in patients with diabetes.[6] These changes can affect the severity and progression of periodontitis in diabetes patients.
Thus, considering that TAOC and SOD levels may have a role in the interaction between diabetes and periodontitis, the aim of this study was to estimate and compare the levels of SOD and TAOC in the serum of diabetes mellitus (DM) type-2 patients and systemically healthy subjects with and without chronic periodontitis (CP).
MATERIALS AND METHODS
The study was designed as a case–control study comprising of 150 subjects, inclusive of both genders, selected from the Department of Periodontics and Department of General Medicine, K.S. Hegde Medical Academy. The study protocol was approved by the Nitte University Ethics Committee and, prior to the study, an informed consent was signed by each patient. The study comprised of three groups of 50 patients each in the range of 35-65 years with
Group 1: DM type-2 patients with CP (DM-CP)
Group 2: Systemically healthy subjects with CP
Group 3: Systemically healthy subjects without periodontitis (PH).
Inclusion criteria
Patients with periodontal disease having probing depth and clinical loss of attachment more than 4 mm in more than 30% of the sites for the DM-CP and CP groups
Subjects with a minimal complement of 20 teeth
Patients are diagnosed to have DM type-2 and who are on oral hypoglycemic agents (metformin) for the DM-CP group
Patients having a gingival index score of less than 2.0, a probing depth of less than 3 mm and no previous history of any periodontal disease for the PH group.
Exclusion criteria
Subjects with any systemic disease or conditions apart from DM type-2 for the DM-CP group
Patients who were on antibiotics/anti-inflammatory drugs/steroids in the past 3 months
Patients who have undergone any periodontal treatment in the last 6 months
Pregnant or lactating women
Subjects who were tobacco or alcohol users
Subjects who had taken vitamins, mineral supplements or antioxidants for the past 3 months.
Each patient was examined using a mouth mirror and a graduated Williams periodontal probe. Patients’ details as shown in Figure 1 were assessed using a standard proforma. Probing depth, clinical attachment level (CAL) and gingival index given by Loe and Silness were the clinical parameters recorded. Periodontitis was established as sites with bleeding on probing having a clinical attachment loss of more than or equal to 4 mm in more than 30% of the sites.[7] CAL was measured on six surfaces of each tooth. Patients with well-controlled diabetes having glycosylated hemoglobin levels of less than 7 were taken. Only follow-up patients who were on oral hypoglycemic agents with or without insulin were included in the study.
Figure 1.
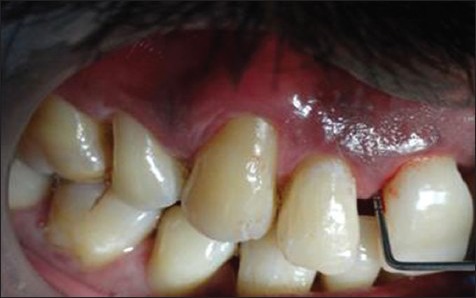
Measurement of pocket probing depth using a graduated Williams periodontal probe
Biochemical analysis
Venous blood was collected from the ante-cubital fossa and the serum was separated by centrifugation and analyzed. Estimation of TAOC for the sample was performed by the phosphomolybdenum method. This quantitative assay is based on the conversion of molybdenum (Mo VI) by reducing agents like antioxidants to molybdenum (Mo V), which further reacts with phosphate under acidic pH, resulting in the formation of a green-colored complex, the intensity of which can be read spectrophotometrically at 695 nm.[8] The estimation of SOD enzyme was carried out by the Beauchamp and Fridovich method. The substrate used for the assay consists of nitrobluetetrazolium chloride (NBT), which reacts with superoxide anions produced on illumination of riboflavin in the presence of methionine as an electron donor, to produce formazan that is a blue-colored complex (The reagents used have been illustrated in Figure 2).[9,10] The decrease in the formation of formazan is directly proportional to the amount of SOD in the sample; 50% decrease in the formation of formazan is taken as one unit of SOD.
Figure 2.
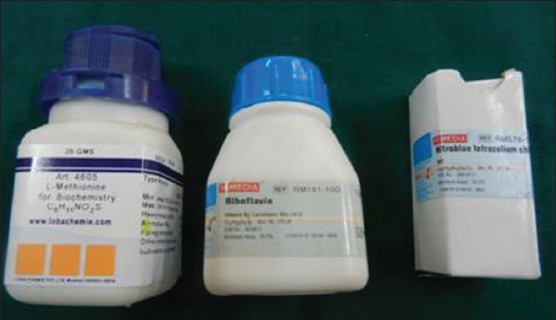
Reagents used for estimation of superoxide dismutase
Statistical analysis
Statistical evaluation of TAOC and SOD serum levels was performed using the Analysis of Variance test (ANOVA). Tukey HSD multiple comparison test was carried out for comparison between the groups. A P-value of less than 0.05 is considered to be significant. Statistical software SPSS Version 17 and MS Excel were used to analyze the data.
RESULTS
Total antioxidant capacity
The mean ± SD values are given in Table 1. The serum TAOC level was found to be highest in the PH group and lowest in the CP group [Figure 3]. The difference in the serum TAOC between the PH group and the group with DM-CP was seen to be statistically significant (P < 0.05). An increase in serum TAOC was also seen in the PH group compared with the CP group, and it was found to be statistically significant (P < 0.05). Serum TAOC was also seen to be elevated in the DM-CP group compared with the CP group [Table 1].
Table 1.
Mean and standard deviation of TAOC and SOD activity levels among the three groups
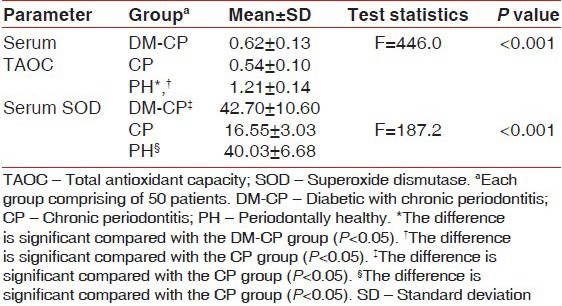
Figure 3.
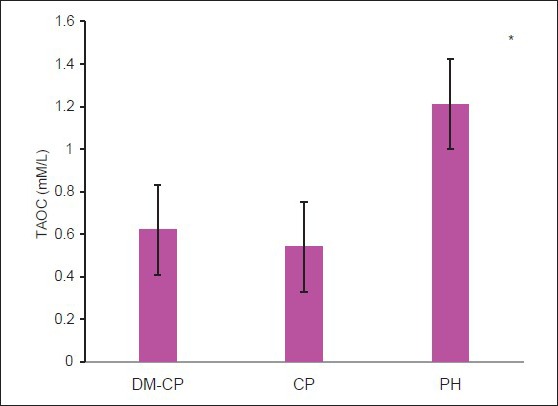
Comparison of serum total antioxidant levels among the three groups. DM-CP -diabetic with chronic periodontitis, CP -chronic periodontitis, PH - periodontally healthy. *The difference is significant compared with the DM-CP and CP groups (P < 0.05)
Superoxide dismutase
The serum levels of SOD were seen to be highest in DM-CP group and lowest in the CP group [Figure 4]. The difference in the serum SOD levels between the DM-CP and CP groups was found to be highly significant (P < 0.05). The difference between the PH and CP groups was also found to be highly significant (P < 0.05). Serum SOD was higher in DM-CP group compared with the PH group; however, the difference was not statistically significant (mean difference = 26.19, P = 0.166).
Figure 4.
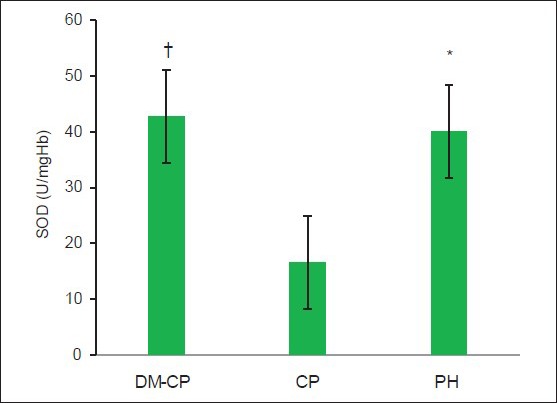
Comparison of serum superoxide dismutase levels among the three groups. DM-CP -diabetic with chronic periodontitis, CP -chronic periodontitis, PH - periodontally healthy. *The difference is significant compared with the CP group (P < 0.05). †The difference is significant compared with the CP group (P < 0.05)
DISCUSSION
In this study, the results demonstrated a significantly lower TAOC value in patients with CP compared with healthy controls. This was in accordance to the study performed by Brock et al., which showed a reduction in systemic and local AO defense in periodontitis.[2]
The disturbed antioxidant status may be explained on the basis of increased ROS production by phagocytic cells in periodontitis. The strongest evidence to implicate ROS in the pathological destruction of the connective tissue during periodontal disease arises in considering Polymorpho Neutrophils infiltration as a key event of the host response against microbial invasion. Such an infiltration in numbers is likely to lead to an increase in the ROS levels[11] causing oxidative stress.[12] Elevated lipid peroxidation and disturbed antioxidant status have also been reported in experimental periodontitis by Tsai.[13] Thus, oxidative stress lies at the heart of the periodontal tissue damage that results from host–microbial interactions, either as a direct result of excess ROS activity/antioxidant deficiency or indirectly as a result of the activation of redox-sensitive transcription factors and the creation of a pro-inflammatory state.[14] Thus, a delicate balance exists between antioxidant defense and repair systems and pro-oxidant mechanisms of tissue destruction, which, if tipped in favor of tissue damage, could lead to significant attachment loss.
In this study, the TAOC in diabetic patients was also found to be significantly lower (P < 0.05) compared with the healthy control group but higher in comparison with the healthy group with periodontitis CP. Oral hypoglycemic agents are known to have potent antioxidant effects.[15] Furthermore, antioxidant benefits have been reported in diabetic patients treated with metformin. The mechanism was shown to be by a decrease in lipid peroxidation.[16] This may explain the higher level of TAOC seen in diabetic patients with periodontitis DM-CP compared with the healthy group with periodontitis CP. It was observed that most of the diabetic patients reporting here in the study were on metformin.
In the present study, a significantly higher serum SOD activity (P < 0.05) was seen in the diabetic group, while the levels were lowest in the healthy group with CP. Statistically, there was no difference between the diabetic patients and the periodontally healthy controls. Studies have shown that in conditions where chronic oxidative damage is formed, SOD enzyme adapts to the situation.[17] A study by Sechi et al. on diabetic rats showed the SOD activity to decrease in the beginning of hyperglycemia, which then adapted and increased to oppose the oxidative stress in the kidneys of the rats.[18] Changes in the glucose level have been demonstrated to influence the Cu, Zn-SOD activity. Hyperglycemia induces superoxide formation, and overproduction of superoxide also appears to be the central mechanism underlying all the major molecular mechanisms implicated in glucose-mediated vascular damage. The overexpression of SOD corrects a variety of hyperglycemia-induced phenotypes in the target cells of diabetic complications.[19] In the present study, increased SOD serum level in the diabetic group may appear as a result of a protective and adaptive mechanism developing in the tissue, and may also be an indicator of the increased generation of ROS in diabetes. Within the periodontal tissues, SOD has been immune-localized predominantly in the periodontal ligament, in association with collagen fibrils and fibroblasts.[20] In the present study, the SOD levels in the healthy group with periodontitis CP was found to be significantly lower than that in the control group. A study done by Ellis et al. has shown a decrease in the SOD activity with an increase in probing depth.[21] Decreased SOD activity and increased number of leukocytes were considered to lead to an increase in the ROS level, resulting in tissue breakdown.
However, in contrast studies done by Akalin (2005), an increased gingival SOD activity level in CP patients was shown. The increase in SOD production was to establish the ROS–AO balance to protect the tissue. The increased SOD levels in the inflamed gingiva were attributed to oxidative stress.[22]
The present study may have important therapeutic implications in terms of the use of antioxidants in periodontal therapy to prevent tissue destruction. Misaki et al., in a interventional study on rats, demonstrated a curative effect of SOD on P. gingivalis-induced inflammation and periodontal wound healing.[23] However, it is only as data becomes available from interventional trials done on humans that we will see whether these compounds live up to their potential for the treatment of periodontal diseases.
Although this study has potential implications, it is not without its limitations. The patients included in this study were from in and around the coastal city of Mangalore and hence it does not allow us to generalize the results to the entire population; therefore, further research should be performed on a larger sample. Most of the oral hypoglycemic agents are known to have potent antioxidant effects; hence, it could have influenced the results. Further studies involving a group of periodontally healthy diabetics may prove better in the identification of oxidative stress markers in diabetes.
CONCLUSION
From the observations made in our study, we can conclude that the serum level of TAOC was found to be highest in the PH group; the antioxidant compromise found in the other two groups may be the result of periodontal inflammation. Our results suggest that serum SOD activity increases in the diabetic group compared with the healthy groups. The increase in DM may be a result of a protective and adaptive mechanism against the oxidative stress developing in the tissue. Finally, the level of serum TAOC and SOD was found to be the least in the CP group. More multicentric, longitudinal studies with a larger sample size are needed to have a better understanding of the interrelationship between oxidative imbalance and chronic disease processes like periodontitis and diabetes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lamster IB, Novak MJ. Host mediators in gingival crevicular fluid: Implications for the pathogenesis of periodontal disease. Crit Rev Oral Biol Med. 1992;3:31–60. doi: 10.1177/10454411920030010501. [DOI] [PubMed] [Google Scholar]
- 2.Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health. J ClinPeriodontol. 2004;31:515–21. doi: 10.1111/j.1600-051X.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaki KT. The neutrophil: Mechanisms of controlling periodontal bacteria. J Periodontol. 1991;62:761–74. doi: 10.1902/jop.1991.62.12.761. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 5.Sies H. Periodontology 2000. Vol. 43. New York: 1991: 2007. Oxidative Stress: Oxidants and Antioxidants; pp. 160–232. [Google Scholar]
- 6.Southerland JH, Taylor GW, Moss K, Beck JD, Offenbacher S. Commonality in chronic inflammatory diseases: Periodontitis, diabetes, and coronary artery disease. Periodontol 2000. 2006;40:130–43. doi: 10.1111/j.1600-0757.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 7.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontology 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 8.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenum complex: Specific application to the determination of vitamin E. AnalBiochem. 1999;269:337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 9.Beauchamp C, Fridovich I. Superoxide dismutase improved assay applicable to acrylamide gels. AnalBiochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. ClinChem. 1988;34:497–500. [PubMed] [Google Scholar]
- 11.Miyasaki KT, Wilson ME, Brunetti AJ, Genco RJ. Oxidative and non-oxidative killing of Actinobacillusactinomycetemcomitans by human neutrophils. Infect Immun. 1986;53:154–60. doi: 10.1128/iai.53.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapira L, Gordon B, Warbington M, Van Dyke TE. Priming effect of Porphyromonasgingivalis lipopolysaccharide on superoxide production by neutrophils from healthy and rapidly progressive periodontitis subjects. J Periodontol. 1994;65:129–33. doi: 10.1902/jop.1994.65.2.129. [DOI] [PubMed] [Google Scholar]
- 13.Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM, et al. Lipid peroxidation. A possible role in the induction and progression of chronic periodontitis. J Periodontal Res. 2005;40:378–84. doi: 10.1111/j.1600-0765.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 14.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 15.Molavi B, Rassouli N, Bagwe S, Rasouli N. A review of thazolidinediones and metformin in the treatment of type-2 diabetes with focus on cardiovascular complications. Vasc Health Risk Manag. 2007;3:967–73. [PMC free article] [PubMed] [Google Scholar]
- 16.Faure P, Rossini E, Wiernsperger N, Richard MJ, Favier A, Halimi S. An insulin sensitizer improves the free radical defence system potential and insulin sensitivity in high fructose-fed rats. Diabetes. 1999;48:353–7. doi: 10.2337/diabetes.48.2.353. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–61. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 18.Sechi LA, Ceriello A, Griffin CA, Catena C, Amstad P, Schambelan M, et al. Renal antioxidant enzyme mRNA levels are increased in rats with experimental diabetes mellitus. Diabetologia. 1997;40:23–9. doi: 10.1007/s001250050638. [DOI] [PubMed] [Google Scholar]
- 19.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 20.Jacoby BH, Davis WL. The electron microscopic immunolocalisation of copper-zinc superoxide dismutase in association with collagen fibres of periodontal soft tissues. J Periodontol. 1991;62:413–20. doi: 10.1902/jop.1991.62.7.413. [DOI] [PubMed] [Google Scholar]
- 21.Ellis SD, Tucci MA, Serio FG, Johnson RB. Factors for progression of periodontal diseases. J Oral Pathol Med. 1998;27:101–5. doi: 10.1111/j.1600-0714.1998.tb01923.x. [DOI] [PubMed] [Google Scholar]
- 22.Akalin FA, Toklu E, Renda N. Analysis of superoxide dismutase activity levels in gingiva and gingival crevicular fluid in patients with chronic periodontitis and periodontally healthy controls. J ClinPeriodontol. 2005;32:238–43. doi: 10.1111/j.1600-051X.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 23.Misaki H, Suzuki M, Yoshie H, Hara K. The effect of superoxide dismutase on the inflammation induced by periodontal pathogenic bacteria and wound healing of gingival incisions. J JpnAssocPeriodontol. 1990;32:93–110. doi: 10.2329/perio.32.93. [DOI] [PubMed] [Google Scholar]


