Abstract
Nucleotides are well known for being the universal currency of intracellular energy transactions, but over the last decade it has become clear that they are also ubiquitous extracellular messenger. In the immune system there is increasing awareness that nucleotides serve multiple roles as stimulants of lymphocyte proliferation, ROS generation, cytokine and chemokine secretion: in one word as pro-inflammatory mediators. However, although often neglected, extracellular nucleotides exert an additional more subtle function as negative modulators of immunity, or as immunedepressants. The more we understand the peculiar biochemical composition of the microenvironment generated at inflammatory sites, the more we appreciate how chronic exposure to low extracellular nucleotide levels affect immunity and inflammation. A deeper understanding of this complex network will no doubt help design more effective therapies for cancer and chronic inflammatory diseases.
Extracellular nucleotides as immunomodulators
Nucleotides are the basic building blocks of nucleic acids, essential components of other biological molecules, intracellular signalling factors and crucially serve as the fundamental currency (ATP) of all cellular energy transactions. Because of the pioneering studies of Geoff Burnstock, we now know that these mediators also have a critical role as extracellular messengers [1]. Initially, it was thought that their messenger role was mainly restricted to the central and peripheral nervous system, but as our knowledge of purinergic physiology increases, we realize that there is no system or apparatus in which these mediators do not have a central role, the immune system included [2],[3]. However, the role for extracellular ATP in the modulation of immunity has gained slow acceptance over the years. Three major advances countered the frank scepticism and disbelief of this concept: a) the cloning and identification in immune cells of basically all P2 receptor subtypes over the past 2 decades, b) the demonstration that ATP is released by immune cells and finally c) that these mediators can be scavenged by specific ectonucleotidases to generate phosphohydrolytic immune suppressive derivatives. It is now clear that purinergic signalling networks are a fundamental component of the immunoregulatory environment [4].
In healthy tissues, ATP is almost exclusively localized intracellularly, whereas the extracellular concentration under basal or quiescent conditions is basically negligible (in the 10-100 nM range). It can be assumed that under these conditions most if not all P2 receptors are inactive, thus ATP-dependent purinergic signalling should be silent at baseline. Maintenance of low or near zero extracellular ATP levels in the pericellular space by ectoenzymes is crucial to avoid P2 receptor desensitization. To this purpose, activity of plasma membrane ecto-ATPases of the E-NTPDase family (e.g. CD39) is of particular importance [5]. However, if stress or injury is applied, parenchymal or resident immune cells release large amounts of ATP together with other nucleotides. For this to happen, we do not need to postulate by necessity the occurrence of membrane damage, as virtually all cells posses pathways for non-lytic ATP release (i.e. ABC transporters, non selective large conductance channels such as connexins and pannexins, constitutive or stimulated exocytosis) which can be activated by mechanical factors or extracellular ligands [6]. Furthermore, in response to stimulation cells may undergo formation of transient breaches in the plasma membrane from which ATP and other nucleotides may easily efflux, driven by the large chemical and electrical gradients. In fact ATP is106 fold more concentrated in the cytosol than in the extracellular milieu (millimolar versus nanomolar), and is negatively charged. As such, it is repelled from the negatively charged intracellular environment. These features make ATP an ideal ubiquitous “danger signal”, or DAMP (Damage Associated Molecular Pattern). Factors that recognize or process these mediators are likely to be markedly upregulated as the injury process is promulgated.
It is clear that the manner by which DAMPs can shape the immune response is complex. Inflammation can have dire consequences on the tissue involved as well as on the whole organism, therefore it is vital that the decision to initiate or abort inflammation be carefully weighed. There is increasing awareness that this choice depends on the integration of “go-ahead” and “stop” signals by cells of innate immunity, notably dendritic cells and macrophages [7]. This is epitomized by response to pathogens. Microrganisms signal their presence in the body by releasing molecules identifying large classes of pathogens (PAMPs, pathogen-associated molecular patterns) [8], however in most cases the mere detection of these pathogen-derived molecules is not sufficient to start inflammation, unless there is concomitant evidence of tissue damage. In other words, the immune system must ensure that the invader is indeed potentially dangerous before initiating a potentially destructive response. In this light, the role of DAMPs is extremely well balanced, because they are thought to give the final “go-ahead” signal for inflammation. In a well-controlled homeostatic system, regulatory mechanisms are vital, thus we can expect that DAMPs provide a feedback or potentially feed forward signals for inflammation that depend on the initiating stimulus, the local or systemic conditions. Not surprisingly, therefore, extracellular nucleotides must balance strong pro-inflammatory actions with an equally relevant, but as yet largely overlooked, anti-inflammatory activity. Extracellular nucleotides are well known for their ability to trigger chemotaxis, endocytosis, proliferation, release of pro-inflammatory cytokines and chemokines, intracellular pathogen killing, or cytotoxicity. Several authoritative reviews are available, to which the reader is referred for update information [9],[10],[11],[12]; [13]. Relatively few communications have highlighted the specific role of nucleotides as immunodepressants (see Boeynaems and coworkers ref [14]. This is to a certain extent surprising in view of the increasing awareness that microbial products (and ATP is no doubt abundantly released by growing bacteria) play a key role in the establishment of immunological tolerance to common antigens [15].
Down-modulation of inflammation by extracellular ATP
The first and most simple explanation of potential inhibitory effects is that down regulation of inflammation is dependent upon biotransformation of nucleosides tri- and di-phosphates to the neutral nucleoside monophosphate that might initiate anergic responses, or to the more classic nucleoside with antagonistic or suppressive/regulatory effects on inflammatory and immune responses. Given the sensitivity to oxidative stress of CD39 and other ectonucleotidase bioactivity within cell membrane lipid rafts, as seen in acute inflammatory responses, other processes might be operative in the immediate minutes of injury or infection. Secondly, therefore is the option of receptor desensitization that precludes repetitive signalling via P2 receptors. Several mechanisms responsible of P2 receptor desensitization have been proposed [16],[17],[18], as well as altered cell responses to different levels of extracellular nucleotides as seen in the ectoATP/ADPase E-NTPD1 (CD39) null mouse [19]. Data from Mizumoto et al. [20]however, suggests that this process largely switches off tissue protective responses, and that unfettered inflammation may result as a consequence. This process occurs not only because of minimal generation of adenosine but also via a specific pattern of selective P2-receptor fall out. It is however clear that such desensitization responses do preclude adaptive immune responses i.e. delayed hypersensitivity where close cell contacts are required for antigen or haptenic presentation. There are certain acute and chronic disorders where the E-NTPD1 null mouse phenotype is paradoxically associated with relative protection. Global hepatic ischemia reperfusion injury in a mutant “immunocompetent” mouse with a E-NTPD1 null vasculature leads to severe inflammation and distant organ perturbation with decreased survival; in contrast, lobar (more restricted injury) and hepatic sequelae are more limited with adoptive transfer of NK cells null for Entpd1 in immunodeficient mice. Thirdly, therefore, there may be a subset of pro-inflammatory cells that are exquisitively sensitive to apoptosis induction by ATP- or NAD-mediated activation of the P2X7 receptor, which is highly resistant to desensitization. There are few doubts that ATP might have an immunodepressant activity by simply deleting P2X7–expressing cells such as dendritic cells (DCs), macrophages or T lymphocytes. Hence, effects of ATP are to delete the very cells expressing high levels of P2X7 that drive inflammation, such as seen in NK and NKT cell mediated injury. An elegant demonstration of a selective immunodepressive effect mediated by cytotoxicity has been recently provided in mice treated with concanavalin-A (ConA) to induce hepatitis [21]. ConA triggers ATP release from natural killer T (NKT) cells, the main effectors of liver damage in this model of inflammation. These cells are characterized by high level of expression of CD39 ecto-ATP/ADPase and of CD73 5′-ecto-nucleotidase. Mice deficient of CD39 are protected from ConA-induced liver injury because failure of cells to degrade ATP by NKT cells causes ATP accumulation to a level that triggers apoptosis of this cell population. However, a few scattered reports clearly show that negative immunomodulation occurred at much lower ATP concentrations than those needed to activate P2X7 and was fully independent of this receptor. Finally, there is the option for detailed cross talk between P2 receptors, P2 receptors and other G-protein coupled receptors. Such heterologous desensitization effects will preclude inflammatory cell recruitment via chemokines [22]. Last and not least, selective interactions triggered on P1 and P2-receptors by adenosine and parent nucleotides might initiate cross talk between intracellular signalling cascades that drive inflammation. For example, neutrophil and microglial chemotaxis are dependent upon both P2 and P1 effects [23],[24]. ATP alone without conversion to adenosine will not permit chemotaxis. Another possibility is the process of conditioning inflammatory responses, as per ischemia and graft protection: here the mechanism is (at least in part) related to induction of ectonucleotidases, as mentioned above. Therefore, ATP alone might be endowed with a specific ability to redirect the immune response towards allergy or tolerance rather than protective inflammation, depending upon the nature of the immune cells, their cellular repertoire of P2 receptors and the state of prior activation [25], [26]. A cynical view may be that ATP might promote either inflammation or non-responsiveness/tolerance by amplifying an originating signal potentially modulated by this mediator at differing concentrations. As an example, chronic (12-24 h) exposure to low ATP concentrations (100-200 μM) of LPS-stimulated human blood monocyte-derived dendritic cells (hBMDC) induces a semi-maturation state characterized by upregulation of co-stimulatory molecules and inhibition of IL-12, IL-6, IL-1β and TNFα production [25];[26]. To the contrary, under these circumstances release of the anti-inflammatory cytokines IL-10 and IL-1Ra is not affected. The exact characterization and identity of the P2 receptors involved is not entirely clear, but more recent data suggest a likely participation of P2Y11 receptors via an increase in intracellular cAMP concentration [27]; [28]. ATP has a strong modulatory activity also on chemokine secretion as it increases constitutive release of CCL22 and decreases the LPS-induced secretion of CXCL10 and CCL5 from hBMDCs. Overall, this complex modulation of cytokine and chemokine secretion results in an impaired ability of hBMDCs to polarize and recruit T helper type 1 (TH1) lymphocytes, and in an enhanced ability to drive T helper type 2 (TH2) differentiation [25;29]. Therefore, exposure of hBMDCs to a pathogen in a microenvironment containing low ATP concentration should induce acquisition of a hBMDC phenotype favouring allergy or tolerance. What it is not known is whether allergy or tolerance will be generalized or restricted to the given antigen processed by the antigen-presenting cells (APCs) in the presence of ATP. Inhibition of inflammatory cytokine release by chronic exposure to low/medium extracellular ATP levels is not restricted to DCs but is also observed in macrophages and unsorted splenocytes at ATP concentrations even lower than those effective in hBNDCs [30]. Quite recently, this negative immunomodulatory effect was also reported in human alveolar macrophages, where pre-treatment with ATP, ADP or the ATP analogue benzoylATP strongly depressed LPS-stimulated IL-12 and TNF-α secretion [31]. Antagonist data from alveolar macrophages suggest that in this cell type the P2Y1 receptor is also involved in negative modulation of immune cell function.
Microarray studies have revealed that in hBMDC ATP upregulates the expression of numerous genes involved in immunosuppression and tolerance, in particular thrombospondin-1 and indoleamine 2,3-dioxygenase [32]; [33]. Thrombospondin-1 inhibits T cell proliferation, down-modulates IL-12 production and stimulates TGF-β secretion. Indoleamine 2,3-dioxygenase, by depleting the aminoacid tryptophan, suppresses T cell proliferation, induces tolerance and may interfere with P2 receptor signalling and desensitization. However, it should also be stressed that activation of hBMDC P2Y receptors also triggers a cAMP-mediated pathway leading to increased expression of the pro-inflammatory cytokine IL-23, which activates memory T cells and plays a role in autoimmune disorders [34]. This process is particularly relevant for differentiation of T helper type 17 (TH17) highly reactive immune cells. Priming hBMDC with ATP does not affect their ability to drive the differentiation of TH17 lymphocytes, in fact ATP might be one of those bacteria-derived factors that promotes TH17 cell differentiation in the gut lamina propria. This view is supported by recent experiments showing that number of lamina propria TH17 cells is strongly reduced in germ-free mice, while total number of CD4+ cells is unaltered [35]. The in vivo final outcome of the complex interaction of extracellular ATP with immune cells might be immunosuppression or tolerance, but also aberrant balance and stimulation of an allergic or autoimmune response. Since the main receptor responsible for these low dose effects of ATP is P2Y11, availability of an animal model would be extremely useful, but unfortunately lack of p2y11 receptor gene in the genome of mice precludes this possibility.
Responses of hBMDCs to ATP are time and dose dependent. Low ATP concentrations not only prime hBMDCs for tolerance, but have also a strong chemotactic effect. Chemotaxis to ATP (and other nucleotides) is completely lost during maturation, despite the fact that mature DCs express P2Y receptors at about the same level as immature DCs. How maturation desensitizes or uncouples P2Y receptors from the cytoskeleton is unknown, but this makes sense in the overall dynamics of the immune response. Indeed, it is desirable that DCs, after they have met the antigen and migrate to regional lymph nodes do not respond to additional differentiation stimuli. Ex vivo or in vivo studies are few, but indirect observations appear to support potential for systemic immunosuppressive effects of ATP. Dagnelie and co-workers have shown that ATP at lower micromolar concentrations inhibits inflammation by blocking TNFα and stimulating IL-10 release [36], [37]. Pharmacological analysis of the P2 receptor subtypes involved suggest an additional role for P2Y11 and P2Y12, and potentially for immune metabolic homeostasis given the potential for nucleoside salvage and cellular rescue. Treatment with ATP ameliorated radiation-induced TNFα release and DNA damage, at the same time increasing IL-10 release [38]. Low-to-medium ATP doses might also protect from oxidative damage and damp down oxygen radicals production in whole blood [13].
Most studies on the immunomodulatory properties of extracellular nucleotides have focused on hBMDCs, while very little attention has been paid to purinergic signalling in plasmacytoid dendritic cells (PDCs). These cells secrete low levels of IL-10, while on the contrary are strong producers of type I IFN (IFN-α, β, ω, and δ). Type I IFNs potentiate cytotoxicity by NK cells and cytotoxic T lymphocytes, stimulate secretion of IFN-γ and differentiation of memory B cells into plasma cells. Therefore, it can be anticipated that agents that downplay type IFNs secretion will also down-modulate the immune response. Schnurr and co-workers have shown that virus-induced IFN-α production by PDCs is strongly decreased by pre-incubation in the presence of several nucleotides (ATP, ADP, UTP, UDP, IDP, UDP-glucose), and this correlates with the expression of P2Y4, P2Y6 and P2Y14 mRNA [39]. P2Y receptor signalling might therefore be an important stop signal to prevent excessive stimulation of inflammation and avoid conditions that might favour autoimmunity.
Increasing the ATP concentration in the incubation medium causes a shift from an immunotolerant towards and immunostimulant hBMDCs phenotype. In the presence of elevated extracellular ATP concentrations above 300-500 μM, hBMDCs are stimulated to secrete IL-12 and IL-1β and down-modulate production of IL-10. This is consistent with the view that while graded exposure to DAMPs may preferentially cause adaptation to the novel condition rather than a frank defense response, a sudden increase of DAMPs in the extracellular space triggers overt inflammation.
In vivo evidence for the immunodepressant activity of ATP
The important role of extracellular nucleotides in differentially shaping immunity is highlighted by distinct responses to skin irritants of mice deleted of CD39, the main ATP/ADP hydrolysing ecto-enzyme of skin Langerhans cells. While CD39-/- animals are extremely sensitive to direct application of irritants, due to their inability to remove extracellular ATP released in response to the noxious agents, they are on the contrary wholly unable to start T cell-mediated allergic contact hypersensitivity Robson [20]. The immunosuppressive or protective activity of ATP might explain the beneficial effects of ATP infusion in patients with advanced non-small-cell lung cancer [40]. In these subjects ATP infusions reduced weight loss, increased muscle strength and ameliorated the overall quality of life. These original observations were recently confirmed by additional studies showing that prolonged incubation of whole human blood with low/medium ATP (100-500 μM) impairs LPS-stimulated IL-12 and IFNγ secretion [41]). A similar effect was exerted by the slowly hydrolysable ATP analogue ATPγS, which suggests that it is the nucleotide signalling moiety that allows this process and down-modulates cytokine secretion and not its degradation product adenosine. Administration of ATP or ATP analogues to mice prior to injection of LPS decreased plasma levels of TNFα and IL-1 and prevented development of endotoxic shock [42]. Unfortunately, no studies have so far investigated the intracellular mechanism underlying this effect, thus we can only speculate as to the molecular basis.
Conclusions and further perspectives
There are no doubts that under certain circumstances ATP can serve as an acute danger signal and drive inflammation when acutely released into the extracellular medium in high concentrations. The outcome may be quite different when cells are intermittently exposed to slow, chronic increases in its extracellular levels. Under this condition, ATP effects are much more subtle, and depending on the cells that are studied, shapes the evolving immune responses towards tolerance, and non-responsiveness rather than cellular reactivity. Dualistic roles for immune mediators are well known, and even in this regard ATP behaves as a classical mediator of inflammation and immunity.
Figure 1.
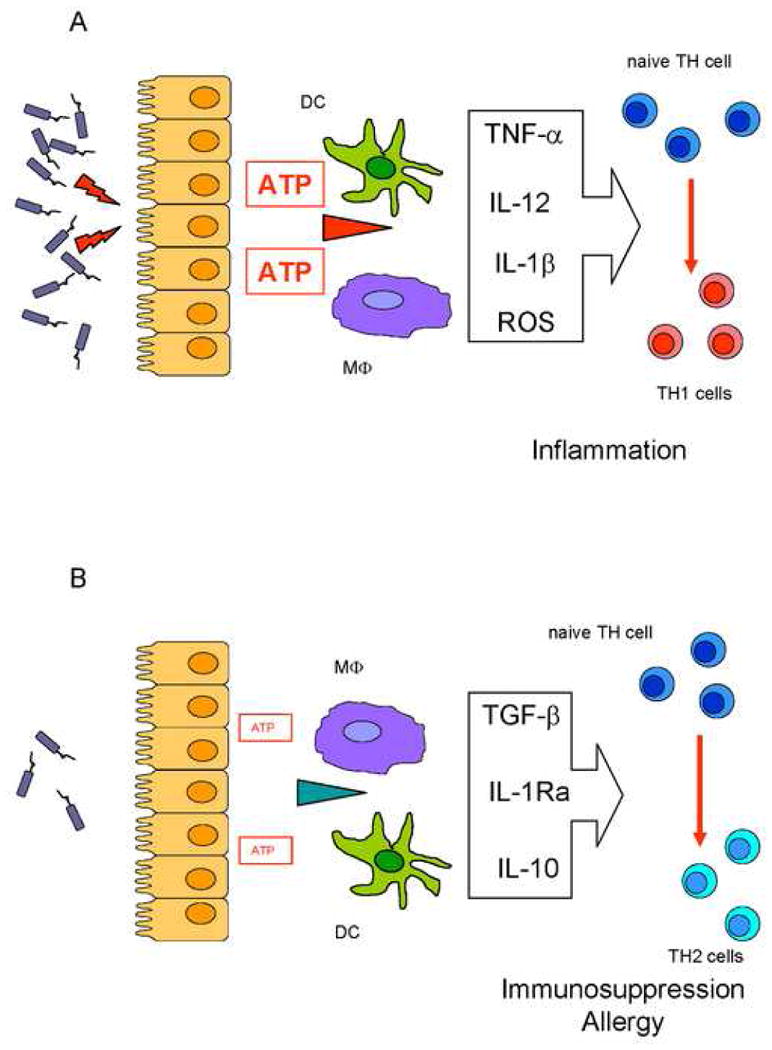
Diverging roles of high or low extracellular ATP concentrations in shaping the immune response. Panel A: Tissue cells release large ATP amounts in response to high pathogen titers. The increase in extracellular ATP level drives inflammatory dendritic cell (DC) and macrophage (Mϕ) differentiation, accompanied by release of pro-inflammatory mediators (TNF-α, IL-12, IL-1β, ROS), naive TH cells to TH1 lymphocyte differentiation and initiation of inflammation. Panel B: In response to low grade stimulation by pathogens, tissue cells release modest amounts of ATP which cause down-modulation of pro-inflammatory cytokines, preferential release of immunodepressive cytokines, differentiation of naive TH cells into TH2 lymphocytes, and a state of immunosuppression or allergy.
Figure 2.

Extracellular ATP drives the differentiation of lamina propria TH17 lymphocytes. Bacteria hosted in the gut lumen continuously release ATP which moulds the differentiation of mucosal dendritic cells (DCs) which are stimulated to secrete IL-6, IL-23 and TGF-β. These cytokines are needed to allow the establishment of a normal population of IL-17-secreting lymphocytes (TH17) lymphocytes in the lamina propria. However, in response to massive releases of ATP, such as those occurring during uncontrolled bacterial growth or epithelial cell damage, secretion of these cytokines can so large to an unphysiological/pathogenic expansion of TH17 lymphocytes. This may trigger an inappropriate local immune response, which eventually causes inflammatory bowel disease.
Acknowledgments
Research in the laboratory of F. Di Virgilio was supported by grants by the Italian Ministry of Education, University and Scientific Research (MIUR), the Italian Association for Cancer Research (AIRC), the Italian Space Agency (ASI-OSMA), Telethon of Italy (n. GGP06070), the Commission of European Communities (7th Framework Program HEALTH-F2-2007-202231), the Regione Emilia Romagna (Research Programs “Innovative approaches to the diagnosis of inflammatory diseases” and “Moniter”), and local funds from the University of Ferrara. Simon C. Robson thanks the NIH for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and suggested readings
• of special interest
•• of outstanding interest
- 1.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 4.Di Virgilio F. Purinergic mechanism in the immune system: a signal of danger for dendritic cells. Purinergic Signalling. 2006;1:205–209. doi: 10.1007/s11302-005-6312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. This is an authoritative concise review summarizing the key role of CD39 in the modulation of immune response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal. 2005;1:311–328. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 8.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 9.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2:e6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 11.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 13.Bours MJ, Swennen EL, Di VF, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Boeynaems JM, Communi D. Modulation of inflammation by extracellular nucleotides. J Invest Dermatol. 2006;126:943–944. doi: 10.1038/sj.jid.5700233. [DOI] [PubMed] [Google Scholar]
- 15.Bjorksten B. Effects of intestinal microflora and the environment on the development of asthma and allergy. Springer Semin Immunopathol. 2004;25:257–270. doi: 10.1007/s00281-003-0142-2. [DOI] [PubMed] [Google Scholar]
- 16.Huang NN, Wang DJ, Heller E, Heppel LA. Homologous desensitization of ATP-stimulated mitogenesis: mechanism involves desensitization of arachidonic acid release and cAMP elevation but not the activation of protein kinase A. J Cell Physiol. 1995;(165):667–675. doi: 10.1002/jcp.1041650326. [DOI] [PubMed] [Google Scholar]
- 17.Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, Leon C, Gachet C. Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol. 2005;67:721–733. doi: 10.1124/mol.104.004846. [DOI] [PubMed] [Google Scholar]
- 18.Tulapurkar ME, Zundorf G, Reiser G. Internalization and desensitization of a green fluorescent protein-tagged P2Y nucleotide receptor are differently controlled by inhibition of calmodulin-dependent protein kinase II. J Neurochem. 2006;96:624–634. doi: 10.1111/j.1471-4159.2005.03594.x. [DOI] [PubMed] [Google Scholar]
- 19.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 20••.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. The first clear in vivo demonstration of the role of extracellular ATP in shaping acute inflammatory reactions or contact dermatitis in the skin. [DOI] [PubMed] [Google Scholar]
- 21.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, Haschemi A, Yegutkin GG, Candinas D, Exley M, Robson SC. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang N, Yang D, Dong H, Chen Q, Dimitrova DI, Rogers TJ, Sitkovsky M, Oppenheim JJ. Adenosine A2a receptors induce heterologous desensitization of chemokine receptors. Blood. 2006;108:38–44. doi: 10.1182/blood-2005-06-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 24.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem. 2008;283:28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166:1611–1617. doi: 10.4049/jimmunol.166.3.1611. The first evidence in support of the role of ATP as an inducer of distorted maturation of DCs. [DOI] [PubMed] [Google Scholar]
- 26•.Wilkin F, Stordeur P, Goldman M, Boeynaems JM, Robaye B. Extracellular adenine nucleotides modulate cytokine production by human monocyte-derived dendritic cells: dual effect on IL-12 and stimulation of IL-10. Eur J Immunol. 2002;32:2409–2417. doi: 10.1002/1521-4141(200209)32:9<2409::AID-IMMU2409>3.0.CO;2-H. An important study describing the opposite effect of extracellular nucleotides on the release of pro-inflammatory and anti-inflammatory cytokines. [DOI] [PubMed] [Google Scholar]
- 27•.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. The first report identifying the P2Y11 receptor as responsible for the immunodepressive effect of extracellular ATP. [DOI] [PubMed] [Google Scholar]
- 28.Schnurr M, Toy T, Stoitzner P, Cameron P, Shin A, Beecroft T, Davis ID, Cebon J, Maraskovsky E. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood. 2003;102:613–620. doi: 10.1182/blood-2002-12-3745. [DOI] [PubMed] [Google Scholar]
- 29.la Sala A, Sebastiani S, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1 T lymphocytes. Blood. 2002;99:1715–1722. doi: 10.1182/blood.v99.5.1715. [DOI] [PubMed] [Google Scholar]
- 30.Hasko G, Kuhel DG, Salzman AL, Szabo C. ATP suppression of interleukin-12 and tumour necrosis factor-alpha release from macrophages. Br J Pharmacol. 2000;129:909–914. doi: 10.1038/sj.bjp.0703134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myrtek D, Muller T, Geyer V, Derr N, Ferrari D, Zissel G, Durk T, Sorichter S, Luttmann W, Kuepper M, Norgauer J, Di VF, Virchow JC, Jr, Idzko M. Activation of human alveolar macrophages via P2 receptors: coupling to intracellular Ca2+ increases and cytokine secretion. J Immunol. 2008;(181):2181–2188. doi: 10.4049/jimmunol.181.3.2181. [DOI] [PubMed] [Google Scholar]
- 32.Marteau F, Gonzalez NS, Communi D, Goldman M, Boeynaems JM, Communi D. Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood. 2005;106:3860–3866. doi: 10.1182/blood-2005-05-1843. [DOI] [PubMed] [Google Scholar]
- 33.Bles N, Horckmans M, Lefort A, Libert F, Macours P, El HH, Marteau F, Boeynaems JM, Communi D. Gene expression profiling defines ATP as a key regulator of human dendritic cell functions. J Immunol. 2007;179:3550–3558. doi: 10.4049/jimmunol.179.6.3550. [DOI] [PubMed] [Google Scholar]
- 34.Schnurr M, Toy T, Shin A, Wagner M, Cebon J, Maraskovsky E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood. 2005;105:1582–1589. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 35••.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. A seminal paper establishing a causal link between ATP secreted by intestinal flora and maturation of mucosal lymphocytes. [DOI] [PubMed] [Google Scholar]
- 36.Swennen EL, Bast A, Dagnelie PC. Immunoregulatory effects of adenosine 5′-triphosphate on cytokine release from stimulated whole blood. Eur J Immunol. 2005;35:852–858. doi: 10.1002/eji.200425423. [DOI] [PubMed] [Google Scholar]
- 37.Swennen EL, Bast A, Dagnelie PC. Purinergic receptors involved in the immunomodulatory effects of ATP in human blood. Biochem Biophys Res Commun. 2006;348:1194–1199. doi: 10.1016/j.bbrc.2006.07.177. [DOI] [PubMed] [Google Scholar]
- 38.Swennen EL, Dagnelie PC, Van den Beucken T, Bast A. Radioprotective effects of ATP in human blood ex vivo. Biochem Biophys Res Commun. 2008;367:383–387. doi: 10.1016/j.bbrc.2007.12.125. [DOI] [PubMed] [Google Scholar]
- 39.Shin A, Toy T, Rothenfusser S, Robson N, Vorac J, Dauer M, Stuplich M, Endres S, Cebon J, Maraskovsky E, Schnurr M. P2Y receptor signaling regulates phenotype and IFN-alpha secretion of human plasmacytoid dendritic cells. Blood. 2008;111:3062–3069. doi: 10.1182/blood-2007-02-071910. [DOI] [PubMed] [Google Scholar]
- 40.Agteresch HJ, Dagnelie PC, van der Gaast A, Stijnen T, Wilson JH. Randomized clinical trial of adenosine 5′-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2000;92:321–328. doi: 10.1093/jnci/92.4.321. [DOI] [PubMed] [Google Scholar]
- 41.Nalos M, Huang S, Sluyter R, Khan A, Santner-Nanan B, Nanan R, McLean AS. “Host tissue damage” signal ATP impairs IL-12 and IFNgamma secretion in LPS stimulated whole human blood. Intensive Care Med. 2008;34:1891–1897. doi: 10.1007/s00134-008-1156-y. [DOI] [PubMed] [Google Scholar]
- 42••.Proctor RA, Denlinger LC, Leventhal PS, Daugherty SK, van de Loo JW, Tanke T, Firestein GS, Bertics PJ. Protection of mice from endotoxic death by 2-methylthio-ATP. Proc Natl Acad Sci U S A. 1994;91:6017–6020. doi: 10.1073/pnas.91.13.6017. The first demonstration, at the time largely ignored, that purinergic agonists might have a potent immunosuppressive activity in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]


