Abstract
Ablative intracranial surgery for Parkinson's disease has advanced to embedding electrodes into precise areas of the basal ganglia. Electrode implantation surgery, referred to as deep brain stimulation (DBS), is preferred in view of its reversibility, adjustability, and capability to be safely performed bilaterally. DBS is been increasingly used for other movement disorders, intractable tremors epilepsy, and sometimes chronic pain. Anesthesiologists need to amalgamate the knowledge of neuroanatomical structures and surgical techniques involved in placement of microelectrodes in defined cerebral target areas. Perioperative verbal communication with the patient during the procedure is quintessential and may attenuate the need for pharmacological agents. This review will endeavor to assimilate the present knowledge regarding the patient selection, available/practiced anesthesia regimens, and perioperative complications after our thorough search for literature published between 1991 and 2013.
Keywords: Deep brain stimulation, electrodes, anaesthesia
Introduction
By the late 1980s, it was found that high-frequency electrical stimulation induces the same functional effect as lesioning,[1] thus rejuvenating the role of surgery in the treatment of Parkinson's disease. Deep brain stimulation (DBS) has been successful in treatment of Parkinson's disease (essential tremors, dystonia, and certain psychiatric conditions).[2,3] DBS is the placement of stimulator electrodes into deep brain structures and clinically testing the patient and connection of the stimulator to an implanted pacemaker. The targets of DBS include the ventralis intermedius nucleus (Vim), the sub-thalamic nucleus (STN), and the globus pallidus (GPi).
DBS incorporates 4 components: The intracranial electrodes, which are inserted surgically inside the brain, a plastic ring and cap seated onto a burr hole to fix the electrodes to the skull, an extension cable that passes subcutaneously and is connected to a pulse generator, which is implanted into the chest in the infraclavicular area or abdomen. The pulse generator's battery usually spans between 2 and 5 years and has to be changed with the generator.
Anesthetic approaches vary depending on the institutions performing these procedures and include monitored care with local anesthesia, sedation, and general anesthesia.[4,5,6,7,8] There are various anesthetic considerations whichever approach is used: DBS was introduced in late 1980 and over time, the anesthetic approach for this procedure changed because of the evolving need of surgical techniques and newly available anesthetics. This review highlights the focus on the recent development in the anesthetic management and the effects of anesthetic technique on DBS. Because of recent evidences have substantially changed the approach to anesthesia for DBS, here we present a systematic literature review of the last 20 years.
A thorough PubMed and Medline literature search was conducted for investigating studies on patients with Parkinson's disease by using the keywords “deep brain stimulation”, “microelectrode recordings”, “macro-stimulation test”, “monitored anesthesia care”, “conscious sedation”, and “general anesthesia”. Complete manuscripts were studied and only those that reported on original studies with human subjects or preclinical studies and were published between 1991 and 2013 were included.
Deep Brain Stimulation: Surgical Technique
DBS surgery is best performed by an experienced surgeon with specific expertise in stereotactic and functional neurosurgery with the help of a professional team consisting of neurologist, neuropsychologist, neuropsychiatrist, and neurophysiologist. DBS surgery involves 2 procedures:
Placement of the electrode(s) into the specified area of the brain, and
The internalization of the lead(s) and implantation of the programmable impulse generator.
Techniques for implanting DBS devices are constantly evolving to target nuclei, which are deep and small in size. Few studies have compared the safety and effectiveness of various surgical techniques. The method of localizing the specific target for electrode placement includes the use of frame-based imaging to visualize brain structures and to establish coordinates, electrophysiologic guidance with micro electrode recordings (MER) and macro-stimulation testing of an awake patient. The whole procedure may be completed on the same day or in a 2-staged procedure with the internalization of the electrode(s) and generator on a different day, typically 3 days to 2 weeks after the procedure, depending on medical center preference. Favoring the best timing of the second stage has not yet been established. The timing depends on many reasons including the duration of the procedure and patient cooperation. Another factor to delay the internalization is the “microlesion” effect caused by edema around the newly implanted electrode. This may cause improvement of the patient's symptoms without any stimulation, and this impairs the ability to check for stimulation-induced benefits.[9] Postoperative brain imaging in the form of computed tomography or magnetic resonance imaging (MRI) is done by most neurosurgeons to check the position of the placed DBS leads and to rule out for the hemorrhage and pneumocephalus.
The surgery starts with the placement of a rigid head frame to the patient's skull and MRI to visualize brain structures and establish references to external coordinates for accurate insertion of the electrode into the specific areas for stimulation. Though different head frames are preferred, in a survey of North American centers performing DBS surgery, the Cosman-Roberts-Wells frame was commonly used, followed by Leksell G.[10] None of these frames gives easy access to the patient's airway. There are also reports of the use of frameless navigation systems for a DBS.[11] After imaging, the patient is transferred to the operating room where he or she is positioned in a supine or semi-sitting position on the operating room table with the stereotactic frame fixed to the bed. A burr hole is made in the cranium for electrode insertion. To localize the target area for stimulation, the neurophysiologist will obtain MERs to detect and amplify the activity of individual neuron cells. The electrode is usually inserted 10-15 mm above the target site and is advanced 0.5-1 mm along its trajectory toward the target nuclei while spontaneous neuronal discharges are recorded. The neurophysiologists use the variations in spontaneous firing rates between specific nuclei (GPi internal and external) and movement-related changes in the firing rates to localize the specific brain target. Macrostimulation involves the clinical testing of the patient's movements, which is used to verify that the stimulation of the electrode at that location will improve the symptoms without causing any side effects. After radiologic confirmation, the electrode is secured and the wound is closed. In bilateral DBS insertions, a second incision is made on the other side and the procedure repeated. The internalization is performed by tunneling the electrode(s) and connecting the extension cable through the scalp and subcutaneously on the side of the neck to an infraclavicular area or abdomen and then connected to the impulse generator.
Anesthetic Considerations
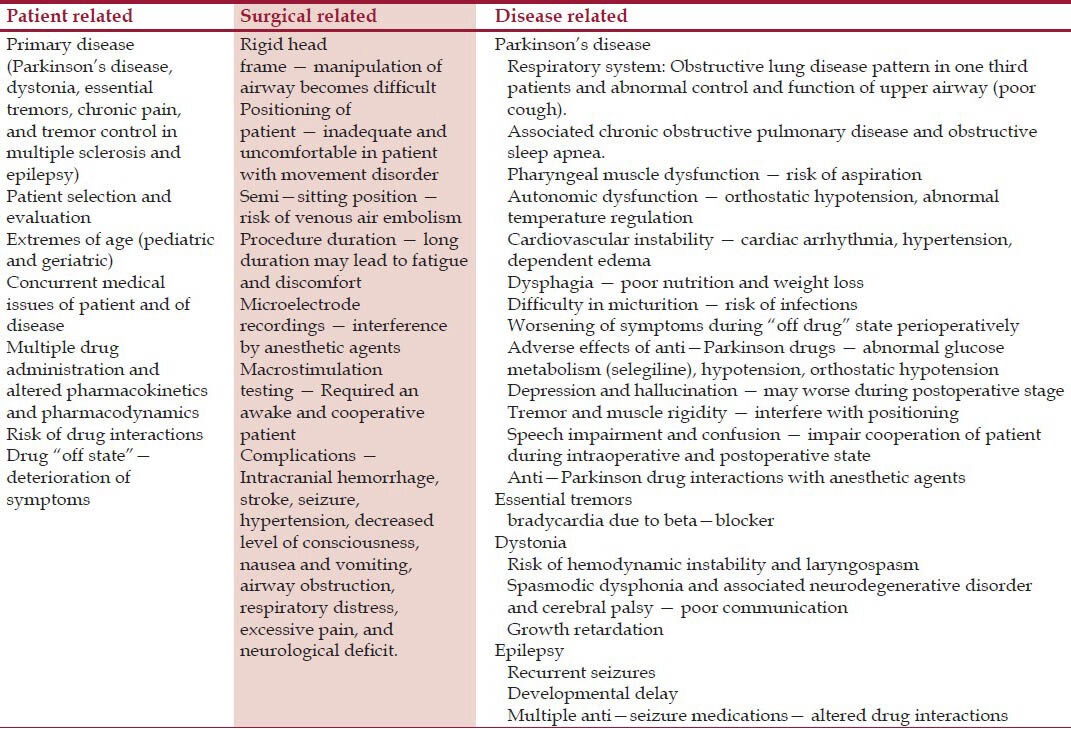
Anesthetic management of patients for DBS placement has specific considerations related to patient and surgical procedure. The anesthesiology team should see these patients before surgery to assess for additional considerations due to associations of much co-morbidity related to the disease processes for which DBS is inserted[12,13,14,15,16,17,18] [Table 1].
Table 1.
Anesthetic considerations of patient undergoing DBS
Anesthetic drug effects on MERs
The anesthesiologists’ role in deep brain electrode insertion is to provide adequate operating conditions and patient comfort, facilitate intraoperative monitoring including neuro-monitoring for target localization, and diagnosing and treating any complications occurring during surgery.
To what extent the anesthetic drugs influence MER is still under debate because the effects of anesthetic drugs are inhomogeneous across different parts of the brain[19] [Table 2]. Propofol is the most commonly used anesthetic drug for sedation and general anesthesia during DBS insertion. Successful MERs have been made from different target sites (GPi, STN, and Vim) using propofol.[5,20] With the use of propofol, different characteristics of neuronal activity among individual target sites and also within the same target site have been seen. Hutchison[20] found that the firing rates in the GPi nucleus were substantially decreased, and long pauses were present in patients with dystonia under general anesthesia with propofol compared with patients mapped under local anesthesia. This was consistent with the finding in animal studies that showed enhancement of GABAergic striatal and GPi external afferents to GPi with propofol.[22] The neuronal firing rates in patients with dystonia may not be affected by the either estimated plasma concentration of propofol or the consciousness level of the patient.[23] Probably because of the overall decreased GPi neuronal firing rates in dystonia. Sanghera studied the effects of general anesthesia with desflurane in 11 patients with dystonia and 6 patients with Parkinson's disease. No differences between awake and anesthetized patients with respect to GPi nuclei firing rates for the dystonia group was observed, but there was a significant decrease in GPi nuclei firing rates in the Parkinson group. They concluded that GPi neuronal firing rates differ between the patients with dystonia and Parkinson's disease, but the effect may be more pronounced in Parkinson's diseases due to anesthesia.
Table 2.
Effects of anesthetic agents on MERs
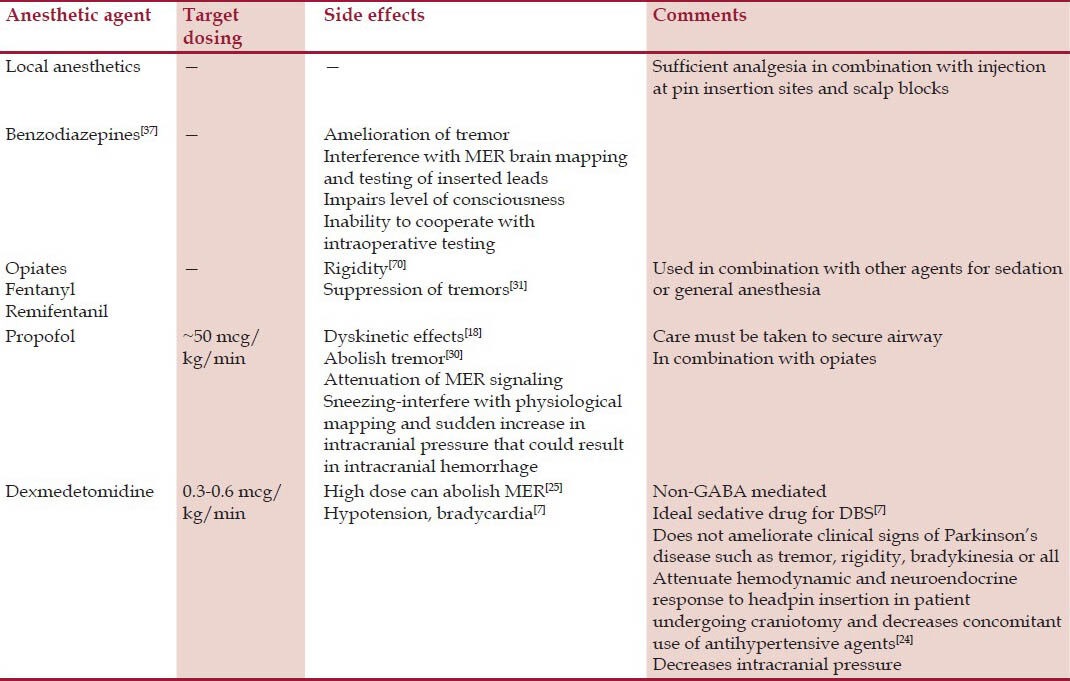
Microelectrode recordings from the STN nuclei during anesthesia have been more successful.[5,24,25,26,27,28] The anesthetic techniques used have varied from monitored anesthesia care with or without sedation, analgesia or both along with conscious sedation with propofol and dexmedetomidine with no airway manipulation to general anesthesia with endotracheal intubation. The anesthetic drug effects on the various target nuclei (STN versus GPi) differ and are explained by the amount of their GABA input. GPi neurons have a higher GABA input compared with STN neurons and are more suppressed by most anesthetic drugs.[29] The effects of anesthetics on the Vim nuclei are not clear.
Anesthetic drugs effect on macrostimulation testing
Clinical stimulation testing is done to observe the clinical benefits and adverse effects of DBS. An awake and cooperative patient is essential. Use of short-acting drugs and stopping them before testing is advantageous for clinical testing. General anesthesia usually interferes with the evaluation of DBS by suppressing the clinical symptoms such as tremors and rigidity.[30,31] Paresthesia or abnormal motor activity associated with stimulation of adjacent structures (internal capsule and medial lemniscus) cannot be assessed in case of general anesthesia. Examination of the optic track with flash visual evoked potentials in patients undergoing stimulation of the GPi nuclei and activation of internal capsule with high intensity stimulus under general anesthesia has been shown to be possible.[32,33] Few medical centers routinely perform DBS implantation under general anesthesia and confirm the location with the help of MRI.[34]
Preoperative evaluation and preparation
Preoperative evaluation starts with careful patient selection, which is a complex process as this determines the successful outcome of DBS surgery. This is the most important step towards better and consistent result for DBS. DBS surgery failures of more than 30% have been seen due to inappropriate indication(s) for surgery.[35] An individual risk-benefit evaluation for each patient must be considered by approaching via a multidisciplinary team, involving a neurosurgeon, a neurologist, a neuropsychologist, an internist, a neuropsychiatrist, and a neurophysiologist. Patients with advanced Parkinson's disease and excellent levodopa (LD) response, younger age, non-LD-responsive motor symptoms, very mild cognitive impairment, and absence of or well-controlled psychiatric disease have shown good outcomes. Application of these rigid criteria may lead to the exclusion of a considerable number of patients with Parkinson's disease.[36]
A thorough airway assessment is required, and options of securing the airway at any stage of the procedure should be planned in advance in patients who are awake for most part of the surgery. A patient with Parkinson's disease may have associated obstructive sleep apnea, and it should be evaluated and managed appropriately. Patients with chronic pain and psychiatric illness have their own specific problems and they need to be managed specially during perioperative stage.
The patient should be assessed for their ability to cooperate during the awake part of the surgery. A full explanation of each step of the procedure is mandatory. Anxiety, preoperatively and during the procedure, may lead to an increase in the arterial blood pressure, causing a risk of intracranial hemorrhage. A careful history of implanted ferrous metals, pacemakers, and aneurysm clips should be taken before MRI. Based on severity of coexisting diseases, a plan for intraoperative positioning and need for invasive monitoring should be determined.
The directions for the continuation or discontinuation of disease-specific drugs should be given after discussion with the neurosurgical team, because some patients have to be in a “drug-off” state to elicit intraoperative mapping and clinical testing. This creates additional concerns to the perioperative care because it may worsen the patient's symptoms, especially in Parkinson's disease and dystonia. In case of severe symptoms, a reduced dose of the regular medication can be administered after discussion with the neurosurgical team. Drugs used for the treatment of motor symptoms should be held in overnight or the morning of surgery. Benzodiazepines, opioids, and other sedatives should be avoided as they interfere with the interpretation of tremor preoperatively and cooperation during surgery in awake condition. In case of patient noncompliance, debilitating medical conditions that preclude safe surgery, dementia, and extensive brain atrophy, DBS surgery is contraindicated.[37]
Anesthetic techniques
In reference to optimal regimen, most medical centers have developed their own techniques to perform the surgery considering neurosurgeons’ need and personal preference. Various techniques have been explained: Local anesthesia either in the form of infiltration at the sites of pin or scalp block with monitored anesthesia care, conscious sedation, and general anesthesia using an asleep-awake-asleep (AAA) technique with or without airway manipulation. Scalp block can be achieved with local anesthetics by blocking the auriculotemporal, zygomaticotemporal, supraorbital, supratrochlear, occipital, and greater occipital nerves. General anesthesia, however, may be required for specific groups of patients who have an increased anxiety for awake surgery, chronic pain syndromes, severe movements due to “off drug” state, severe dystonia or choreoathetosis, and pediatric patients. With adequate patient education, anxiety can be minimized. An awake technique has many advantages and most centers avoid general anesthesia at least during the mapping phase in order to best detect cellular activity and movement-related responses to neurostimulation. Options include monitored anesthetic care with or without sedation, analgesia, or both.[6]
Many airway instrumentation adjuncts have been described for the awake craniotomy, including nasal cannula, facemask, awake fiberoptic endotracheal intubation with local anesthetic infiltration, cuffed oropharyngeal airway, and the laryngeal mask airway. Airway intervention must be required due to limited access to the restricted airway and tunneling of the cable on the side of the neck. Rigid stereotactic frame is normally placed on the patient's head with the use of local anesthesia for the pin sites or scalp block, which is followed by MRI for localization and tabulating the variables for DBS placement. Sometimes patients may require sedation for frame placement or for MRI. If intravenous sedation or general anesthesia has to be given in the radiology room, adequate equipment and support to provide anesthesia care must be present. Airway interventions should precede placement of the rigid stereotactic frame. Conventional laryngoscopy is difficult with stereotactic frame in place and patient positioning. In such cases, the airway should be secured with fiberoptic endotracheal intubation or laryngeal mask airway (LMA). Recently, LMA has become a popular device for craniotomies. LMA is well tolerated at lighter planes of anesthesia, easy to insert and remove, and enables ventilation to be controlled. Increased risk of regurgitation and aspiration should be considered in patients with Parkinson's disease before inserting LMA.
Monitored anesthesia care
In an “awake” technique, an anesthesiologist provides patient comfort and helps eliciting clinical testing. Subcutaneous infiltration with local anesthetic is used at the pin sites and at the site of incision(s) for the bur hole(s) for electrode insertion. Cranial nerve blocks (supraorbital and greater occipital) are an alternative because they have been shown to be less painful than subcutaneous infiltration, although they did not result in any difference in pain at the time of pin placement or during surgery.[38] The local anesthetic drugs frequently used include bupivacaine, ropivacaine, and lidocaine with and without epinephrine.[39] Local anesthetic complications include toxic blood levels resulting in seizures and respiratory and cardiac arrest. If the procedure has been long, additional infiltration may be required for closure.
Standard monitoring includes an electrocardiogram, noninvasive arterial blood pressure, oxygen saturation, and end-tidal CO2. Invasive blood pressure monitoring may be indicated for blood pressure control. Patients with severe movement disorders and spasticity pose difficulty in monitoring. Supplemental oxygen is delivered through nasal prongs or a face mask with an outlet for end-tidal CO2 and respiratory rate monitoring. For awake patients, proper positioning is an important step to ensure maximal comfort and cooperativeness. The head and neck should be positioned with some degree of flexion at the lower cervical spine and extension at the atlanto-occipital joint to make the patient's airway patent. The legs should be flexed and supported under the knees to maintain stability when the head and back are elevated to a sitting position. To aid in positioning, special treatment modalities have been used such as physiotherapy, small doses of levodopa, and intrathecal hydromorphone.[40,41] Adequate pain control, meticulous patient positioning and padding, attention to thermal control, and avoidance of excessive fluid administration to prevent bladder distension are very important. Patients should be encouraged to void before surgery, in case required sheath catheter is a good alternative.
Obstructive sleep apnea patients may require continuous positive airway pressure therapy intra-operatively. The use of clear plastic drapes will make it easy for the anesthesiologist to maintain verbal and eye contact with the patient throughout the case. A good patient communication, reassurance and motivation are all necessary for better outcome in awake patients. In a recent prospective trial of 200 patients, an awake craniotomy with DBS lead placement was well tolerated, with reduced intensive care time and hospital stay.[42]
Conscious sedation
In some medical centers conscious sedation is used for electrode insertion, especially during the opening and closure of the procedure. Propofol is one of the most commonly used drugs. Other drugs include midazolam, opioids such as fentanyl or remifentanil, and dexmedetomidine.[6,7,8,24,25,43,44] The use of benzodiazepines is usually avoided.[45] Propofol is generally used as a target-controlled infusion and typically combined with remifentanil or fentanyl. The mean infusion rates of propofol reported in the literature are approximately 50 mcg/kg/min.[8,41] Propofol provides titratable sedation and a rapid, smooth recovery. In a study of 50 patients, comparing propofol infusion supplemented with remifentanil or fentanyl for conscious sedation, Manninen found that there was no difference in outcomes among the groups, and most patients were completely satisfied.[46] A retrospective analysis of AAA technique using propofol and remifentanil found that adequate conditions were obtained in 98% of patients, with a median wake-up time of 9 min.[47] Fifteen patients were examined for the effects of propofol on MER signals and it was found that adequate sedation was achieved with a mean infusion dose of 50 mg/kg/min. This significantly decreased the spontaneous activity of the STN neurons and therefore interfered with optimal lead placement. However, the effect of propofol was short-lived and neuronal activity returned to baseline in 9.4 ± 4.2 min after propofol administration was terminated.[48] Khatib retrospectively evaluated the perioperative risk for 250 patients undergoing DBS procedure. All patients received local anesthetic before scalp incision. In most cases, propofol was used primarily only during the first 30-45 min of the case to facilitate head-frame placement, with a mean infusion dose of propofol of 67.2 ± 53.2 mg/kg/min.[8] They found total complication rate of 11.6%, and the most common complications were neurologic (3.6%) and psychological/psychiatric complication (3.2%). The limitations of this study were that this was a solitary institutional experience using predominately one anesthetic technique, where more than 90% of DBS procedures were performed under propofol sedation, and only 2.3% of cases were performed without supplemental sedatives or anesthetic drugs.
Low-dose infusion rates (0.3-0.6 mcg/kg/h) of dexmedetomidine may be a better choice due to its non-GABA-mediated mechanism of action allowing for MER, hemodynamic stability, and analgesic properties.[7,24,25] Optimal conditions for MER or stimulation testing can be achieved with the use of conscious sedation as long as short-acting drugs are used and stopped before the recordings and testing. Dexmedetomidine is a central-acting alpha2 agonist that offers sedation and anxiolysis and helps maintain hemodynamic stability.[49] Dexmedetomidine has an anesthetic-sparing effect and preserves respiration with minimal respiratory depression, even with infusions at the higher end of the dose range.[50,51] Patients can also be awakened easily by verbal stimulation after administration of dexmedetomidine. The cerebral effects are consistent with a desirable neurophysiologic profile, including neuroprotective characteristics.[52] Dexmedetomidine is particularly valuable when eloquent areas (those involved in communication) of the brain are stimulated and an awake, cooperative patient is required for neurocognitive testing. Dexmedetomidine can be used as a sole agent, an adjunct, or a rescue drug for the awake craniotomy.[24,53] A retrospective study found that dexmedetomidine provided patient comfort, did not interfere with electrophysiological mapping, and provided hemodynamic stability, thereby significantly reducing the use of antihypertensive medication, although supplemental antihypertensives were given.[24] A retrospective chart review of 28 pediatric cases of patients who underwent DBS implantation for dystonia using combinations of dexmedetomidine- and propofol-based anesthesia found that the patients tolerated the procedure well and appeared comfortable.[54] The anesthesia was performed with a high degree of safety and minimal side effects or intraoperative complications were noted.
Recently, Sassi conducted a study on 23 randomized patients for DBS surgery under dexmedetomidine.[55] He found that there were no hemodynamic instability, respiratory depression, and other side effects. They concluded that dexmedetomidine should be considered as a valuable option for sedation in poorly collaborating patients undergoing DBS surgery.
The depth of anesthesia is usually monitored to titrate sedation and the states of arousal during DBS insertion have shown conflicting outcomes. The accuracy of bispectral index (BIS) monitoring during MER is questionable because the effects of anesthetics are heterogeneous across the different parts of the brain, and there is dissociation between the neocortical and subcortical effects of IV and inhaled drugs.[19] Schulz found that the use of BIS did not offer any advantages regarding the time to arousal, total propofol consumption, and cardiopulmonary stability.[56] They have not studied about effects on MER. Elias showed a positive result for the use of BIS monitoring for titrating dexmedetomidine sedation.[25] They found that the subthalamic MER signals were equivalent to the awake state when sedation was titrated to an easily arousable state (BIS value >80) in patients with Parkinson's disease. However, deep sedation (BIS <80) suppressed MER signals.
General anesthesia
General anesthesia for DBS surgery may be a good alternative in patients who cannot tolerate local anesthesia or monitored anesthetic care due to excessive fear, anxiety, reduced cooperation, or severe “off-medication” effects. General anesthesia may provide a higher level of acceptance for DBS surgery by some patients. Intraoperative mapping and stimulation testing will be difficult under general anesthesia. There has been a hesitation for general anesthesia, as optimal targeting of the STN with intraoperative MERs is usually performed under local anesthesia.[5] Experience in intraoperative micro-recording of STN under GA is sparse, and neuronal firing patterns are not well characterized.[27] Yamada[26] in a case control study of 25 patients compared a nitrous-sevoflurane-opioid technique versus local anesthesia and found that general anesthesia in patients with Parkinson's disease did not adversely affect postoperative improvements in motor and daily activity scores, except for “off-medication” bradykinesia. A year later, Lin[28] found that desflurane anesthesia allowed adequate MERs for successful DBS insertion. Braun[57] retrospectively studied the effect of general anesthesia during electrode placement on the clinical outcome of DBS of the subthalamic nucleus (DBS-STN). They analyzed 47 consecutive patients (grouped in 5) who underwent DBS-STN and found that there were no significant differences between the 5 groups of patients in respect to improvement of Parkinsonian symptoms.
Recently, Lettieri[58] evaluated changes in subthalamic nucleus’ neuronal activity in Parkinson's disease patients during DBS surgery under general anesthesia and compared this data with those recorded in the same subjects during previous surgery under local anesthesia. They used ketamine and remifentanil bolus for induction and infusion for maintenance. There was no statistically significant difference between the first and second surgical procedures in any of the neurophysiological parameters analyzed. The authors also concluded that bilateral STN-DBS for advanced Parkinson's disease with MER guidance is possible and reliable under a ketamine-based anesthetic protocol. Thus, DBS insertion under general anesthesia is possible with careful titration of anesthetics and with the use of limited electro-physiologic mapping. Randomized controlled studies are needed to compare the long-term clinical benefits of patients undergoing DBS insertion under general anesthesia with that of local anesthesia.
Perioperative complications
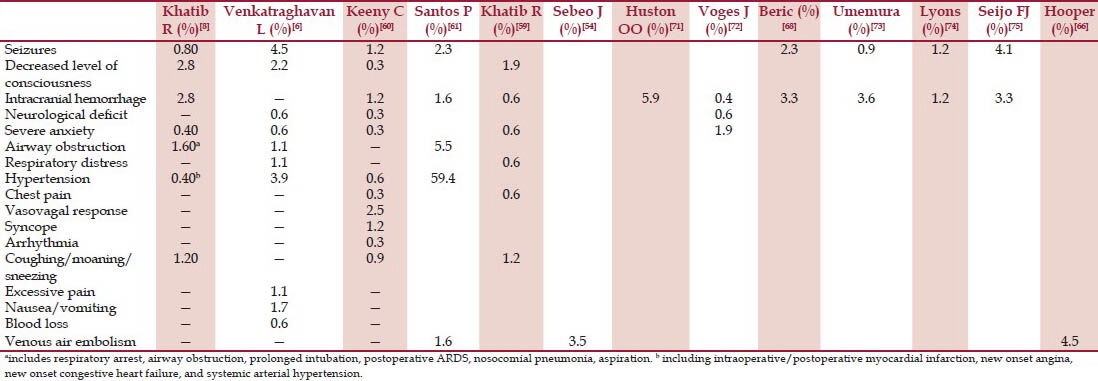
The placement of DBS is potential for adverse outcomes during perioperative period [Table 3]. The DBS surgery merits utmost vigilance and rapid recognition and treatment of the complications. An intraoperative incidence of adverse events of 6.96% was seen in a series of 158 cases reviewed by Khatib for DBS surgery under sedation with propofol or dexmedetomidine.[59] These were coughing, sneezing, aspiration, pulmonary edema, combative behavior/agitation/confusion, bronchospasm, angina, and intracranial hemorrhage. In another study by Venkatraghvan, the intraoperative adverse complications were noted in 16% cases (out of 172 DBS cases), and the most frequent complications were seizure (4.5%) and hypertension (3.9%).[60] At Baylor College of Medicine Parkinson's Disease Center and Movement Disorders Clinic, Kenny retrospectively reviewed a total of 319 patients who underwent DBS from 1995 to 2005.[60] They found that over all neurological adverse events were 3% and cardiovascular adverse events were 4.9%. Khatib again reviewed 258 electrode insertion procedures under a variety of techniques and observed that the most common neurological complications were intracranial hemorrhage and seizure.[8] Overall, intraprocedure complications were reported to occur in 12-16% of patients.[6,8]
Table 3.
Intraoperative complications of DBS insertion
Airway compromise is an important consideration with the conscious sedation. Intraoperative respiratory complications occur in 1.6-2.2% of patients.[6,8] A stereotactic head frame makes airway access limited. A gradual shift of the body with neck flexion often occurs during surgery and may make talking difficult and even obstruct the airway. In the awake patient, this may be aggravated with sedation or intracranial events such as seizures or hemorrhage leading to a decreased level of consciousness. Appropriate airway equipment should be readily available because managing the airway in a patient with a rigid head frame poses a daunting challenge. If possible, one should secure the airway without the removal of the patient's head frame, so the surgery could be continued. In an emergency situation, securing the airway with LMA may be the most appropriate action.
Other respiratory complications are related to the patient's specific diseases. Patients with Parkinson's disease may have pulmonary dysfunction from poor respiratory muscle function resulting in reduced forced vital capacity and reduced baseline arterial oxygen saturation, upper airway obstruction, dysarthria, and obstructive sleep apnea.[8,12,45,61] Respiratory insufficiency may be caused by associated chronic obstructive pulmonary disease or the absence of anti-Parkinson medications in the postoperative period.
Cardiovascular adverse events can lead to devastating complications. Hypertension is a common intraoperative event and can be related to poor preoperative control, patient distress, or anxiety during the procedure, or can be secondary to other events. Hypertension has been associated with increased risk of intracerebral hemorrhages.[62,63] Arterial blood pressure must be controlled before the insertion of the electrode to prevent intracranial hemorrhages. During procedure, comfortable positioning and reassurance may ease distress. Dexmedetomidine sedation is advantageous.[7] Frequently used drugs include labetalol, hydralazine, nitroglycerine, sodium nitroprusside, and esmolol. The optimal level of blood pressure is not well-defined; one may use a systolic blood pressure of <140 mmHg or a 20% increase of the patient's usual range.[63] Few studies have suggested decreased incidence of intacranial hemorrhages in DBS surgery under general anesthesia.[64] Orthostatic hypotension may result from Parkinson's disease itself or anti-Parkinson medications and might be further aggravated by the vasodilating effects of anesthetics and perioperative hypovolemia. Glossop and Dobbs[65] reported two patients who experienced chest pain, tachycardia, hypertension, and oxygen desaturation during insertion of a DBS under local anesthesia. This was accompanied with ST segment changes and increased troponins, although further testing showed normal coronary arteries. They attributed the symptoms to abnormal vasoactive responses resulting in coronary vasospasm.
Other less cardiovascular complications include venous air embolism.[66] Semi-sitting position in a hypovolemic patient increases the risk. During creation of the bur hole in awake patients, sudden vigorous coughing may be a sign of venous air embolism. Other signs are unexplained hypoxia, tachycardia, tachpnea, chest discomfort, and hypotension. Early detection may be possible with precordial Doppler monitoring. The incidence of venous air embolism as detected by a precordial Doppler ultrasound has been reported to be 4.5%. Hooper et al.[66] in their small study of 21 patients noted 1 venous air embolism (1 of 22 lead insertions), and the important predictors were patient positioning and the occurrence of coughing. Tension pneumocephalus has also been reported during DBS insertion, which occurs due to cerebrospinal fluid leakage from burr holes.[67]
DBS insertion may cause neurologic complications during or after the procedure.[68] Focal deficits such as extremity weakness or confusion may not require any acute treatment by the anesthesiologist. Seizures have been reported to occur in 0.5-4.5% of patients and often occur during stimulation testing.[6,8] Most seizures that occur during procedure are focal and self-limited. Few patients may require small doses of midazolam or propofol. A sudden loss of consciousness resulting from an intracranial bleed or neurologic injury will require rapid and more aggressive treatment.
Themistocleous[69] has also reported occurrence of neurolept malignant syndrome in patient for DBS surgery and he suggested that it occurs due to discontinuation of anti-Parkinson medication.
Conclusion
Ablative procedures can be effective for the symptoms of movement disorders but they may render a permanent lesion in the targeted nuclei and are therefore not reversible. For more than 2 decades, DBS has increasingly substituted ablative procedures such as thalamotomy and pallidotomy. Most medical centers have developed their techniques of performing DBS procedure depending upon neurosurgeons’ need and patients’ status. An option includes monitored anesthesia care with or without sedation, conscious sedation, and general anesthesia. Most of the recent studies recommend use of low dose dexmedetomidine and remifentanil along with scalp block for adequate sedation and analgesia with least interference to MERs, early recovery, and less perioperative complications. However, general anesthesia may be required for specific groups of patients such as increased anxiety for awake surgery, chronic pain syndromes, severe movements due to “off drug” state, severe dystonia, or choreoathetosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–6. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 2.Pereira EA, Green AL, Nandi D, Aziz TZ. Deep brain stimulation: Indications and evidence. Expert Rev Med Devices. 2007;4:591–603. doi: 10.1586/17434440.4.5.591. [DOI] [PubMed] [Google Scholar]
- 3.Halpern C, Hurtig H, Jaggi J, Grossman M, Won M, Baltuch G. Deep brain stimulation in neurologic disorders. Parkinsonism Relat Disord. 2007;13:1–16. doi: 10.1016/j.parkreldis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Ollinet C, Bedague D, Carcey J, Oddoux ME, Payen JF. Functional surgery for movement disorders: Implications for anesthesia. Ann Fr Anesth Reanim. 2004;23:428–32. doi: 10.1016/j.annfar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 5.Maltête D, Navarro S, Welter ML, Roche S, Bonnet AM, Houeto JL, et al. Subthalamic stimulation in Parkinson disease: With or without anesthesia? Arch Neurol. 2004;61:390–2. doi: 10.1001/archneur.61.3.390. [DOI] [PubMed] [Google Scholar]
- 6.Venkatraghavan L, Manninen P, Mak P, Lukitto K, Hodaie M, Lozano A. Anesthesia for functional neurosurgery: Review of complications. J Neurosurg Anesthesiol. 2006;18:64–7. doi: 10.1097/01.ana.0000181285.71597.e8. [DOI] [PubMed] [Google Scholar]
- 7.Rozet I. Anesthesia for functional neurosurgery: The role of dexmedetomidine. Curr Opin Anaesthesiol. 2008;21:537–43. doi: 10.1097/ACO.0b013e32830edafd. [DOI] [PubMed] [Google Scholar]
- 8.Khatib R, Ebrahim Z, Rezai A, Cata JP, Boulis NM, John Doyle D, et al. Perioperative events during deep brain stimulation: The experience at Cleveland clinic. J Neurosurg Anesthesiol. 2008;20:36–40. doi: 10.1097/ANA.0b013e318157a15a. [DOI] [PubMed] [Google Scholar]
- 9.Rezai AR, Kopell BH, Gross RE, Vitek JL, Sharan AD, Limousin P, et al. Deep brain stimulation for Parkinson's disease: Surgical issues. Mov Disord. 2006;21:S197–218. doi: 10.1002/mds.20956. [DOI] [PubMed] [Google Scholar]
- 10.Ondo WG, Bronte-Stewart H DBS Study Group. The North American survey of placement and adjustment strategies for deep brain stimulation. Stereotact Funct Neurosurg. 2005;83:142–7. doi: 10.1159/000088654. [DOI] [PubMed] [Google Scholar]
- 11.Gumprecht HK, Widenka DC, Lumenta CB. Brainlab VectorVision Neuronavigation System: Technology and clinical experiences in 131 cases. Neurosurgery. 1999;44:97–105. doi: 10.1097/00006123-199901000-00056. [DOI] [PubMed] [Google Scholar]
- 12.Mason LJ, Cojocaru TT, Cole DJ. Surgical intervention and anesthetic management of the patient with Parkinson's disease. Int Anesthesiol Clin. 1996;34:133–50. doi: 10.1097/00004311-199603440-00010. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson G, Pereira AC, Hall GM. Parkinson's disease and anesthesia. Br J Anaesth. 2002;89:904–16. doi: 10.1093/bja/aef268. [DOI] [PubMed] [Google Scholar]
- 14.Fikkers BF, Zandstra DF. Primary Laryngospasm in a patient with Parkinson's disease: Ttreatment with CPAP via a minitracheostomy following extubation. Intensive Care Med. 1995;21:863–4. doi: 10.1007/BF01700975. [DOI] [PubMed] [Google Scholar]
- 15.Frost EA, Osborn I. Deep brain stimulation—surgery for movement disorders and Parkinson's disease. Int Anesthesiol Clin. 2009;47:57–68. doi: 10.1097/AIA.0b013e31819342e9. [DOI] [PubMed] [Google Scholar]
- 16.Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonist for Parkinson's disease. N Engl J Med. 2007;356:39–46. doi: 10.1056/NEJMoa054830. [DOI] [PubMed] [Google Scholar]
- 17.Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth. 2005;95:434–41. doi: 10.1093/bja/aei210. [DOI] [PubMed] [Google Scholar]
- 18.Krauss JK, Akeyson EW, Giam P, Jankovic J. Propofol-induced dyskinesias in Parkinson's disease. Anesth Analg. 1996;83:420–2. doi: 10.1097/00000539-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 19.Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, Regis JM, et al. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–12. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- 20.Hutchison WD, Lang AE, Dostrovsky JO, Lozano AM. Pallidal neuronal activity: Implications for models of dystonia. Ann Neurol. 2003;53:480–8. doi: 10.1002/ana.10474. [DOI] [PubMed] [Google Scholar]
- 21.Peduto VA, Concas A, Santoro G, Biggio G, Gessa GL. Biochemical and electrophysiologic evidence that propofol enhances GABAergic transmission in the rat brain. Anesthesiology. 1991;75:1000–9. doi: 10.1097/00000542-199112000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Steigerwald F, Hinz L, Pinsker MO, Herzog J, Stiller RU, Kopper F, et al. Effects of propofol anesthesia on pallidal neuronal discharges in generalized dystonia. Neurosci Lett. 2005;386:156–9. doi: 10.1016/j.neulet.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Sanghera MK, Grossman RG, Kalhorn CG, Hamilton WJ, Ondo WG, Jankovic J. Basal ganglia neuronal discharge in primary and secondary dystonia in patients undergoing pallidotomy. Neurosurgery. 2003;52:1358–73. doi: 10.1227/01.neu.0000064805.91249.f5. [DOI] [PubMed] [Google Scholar]
- 24.Rozet I, Muangman S, Vavilala MS, Lee LA, Souter MJ, Domino KJ, et al. Clinical experience with dexmedetomidine for implantation of deep brain stimulators in Parkinson's disease. Anesth Analg. 2006;103:1224–8. doi: 10.1213/01.ane.0000239331.53085.94. [DOI] [PubMed] [Google Scholar]
- 25.Elias WJ, Durieux ME, Huss D, Frysinger RC. Dexmedetomidine and arousal affect of subthalamic neurons. Mov Disord. 2008;23:1317–20. doi: 10.1002/mds.22080. [DOI] [PubMed] [Google Scholar]
- 26.Yamada K, Goto S, Kuratsu J, Matsuzaki K, Tamura T, Nagahiro S, et al. Stereotactic surgery for subthalamic nucleus stimulation under general anesthesia: A retrospective evaluation of Japanese patients with Parkinson's disease. Parkinsonism RelatDisord. 2007;13:101–7. doi: 10.1016/j.parkreldis.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Hertel F, Züchner M, Weimar I, Gemmar P, Noll B, Bettag M, et al. Implantation of electrodes for deep brain stimulation of the subthalamic nucleus in advanced Parkinson's disease with the aid of intraoperative microrecording under general anesthesia. Neurosurgery. 2006;59:1138–45. doi: 10.1227/01.NEU.0000245603.77075.55. [DOI] [PubMed] [Google Scholar]
- 28.Lin SH, Chen TY, Lin SZ, Shyr MH, Chou YC, Hsieh WA, et al. Subthalamic deep brain stimulation after anesthetic inhalation in Parkinson disease: A preliminary study. J Neurosurg. 2008;109:238–44. doi: 10.3171/JNS/2008/109/8/0238. [DOI] [PubMed] [Google Scholar]
- 29.Benarroch EE. Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology. 2008;70:1991–5. doi: 10.1212/01.wnl.0000313022.39329.65. [DOI] [PubMed] [Google Scholar]
- 30.Anderson BJ, Marks PV, Futter ME. Propofol-contrasting effects in movement disorders. Br J Neurosurg. 1994;8:387–8. doi: 10.3109/02688699409029633. [DOI] [PubMed] [Google Scholar]
- 31.Bohmdorfer W, Schwarzinger P, Binder S, Sporn P. Temporary suppression of tremor by remifentanil in a patient with Parkinson's disease during cataract extraction under local anesthesia. Anaesthesist. 2003;52:795–7. doi: 10.1007/s00101-003-0522-y. [DOI] [PubMed] [Google Scholar]
- 32.Lozano AM, Kumar R, Gross RE, Giladi N, Hutchison WD, Dostrovsky JO, et al. Globus pallidus internus pallidotomy for generalized dystonia. Mov Disord. 1997;12:865–70. doi: 10.1002/mds.870120606. [DOI] [PubMed] [Google Scholar]
- 33.Krause M, Fogel W, Kloss M, Rasche D, Volkmann J, Tronnier V. Pallidal stimulation for dystonia. Neurosurgery. 2004;55:1361–8. doi: 10.1227/01.neu.0000143331.86101.5e. [DOI] [PubMed] [Google Scholar]
- 34.Coubes P, Vayssiere N, El Fertit H, Hemm S, Cif L, Kienlen J, et al. Deep brain stimulation for dystonia. Surgical technique. Stereotact Funct Neurosurg. 2002;78:183–91. doi: 10.1159/000068962. [DOI] [PubMed] [Google Scholar]
- 35.Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, et al. Management of referred deep brain stimulation failures: A retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005;62:1250–5. doi: 10.1001/archneur.62.8.noc40425. [DOI] [PubMed] [Google Scholar]
- 36.Morgante L, Morgante F, Moro E, Epifanio A, Girlanda P, Ragonese P, et al. How many parkinsonian patients are suitable candidates for deep brain stimulation of subthalamic nucleus? Results of a questionnaire. Parkinsonism Relat Disord. 2007;13:528–31. doi: 10.1016/j.parkreldis.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Deiner S, Hagen J. Parkinson's disease and deep brain stimulator placement. Anesthesiology Clin. 2009;27:391–415. doi: 10.1016/j.anclin.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Watson R, Leslie K. Nerve blocks versus subcutaneous infiltration for stereotactic frame placement. Anesth Analg. 2001;92:424–7. doi: 10.1097/00000539-200102000-00028. [DOI] [PubMed] [Google Scholar]
- 39.Heavner JE. Local anesthetics. Curr Opin Anaesthesiol. 2007;20:336–42. doi: 10.1097/ACO.0b013e3281c10a08. [DOI] [PubMed] [Google Scholar]
- 40.Chevrier E, Fraix V, Krack P, Chabardes S, Benabid AL, Pollak P. Is there a role for physiotherapy during deep brain stimulation surgery in patients with Parkinson's disease? Eur J Neurol. 2006;13:496–8. doi: 10.1111/j.1468-1331.2006.01298.x. [DOI] [PubMed] [Google Scholar]
- 41.Lotto M, Boulis N. Intrathecal opioids for control of chronic low back pain during deep brain stimulation procedures. Anesth Analg. 2007;105:1410–2. doi: 10.1213/01.ane.0000286169.02429.11. [DOI] [PubMed] [Google Scholar]
- 42.Taylor MD, Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: A prospective trial of 200 cases. J Neurosurg. 1999;90:35–41. doi: 10.3171/jns.1999.90.1.0035. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda M, Kameyama S, Noguchi R, Tanaka R. Intraoperative monitoring for functional neurosurgery during intravenous anesthesia with propofol. No Shinkei Geka. 1997;25:231–7. [PubMed] [Google Scholar]
- 44.Murata J, Sawamura Y, Kitagawa M, Saito H, Kikuchi S, Tashiro K. Minimally invasive stereotactic functional surgery using an intravenous anesthetic propofol and applying image Fusion and Atlas Plan. No To Skinkei. 2001;53:457–62. [PubMed] [Google Scholar]
- 45.Davies A. Midazolam induced dyskinesias. Palliat Med. 2000;14:435–6. doi: 10.1177/026921630001400513. [DOI] [PubMed] [Google Scholar]
- 46.Manninen PH, Balki M, Lukitto K, Bernstein M. Patient satisfaction with awake craniotomy for tumor surgery: A comparison of remifentanil and fentanyl in conjunction with propofol. Anesth Analg. 2006;102:237–42. doi: 10.1213/01.ANE.0000181287.86811.5C. [DOI] [PubMed] [Google Scholar]
- 47.Keifer JC, Dentchev D, Little K, Warner DS, Friedman AH, Borel CO. A retrospective analysis of a remifentanil/propofol general anesthetic for craniotomy before awake functional brain mapping. Anesth Analg. 2005;101:502–8. doi: 10.1213/01.ANE.0000160533.51420.44. [DOI] [PubMed] [Google Scholar]
- 48.Raz A, Eimerl D, Bergman H. Propofol induced changes in the neuronal activity of sub-thalamic nucleus neurons. Anesthesiology. 2008;109:A838. [Google Scholar]
- 49.Bekker A, Sturaitis MK. Dexmedetomidine for neurological surgery. Neurosurgery. 2005;57:1–10. doi: 10.1227/01.neu.0000163476.42034.a1. [DOI] [PubMed] [Google Scholar]
- 50.Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, et al. Dexmedetomidine pharmacodynamics: Part I: Crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–76. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Bustillo MA, Lazar RM, Finck AD, Fitzsimmons B, Berman MF, Pile-Spellman J, et al. Dexmedetomidine may impair cognitive testing during endovascular embolization of cerebral arteriovenous malformations: A retrospective case report series. J Neurosurg Anesthesiol. 2002;14:209–12. doi: 10.1097/01.ANA.0000017492.93942.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuhmonen J, Haapalinna A, Sivenius J. Effects of dexmedetomidine after transient and permanent occlusion of the middle cerebral artery in the rat. J Neural Transm. 2001;108:261–71. doi: 10.1007/s007020170071. [DOI] [PubMed] [Google Scholar]
- 53.Moore TA, 2nd, Markert JM, Knowlton RC. Dexmedetomidine as rescue drug during awakecraniotomy for cortical mapping and tumor resection. Anesth Analg. 2006;102:1556–8. doi: 10.1213/01.ane.0000200286.15825.6c. [DOI] [PubMed] [Google Scholar]
- 54.Sebeo J, Deiner SG, Alterman RL, Osborn IP. Anesthesia for pediatric deep brain stimulation. Anesthesiol Res Pract 2010. 2010:401419. doi: 10.1155/2010/401419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sassi M, Zekaj E, Grotta A, Pollini A, Pellanda A, Borroni M, et al. Safety in the use of dexmedetomidine (Precedex) for deep brain stimulation surgery: Our experience in 23 randomized patients. Neuromodulation. 2013;16:401–6. doi: 10.1111/j.1525-1403.2012.00483.x. [DOI] [PubMed] [Google Scholar]
- 56.Schulz U, Keh D, Barner C, Kaiser U, Boemke W. Bispectral index monitoring does not improve anesthesia performance in patients with movement disorders undergoing deep brain stimulating electrode implantation. Anesth Analg. 2007;104:1481–7. doi: 10.1213/01.ane.0000261516.45687.ee. [DOI] [PubMed] [Google Scholar]
- 57.Braun M, Winkler D, Wehner M, Busch T, Schwarz J. Deep brain stimulation and general anesthesia. Basal Ganglia. 2011;1:79–82. [Google Scholar]
- 58.Lettieri C, Rinaldo S, Devigili G, Pauletto G, Verriello L, Budai R, et al. Deep brain stimulation: Subthalamic nucleus electrophysiological activity in awake and anesthetized patients. Clin Neurophysiol. 2012;123:2406–13. doi: 10.1016/j.clinph.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 59.Khatib R, Ebrahim Z, Rezai A. Anesthetic complications during deep brain stimulation. Anesthesiology. 2004;101:A379. [Google Scholar]
- 60.Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A, et al. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg. 2007;106:621–5. doi: 10.3171/jns.2007.106.4.621. [DOI] [PubMed] [Google Scholar]
- 61.Santos P, Valero R, Arguis MJ, Carrero E, Salvador L, Rumia J, et al. Preoperative adverse events during stereotactic microelectrode-guided deep brain surgery in Parkinson's disease. Rev Esp Anestesiol Reanim. 2004;51:523–30. [PubMed] [Google Scholar]
- 62.Binder DK, Rau GM, Starr PA. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery. 2005;56:722–32. doi: 10.1227/01.neu.0000156473.57196.7e. [DOI] [PubMed] [Google Scholar]
- 63.Gorgulho A, De Salles AA, Frighetto L, Behnke E. Incidence of hemorrhage associated with electrophysiological studies performed using macroelectrodes and microelectrodes in functional neurosurgery. J Neurosurg. 2005;102:888–96. doi: 10.3171/jns.2005.102.5.0888. [DOI] [PubMed] [Google Scholar]
- 64.Maldonado IL, Roujeau T, Cif L, Gonzalez V, El-Fertit H, Vasques X, et al. Magnetic resonance-based deep brain stimulation technique: A series of 478 consecutive implanted electrodes with no perioperative intracerebral hemorrhage. Neurosurgery. 2009;65:196–201. doi: 10.1227/01.NEU.0000342404.14347.FB. [DOI] [PubMed] [Google Scholar]
- 65.Glossop A, Dobbs P. Coronary artery vasospasm during awake deep brain stimulation surgery. Br J Anaesth. 2008;101:222–4. doi: 10.1093/bja/aen149. [DOI] [PubMed] [Google Scholar]
- 66.Hooper AK, Okun MS, Foote KD, Haq IU, Fernandez HH, Hegland D, et al. Venous air embolism in deep brain stimulation. Stereotact Funct Neurosurg. 2008;87:25–30. doi: 10.1159/000177625. [DOI] [PubMed] [Google Scholar]
- 67.Jain V, Prabhakar H, Rath GP, Sharma D. Tension pneumocephalus following deep brain stimulation surgery with bispectral index monitoring. Eur J Anaesthesiol. 2007;24:203–4. doi: 10.1017/S0265021506001736. [DOI] [PubMed] [Google Scholar]
- 68.Beric A, Kelly PJ, Rezai A, Sterio D, Mogilner A, Zonenshayn M, et al. Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg. 2001;77:73–8. doi: 10.1159/000064600. [DOI] [PubMed] [Google Scholar]
- 69.Themistocleous MS, Boviatsis EJ, Stavrinou LC, Stathis P, Sakas DE. Malignant neuroleptic syndrome following deep brain stimulation surgery: A case report. J Med Case Rep. 2011;5:255. doi: 10.1186/1752-1947-5-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klausner JM, Caspi J, Lelcuk S, Khazam A, Marin G, Hechtman HB, et al. Delayed muscular rigidity and respiratory depression following fentanyl anesthesia. Arch Surg. 1988;123:66–7. doi: 10.1001/archsurg.1988.01400250076013. [DOI] [PubMed] [Google Scholar]
- 71.Huston OO, Watson RE, Bernstein MA, McGee KP, Stead SM, Gorman DA, et al. Intraoperative magnetic resonance imaging findings during deep brain stimulation surgery. J Neurosurg. 2011;115:852–7. doi: 10.3171/2011.5.JNS101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voges J, Waerzeggers Y, Maarouf M, Lehrke R, Koulousakis A, Lenartz D, et al. Deep-brain stimulation: Long-term analysis of complications caused by hardware and surgery — experiences from a single centre. J Neurol Neurosurg Psychiatry. 2006;77:868–72. doi: 10.1136/jnnp.2005.081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB, et al. Deep brain stimulation for movement disorders: Morbidity and mortality in 109 patients. J Neurosurg. 2003;98:779–84. doi: 10.3171/jns.2003.98.4.0779. [DOI] [PubMed] [Google Scholar]
- 74.Lyons KE, Wilkinson SB, Overman J, Pahwa R. Surgical and hardware related complications of subthalamic stimulation: A series of 160 procedures. Neurology. 2004;63:612–6. doi: 10.1212/01.wnl.0000134650.91974.1a. [DOI] [PubMed] [Google Scholar]
- 75.Seijo FJ, Alvarez-Vega MA, Gutierrez JC, Fdez-Glez F, Lozano B. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson's disease. Review of 272 procedures. Acta Neurochir (Wien) 2007;149:867–75. doi: 10.1007/s00701-007-1267-1. [DOI] [PubMed] [Google Scholar]


