Abstract
Background:
In Iranian traditional medicine, Ferula assa-foetida oleo gum resin (asafoetida) have been used as anti-convulsant agents.
Aims:
This study was designed to evaluate the anti-convulsant effect of asafoetida on chemical and amygdala -kindled rats.
Materials and Methods:
In chemical model, rats received orally asafoetida at dose of 50 and 100 mg/kg 90 minutes prior to Pentylenetetrazol injection in dose of 35 mg/kg intraperitoneally (i.p.) and control group received normal saline. Convulsive behavior was recorded for 30 minutes. For amygdala kindle model, bipolar stimulating and monopolar recording electrodes were implanted stereotaxically. After kindling, the effect of asafoetida (50 and 100mg/kg) on after discharge duration, duration of stage 5 seizure and latency to the onset of bilateral forelimb clonuses was measured.
Results:
Pretreatment animals with asafoetida significantly reduced the mean seizure stage during the 20 kindling injection of Pentylenetetrazol. Seizure parameters in amigdala kindle model improved in treatment animals at both dose 50 and 100 mg/kg. The number of stimulations in stage 3, 4, and 5 in asafoetida-treated rats at both doses significantly increased.
Conclusions:
These results showed that asafoetida could prevent seizure in both chemical and electrical kindling model and this effect may partially be related to the terpenoids compounds.
Keywords: Amygdala kindling, Asafoetida, PTZ, Seizure
Introduction
Epilepsy is a brain disorder which is characterized by seizures that induced by a complex of neurotransmitter systems. This disorder is one of the most common neurological impair which affect about 50 million people in the entire world.[1] Approximately 5-10% of the world population experience epilepsy in their lifetime that occurring during early childhood or late adulthood.[2] This disorder, if untreated, can lead to impaired intellectual function or death and is typically accompanied by psychopathological consequences. Currently, many synthetic drugs like carbamazepine, ethosuximide, gabapentin, oxcarbazepine, phenobarbital, phenytoin, valproic acid, felbamate, etc. are used as potent anti-epileptic agents, but they are not free from marked side effects.[3] There is some difficulty in the treatment procedure of epilepsy, for example, approximately 30% of the patients continue to have seizures with current anti-epileptic drug therapy and these drugs have the serious side effects and chronic toxicity.[4] Hence, there is a need to address a potent alternative as anti-epileptic agent with minimal side effects. In the recent years, there is an increasing interest to use natural sources which would be a valuable agent for finding the new therapeutic drugs. In Iranian traditional medicine, there are some medicinal plants which were used for the treatment of epilepsy.[5] Plants of the genus Ferula belongs to the family of Apiaceae include about 130 species that distributed throughout central Asia and Mediterranean area.[6] Ferula assa-foetida L. is one of these species that grows wildly in central area of Iran. The part used of this plant and several other species of Ferula is an oleo gum resin (asafoetida) that obtained by incision of stem and root. This oleo gum resin is considered as an important matter of pharmacological and industrial application. The gum fraction of asafoetida contains the glucuronic acid, galactose, arabinose, and rhamnose and its resin is consisting of umbelliferone, ferulic acid and its esters, coumarins, sesquiterpene coumarins, and other terpenoids.[7] In Iranian folk medicine, asafoetida have been used for anti-convulsant, anti-spasmodic, carminative, digestive, expectorant, sedative, anti-hysteric, laxative, aphrodisiac, anti-septic, and analgesic activities.[5] Recent pharmacological and biological studies have also shown several activities, such as anti-oxidant, anti-viral, anti-fungal, cancer chemopreventive, anti-diabetic, anti-spasmodic, hypotensive, and molluscicidal from this oleo gum resin.[7] In some previous studies; possible anti-convulsant effect of some Ferula species has been investigated. Studies on F gummosa acetone root extract showed that this extract has anti-convulsant activity in generalized epilepsy in mice.[8] Our previous study showed that methanolic extract of some different Ferula species did not any effect on generalized epileptic behavior in mice.[6] In this study, to confirm the evidence of this folk medicine for the anti-convulsant activity, we experiment anti-convulsant effect of asafoetida in chemical and amygdala-kindled rats.
Materials and Methods
Animals
Male Wistar rats (aged 8 weeks at the beginning of the experiments), 180-250 g, were reared in the animal house and kept under controlled standard condition of 20 ± 2ºC and 12-L/12-D photoperiod. Rats were housed and maintained on a standard tap water and commercial rat pellet diet. The experimental protocol was approved by the Ethic Committee of Shahid Sadoghi University of Medical Sciences.
Plants material and chemical
Ferula assa-foetida oleo gum resin (asafoetida) was collected from Tabas region (Yazd province, Iran) during the summer and the plant species was botanically identified by Dr. Abbas Zarezadeh in Yazd Agricultural Research Center. The dried powder of asafoetida was soaked in distilled water overnight at room temperature and the yielded suspension was used orally. Concentrations and dosages of the extract were expressed as crude amount of the dried oleo gum resin used in preparing the stock solution. Pentilen tetrazol (PTZ) (Sigma, USA) were dissolved in saline and prepared to use intraperitoneally.
Experiment 1
PTZ kindling procedure
Twenty-four male Wistar rats were divided equally and randomly to four groups (one control and three test groups). Treatment animals received orally asafoetida in dose of 50 and 100 mg/kg 90 minute prior to PTZ injection in a subconvulsive dose of 35 mg/kg i.p. Control group received saline 90 minute before injection of PTZ (35 mg/kg i.p). After each injection of PTZ, the animals were placed individually in plastic boxes. Convulsive behavior was recorded for 30 minutes and behavioral changes were scored according to Racine's criteria as follows: Stage 0: No response, stage 1: Ear and facial twitching, stage 2: Myoclonic jerks without rearing, stage 3: Myoclonic jerks with rearing, stage 4: Turn over into side position, clonic–tonic seizures, stage 5: Turn over into back position, generalized clonic–tonic seizures.[9]
Experiment 2: Electrical kindling
Surgical procedure
Eighteen rats were anesthetized i.p. with ketamine hydrochloride (100 mg/kg) and xylazine (10 mg/kg) and placed in a Stoelting stereotaxic instrument. Bipolar stimulating and monopolar recording electrodes were stereotaxically implanted in the basolateral amygdala of the right the hemisphere (coordinates: anteroposterior (AP), −2.5 mm; mediolateral (ML), 4.8 mm; dorsoventral (DV), 7.5 mm) according to Paxinos and Waston.[10] The electrodes (Teflon-coated, 125 μm in diameter; A.M. system Inc., USA) were insulated except at their tips. Two other electrodes were connected to the skull screws and placed above the left cortical surface as earth and differential electrodes. The pines attached to the electrodes were inserted into a socket which was fixed on the skull. A 23-gauge guide cannula was also implanted in the right lateral ventricle (coordinates: AP, −1.0 mm; ML, 1.2 mm; DV, 3.6 mm).
Kindling procedure
One week after the surgery, after discharge (AD) threshold was determined in the amygdala by 2 second, 60 Hz monophasic square wave stimulus of 1 ms per wave. Stimulation was provided by a stimulator and constant current stimulus isolation unit (WSI, Iran). The Stimulation were initially delivered at 10 μA and then at 5-minutes intervals increasing stimulus intensity in increments of 10 μA until at least 5 second of after discharges were recorded as previously described.[11] Then, animals were stimulated daily at AD threshold intensity until three consecutive stage 5 seizures (fully kindled) according to Racine scales were elicited.[9] Subsequent to amygdala stimulation, convulsive behaviors and electrophysio-logical parameters including after discharge duration (ADD), stage 4 latency (S4L), stage 5duration (S5D) were recorded.
Experimental groups and treatments
The effect of Ferula assa-foetida oleo gum resin on the kindled rats and kindling development was determined. Rats were first fully kindled with daily amygdala stimulations and then, seizure parameters of fully kindled rats in the last stimulation session were recorded as control data. One day later, rats received asafoetida orally in dose of 50, 100 mg/kg 90 min prior to electrical stimulation. After each electrical stimulation, S4L, S5D, SD, and ADD were recorded. Effects of asafoetida on the kindling development were evaluated in separate groups; rats received asafoetida at the doses of 50, 100 mg/kg 90 minute prior to each electrical stimulation. After amygdala stimulation, epileptic behaviors, and electrophysiological parameters were monitored and scored for 30 minutes.
Statistical analysis
Scores of seizure stages were compared using Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks followed by multiple comparison tests. Results of S4L and S5D were compared using one-way and repeated measure ANOVA. Tukey's test was employed to compare the differences among treated groups using Graph pad prism version 5. The P-value less than 0.05 were considered to be statistically significant.
Results
Effects of asafoetida on PTZ kindling development
Pretreatment animals with asafoetida at 50 and 100 mg/kg significantly reduced the mean seizure stage during the 20 kindling injection as compared with the saline control animals [P < 0.05, Figure 1].
Figure 1.
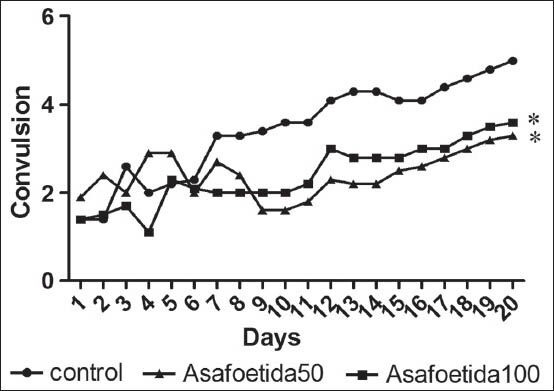
Effect of asafoetida pretreatment on the PTZ-induced kindling intensity. *P < 0.05 indicates significant differences as compared to PTZ-kindled group
Effects of asafoetida in amygdala-kindled rats
In amygdale-kindled rats, asafoetida at the doses of 50 and 100 mg/kg led to decrease in SD, S5D, and ADD [P < 0.05, Figure 2]. S4L was significantly longer in the asafoetida treated-than saline treated rats (P < 0.05). In the groups that treated with asafoetida before each amygdala stimulation, cumulative S4L was significantly longer than control group. Cumulative S5D, cumulative SD, and cumulative ADD were significantly decreased in asafetida-treated rats [P < 0.05, Figure 3]. Figure 4 showed the effect of asafoetida administration on the number of stimulations needed to achieve different seizure stages. There is a significant increase in the number of stimulations to seizure stage 3, 4, and 5 in asafetida-treated rats at both doses (P < 0.05).
Figure 2.
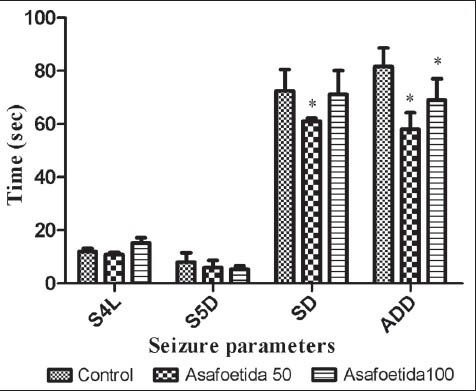
Effect of asafoetida at the doses of 50 and 100 mg/kg on SD, S4L, S5D, and ADD. *P < 0.05 indicates significant differences as compared to control. ADD after discharge duration, S5D stage 5 duration, SD seizure duration, S4L stage 4 latency
Figure 3.
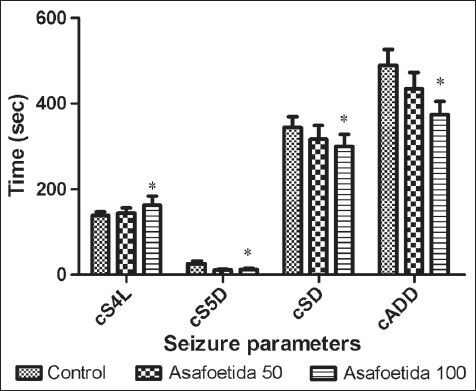
Effect of asafoetida at the doses of 50 and 100 mg/kg on cSD, cS4L, cS5D, and cADD. *P < 0.05 indicates significant differences as compared to control. cSD cumulative seizure duration. cS4L cumulative stage 4 latency. cS5D cumulative stage 5 duration. cADD cumulative after discharge duration
Figure 4.
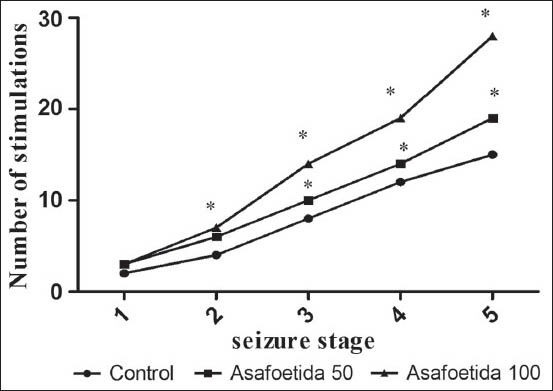
Effect of asafoetida administration on the number of stimulations. *P < 0.05 indicates significant differences as compared to control group
Discussion
In this study, our results showed that asafoetida had an obvious anti-convulsant effect in PTZ and amygdala-kindled rats. In Ayurveda, asafoetida is introduced as a valuable remedy for hysteria and nervous disorders and American people also orally use it as a stimulant to the brain and nerves.[7] In Iranian traditional medicine, the gum obtained from F. gumosa, F. assa-foetida, F. badrakema, and F. diversivittata have been used as an anti-epileptic remedy.[5] There are several studies that investigated anti-convulsant activity of some Ferula species. Sayyah et al., reported that seed and root acetone extracts of Ferula gummosa have anti-convulsant activity in mice.[8,12] They proposed that the anti-seizure profile of F. gummosa seed acetone extract may be related in part to monoterpenes and terpenoid compound present in the extract. They also mentioned that fruit essential oil of F. gummosa has anti-epileptic potential.[13] In previous study, we reported that some methanolic extract of Ferula species could not prevent PTZ-induced seizure.[6] The controversy results about the anti-epileptic effect of these extracts may be related to epileptic model and/or extracting method. Kiasalari et al., showed that hydro-alcoholic extract of asafoetida is able to reduce seizure duration and its intensity and this anti-seizure effects is dependent to dosage.[14] They concluded that anti-oxidant activity of this extract maybe has a role to preventive of seizure. They also showed that malondialdehyde (MDA) and nitric oxide (NO) levels decreased in the brain tissue. The results of this research are consistent with the results obtained in our study. Our data showed that asafoetida with dose 100mg/kg has generally had a better effect in reducing the severity convulsive and other parameters of seizures. In this group, number of stimulations that needed to achieve different seizure stages increased significantly [Figure 4]. Cumulative S4L, cS5D, cSD, and cADD were significantly longer than control group and these parameters were more effective at asafoetida100mg/kg. PTZ kindling model is a common model and is valid model for human generalized myoclonic seizures and also generalized seizures of the absence type.[15] As shown in Figure 1, our results also indicated that asafoetida could decrease the intensity of seizure in PTZ kindle model. This type of seizures can be prevented by drugs that enhance gamma amino butyric acid type A (GABAA) receptor mediated inhibitory neurotransmission, such as benzodiazepines and Phenobarbital and perhaps valproate and felbamate.[8] Drugs that reduce T-type calcium current, such as ethosuximide can also prevent seizures induced by PTZ. Although the anti-convulsant mechanism of Ferula genus has not been identified, there are some hypotheses about these effects. Sayyah et al., suggested that maybe existence of monoterpenes including α-Pinene and β-Pinene (with anti-epileptic properties) ceased anti-convoulsant property.[13] Linalool is another monoterpene compound, which has protective effect against PTZ-, picrotoxin-, and N-methyl-D-aspartate (NMDA)-induced convulsions.[16] Moreover, pinene, eugenol and methyleugenol exhibited anti-convulsant profile in some experimental seizures such as maximum electroshock (MES) and PTZ tests.[17,18] Modulation of glutamatergic and GABAergic transmission is some mechanisms indicated for anti-convulsant action of the monoterpenes like linalool and eugenol.[16,19,20] Therefore, it seems that the anti-seizure profile of asafoetida may be related in part to monoterpens and terpenoid compounds present in the root because these compounds were also detected in Ferula assa- foetida.[21,22] It was determined that pinene analogs prevent idiopathic epilepsy in prone mice.[23] In addition, flavonoid compounds with anti-oxidant properties are among the fractions of Ferula plants and could be another candidate by which the anti-convulsant effect of Ferula is occurred. Phytochemistry of asafoetida demonstrated that this oleo gum resin is the rich presence of alkaloids, flavonoids and acidic compounds. A large number of different sesquiterpene coumarins have been reported from asafoetida. The compounds of Ferula assa-foetida are not limited to sesquiterpene coumarins; it also contains some other compounds belonging to different classes of natural products, such as diterpenes phenolics, acetylenes, sesquiterpenes, and sulfur compounds.[24]
Conclusion
According to the present study, we concluded that asafoetida has an anti-convulsant effect that this observation confirms the use of this plant in traditional medicine. However, further investigations including chronic toxicity studies and activity-guided fractionation must be performed in order to assess the real toxicological profile and the active compounds and mechanism action of this oleo gum resin.
Acknowledgements
The authors are pleased to thank Dr. Abbas Zarezadeh from Yazd Agricultural Research Center for his assistance in providing the asafoetida used in this research.
Footnotes
Source of Support: This research was supported by Vice Chancellery of Research of Shahid Sadoughi University of Medical Sciences and Health Services.
Conflict of Interest: None declared.
References
- 1.Moshi MJ, Kagashe GA, Mbwambo ZH. Plants used to treat epilepsy by Tanzanian traditional healers. J Ethnopharmacol. 2005;97:327–36. doi: 10.1016/j.jep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Lowenstein DH. Harrison's Principles of Internal Medicine. 17th ed. Churchill: Livingstone Medical Publications; 2010. Seizures and epilepsies; pp. 2124–57. [Google Scholar]
- 3.Engel J., Jr Surgery for seizures. N Engl J Med. 1996;334:647–52. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- 4.Poole K, Moran N, Bell G, Solomon J, Kendall S, McCarthy M, et al. Patients’ perspectives on services for epilepsy: A survey of patient satisfaction, preferences and information provision in 2394 people with epilepsy. Seizure. 2000;9:551–8. doi: 10.1053/seiz.2000.0450. [DOI] [PubMed] [Google Scholar]
- 5.Zargari A. 6th ed. Tehran: Tehran University Publications; 1996. Medicinal Plants; pp. 592–602. [Google Scholar]
- 6.Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol. 2010;48:242–6. doi: 10.3109/13880200903081796. [DOI] [PubMed] [Google Scholar]
- 7.Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)- a review. J Ethnopharmacol. 2011;134:1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 8.Sayyah M, Mandgary A, Kamalinejad M. Evaluation of the anticonvulsant activity of the seed acetone extract of Ferula gumosa Boiss. against seizures induced by pentylenetetrazole and electroconvulsive shock in mice. J Ethnopharmacol. 2002;82:105–9. doi: 10.1016/s0378-8741(02)00166-6. [DOI] [PubMed] [Google Scholar]
- 9.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and Clinical Neurophysiology. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 10.Paxinos G, Watson C. 4th ed. San Diego: Academic Press; 1998. The Rat Brain in Stereotaxic Coordinates; pp. 155–71. [Google Scholar]
- 11.Rezvani ME, Roohbakhsh A, Allahtavakoli M, Shamsizadeh A. Anticonvulsant effect of aqueous extract of Valeriana officinalis in amygdala-kindled rats: Possible involvement of adenosine. J Ethnopharmacol. 2010;127:313–8. doi: 10.1016/j.jep.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Sayyah M, Mandgary A. Anticonvulsant effect of Ferula gummosa Boiss. root against experimental seizures. Iran Biomed J. 2003;7:139–43. [Google Scholar]
- 13.Sayyah M, Kamalinejad M, Bahrami Hidage R, Rustaiyan A. Antiepileptic potentional and composition of the fruit essential oil of ferula gummosa boiss. Iranian Biomed J. 2001;2:69–72. [Google Scholar]
- 14.Kiasalari Z, Khalili M, Roghani M, Heidari H, Azizi Y. Antiepileptic and antioxidant effect of hydroalcoholic extract of ferula assa foetida gum on pentylentetrazole-induced kindling in male mice. Basic Clin Neurosci. 2013;4:21–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Loscher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988;2:145–81. doi: 10.1016/0920-1211(88)90054-x. [DOI] [PubMed] [Google Scholar]
- 16.Brum LF, Elisabetsky E, Souza D. Effects of linalool on. [(3)H] MK801 and [(3)H] Phytother Res. 2001;15:422–5. doi: 10.1002/ptr.973. [DOI] [PubMed] [Google Scholar]
- 17.Consroe P, Martin A, Singh V. Antiepileptic potential of cannabinoid analogs. J Clin Pharmacol. 1981;21:428–36S. doi: 10.1002/j.1552-4604.1981.tb02623.x. [DOI] [PubMed] [Google Scholar]
- 18.Dallmeier K, Carlini EA. Anesthetic, hypothermic, myorelaxant and anticonvulsant effects of synthetic eugenol derivatives and natural analogue. Pharmacology. 1981;22:113–27. doi: 10.1159/000137479. [DOI] [PubMed] [Google Scholar]
- 19.Szbadics J, Erdelyi L. Pre-and postsynaptic effects of eugenol and related compounds on Helix pomatia L. neurons. Acta Biol Hung. 2000;51:265–73. [PubMed] [Google Scholar]
- 20.Wie MB, Won MH, Lee KH, Shin JH, Lee JS, Suh HW, et al. Eugenol protects neuronal cells from excitotoxic and oxidative injury in primary cortical cultures. Neurosci Lett. 1997;225:93–6. doi: 10.1016/s0304-3940(97)00195-x. [DOI] [PubMed] [Google Scholar]
- 21.Kavoosi G, Rowshan V. Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assa-foetida oleo-gum-resin: Effect of collection time. Food Chem. 2013;138:2180–7. doi: 10.1016/j.foodchem.2012.11.131. [DOI] [PubMed] [Google Scholar]
- 22.Kanani MR, Rahiminejad MR, Sonboli A, Mozaffarian V, Kazempour Osaloo S, Nejad Ebrahimi S. Chemotaxonomic significance of the essential oils of 18 Ferula species (Apiaceae) from Iran. Chem Biodivers. 2011;8:503–17. doi: 10.1002/cbdv.201000148. [DOI] [PubMed] [Google Scholar]
- 23.Guzmán-Gutiérrez SL, Gómez-Cansino R, García-Zebadúa JC, Jiménez-Pérez NC, Reyes-Chilpa R. Antidepressant activity of Litsea glaucescens essential oil: Identification of β-pinene and linalool as active principles. J Ethnopharmacol. 2012;143:673–9. doi: 10.1016/j.jep.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Iranshahi M. A review of volatile sulfur-containing compounds from terrestrial plants: Biosynthesis, distribution and analytical methods. J Essent Oil Res. 2012;24:393–434. [Google Scholar]


