Abstract
Background
Despite an increasing number of procedures being performed, there is no consensus on an optimal approach to EUS-guided FNA (EUS-FNA) or interventions.
Objective
Validate an algorithmic approach to EUS-FNA/interventions with the objective of improving technical outcomes and resource use.
Design
Prospective study.
Setting
Tertiary-care referral center.
Patients
Consecutive patients undergoing EUS-FNA and/or interventions.
Intervention
Phase I was a retrospective analysis of EUS-FNA/interventions performed in 548 patients. The 19-gauge needle was used for interventions, and the 22- or 25–gauge needle was used interchangeably for performing FNAs. At phase I, the technical failure rate was 11.5%. Based on these observations, an algorithm was proposed by which all transduodenal FNAs were performed by using a 25-gauge needle and other FNAs with a 22-gauge needle. All transduodenal interventions were performed with a Flexible 19-gauge needle and others with a standard 19-gauge needle. This algorithm was tested prospectively in phase II on 500 patients.
Main Outcome Measurements
Compare technical failure, diagnostic adequacy, procedural complications, and average needle costs between both phases.
Results
The technical failure rate was significantly less in phase II compared with that of phase I (1.6% vs 11.5%; P < .001) for both FNA (1.8% vs 10.9%; P < .001) and therapeutic interventions (0% vs 16.4%; P = .001). Although there was no difference in diagnostic adequacy (97.1% vs 98.4%; P =.191) or complications (0.4% vs 0.2%; P = 1.0) between phases I and II, the average cost per case was significantly less in phase II ($199.59 vs $188.30; P =.008).
Limitations
Single-center study.
Conclusion
An algorithmic approach to EUS-FNA/interventions yielded better technical outcomes and cost savings without compromising diagnostic adequacy.
EUS-guided FNA (EUS-FNA) is a very sensitive technique for establishing tissue diagnosis in patients with suspected GI malignancies and periluminal lesions.1,2 Several factors determine the technical outcomes of an FNA procedure: location and nature of the lesion, presence of an on-site cytopathologist, and the experience of the endosonographer.3–5 Studies have shown that more FNA passes are required to establish a definitive diagnosis in patients with pancreatic masses compared with other lesions, particularly in the absence of an on-site cytopathologist.6 However, routinely performing more than 5 passes in every patient with a pancreatic mass represents a substantial burden in terms of procedural duration, need for adjunctive sedation, increased risk of complications, and, more importantly, use of additional needles per case. Although several studies have evaluated the technical aspects of an FNA procedure,7–9 to our knowledge, no study has examined the relationship between technical outcomes and resource use. Given the increasing number of EUS procedures being performed and the need to use more than one needle in some patients because of technical failure,10,11 this study attempted to develop an algorithm with the objective of improving technical outcomes and optimizing resource use for FNA procedures and interventions.
PATIENTS AND METHODS
Given the lack of adequate data on resource use during EUS procedures, this study was executed in two phases: phase I for retrospective data analysis to assess technical outcomes and resource use during EUS-FNA/interventions and phase II for prospective validation of an algorithm designed to improve technical outcomes and resource use. In both phases, we excluded patients who underwent sampling of more than one lesion in a single endoscopic session and those enrolled in clinical trials evaluating specific FNA needles.
Phase I study
This involved retrospective analysis of all EUS-FNA procedures/interventions performed over a 7-month period from January to July 2010 at the University of Alabama at Birmingham. The EUS database was queried for patient demographics, procedural indications, lesion sampled, FNA route, type and number of needles used per procedure, diagnostic adequacy, and complications. All procedures were performed by two endosonographers who used the standard 19-gauge needles (EchoTip, Cook Medical, Winston-Salem, NC) for interventions and the 22 or 25–gauge needles interchangeably for performing FNAs. A 22-gauge needle was used to aspirate cysts measuring ≤2 cm in size and a standard 19-gauge needle for cysts measuring >2 cm in size. A 20-gauge celiac plexus neurolysis (CPN) needle or a standard 19-gauge needle was used for performing celiac plexus block or neurolysis. An on-site cytopathologist was available for rendering diagnosis in all cases. Diagnostic adequacy was defined as the ability to establish a preliminary diagnosis based on on-site analysis of FNA specimens. Technical failure was defined as the need for use of more than one needle because of its dysfunction or the inability to successfully access and/or sample an organ or a lesion in an individual patient.
Phase I outcomes
At phase I, 625 needles were used in 548 patients (diagnostic FNAs = 487, interventions = 61), with an overall technical failure rate of 11.5% (Tables 1 and 2). Of the 63 technical failures, 53 were FNAs and 10 were therapeutic interventions. Reasons for technical failure in the 53 diagnostic FNA cases were failure to deploy the needle out of the sheath in 38, kinking of the biopsy needle at the handle in 3, bent needle tip that precluded adequate needle visualization in 9 (FNA of solid masses), and stylet dysfunction in 3. Reasons for technical failure in the 10 interventions were inability to deploy the needle out of the sheath in 7 and the needle being bent out of shape, thereby precluding adequate visualization in 3. Overall, more technical failures were observed with the use of 19-gauge versus 22- and/or 25–gauge needles (19.7% vs 8.8%; P = .004) and with transduodenal versus other routes (24.4% vs 5.2%; P = .001) for both diagnostic (technical failure in 10.9%) and therapeutic (technical failure in 16.4%) procedures. Of the 63 technical failures, 44 (70%) were encountered during transduodenal procedures. When evaluating technical failures by the type of needle and route, compared to 25-gauge, a higher proportion of failures were observed with 19- and 22–gauge needles when the transduodenal route was navigated: 15 of 28 (53.6%) versus 12 of 14 (85.7%) and 17 of 21 (81.0%), respectively (P = .012). The overall diagnostic adequacy was 97.1%.
TABLE 1.
Patient characteristics for phases I and II
| Phase I (n = 548) |
Phase II (n = 500) |
P value |
|
|---|---|---|---|
| Age, y | |||
| Mean (SD), median | 66 (17.5), 61 | 61.9 (14.1), 63 | < .001 |
| IQR (range) | 54–79 (8–97) | 53–72 (10–93) | |
| Sex, no. (%) | .150 | ||
| Female | 254 (46.4) | 254 (50.8) | |
| Male | 294 (53.6) | 246 (49.2) |
SD, Standard deviation; IQR, interquartile range.
TABLE 2.
Procedure characteristics and comparison of technical outcomes in phases I and II
| Phase I (n = 548) |
Phase II (n = 500) |
P value |
|
|---|---|---|---|
| Procedure indications, no. (%) | .590 | ||
| Diagnostic | 487 (88.9) | 439 (87.8) | |
| Interventional | 61 (11.1) | 61 (12.2) | |
| Route (all procedures), no. (%) | .018 | ||
| Transduodenal | 180 (32.8) | 199 (39.8) | |
| Transesophageal | 110 (20.1) | 69 (13.8) | |
| Transgastric | 238 (43.4) | 218 (43.6) | |
| Transrectal | 20 (3.6) | 14 (2.8) | |
| Needle type (all procedures), no. (%) | < .001 | ||
| 19 gauge | 71 (11.4) | 132 (25.3) | |
| 22 gauge | 171 (27.4) | 212 (40.6) | |
| 25 gauge | 383 (61.3) | 178 (34.1) | |
| Diagnostic procedures, route, no. (%) | .023 | ||
| Transduodenal | 170 (34.9) | 185 (42.1) | |
| Transesophageal | 109 (22.4) | 69 (15.7) | |
| Transgastric | 197 (40.5) | 179 (40.8) | |
| Transrectal | 11 (2.3) | 6 (1.4) | |
| Diagnostic procedures, site, no. (%) | .312 | ||
| Pancreaticobiliary/cysts | 313 (64.3) | 303 (69.0) | |
| Liver/adrenal | 15 (3.1) | 8 (1.8) | |
| Luminal/submucosal | 49 (10.1) | 35 (8.0) | |
| LN/mediastinal/lung | 110 (22.6) | 93 (21.2) | |
| Interventional procedures, route, no. (%) | .687 | ||
| Transduodenal | 10 (16.4) | 14 (23.0) | |
| Transesophageal | 1 (1.6) | 0 (0) | |
| Transgastric | 41 (67.2) | 39 (63.9) | |
| Transrectal | 9 (14.8) | 8 (13.1) | |
| Interventional procedures, site, no. (%) | .995 | ||
| Abscess drainage | 9 (14.8) | 10 (16.4) | |
| CBD drainage/fiducial/pancreatogram | 11 (18.0) | 11 (18.0) | |
| CPN | 13 (21.3) | 13 (21.3) | |
| PFC drainage | 28 (45.9) | 27 (44.3) | |
| Needle failure (all procedures), no. (%) | 63 (11.5) | 8 (1.6) | < .001 |
| Needle failure by procedure type, no. (%) | |||
| Diagnostic | 53 (10.9) | 8 (1.8) | < .001 |
| Interventional | 10 (16.4) | 0 | .001 |
| Needle failure by needle type, no. (%) | |||
| 19 gauge | 14 (19.7) | 1 (0.8) | < .001 |
| 22 gauge | 21 (12.3) | 0 (0) | < .001 |
| 25 gauge | 28 (7.3) | 7 (3.9) | .124 |
| Needle failure by route, no. (%) | |||
| Transduodenal | 44 (24.4) | 7 (3.5) | < .001 |
| Transesophageal | 0 | 0 | 0 |
| Transgastric | 18 (7.6) | 1 (0.5) | < .001 |
| Transrectal | 1 (5.0) | 0 | 1.000 |
| Needle cost per procedure (U.S. $) | 199.59 | 188.30 | .008 |
LN, Lymph node; CBD, common bile duct; CPN, celiac plexus neurolysis; PFC, pancreatic fluid collection.
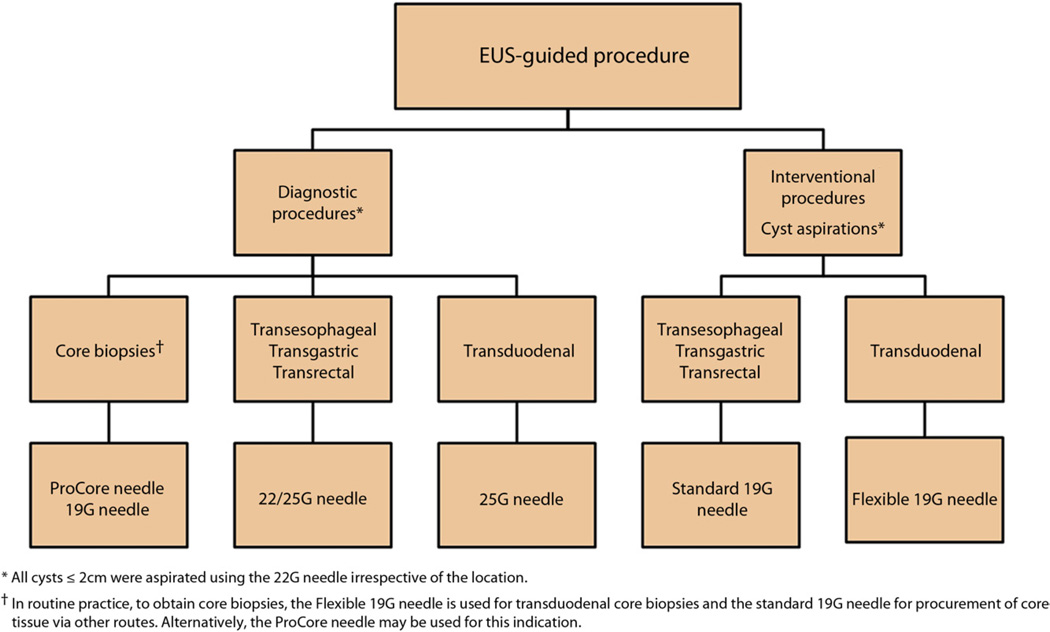
Proposed new algorithm for FNA and interventions
Based on these observations, an algorithm (Fig. 1) was developed with the objective of improving technical outcomes and resource use. As in phase I, all FNAs for tissue acquisition via the duodenum were performed by using the same 25-gauge needle and all other routes with a 22-gauge needle. Although all cyst aspirations (>2 cm in size) and interventions via the duodenum were performed by using the newly developed Flexible 19-gauge needle (Boston Scientific, Natick, Mass), a standard 19-gauge needle was used to perform these indications via other routes. Cyst lesions ≤2 cm in size were aspirated by using a 22-gauge needle, irrespective of its location. As in phase I, all celiac plexus blocks and neurolysis were undertaken by using a 20-gauge CPN or standard 19-gauge needle.
Figure 1.
Proposed new algorithm for EUS-guided FNA and interventions.
Phase II study
This algorithm was then applied prospectively in phase II (September 2011 to April 2012) by 3 endosonographers. Data collected were patient demographics, procedural indications, type of needle used, route of FNA and/or intervention, technical failure, reasons for failure, diagnostic adequacy, and procedural complications.
The institutional review board of the University of Alabama at Birmingham approved the study and granted a waiver of written informed consent, given that standard U.S. Food and Drug Administration–approved accessories were used for approved indications, and the only technical function was assessed during standard-of-care procedures.
Outcome measures
The main outcome measure was to compare rates of technical failure between phases I and II. The secondary measures were to compare the rates of diagnostic adequacy and procedural complications and the average cost of an FNA needle per individual patient.
Statistical analysis
Baseline patient characteristics, procedure outcomes, and average cost of needle per individual patient were calculated for phases I and II. For comparison of categorical data between the two phases, a chi-square or Fisher exact test was used as indicated. For continuous data, the 2-sample t test was performed for comparison of patient age, and the Wilcoxon rank-sum test was used for comparison of the needle cost data. Statistical significance was determined to be a P value of less than .05. Datasets were compiled by using Microsoft Excel (Microsoft, Redmond, WA, USA), and all statistical analyses were performed by using Stata 10 (StataCorp, College Station, TX, USA).
RESULTS
In phase II, 500 consecutive patients underwent EUS-FNA and/or interventions over the 7-month period. With the exception of age, there was no difference in patient demographics or procedural indications between phases I and II (Tables 1 and 2). By adapting the algorithm, compared to phase I, more 19- and 22–gauge needles were used in phase II (Table 2). More patients in phase II underwent transduodenal FNAs compared with patients in phase I. After exclusion of patients who required sampling of more than one site (n = 4), the overall rate of technical failure in phase II was found to be significantly less compared with that of phase I, 1.6% versus 11.5% (P < .001). This difference in technical failure was significant for both diagnostic FNAs (10.9% vs 1.8%; P < .001) and therapeutic interventions (16.4% vs 0%; P = .001) between phases I and II, respectively. All 8 technical failures in phase II were encountered during diagnostic FNA procedures that included stylet dysfunction in 1 patient who underwent a transgastric cyst aspiration by using a standard 19-gauge needle and the 25-gauge needle not being able to exit the sheath during transduodenal FNAs in 7 patients.
When technical failures were evaluated based on needle type, compared with phase I, needle dysfunction was less common for both 19- and 22–gauge needles in phase II, 19.7% versus 0.8% (P < .001) and 12.3% versus 0% (P < .001), respectively. There was no difference in rates of technical failure for the 25-gauge needle between phases I and II at 7.3% versus 3.9% (P = .124). When technical failures were evaluated based on the route of access, compared with phase I, there were significantly less failures in phase II for both transduodenal (24.4% vs 3.5%; P < .001) and transgastric (7.6% vs 0.5%; P < .001) procedures. There was no difference in the overall rates of diagnostic adequacy between phases I and II at 97.1% versus 98.4% (P = .191), respectively (Table 3). Also, there was no difference in rates of procedural complications between phase I and II procedures (0.4% vs 0.2%; P = 1.0), respectively. Two patients in phase I after FNA of pancreatic masses encountered procedural complications that included mild pancreatitis in one and abdominal pain in the other. The patient with pancreatitis required hospitalization for 2 days, and the patient with abdominal pain was managed conservatively. One patient in phase II developed bleeding after FNA of a common bile duct mass that was managed conservatively with the patient as an outpatient. The average cost of one FNA needle per patient was significantly less in phase II compared with phase I at $188.30 versus $199.59 (P = .008).
TABLE 3.
Comparison of diagnostic adequacy of FNA samples in phases I and II
| Phase I (n = 487) |
Phase II (n = 439) |
P value* |
|
|---|---|---|---|
| Adequate for diagnosis, no. (%) | .191 | ||
| Overall | 473 (97.1) | 432 (98.4) | |
| Pancreaticobiliary | |||
| Neoplastic† | 180 (37.0) | 209 (47.6) | |
| Non-neoplastic‡ | 109 (22.4) | 98 (22.3) | |
| Luminal/submucosal | |||
| Neoplastic§ | 29 (6.0) | 20 (4.6) | |
| Non-neoplastic‖ | 11 (2.3) | 5 (1.1) | |
| Peri-intestinal | |||
| Neoplastic¶ | 56 (11.5) | 38 (8.7) | |
| Non-neoplastic** | 88 (18.1) | 62 (14.1) | |
| Indeterminate/nondiagnostic, no. (%) | |||
| Overall | 14 (2.9) | 7 (1.6) | |
| Pancreaticobiliary | 7 (1.4) | 4 (0.9) | |
| Luminal/submucosal | 2 (0.4) | 1 (0.2) | |
| Peri-intestinal | 5 (1.0) | 2 (0.5) |
P value is for comparison of the overall diagnostic adequacy between phases I and II.
Neoplastic pancreaticobiliary lesions include ampullary cancer, cholangiocarcinoma, cystic neoplasm of the pancreas, intraductal papillary mucinous neoplasm, metastatic lesion to the pancreas, neuroendocrine tumor, and pancreatic adenocarcinoma.
Nonneoplastic pancreaticobiliary lesions include acute/chronic pancreatitis, benign bile duct/hepatic/splenic mass, benign pancreatic cyst (including pseudocyst, infected cyst, and pancreatic necrosis), liver cirrhosis, serous cystadenoma, and pancreatic lymphocele.
Neoplastic luminal/submucosal lesions include carcinoid tumor, esophageal/gastric/small/large bowel cancer, gastric lymphoma, GI stromal tumor, and neuroendocrine tumor.
Nonneoplastic luminal/submucosal lesions include gastric ulcer, benign mass, duplication cyst, and hyperplastic polyp.
Neoplastic peri-intestinal lesions include mediastinal/lung cancer, metastatic lymph node, and lymphoma.
Non-neoplastic peri-intestinal lesions include benign/inflammatory lymph node, bronchogenic or duplication cyst, and periluminal abscess.
DISCUSSION
In this study, we validated a simple algorithm for better technical outcomes and resource use at EUS. These findings are important, given the increasing number of EUS-FNA procedures and/or interventions being performed and decreasing reimbursements from insurance carriers for endoscopic procedures. Although not well-studied, technical failure due to needle dysfunction is not an uncommon occurrence during EUS procedures. Although there are no studies that have specifically compared the relationship between technical outcomes and needle caliber as the main outcome measure, in a prospective trial that evaluated the 19-gauge Tru-Cut biopsy, 22-gauge, and 25-gauge needles for EUS-FNA of pancreatic mass lesions, the technical success rates of the 19-, 22-, and 25–gauge needles were 0%, 33.3%, and 100% for lesions in the uncinate process, 33.3%, 83.3%, and 100% for lesions in the pancreatic head, and 83.3%, 100%, and 100% for pancreatic body and/or tail lesions, respectively.3 The superiority of the 25-gauge needle assembly for transduodenal FNA stems from its thin caliber because it enables easy exit from the biopsy channel even when the tip of the echoendoscope is acutely angulated. Based on published literature3 and our observations, in phase II of this study, we used the 25-gauge needle exclusively for transduodenal FNAs and the 22-gauge needle for other FNAs. In 3 randomized trials that compared the performance of the 22- and 25–gauge needles, there was no statistical difference in technical performance or diagnostic yield between the two needle types.12–14 However, in two of the studies, there was a trend toward better performance of the 25-gauge needle, particularly for pancreatic head and/or uncinate lesions. Also, in phase I of the present study, we observed that the 25-gauge needle performed better than the 22-gauge needle for transduodenal FNAs. We used the 22-gauge needle for FNAs other than transduodenal procedures for two reasons: first, the 22-gauge needle has the added advantage of procuring better histologic samples than do 25-gauge needles,3 and second, its technical performance equals that of the 25-gauge needle for all FNAs except transduodenal cases. However, because of limited data, the decision to use a 22- or 25-gauge needle for FNA of lesions that do not require a transduodenal route should be based on operator preference and experience. Despite the disadvantages that are inherent in its size, 19-gauge needles are indispensible for certain indications: (1) for therapeutic procedures that require the passage of a 0.035-inch guidewire; (2) for aspiration of large cyst lesions, particularly if they are mucoid; and (3) for procurement of core tissue. In phase I of the present study, we had technical difficulty with the 19-gauge needle when therapeutic interventions or cyst aspirations were undertaken via the transduodenal route. This was because it was either difficult to exit the needle out of the sheath, the needle was severely bent precluding good sonographic visualization, or it was difficult to remove the stylet from the needle assembly once the lesion was accessed. In order to circumvent this problem, in phase II of the study, we used the newly developed Flexible 19-gauge needle for all transduodenal interventions and cyst aspirations. This new needle is made of nitinol, which enhances the flexibility of the FNA assembly and facilitates ease of access for interventions and tissue procurement via the transduodenal route.15 Although the Flexible 19-gauge needle also can be used for any transgastric and/or esophageal or transrectal procedure, the cost of the needle is more than that of a standard 19-gauge needle and does not confer any added benefit. With regard to CPN, although 22-gauge needles can be used, in both phases of this study we used the standard 19-gauge FNA and 20-gauge CPN needles and found no difference in technical performance between needle types.
There are a few limitations to this study. First, this is a single-center study in which all procedures were performed by expert endosonographers, and the findings therefore may not be applicable to less-experienced endoscopists. However, the technical outcomes, even within our center, were significantly better after incorporation of this algorithm. Some practice patterns, such as use of a 19-gauge needle for diagnostic cyst aspiration, are unique to endosonographers and institutions. We prefer the 19-gauge needle because it is more time efficient and can aspirate mucin better, whereas other endosonographers might prefer to use the 22-gauge needle for the same indication. Second, the diagnostic adequacy reported was based on on-site analysis and not on long-term clinical follow-up. Third, we did not compare the performance of different needles for core tissue procurement in this algorithm because this indication is still evolving. In our unit, we used the Flexible 19-gauge needle for core tissue acquisition via the duodenum and the standard 19-gauge needle for core tissue acquisition via other routes. In a recent study, we showed that the Flexible 19-gauge needle is able to procure histologic samples in >90% of patients.15 Another needle with reverse bevel technology can acquire core tissue in nearly 90% of patients.16 Fourth, the needle costs reported in this study pertain only to our institution and may not be applicable to other centers. We did not estimate the total cost savings because all patients had only one procedure in a single endoscopy session, and the additional costs incurred were therefore attributed only to the use of more needles. Last, because the investigators were not blinded, an element of bias cannot be excluded.
In conclusion, we present a simple algorithm for a clinical approach to FNAs and interventions. In our hands, this algorithm yielded better technical outcomes for both diagnostic and therapeutic interventions and resulted in significant cost savings without compromising the diagnostic adequacy of FNA samples. If validated by other investigators, incorporating the proposed algorithm in routine clinical care is likely to improve the practice of EUS-FNA and interventions.
Take-home Message.
An algorithmic approach based on using specific needles for different routes during FNA or interventions improves the technical outcomes and resource use of EUS procedures.
Abbreviations
- CPN
celiac plexus neurolysis
- EUS-FNA
EUS-guided FNA
Footnotes
DISCLOSURE: S. Varadarajulu is a consultant for Boston Scientific Corporation and Olympus Medical Systems. No other financial relationships relevant to this publication were disclosed.
REFERENCES
- 1.Othman MO, Wallace MB. The role of endoscopic ultrasonography in the diagnosis and management of pancreatic cancer. Gastroenterol Clin North Am. 2012;41:179–188. doi: 10.1016/j.gtc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Gill KR, Ghabril MS, Jamil LH, et al. Endosonographic features predictive of malignancy in mediastinal lymph nodes in patients with lung cancer. Gastrointest Endosc. 2010;72:265–271. doi: 10.1016/j.gie.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 4.Klapman JB, Logrono R, Dye CE, et al. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 5.Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33–37. doi: 10.1016/s0016-5107(03)02028-5. [DOI] [PubMed] [Google Scholar]
- 6.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–1710. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 7.Wani S, Gupta N, Gaddam S, et al. A comparative study of endoscopic ultrasound guided fine needle aspiration with and without a stylet. Dig Dis Sci. 2011;56:2409–2414. doi: 10.1007/s10620-011-1608-z. [DOI] [PubMed] [Google Scholar]
- 8.Puri R, Vilmann P, Săftoiu A, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 9.Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–327. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erikson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 11.Erikson RA, Sayage-Rabie L, Avots-Avotins A. Clinical utility of endoscopic ultrasound-guided fine needle aspiration. Acta Cytol. 1997;41:1647–1653. doi: 10.1159/000333155. [DOI] [PubMed] [Google Scholar]
- 12.Fabbri C, Polifemo AM, Luigiano C, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: a prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647–652. doi: 10.1016/j.dld.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Camellini L, Carlinfante G, Azzolini F, et al. A randomized clinical trial comparing 22G and 25G needles in endoscopic ultrasound-guided fine-needle aspiration of solid lesions. Endoscopy. 2011;43:709–715. doi: 10.1055/s-0030-1256482. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui UD, Rossi F, Rosenthal LS, et al. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–1097. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012;76:336–343. doi: 10.1016/j.gie.2012.04.455. [DOI] [PubMed] [Google Scholar]
- 16.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–1196. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]