Abstract
Background:
Obese subjects are at risk of multiple comorbidities including stroke and coronary heart disease (CHD), which is partly due to disturbances in the hemostatic system.
Aims:
The aims of the present study were to determine the effects of a weight-loss program on fibrinogen and fibrinolytic markers.
Materials and Methods:
Twenty-eight obese subjects were involved in a weight-loss program consisted of exercise and nutritional education for 12-weeks duration. Physical parameters were documented and blood specimen was tested at pre and post-intervention for fibrinogen, tissue plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1), and thrombin activatable fibrinolysis inhibitor (TAFI). Paired t-test was used for statistical analysis.
Results:
There was a significant decline in the levels of t-PA, PAI-I, TAFI and fibrinogen following the weight-loss program (P < 0.01 for each). A significant positive correlation between tPA levels and body weight, body mass index (BMI), waist circumference, and fat-free mass were found. There was also a significant correlation betwen BMI and other blood parameters.
Conclusion:
Reduced fibrinogen, fibrinolytic, and physical parameters were demonstrated in obese subjects following the weight reduction program. These findings suggest the possible beneficial effects of this program on the hemostatic burden particularly on the fibrinolytic biomarkers.
Keywords: Exercise, Fibrinogen, Obese, Plasminogen activator, Plasminogen activator inhibitor-1, Thrombin activatable fibrinolysis inhibitor
Introduction
Obesity is one of the most serious public health problems and a leading preventable cause of death worldwide.[1] Various studies have suggested that obesity per se is an independent cardiovascular risk factor, as well as predisposing to type 2 diabetes, hypertension, and dyslipidemia.[2,3,4]
Obese subjects are at risk to develop coronary heart diseases (CHD) and this is partly due to disturbances in the hemostatics and fibrinolytic system. Indeed, obese subjects tend to have higher values of fibrinogen, factor VII, factor VIII, and plasminogen activator inhibitor -1 (PAI-1) compared to non-obese subjects.[5,6] CHD is a multifactorial disease and its development involves formation of atheromatous plaques progressing over the lifetime of any individual. The acute phase of CHD consists of rupture of the plaque and formation of an acute thrombus.[7] The fibrinolytic system is responsible for the degradation of the solid phase fibrin network which constitutes the major protein component of the thrombus. The system is involved in the dissolution of blood clots and thus ensures an unobstructed circulation. Impaired fibrinolysis in obesity is associated with higher plasminogen activator inhibitor (PAI-1) and plasminogen activator (t-PA -PA) levels (in-complex) and closely linked to the development of CHD and coronary events.[8]
There is an association between body mass index, waist to hip ratio with hemostatic factors and impaired fibrinolysis, which suggests that obesity is a risk factor whose effect is mediated in part by a prothrombotic state.[6] These factors include over-expression of vascular endothelial PAI-1, down-regulation of fibrinolysis due to disturbed thrombin activatable fibrinolytic inhibitor (TAFI), elevation of plasma coagulation proteins (factor VII, factor X, fibrinogen), and enhanced platelet activation.[9]
Exercise and dietary modification for promoting weight loss in obese individuals are expected to reduce the risk of developing CHD.[10] Most of the studies which have looked at the effects of exercise and weight-loss program used biochemical markers or physical parameters to assess the risk of cardiovascular disease in obesity. It was suggested that effective interventions should be considered in improving the obesity-associated thrombotic risk profile. There are sparse data describing the effects of fibrinolytic system which contribute to the prothrombotic state following weight reduction in obese subjects.[11] Developing effective physical activity and lifestyle modification are the strategies to decrease obesity and is expected to be beneficial in limiting obesity-associated long-term health and societal impact.
The aims of the present study were to determine the effects of a weight-loss program at Obesity Clinic, Hospital Universiti Sains Malaysia on fibrinogen and fibrinolytic markers and to determine the correlation of these parameters with the physical predictors associated with CHD. The present study looked at the correlation of the fibrinogen and fibrinolytic markers with physical parameters which are known predictors associated with cardiovascular diseases. Improvement of the physical predictors are expected to improve the level of harmful fibrinolytic markers and fibrinogen suggesting the beneficial effecs of weight loss on cardiovascular system.
Materials and Methods
Subjects
This was an interventional study conducted at Obesity Clinic, Hospital Universiti Sains Malaysia (HUSM), Kubang Kerian, Kelantan. The obese subjects in this study voluntarily registered at Obesity Clinic to enter the weight loss program which consisted of dietary and exercise interventions. Subjects were given verbal explanation regarding the study and all subjects had given a written consent before enrolling into the study.
A total of 28 obese subjects successfully completed this study. Subjects who were included in this study had the following criteria: Adult (above 21-years old), body mass index ≥30 kg/m2, fit to participate or join the program after endorsed by physicians and for females, they should not be pregnant throughout the study period. Subjects who were on weight-loss supplements, regularly engaged in physical activities or taking drugs such as warfarin and antithrombotic drugs or having bleeding and thrombophilic disorders were excluded from this study. In this study, the medical co-morbidities were not counted in the inclusion and exclusion criteria and all the participants included were medically fit subjects. Two of the subjects were confirmed diabetic but they were included in the study as their blood sugar was well controlled with diabetic diet.
Weight-loss program
The research design had been approved by Research and Ethics Committee of University Sains Malaysia. The study was carried out between 2009 and 2012.
Once enrolled in the program, subjects underwent complete medical and physical examinations to ensure that they were fit to go through the exercise intervention period. A diary was given to each subject and they were asked to self monitor and record their daily food intake and activities. About 5 ml of blood specimen was drawn from all the subjects before intervention (as the baseline) and after completing the program (post-intervention). The blood was tested for fibrinogen, plasminogen activator inhibitor type-1 (PAI-1), and tissue-type plasminogen activator (t-PA) antigen levels. Physical parameters which were measured included the body weight, body mass index (BMI), waist circumference, waist hip ratio, and fat-free mass.
Body weights were measured to the nearest 0.1 kilogram using a digital scale (SECA model). Height was measured by using a Body meter (SECA Model 208), which has a precision of up to 0.1 cm. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Respondents were classified as obese if their BMI was 30 kg/m2 or higher, in accordance with World Health Organization recommendation. Waist measurement was taken midway between the inferior margin of the last rib and the crest of the ileum in a horizontal plane. Hip circumference was measured around the pelvis at the point of maximal protrusion of the buttocks. Fat-free mass was determined using a hand-held bioelectrical impedance machine (Bodystat-1500, Bodystat Ltd, UK).
Subjects were instructed to attend the weekly exercise program (every Thursday) from 8.00 am until 12.45 pm, for 12-weeks duration. The program consisted of brisk walk for 2-4 km (approximately 1 hour per session), 30 minutes dumb bell (4 kg) resistance exercise and 30 minutes easy style of aerobic dance exercise accompanied with nutrition education modules for 12 weeks. Nutrition education was based on portion control method where participants reduced their calorie intake daily. One of the method was reducing the main meals into three meals per day without snacking except for diabetic patient. Subjects were instructed to reduce carbohydrate intake such as white rice for only one cup (one serving) for every main meal. Apart from white rice, subjects were also intructed to consume only one portion of protein (such as beef, fish, or chicken) with two portions of vegetables and fruits. Apart from the weekly program, subjects were instructed to do daily moderate intensity physical activities such as brisk walking, dumb bell at home, etc. The subjects were requested to gradually increase the duration and frequency of their physical activities day by day at home.
The weekly dietary program consisted of 12 nutritional modules thats were given to the subjects. Each module incorporated recommendations for healthful eating, behavior modification, and physical activity.
Determination of t-PA level
Measurement of human t-PA was done using enzyme immunoassay (IMUBIND® t-PA, Germany). Plasma samples and t-PA standards were added to the wells which were coated with t-PA polyclonal antibody. Sufficient incubation time was given to allow binding of t-PA to the antibodies before adding peroxidase of anti-t-PA. Following this process, the unbound material was washed away. Subsequently, ortho-phenylenediamine (OPD) was added to the wells and a chemical reaction was produced and turned the solution into orange color. The absorbance was measured and its value was directly proportional to the amount of t-PA present in the sample. The result was expressed in ng/ml. The procedure was done according to the manufacturer's recommendation.
Determination of PAI-1 level
Measurement of human PAI-1 was done using Technozym® PAI-1 Antigen by enzyme immunoassay (Technozym® PAI-1, Austria). The test utilized the double antibody principle. Plasma sample or PAI-1 standards were added to the wells which were coated with anti-PAI-1 monoclonal antibody and contained soluble non-immune IgG. The incubation time was to allow PAI-1 to bind to the captured antibodies. After incubation, HRP-labelled Fab fragments of anti-PAI-1 IgG was added. These substances were allowed to react with the bound PAI-1. The wells were emptied and washed to remove unbound conjugate after which stopping solution was added. The amount of yellow color developed was directly proportional to the amount of PAI-1 present in the sample. The result was expressed in ng/ml. The procedure was done according to the manufacturer's recommendation (Technozym® PAI-1 Catalogue).
Determination of TAFI level
Measurement of TAFI level was performed by using STA® - Stachrom TAFI kit. The assay was carried out in three steps: Activation of TAFI into TAFIa by the thrombin-thrombomodulin complex (Reagent 1) and this process was followed by partial hydrolysis of a specific chromogenic substrate (Reagent 2) by the TAFIa. The last process involved specific hydrolysis of the partially hydrolysed substrate by carboxypeptidase A and this event led to discolouration of the substrate. Thus, measurement of the discolouration at 405 nm was directly proportional to the TAFI level of the sample.
Determination of fibrinogen
Fibrinogen level was quantitatively determined by the Clauss method assay using Compact Stago Coagulation Analyzer STA®, France. The commercial reagent, STA®-Fib used was from Diagnostica Stago, France. A high concentration of thrombin was added to dilute test plasma and the clotting time then measured. The test result was compared with a calibration curve prepared by clotting a series of dilutions of a reference plasma sample of known fibrinogen concentration, and a result in g/l was obtained. The reference normal range for fibrinogen was 1.5-4 g/l.
Statistical analysis
Data entry and analysis were done using Statistical Package for Social Sciences version 18.0 for windows (SPSS Inc, Chicago, IL, USA). The data distribution of each parameter was initially determined before running any test and parametric test was employed for normally distributed data. Physical paramaters and level of fibrinolytic markers were analysed using paired t-test. In order to determine the correlation of the fibrinolytic markers with other parameters, Spearman's rho correlation analysis was used. The P-value <0.05 was considered significant. The correlation analysis was conducted using the pre and post-interventional parameters during the study period. Significant differences which were observed in the paramaters investigated remained significant even when the data from the two diabetic subjects were excluded from the calculation.
Results
A total of 28 subjects were recruited for this study from April 2009 till May 2012. Majority of the subjects were female (85.71%) and the rest were male (14.29%). The age range of the subjects was between 22 and 62-years old and the mean age was 43.68 years. All of them were from Malay ethnic group.
Physical parameters
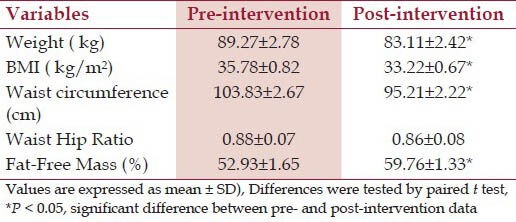
The mean weight of the subjects at baseline was 89.27 ± 2.78 kg and reduced to 83.11 ± 2.42 kg at post-intervention. Majority of the subjects had loss between 5 and 10% of their body weight. Interestingly, there were four subjects (14.3%) who had lost more than 10% of their body weight. Overall mean weight loss for all the subjects was 6.7%. Apart from weight and BMI, the study also measured other parameters such as waist circumference, waist-to-hip ratio, and fat-free mass (percentage of total body weight). There was a significant reduction of waist circumference, BMI and fat-free mass at post-intervention [Table 1]. However, no significant difference was seen in waist to hip ratio at post-intervention when compared to pre-intervention [Table 1].
Table 1.
Changes in physical parameter at pre- and post-intervention
t-PA, PAI-1, and fibrinogen
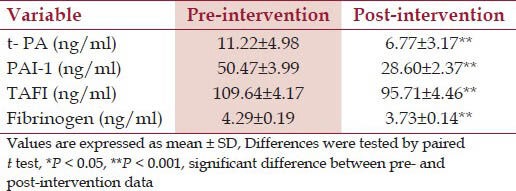
The mean t-PA, PAI-1, TAFI, and fibrinogen levels were reduced significantly at post-intervention when compared with pre intervention value [Table 2].
Table 2.
Mean t-PA, PAI-1, TAFI and fibrinogen levels at pre- and at post-intervention
Correlation between fibrinolytic markers and physical parameters
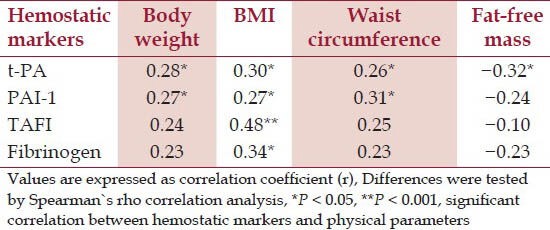
Based on Spearman's rho Correlation, there was a significant (P < 0.05), positive and fair correlation between the anthropometric data with t-PA and PAI-1 [Table 3]. For TAFI, the fair and significant correlation was only observed with BMI. Similar degree of correlation was also shown between BMI and fibrinogen [Table 3]. However, there was poor correlation between body weight and waist circumference with fibrinogen and TAFI. Poor correlation was also observed among PAI-1, TAFI, and fibrinogen with fat-free mass; however, there was significant and fair correlation between t-PA and fat-free mass. Correlation analysis was not done between the hematological parameters and waist to hip ratio as there was no significant difference in the ratio before and after the intervention.
Table 3.
Correlation between hemostatic marker and physical parameter
Discussion
Various studies have shown the association of hemostatic and fibrinolytic factors with an increased risk of cardiovascular disease.[12,13,14] The fibrinolytic system is responsible for degradation of fibrin in the blood vessels with the enzyme plasmin playing a central role. Plasmin is formed from plasminogen with the involvement of tPA on the surface of fibrin. In the present study, the levels of fibrinolytic markers were high at the baseline. Increased plasma t-PA is associated with inhibition of endogenous fibrinolysis.[15] Free t-PA which is released into blood from endothelial cells immediately forms a complex with circulating PAI-1. Thus ordinary assays of t-PA antigen in the plasma did not measure free and active t-PA, but mainly in the complex form with PAI-1. Thereby, increased t-PA plasma levels could be a consequence of increased PAI-1 plasma levels. This fibrinolytic activity is dependent on the balance between tPA and PAI-1.[16] Impaired function of fibrinolytic system is expected to cause progression of vascular disease and predispose to vascular thrombosis.
Fibrinogen, an acute-phase protein, plays an essential role in the blood coagulation system. The potential mechanisms whereby higher fibrinogen could contribute to risk of CHD include increased fibrin formation, platelet aggregation, plasma viscosity, and through-binding of leucocytes to endothelial cells.[17]
This study showed significant reduction in PAI-1 and t-PA levels at post-intervention, and the parameters had positive correlations with BMI, body weight, and waist circumference. Importantly, it was reported that weight loss led to a decrease in plasma PAI-1 and t-PA irrespective of diet regiment presecribed, which rose again if weight was regained.[18,19,20] The decrease in the plasma PAI-1 and t-PA was also reported following hypocaloric diet combined with light to moderate exercise.[21] Previous clinical studies supported the possibility that adipose tissue contributed directly to elevated plasma PAI-1, a hypothesis that emerged strongly when it was observed that a mouse with higher adipose tissue expressed high levels of PAI-1.[22] These findings and the emerging role of adipose tissue as an organ secreting proteins into the blood suggested the important role of this tissue in the elevation of plasma PAI-1.[23] The potentially large mass of adipose tissue in obese subjects would have a capacity to synthesize PAI-1. In this study, the parallel reduction of t-PA and PAI-1 at post-intervention was most likely due to measurement of t-PA which has formed complexes with PAI-1 antigen as described earlier.
This program has shown a reduction in the TAFI level although TAFI level was within the reported normal reference range at pre and post intervention. TAFI level was fairly correlated with BMI. TAFI levels in healthy individuals vary widely compared to the reference values and the level of TAFI antigen does not differ between men and women.[24] Increased TAFI antigen levels has been observed in venous and coronary artery blood from patients with coronary artery disease. In addition, an elevated TAFI antigen level has been identified as a risk factor for angina pectoris and is associated with acute ischemic stroke.[24] These findings may indicate an important role for TAFI in the development of atherothrombotic changes in patients with metabolic disturbances associated with hyperlipidemia.
In keeping the role of TAFI as an antifibrinolytic factor, several clinical studies such as Van Tilburg et al. and Eichinger et al. have shown that high plasma concentration of TAFI as a risk factor for thrombotic disorders.[25,26] Taken together, the data seem to agree that high plasma concentration of TAFI is a risk factor for venous thrombosis, although more studies will be required to confirm the magnitude of the risk and its interaction with other prothrombotic risk factors e.g., in arterial thrombosis.[27] To date, there are limited data investigating the associations between obesity and TAFI.
The present study has shown significant reduction in fibrinogen at post-intervention. Increased level of coagulation factors have been found in obese subjects.[28] A study has reported that fibrinogen level showed a direct correlation with WHR.[29] The relationship indicates an association between body fat distribution with changes in coagulation activity. These data may be of interest, since general epidemiological studies suggest that high level of fibrinogen may be one of the important factors involved in the development of ischemic heart disease.[29] Fibrinogen's association with increased mortality is probably directly related to its ability to promote thromboses or clots inside the blood vessels.[30] This is one of the main mechanisms underlying ischemia and heart attack.
In the present study, there was a positive correlation between fibrinogen and BMI (r = 0.34, P = 0.01). Marckmann et al reported that fibrinogen level was 6% lower compared to baseline after 24 weeks of weight-loss program.[19] They have shown that body weight changes were significantly correlated with changes in plasma fibrinogen. Their study also showed a strong association existed between changes in waist circumference and fibrinogen (r = 0.53), and a lower association between WHR and fibrinogen (r = 0.40). In the present study, there is no direct correlation between fibrinogen level with body weight, waist circumference and fat-free mass. Fibrinogen is an acute phase protein and its effect may vary depending on the host factors. Its level is most probably more influenced by BMI compared to other physcial parameters.
In conclusion, reduced fibrinogen, fibrinolytic and physical parameters were noted in obese subjects following weight-reduction program. The physical parameters are known predictors associated with cardiovascular morbidity while the hemostatic system contributes to the progression of vascular atherothrombotic diseases. The positive correlation between the fibrinolytic markers and the physical predictors suggest the potential role of the fibrinolytic biomarkers in the risk assessment of cardiovascular morbidities in parallel with the conventional predictors.
Acknowledgment
This study was supported partly by Universiti Sains Malaysia Research University grant (101/PPSP/812031). A special thanks to Associate Prof. Dr. Shamsul Kamalrujan for his contribution during the progress of the study.
Footnotes
Source of Support: Sponsored partly by Universiti Sains Malaysia Research University grant (101/PPSP/812031)
Conflict of Interest: None declared.
References
- 1.Barnes AS, Goodrick GK, Parlik V, Markesino J, Laws DY, Taylor WC. Weight loss maintenance in African-American Women: Focus groups results and questionnaire development. J Gen Intern Med. 2007;22:915–22. doi: 10.1007/s11606-007-0195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallistl S, Sudi KM, Borkenstein M, Troebinger M, Weihandl G, Muntean W. Determinants of haemostatic risk factors for coronary heart disease in obese children and adoslescents. Int J Obes Relat Metab Disord. 2000;24:1459–64. doi: 10.1038/sj.ijo.0801427. [DOI] [PubMed] [Google Scholar]
- 3.Masahiko S, Priscilla YH, David DW. Role of thrombotic and fibrinolytic factors in acute coronary syndromes. Prog Cardiovasc Dis. 2004;46:524–38. doi: 10.1016/j.pcad.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Calderon KS, Yucha CB, Schaffer SD. Obesity–related cardiovascular risk factors: Intervention recommendations to decrease adoslescent obesity. J Pediatr Nurs. 2005;20:3–14. doi: 10.1016/j.pedn.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–9. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 6.Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3:85–101. doi: 10.1046/j.1467-789x.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 7.Abbate R, Cioni G, Ricci I, Miranda M, Gori AM. Thrombosis and acute coronary syndrome. Thromb Res. 2012;129:235–40. doi: 10.1016/j.thromres.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Cooper JA, Miller GJ, Bauer KA, Morrissey JH, Meade TW, Howarth DJ, et al. Comparison of novel hemostatic factors and conventional risk factors for prediction of coronary heart disease. Circulation. 2000;102:2816–22. doi: 10.1161/01.cir.102.23.2816. [DOI] [PubMed] [Google Scholar]
- 9.Meade TW, Cooper JA, Chakrabarti R, Miller GJ, Stirling Y, Howarth DJ. Fibrinolytic activity and clotting factors in ischaemic heart disease in women. BMJ. 1996;312:1581. [PMC free article] [PubMed] [Google Scholar]
- 10.Pritchett AM, Foreyt JP, Mann DL. Treatment of the metabolic syndrome: The impact of lifestyle modification. Curr Atheroscler Rep. 2005;7:95–102. doi: 10.1007/s11883-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 11.Calles-Escandon J, Ballor D, Harvey-Berino J, Ades P, Tracy R. Amelioration of the inhibition of fibrinolysis in elderly, obese subjects by moderate energy intake restriction. Am J Clin Nutr. 1996;64:7–1. doi: 10.1093/ajcn/64.1.7. [DOI] [PubMed] [Google Scholar]
- 12.Jansson JH, Nilsson TK, Johnson O. Von Willebrand factor in plasma: A novel risk factor for recurrent myocardial infarction and death. Br Heart J. 1991;66:351–5. doi: 10.1136/hrt.66.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamsten A, Erihson P. Fibrinolysis and atherosclerosis. Baillicbes Clin Haematol. 1995;8:345–63. doi: 10.1016/s0950-3536(05)80272-0. [DOI] [PubMed] [Google Scholar]
- 14.Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. N Engl J Med. 1995;332:635–41. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- 15.Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1997;69:381–7. [PubMed] [Google Scholar]
- 16.Vague P, Juhan-Vague I, Aillaud MF, Badier C, Viard R, Alessi MC, et al. Correlation between fibrinolytic activity, plasminogen activator inhibitor level, plasma insulin level, and relative body weight in normal and obese subjects. Metabolism. 1986;35:250–3. doi: 10.1016/0026-0495(86)90209-x. [DOI] [PubMed] [Google Scholar]
- 17.Kamath S, Lip GY. Fibrinogen: Biochemistry, epidemiology and determinants. QJM. 2003;96:711–29. doi: 10.1093/qjmed/hcg129. [DOI] [PubMed] [Google Scholar]
- 18.Sundell IB, Dahlgren S, Ranby M, Lundin E, Stenling R, Nilsson TK. Reduction of elevated plasminogen activator inhibitor levels during modest weight loss. Fibrinolysis. 1989;3:51–3. [Google Scholar]
- 19.Marckmann P, Toubro S, Astrup A. Sustained improvement in blood lipids, coagulation, and fibrinolysis after major weight loss in obese subjects. Eur J Clin Nutr. 1998;52:329–33. doi: 10.1038/sj.ejcn.1600558. [DOI] [PubMed] [Google Scholar]
- 20.Mavri A, Stegnar M, Krebs M, Sentocnik JT, Geiger M, Binder BR. Impact of adipose tissue on plasma plasminogen activator inhibitor-1 in dieting obese women. Arterioscler Thromb Vasc Biol. 1999;19:1582–7. doi: 10.1161/01.atv.19.6.1582. [DOI] [PubMed] [Google Scholar]
- 21.Folsom AR, Qamhieh HT, Wing RR, Jeffery RW, Stinson VL, Kuller LH, et al. Impact of weight loss on plasminogen activator inhibitor (PAI-1), factor VII, and other hemostatic factors in moderately overweight adults. Arterioscler Thromb. 1993;13:162–9. doi: 10.1161/01.atv.13.2.162. [DOI] [PubMed] [Google Scholar]
- 22.Sawdey MS, Loskutoff DJ. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo: Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-a, and transforming growth factor-ß. J Clin Invest. 1991;88:1346–53. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegelman BM, Flier JS. Adipogenesis and obesity: Rounding out the big picture. Cell. 1996;87:377–89. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 24.Juhan-Vague I, Renucci JF, Grimaux M, Morange PE, Gouvernet J, Gourmelin Y, et al. Thrombin-activatable fibrinolysis inhibitor antigen levels and cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2000;20:2156–61. doi: 10.1161/01.atv.20.9.2156. [DOI] [PubMed] [Google Scholar]
- 25.Van Tilburg NH, Rosendaal FR, Bertina RM. Thrombin activatable fibrinolysis inhibitor and the risk for deep vein thrombosis. Blood. 2000;95:2855–9. [PubMed] [Google Scholar]
- 26.Eichinger S, Schonauer V, Weltermann A, Minar E, Bialonczyk C, Hirschl M, et al. Thrombin-activatable fibrinolysis inhibitor and the risk for recurrent venous thromboembolism. Blood. 2004;103:3773–6. doi: 10.1182/blood-2003-10-3422. [DOI] [PubMed] [Google Scholar]
- 27.Boffa MB, Koschinsky ML. Curiouser and curiouser: Recent advances in measurement of thrombin-activatable fibrinolysis inhibitor (TAFI) and in understanding its molecular genetics, gene regulation, and biological roles. Clin Biochem. 2007;40:431–42. doi: 10.1016/j.clinbiochem.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Rissanen P, Vahtera E, Krusius T, Uusitupa M, Rissanen A. Weight change and blood coagulability and fibrinolysis in healthy obese women. Int J Obes Relat Metab Disord. 2001;25:212–8. doi: 10.1038/sj.ijo.0801540. [DOI] [PubMed] [Google Scholar]
- 29.Licata G, Scaglione R, Avellone G, Ganguzza A, Corrao S, Arnone S, et al. Hemostatic function in young subjects with central obesity: Relationship with left ventricular function. Metabolism. 1995;44:1417–21. doi: 10.1016/0026-0495(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 30.De Moerloose P, Boehlen F, Neerman-Arbez M. Fibrinogen and the risk of thrombosis. Semin Thromb Hemost. 2010;36:7–17. doi: 10.1055/s-0030-1248720. [DOI] [PubMed] [Google Scholar]


