Abstract
Antibiotics are systemically and locally used extensively in endodontics. However, local antibiotic application mode is considered more effective than systemic administration. The local mode enables the dentist to target bacteria in every nook and corner of root canal system, which is otherwise beyond reach if targeted by instrumentation or conventional root canal treatment protocols. Therefore, they are an important adjunct to conventional treatment of root canal. The present study reviews the various antibiotic containing dental agents used in endodontics. A web-based research on MedLine was performed with terms Review Articles published in the last 10 year's dental journals in English for literature researching, extracting, and synthesizing data. Relevant articles were shortlisted. Important cross-reference articles were also reviewed.
Keywords: Antibiotics, Intracanal medicaments, Irrigants, Obturation, Regeneration
Introduction
Antibiotics have revolutionized the entire health-care system, since the discovery of penicillin in 1928 by Fleming. For many decades, antibiotics are prescribed in numerous disciplines of medicine and dentistry.[1] During endodontic treatment, antibiotics may be given systemically or regionally (i.e., intra-dental use). Systemic antibiotics should be used to treat dental infections on the basis of a defined indication.[2] The local application of antibiotics is an effective mode of disinfection in endodontics[3], as systemic antibiotics fail to reach the necrotic pulpless teeth due to absence of blood supply.
The purpose of this article is to review the antibiotic compounds used during endodontic treatment. A web-based research on MedLine was done to collect relevant articles by using term Review published in the last 10 year's dental journals in English language papers. The keywords searched on MedLine were “Antibiotics and Endodontics,” “Intracanal medicaments,” “Antibiotics and Root Canal Irrigation,” “Antibiotics and Obturation,” “Root Canal Sealers,” “Tooth Reimplantation,” and “Root Canal Revascularization.” In addition, important cross-reference articles were reviewed. The present review screened about 100 articles, relevant articles were shortlisted and facts were compiled.
History
Mixtures with antimicrobial properties utilized in the treatment of infections were described over 2,000 years ago.[4] Traditionally, plant materials and extracts[5], honey[6], moldy soybean curd, warm earth rich in molds and fungi were used to treat infections. These folk remedies tell the tale of inadvertent use of antibiotics since ages, although the concept of antibiotics is relatively recent. Physician Paul Ehrlich laid down the foundation of idea of antibiotics by introducing the term “magic bullets” for a chemical that would attach itself to the germ and kill it. Jean Paul Vuillemin used the term “antibiosis” that means “against life” for early antibacterial drugs.[7] Antibiosis was first described in 1877 by Louis Pasteur and Robert Koch.[8] In 1942, Selman Waksman referred antibacterial drugs as antibiotics to describe any substance produced by a micro-organism that is antagonistic to the growth of other micro-organisms in a high dilution.[9] Grossman, known as father of endodontics, in 1951 proposed PBSC (polyantibiotic paste which suspended in a silicone vehicle, and was a combination of penicillin, bacitracin, streptomycin and caprylate sodium), that was the firstly reported local use of an antibiotic in endodontics.[10] Polyantibiotic paste showed therapeutic potential, but owing to the drawbacks including ineffectiveness against anaerobic species and allergic reactions, the Food and Drug Administration (FDA) prohibited PBSC for endodontic use in 1975. Later, an antifungal version of PBSC named with PBSN, in which Nystatin substituted caprylate sodium, was released.[11]
Discussion
The various modalities, which contain antibiotic compounds for the treatment in Endodontics, are discussed below:
Pulp capping
Several topical antibiotic-containing preparations have been evaluated for their use as pulp capping agents but without a precise efficacy.
Ledermix containing both triamcinolone (a steroid) and demethylchlortetracycline (an antibiotic)[12] is a topical preparation that is used as pulp capping agent. It is available in kits containing dental paste, dental cement powder, hardener “F” dental liquid (fast setting time) and hardener “S” dental liquid (slow setting time). The paste form is preferred in the treatment of exposed pulp, as it contains one third more steroid than the cement form. Thus, it is effective in the control of inflammation after tooth preparation, and reduces the need to rely on analgesics for pain relief. For emergency management of irreversible pulpitis, the paste relieves pain till definitive root canal therapy is carried out. The cement can be used as a pulp-capping agent in case of small pulp exposure and as an excellent subliner for deep cavities where no exposure has occurred but hypersensitive dentine is present. Although Ledermix has shown to be an efficacious pulp capping agent[13], since it is a pulp irritant[14], its use as a pulp capping agent is not recommended.[15]
Pulpomixine is composed of dexamethasone acetate, polymyxin B sulfate and framycetin sulfate. Its use is contraindicated in patients allergic to any of its ingredients. Pulpoximine is applied to the floor of the cavity in deep cavities without pulp exposure, acute pulpitis and recent pulp exposure with recent pulpitis. Preparations containing erythromycin estolate[16] and vancomycin[17] have also been used, but they are proved to be ineffective with some side effects.
Intracanal medicaments
Intracanal medicaments are defined as antiseptic agents in the chemical form applied to the walls of the root canals with the objective of eliminating microorganisms present before or even after cleaning and irrigating the root canal system. Various polyantibiotic intracanal pastes that have been in use are discussed below:
Ledermix paste
Schroeder and Triadan developed Ledermix in 1960, which was made commercially available by Lederle Pharmaceuticals in 1962.[18] Ledermix paste contains an antibiotic demeclocycline—HCl (3.2%) and a corticosteroid, triamcinolone acetonide (1%), in a polyethylene glycol base.[18] The paste utilizes corticosteroids to control pain and inflammation related with pulp and periapical diseases.[18] Antibiotic is added to Ledermix to compensate for the perceived corticoid-induced reduction in the host immune response. Both triamcinolone and demeclocycline are capable of diffusing through dentinal tubules and cementum to reach the periradicular and periapical tissues.[19] Various studies have confirmed the effectiveness of Ledermix as intracanal medicament.[20,21,22] Perfect for use in endodontic therapy and between appointments, Ledermix is water soluble, well rinsed out easily, and does not result in any systemic side effect in the intradental use.[23]
Odontopaste
Released in February 2008, odontopaste is a zinc oxide-based root canal paste with 5% clindamycin hydrochloride and 1% triamcinolone acetonide. Clindamycin is effective against many endodontic pathogens including Streptococci, Peptostreptococcus, Actinomyces, Fusobacterium, Eubacterium, Propionobacterium, Microaerophilic, Peptococcus, Porphyromonas, Veillonella and Prevotella. Clindamycin paste as an intracanal dressing has good antibacterial effect[24,25], however, it does not have the anti-resorptive properties.[25] This antibiotic provides a bacteriostatic activity, and acts as interim dressing material preventing bacterial repopulation within the root canal. Additionally, the steroid part, triamcinolone acetonide, can temporarily reduce inflammation and postoperative pain. No bleaching is required following the use of odontopaste, as it does not stain teeth.[26] Mixing of additional calcium hydroxide in a 50:50 combination with Odontopaste is not recommended because the steroid component gets destroyed immediately by increased alkalinity. Odontopaste contains calcium hydroxide at 0.5% level, which has been proven to be optimal for the preservation of the steroid component.[27]
Septomixine forte
Septomixine Forte paste contains dexamethasone, halethazole tartrate, neomycin sulfate, polymyxin B sulfate, and tyrothricin. Septomixine Forte paste, however, is no longer recommended because the antibiotics (neomycin and polymyxin B sulfate) are unsuitable for use against endodontic bacteria due to their inappropriate spectra of activity.[19]
Sulfonamides
Sulfanilamide and sulfathiazole were initially used as root canal medicaments,[28] but now are no longer used as they tend to cause a yellowish discoloration of the teeth.[29]
Irrigants Containing Antibiotics
Irrigation is an indispensable part of root canal treatment as it cleans much better than root canal instrumentation alone does.[30] A variety of chemicals has been used as root canal irrigants, including citric and phosphoric acids, chelating agent (EDTA), proteolytic enzymes, alkaline solutions (sodium hypochlorite, sodium hydroxide, urea and potassium hydroxide), oxidative agents (hydrogen peroxide and Gly-Oxide), local anaesthetic solutions and normal saline.[31] Two new root canal irrigants, Mixture of Tetracycline, Citric Acid and Detergent (MTAD) and Tetraclean, containing antibiotics have recently been introduced.
BioPure MTAD
Root canal irrigant MTAD commercialized as BioPure MTAD was introduced by Torabinejad and Johnson[32] in 2003.
MTAD is commercially available as Powder-Liquid system. Part A is a liquid containing 4.25% citric acid and 0.5% polysorbate 80 detergent (Tween 80). Citric acid removes organic and inorganic materials from the surfaces of roots. Tween 80 (polyoxyethylene sorbitan mono-oleate) is a non-ionic surfactant, which helps reducing the surface tension thereby enhancing the flow and penetration of irrigating solutions. Part B is a powder containing 3% doxycycline hyclate, which is a broad spectrum antibiotic, and is supplied in bottles. Doxycycline hyclate is used to increase the water solubility, instead of its free-base doxycycline monohydrate.[33] It disinfects the internal surface of root, is bacteriostatic and shows the property of substantivity and anticollagenase activity. The powder and liquid are mixed by following the manufacturer's instructions to obtain the final ready to use MTAD.
MTAD is clinically effective and biocompatible, and has proved its antimicrobial effectiveness against E. faecalis[34,35] and over standard irrigants.[36,37] The bacteriostatic property of doxycycline is advantageous, because in the absence of bacterial cell lysis, endotoxins are not released[38] and the substantivity of doxycycline provides a prolonged antibacterial effect.[38] Doxycycline additionally helps MTAD to get rid of smear layer by acting as a calcium chelator, thereby, resulting in root surface demineralization.[39] The exact mechanism of antimicrobial action of citric acid is not known yet, however, it is known to extend the antibacterial impact of varied substances.[40]
Tetraclean
Tetraclean is a mixture of an antibiotic, acid and detergent like MTAD, but differs in the concentration of doxycycline (50 mg/ml) and type of detergent (polypropylene glycol).[41] It shows a high action against strict anaerobic as well as facultative anaerobic bacteria, and also removes the smear layer. Giardino et al.[41] showed that both cetrexedin and tetraclean had the lowest value of surface tension when compared to 17% EDTA, Smear Clear, 5.25% NaOCl and MTAD. Tetraclean is more effective than MTAD against E. faecalis in the planktonic culture and the mixed species in vitro biofilm.[42] The another study by Giardino et al. again proved a higher antimicrobial efficacy of tetraclean, as it resulted a high degree of biofilm disaggregation on cellulose nitrate membrane filters when compared to MTAD and 5.25% NaOCl.[43]
Obturation with medicated gutta percha points
If root-canal obturation material possesses some antimicrobial activity, it can assist eliminate microbes residing in anatomical complexities of root canals such as cementum crypts, secondary canals, isthmus, and dentin tubules, and survives biomechanical preparation of root canal space.
Howard Martin developed medicated gutta-percha (MGP)[44] containing 10% iodoform and tetracycline-impregnated gutta percha (TGP) containing 10% tetracycline[45], which intent to retard the growth of bacteria inside the obturated root canal. TGP acts as an antimicrobial reservoir with a capability of diffusing onto the surface of the gutta percha, inhibiting colonization of bacteria on the gutta percha points and within the root canals.[46] TGP has been advocated as an inter-appointment intracanal medicament and final obturating material.[47] Gutta Percha points containing metronidazole for root canal disinfection have been investigated, and may be considered as an ideal method for clinical application.[48,49] This concept needs in-vivo studies to evaluate the toxicity and antibacterial and antifungal effects.
Medicated Sealers
The addition of antibiotics to the root canal sealer is beneficial to prevent re-infection and impart antimicrobial property for an extended period of time. Hoelscher et al. found that, except for metronidazole, amoxicillin, penicillin, clindamycin and doxycycline enhanced the antimicrobial efficacy of Kerr Pulp Canal Sealer (PCS) against E. faecalis.[50] Another study showed that addition of amoxicillin, doxycycline and metronidazole to Kerr PCS (extended working time) improves both the antibacterial property and apical sealing ability.[51] Hasan R et al. found that AH26 sealer in combination with amoxicillin and doxycycline individually was effective in killing E.faecalis in dentinal tubules.[52]
Tooth reimplantation
Avulsed teeth with immature roots are subject to root resorption, but also possess the potential for pulpal revascularization.[53] Bacterial contamination of root surfaces may occur during the extra-oral time that could inhibit healing of the recently replanted teeth, therefore, a protocol for the topical treatment of exposed roots with doxycycline before reimplantation was developed.[54] Avulsed teeth with an open apex should be soaked for 5 minutes in a 1 mg/20 mL doxycycline solution, and then reimplanted.[55] This resulted in up to 40% to 60% increase in pulp revascularization of the reimplantation cases along with decrease in frequency of external replacement resorption, ankylosis, and external inflammatory resorption.[54] The beneficial effect of soaking a tooth in doxycycline has been confirmed by Yanpiset et al.[56] Along with this it is recommended to prescribe oral antibiotic therapy to avoid infection and external root resorption.[57] The antibiotic choice is amoxicillin,[57] although doxycycline is advantageous.[58]
Antibiotics in regenerative endodontics
Sterile environment is the prerequisite for success of any regenerative endodontic procedures. To achieve this, triple antibiotic paste (TAP) based on lesion sterilization and tissue repair therapy concept is most commonly used. The lesion sterilization and tissue repair therapy utilizes a combination of antibacterial drugs for the disinfection of pulpal lesions.[59] TAP, first used by Sato et al.[60], contains metronidazole, ciprofloxacin, and minocycline, and is commercially available as 3 MIX MP. Hoshino et al. recommended metronidazole (500 mg), minocycline (100 mg) and ciprofloxacin (200 mg) at a ratio of 1:1:1 for the 3Mix formulation.[61] The carrier (MP) recommended was propylene glycol and macrogol ointment[61] at the ratio of 1:1. The formulation was modified by Takushige et al., and recommended metronidazole, minocycline and ciprofloxacin antibiotics mixed in the ratio at 3:3:1. This can be mixed with MP or root canal sealers, although the mixture with sealers is presently not recommended.[62]
A recent study showed that the administration of single antibiotic augmentin paste for 5 weeks as an intracanal medicament resulted in excellent infection control, and gave complete osseous healing of the periapical lesion and formation of the root apex.[63]
Antibiotic-containing scaffolds
Although TAP is established antibiotic paste, but it has its own drawbacks. TAP is radiolucent[61], propylene glycol as a vehicle of TAP may be difficult to remove from the dentin surface, an additional appointment is required to remove TAP and re-opening the tooth to remove TAP introduces a risk of recontamination. Antibiotic containing scaffolds can solve the problems.
Bottino MC et al.[64] have suggested that the polymer-based antibiotic-containing electrospun scaffolds may act as a biologically safe antimicrobial drug delivery system for regenerative endodontics. This can improve drug delivery due to high surface area fibers arranged in an interconnecting structure that allows controlled drug release[65] and improve drug adaptation to the canal wall in the regeneration procedure. As the scaffold degrades over time[66], it does not required to be removed, thus, reduces appointments and a subsequent risk of bacterial contamination. In addition, the drug release can be manipulated in a mode-rapid, intermediate or delayed, depending on the polymer used.[67] The effectiveness of an electrospun scaffold capable of drug release has been reported in the literature.[65,68] Bottino MC et al.[69] suggested the electrospun nanocomposite fibrous material to act as a scaffold for regenerative endodontics, and a drug-delivery device to aid in root maturogenesis and the regeneration of the pulp-dentine complex. Various other synthetic electrospun polymeric nanofibers are under investigation for use as drug delivery devices in the medicine and other applications for tissue engineering.[65]
Demerits of Local Antibiotic Application
Although a local antibiotic medication in endodontics offers advantages including an efficient and predictable disinfection, and a high drug concentration at the local site, reducing systemic complications of antibiotic medication, this mode has some drawbacks, including development of bacterial resistant strains[70], allergic reactions[71,72], inhibition of angiogenesis[73], and tooth staining or discoloration.[74,75] In addition to these drawbacks, the development of antibiotic resistance is alarming, and should be checked. One major cause contributing to antibiotic resistance is the use of antibiotics in an improper manner, which leads to resistant microorganisms and increases transfer of resistance genes from antibiotic-resistant to antibiotic-susceptible microorganisms.[76] Antibiotics should be used very selectively, but, dentists unfortunately prescribe antibiotics for dental conditions which should be managed by analgesics and local measures. The profession needs to realize the importance of using antibiotics correctly, and routine prescribing antibiotics for endodontic procedure must be discouraged. Antibiotic resistance threatens mankind with the prospect of a return to the pre-antibiotic era.[77] It is strongly suggested that bacterial resistance to antibacterial agents is reversed by avoiding the use of antibiotics until extremely necessary.[78]
Can Intracanal Antibiotics be Substituted?
The sole purpose behind a local application of antibiotic compounds is to eliminate microbes. If this motive is achieved by some other means, then antibiotics can be avoided. The EndoVac apical negative-pressure system of irrigation can be one of such ways, in which the irrigating agents are safely delivered to the full extent of the root-canal terminus, resulting in a better removal of organic tissue, microbial contaminants[79] and a better cleaning of the isthmus area.[80] Thus, optimum conditions are created to promote healing without the use of antibiotics. Hockett et al.[81] showed that the apical negative pressure eradicated biofilms of Enterococcus faecalis within 48 hours, not only from the walls of the root canal system, but also from the dentinal tubules. Various other studies have also shown that apical negative pressure with sodium hypochlorite irrigation results in similar bacterial reductions, as with use of apical positive pressure irrigation and a triple antibiotic in immature teeth[82] and equivalent repair process resulted.[83] Thus, the use of intracanal antibiotics is not necessary.[83] Additionally, using negative apical pressure and sodium hypochlorite also avoids the risk of drug resistance, tooth discoloration, and allergic reactions.[74,84]
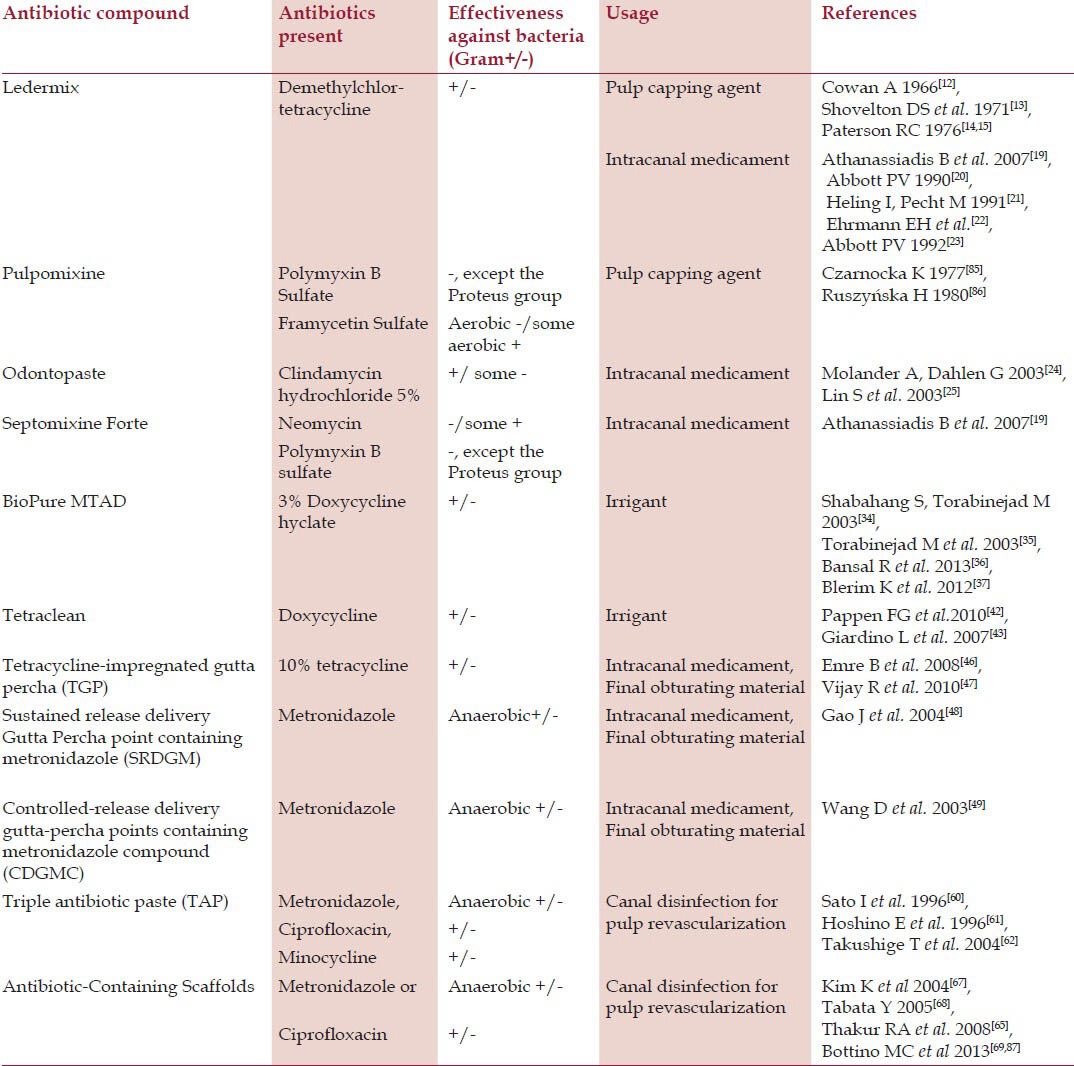
Various antibiotic containing agents, applications, and bacterial spectrum along with the literature are given in Table 1.
Table 1.
Antibiotics containing agents used in endodontics
Conclusion
Use of antibiotics both systemic and topical is common in the endodontic treatment, particularly for patients with pain or swelling. The antibiotic containing dental agents are used to eliminate bacteria from the root canal system, and provide a favorable healing environment. The use of antibiotic containing dental agents should be carefully justified, in order to avoid a bacterial resistance.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Abbott PV. Selective and intelligent use of antibiotics in endodontics. Aust Endod J. 2000;26:30–9. doi: 10.1111/j.1747-4477.2000.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 2.Miles M. Anaesthetics, analgesics, antibiotics and endodontics. Dent Clin North Am. 1984;28:865–82. [PubMed] [Google Scholar]
- 3.Gilad JZ, Teles R, Goodson M, White RR, Stashenko P. Development of a Clindamycin impregnated fiber as an intracanal medication in endodontic therapy. J Endod. 1999;25:722–7. doi: 10.1016/S0099-2399(99)80117-4. [DOI] [PubMed] [Google Scholar]
- 4.Lindblad WJ. Considerations for determining if a natural product is an effective wound-healing agent. Int J Low Extrem Wounds. 2008;7:75–81. doi: 10.1177/1534734608316028. [DOI] [PubMed] [Google Scholar]
- 5.Forrest RD. Early history of wound treatment. J R Soc Med. 1982;75:198–205. doi: 10.1177/014107688207500310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molan PC. Honey as a topical antibacterial agent for treatment of infected wounds. Nurs Times. 2001;49:96. [Google Scholar]
- 7.Foster W, Raoult A. Early descriptions of antibiosis. J R Coll Gen Pract. 1974;24:889–94. [PMC free article] [PubMed] [Google Scholar]
- 8.Landsberg H. Prelude to the discovery of penicillin. Isis. 1949;40:225–7. [Google Scholar]
- 9.Waksman SA. What is an antibiotic or an antibiotic substance? Mycologia. 1947;39:565–9. [PubMed] [Google Scholar]
- 10.Grossman LI. Polyantibiotic treatment of pulpless teeth. J Am Dent Assoc. 1951;43:265–78. doi: 10.14219/jada.archive.1951.0213. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadi Z. An update on the antibiotic-based root canal irrigation solutions. Iran Endod J. 2008;3:1–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan A. Treatment of exposed vital pulps with a corticosteroid antibiotic agent. Br Dent J. 1966;120:521–32. [PubMed] [Google Scholar]
- 13.Shovelton DS, Friend LA, Kirk EE, Rowe AH. The efficacy of pulp capping materials. A comparative trial. Br Dent J. 1971;130:385–91. doi: 10.1038/sj.bdj.4802670. [DOI] [PubMed] [Google Scholar]
- 14.Paterson RC. Corticosteroids and the exposed pulp. Br Dent J. 1976;140:174–7. doi: 10.1038/sj.bdj.4803726. [DOI] [PubMed] [Google Scholar]
- 15.Paterson RC. Bacterial contamination and the exposed pulp. Br Dent J. 1976;140:231–6. doi: 10.1038/sj.bdj.4803741. [DOI] [PubMed] [Google Scholar]
- 16.Barker RG, Mitchell DF. Topical antibiotic treatment of infected dental pulps of monkeys. J Dent Res. 1969;48:351–5. doi: 10.1177/00220345690480030401. [DOI] [PubMed] [Google Scholar]
- 17.Gardner DE, Mitchell DF, McDonald RE. Treatment of pulps of monkeys with vancomycin and calcium hydroxide. J Dent Res. 1971;50:1273–7. doi: 10.1177/00220345710500053001. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed HM, Abbott PV. Discolouration potential of endodontic procedures and materials — a review. Int Endod J. 2012;45:883–97. doi: 10.1111/j.1365-2591.2012.02071.x. [DOI] [PubMed] [Google Scholar]
- 19.Athanassiadis B, Abbott PV, Walsh LJ. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust Endod J. 2007;52:S64–82. doi: 10.1111/j.1834-7819.2007.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 20.Abbott PV. Medicaments: Aids to success in endodontics. Part 1. A review of literature. Aust Endod J. 1990;35:438–48. doi: 10.1111/j.1834-7819.1990.tb05427.x. [DOI] [PubMed] [Google Scholar]
- 21.Heling I, Pecht M. Efficacy of Ledermix paste in eliminating Staphylococcus aureus from infected dentinal tubules in vitro. Endod Dent Traumatol. 1991;7:251–4. doi: 10.1111/j.1600-9657.1991.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 22.Ehrmann EH, Messer HH, Adams GG. The relationship of intracanal medicaments to postoperative pain in endodontics. Int Endod J. 2003;36:868–75. doi: 10.1111/j.1365-2591.2003.00735.x. [DOI] [PubMed] [Google Scholar]
- 23.Abbott PV. Systemic release of corticosteroids following intradental use. Int Endod J. 1992;25:189–91. doi: 10.1111/j.1365-2591.1992.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 24.Molander A, Dahlen G. Evaluation of the antibacterial potential of tetracycline or erythromycin mixed with calcium hydroxide as intracanal dressing against E. faecalis in vivo. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:744–50. doi: 10.1016/s1079-2104(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 25.Lin S, Levin L, Peled M, Weiss EI, Fuss Z. Reduction of viable bacteria in dentinal tubules treated with clindamycin or tetracycline. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:751–6. doi: 10.1016/s1079-2104(03)00355-x. [DOI] [PubMed] [Google Scholar]
- 26.Joiner A. The bleaching of teeth: A review of the literature. J Dent. 2006;34:412–9. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Athanassiadis M, Jacobsen N, Parashos P. The effect of calcium hydroxide on the steroid component of Ledermix and Odontopaste. Int Endod J. 2011;44:1162–9. doi: 10.1111/j.1365-2591.2011.01940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fouad AF. Are antibiotics effective for endodontic pain? An evidence-based review. Endod Top. 2002;3:52–66. [Google Scholar]
- 29.Weine FS, editor. 5th ed. St. Louis: Mosby; 1996. Endodontic Therapy; pp. 378–9. [Google Scholar]
- 30.Gulabivala K, Patel B, Evans G, Ng YL. Effects of mechanical and chemical procedures on root canal surfaces. Endo Top. 2005;10:103–22. [Google Scholar]
- 31.Becker TD, Woollard GW. Endodontic irrigation. Gen Dent. 2001;49:272–6. [PubMed] [Google Scholar]
- 32.Torabinejad M, Johnson WB. United States Patent Application. Philadelphia: US Patent & Trade Mark Office; 2003. Irrigation solution and methods of use. [Google Scholar]
- 33.Bogardus JB, Blackwood RK., Jr Solubility of doxycycline in aqueous solution. J Pharm Sci. 1979;68:188–94. doi: 10.1002/jps.2600680218. [DOI] [PubMed] [Google Scholar]
- 34.Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis-contaminated root canals of extracted human teeth. J Endod. 2003;29:576–9. doi: 10.1097/00004770-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Torabinejad M, Shabahang S, Aprecio R, Kettering JD. The antimicrobial effect of MTAD: An in vitro investigation. J Endod. 2003b;29:400–3. doi: 10.1097/00004770-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Bansal R, Jain A, Mittal S, Kumar T, Jindal N, Kaur D. A comparison of the antibacterial efficiency of MTAD (mixture of tetracycline, citric acid and detergent), 2.5% sodium hypochlorite and 2% chlorhexidine root canal irrigants against Enterococcus Faecalis in root canals of single rooted mandibular premolars-an in vitro study. IOSR – JDMS. 2013;5:47–53. [Google Scholar]
- 37.Blerim K, Donika B, Miranda S, Shuhreta O, Fatmir D, Ferit K. The Antibacterial Efficacy of Biopure MTAD in Root Canal Contaminated with Enterococcus faecalis. ISRN Dent 2012. 2012:390526. doi: 10.5402/2012/390526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. A new solution for the removal of smear layer. J Endod. 2003;29:170–5. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Beltz RE, Torabinejad M, Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentin. J Endod. 2003;29:334–7. doi: 10.1097/00004770-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Kurup TR, Wan LS, Chan LW. Effect of surfactants on the antibacterial activity of preservatives. Pharm Acta Helv. 1991;66:274–80. [PubMed] [Google Scholar]
- 41.Giardino L, Ambu E, Becce C, Rimondini L, Morra M. Surface tension comparison of four common root canal irrigants and two new irrigants containing antibiotic. J Endod. 2006;32:1091–3. doi: 10.1016/j.joen.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Pappen FG, Shen Y, Gian W, Leonardo MR, Giardino L, Haapasalo M. In vitro antibacterial action of Tetraclean, MTAD and Five experimental irrigation solutions. Int Endod J. 2010;43:528–35. doi: 10.1111/j.1365-2591.2010.01712.x. [DOI] [PubMed] [Google Scholar]
- 43.Giardino L, Ambu E, Savoldi E, Rimondini R, Cassanelli C, Debbia EA. Comparative evaluation of antimicrobial efficacy of sodium hypochlorite, MTAD, and Tetraclean against enterococcus faecalis biofilm. J Endod. 2007;33:852–5. doi: 10.1016/j.joen.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Martin H, Martin TR. Iodoform gutta percha: MGP, a new endodontic paradigm. Dent Today. 1999;18:76–81. [PubMed] [Google Scholar]
- 45.Martin H. Antibiotic/Medicated Gutta Perch Point. US Patent. 2003 6,602,516. [Google Scholar]
- 46.Emre B, Tayfun A, Mustafa S. The antimicrobial and antifungal efficacy of tetracycline integrated gutta-percha. Indian J Dent Res. 2008;19:112–5. doi: 10.4103/0970-9290.40464. [DOI] [PubMed] [Google Scholar]
- 47.Vijay R, Suman M, Shashikala K. Evaluation of antimicrobial efficacy of tetracycline gutta percha and calcium hydroxide impregnated gutta percha against enterococcus faecalis- an in vitro study. IJDA. 2010;2:248–52. [Google Scholar]
- 48.Gao J, Wang ZP, Li XG, Wang D, Zhang L. The preparation and in vitro release test of sustained release delivery gutta-percha point containing metronidazole. Shanghai Kou Qiang Yi Xue. 2004;13:557–60. [PubMed] [Google Scholar]
- 49.Wang D, Wang Z, Gao J. The development and in vitro release rate determination of controlled-release delivery gutta-percha point containing metronidazole compound. Hua Xi Kou Qiang Yi Xue Za Zhi. 2003;21:361–3. [PubMed] [Google Scholar]
- 50.Hoelscher AA, Bahcall JK, Maki JS. In vitro evaluation of the antimicrobial effects of a root canal sealer-antibiotic combination against Enterococcus faecalis. J Endod. 2006;32:145–7. doi: 10.1016/j.joen.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 51.Shrestha S, Mala K. Evaluation of sealing ability of a root canal sealer with various antibiotic additives: An in vitro study. J Interdiscip Dent. 2013;3:21–4. [Google Scholar]
- 52.Hasan R, Kazem AY, Fereshteh J, Shaghayegh P. Antimicrobial effects of AH26 sealer/antibiotic combinations against Enterococcus faecalis. Iran Endod J. 2008;3:107–12. [PMC free article] [PubMed] [Google Scholar]
- 53.Kling M, Cvek M, Mejare I. Rate and predictability of pulp revascularization in therapeutically reimplanted permanent incisors. Endo Dent Traumatol. 1986;2:83–8. doi: 10.1111/j.1600-9657.1986.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 54.Cvek M, Cleaton-Jones P, Austin J, Lownie J, King M, Fatti P. Effect of topical application of doxycycline on pulp revascularization and periodontal healing in reimplanted monkey incisors. Endod Dent Traumatol. 1990;6:170–6. doi: 10.1111/j.1600-9657.1990.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 55.Krasner P, Rankow H. New philosophy for the treatment of avulsed teeth. Oral Surg Oral Med Oral Pathol. 1995;79:616–23. doi: 10.1016/s1079-2104(05)80105-2. [DOI] [PubMed] [Google Scholar]
- 56.Yanpiset K, Trope M. Pulp revascularization of replanted immature dog teeth after different treatment methods. Endod Traumatol. 2000;16:211–7. doi: 10.1034/j.1600-9657.2000.016005211.x. [DOI] [PubMed] [Google Scholar]
- 57.Hammarstrom L, Blomloff L, Feiglin B, Andersson L, Lindskog S. Replantation of teeth and antibiotic treatment. Endod Dent Traumatol. 1986;2:51–7. doi: 10.1111/j.1600-9657.1986.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 58.Sae-Lim V, Wang CY, Trope M. The effect of systemic tetracycline on resorption of dried replanted dogs teeth. Endod Dent Traumatol. 1998;14:216–20. doi: 10.1111/j.1600-9657.1998.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 59.Hoshino E, Takushige T. LSTR 3Mix-MP method-better and efficient clinical procedures of lesion sterilization and tissue repair (LSTR) therapy. Dent Rev. 1998;666:57–106. [Google Scholar]
- 60.Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118–24. doi: 10.1111/j.1365-2591.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 61.Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125–30. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 62.Takushige T, Cruz EV, Moral AA, Hoshino E. Endodontic treatment of primary teeth using a combination of antibacterial drugs. Int Endod J. 2004;37:132–8. doi: 10.1111/j.0143-2885.2004.00771.x. [DOI] [PubMed] [Google Scholar]
- 63.Nosrat A, Li KL, Vir K, Hicks ML, Fouad AF. Is pulp regeneration necessary for root maturation? J Endod. 2013;39:1291–5. doi: 10.1016/j.joen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 64.Thakur RA, Florek CA, Kohn J, Michniak BB. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int J Pharm. 2008;364:87–93. doi: 10.1016/j.ijpharm.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 65.Cui W, Zhou Y, Chang J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater. 2010;11:014108. doi: 10.1088/1468-6996/11/1/014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moioli EK, Clark PA, Xin X, Lal S, Mao JJ. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv Drug Deliver Rev. 2007;59:308–24. doi: 10.1016/j.addr.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Tabata Y. Nanomaterials of drug delivery systems for tissue regeneration. Method Mol Biol. 2005;300:81–100. doi: 10.1385/1-59259-858-7:081. [DOI] [PubMed] [Google Scholar]
- 69.Bottino MC, Yassen GH, Platt JA, Labban N, Windsor LJ, Spolnik KJ, et al. A novel three-dimensional scaffold for regenerative endodontics: Materials and biological characterizations. J Tissue Eng Regen Med “in press”. 2013 doi: 10.1002/term.1712. [DOI] [PubMed] [Google Scholar]
- 70.Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodontal Res. 2002;37:389–98. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 71.Hausermann P, Scherer K, Weber M, Bircher AJ. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277–80. doi: 10.1159/000087024. [DOI] [PubMed] [Google Scholar]
- 72.de Paz S, Perez A, Gomez M, Trampal A, Domínguez Lázaro A. Severe hypersensitivity reaction to minocycline. J Investig Allergol Clin Immunol. 1999;9:403–4. [PubMed] [Google Scholar]
- 73.Tamargo RJ, Bok RA, Brem H. Angiogenesis inhibition by minocycline. Cancer Res. 1991;51:672–5. [PubMed] [Google Scholar]
- 74.Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G. Tooth discoloration induced by endodontic materials: A laboratory study. Int Endod J. 2012;45:942–9. doi: 10.1111/j.1365-2591.2012.02053.x. [DOI] [PubMed] [Google Scholar]
- 75.Kim JH, Kim Y, Shin SJ, Park JW, Jung IY. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: A case report. J Endod. 2010;36:1086–91. doi: 10.1016/j.joen.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 76.ADA Council on Scientific Affairs. Antibiotic use in dentistry. J Am Dent Assoc. 1997;128:648. [PubMed] [Google Scholar]
- 77.Doherty R. In the news: Antibiotic resistance. Br Dent J. 2014;216:56. doi: 10.1038/sj.bdj.2014.44. [DOI] [PubMed] [Google Scholar]
- 78.Callahan JR. Fear of flesh eating Bacteria. In: Callahan JR, editor. 50 Health Scares that fizzled. 1st Ed. Santa Barbara, California: 2011. pp. 65–69. [Google Scholar]
- 79.Glassman G. Endodontic irrigants and irrigant delivery systems. Roots. 2013;1:30–7. [Google Scholar]
- 80.Susin L, Liu Y, Yoon JC, Parente JM, Loushine RJ, Ricucci D, et al. Canal and isthmus debridement efficacies of two irrigant agitation techniques in a closed system. Int Endod J. 2010;43:1077–90. doi: 10.1111/j.1365-2591.2010.01778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hockett JL, Dommisch JK, Johnson JD, Cohenca N. Antimicrobial efficacy of two irrigation techniques in tapered and Nontapered canal preparations: An in vitro study. J Endod. 2008;34:1374–7. doi: 10.1016/j.joen.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 82.Cohenca N, Heilborn C, Johnson JD, Flores DS, Ito IY, da Silva LA. Apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing on root canal disinfection in dog teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e42–6. doi: 10.1016/j.tripleo.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 83.da Silva LA, Nelson-Filho P, da Silva RA, Flores DS, Heilborn C, Johnson JD, et al. Revascularization and periapical repair after endodontic treatment using apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing in dogs’ teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:779–87. doi: 10.1016/j.tripleo.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 84.Eickholtz P, Kim TS, Burklin T, Schacher B, Renggli HH, Schaecken MT, et al. Nonsurgical periodontal therapy with adjunctive topical doxycycline: A double-blind randomized controlled multicenter study. J Clin Periodontol. 2002;29:108–17. doi: 10.1034/j.1600-051x.2002.290204.x. [DOI] [PubMed] [Google Scholar]
- 85.Czarnocka K. Clinical observations on Pulpomixine used for dental pulp capping in deciduous teeth. Czas Stomatol. 1977;30:300–1. [PubMed] [Google Scholar]
- 86.Ruszyńska H. Evaluation of the effect of the preparation, Pulpomixine, used for indirect covering of inflamed dental pulp in dogs. Czas Stomatol. 1980;33:1053–9. [PubMed] [Google Scholar]
- 87.Bottino MC, Kamocki K, Yassen GH, Platt JA, Vail MM, Ehrlich Y, et al. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res. 2013;92:963–9. doi: 10.1177/0022034513505770. [DOI] [PMC free article] [PubMed] [Google Scholar]


