Abstract
Negative functional magnetic resonance imaging (fMRI) response in the striatum has been observed in several studies during peripheral sensory stimulation, but its relationship between local field potential (LFP) remains to be elucidated. We performed cerebral blood volume (CBV) fMRI and LFP recordings in normal rats during graded noxious forepaw stimulation at nine stimulus pulse widths. Albeit high LFP–CBV correlation was found in the ipsilateral and contralateral sensory cortices (r=0.89 and 0.95, respectively), the striatal CBV responses were neither positively, nor negatively correlated with LFP (r=0.04), demonstrating that the negative striatal CBV response is not originated from net regional inhibition. To further identify whether this negative CBV response can serve as a marker for striatal functional recovery, two groups of rats (n=5 each) underwent 20- and 45-minute middle cerebral artery occlusion (MCAO) were studied. No CBV response was found in the ipsilateral striatum in both groups immediately after stroke. Improved striatal CBV response was observed on day 28 in the 20-minute MCAO group compared with the 45-minute MCAO group (P<0.05). This study shows that fMRI signals could differ significantly from LFP and that the observed negative CBV response has potential to serve as a marker for striatal functional integrity in rats.
Keywords: fMRI, isoflurane, local field potential, pain, striatum, stroke
Introduction
The striatum receives afferent inputs from cerebral cortex, ventromedial, ventrolateral, and mediodorsal thalamic nuclei and projects major efferent outputs to globus pallidus interna/externa and substantia nigra pars reticulata.1 It plays a pivotal role in several neurodegenerative disorders and regulating motor behavior. Recent reports showed that unilateral noxious forepaw electrical stimulation in rats evoked sustained negative blood oxygenation level dependent (BOLD), cerebral blood flow (CBF), and cerebral blood volume (CBV) functional magnetic resonance imaging (fMRI) responses in the bilateral striatum with no significant difference between two hemispheres,2, 3, 4, 5, 6 while the neuronal spike and c-Fos activities increased.6 This negative response has also been observed in epileptic rats during whisker stimulation7 or normal rats during direct nerve stimulation.8 Functional magnetic resonance imaging response has been shown to correlate better with local field potential (LFP) instead of spike;9 however, whether this particular negative fMRI response matches with LFP changes remains unclear.
Nociceptive stimuli have been shown to reliably evoke this intriguing negative fMRI responses in the rat striatum under alpha-chloralose anesthesia,2, 5, 6, 10 medetomidine sedation,4 or medetomidine combined with light isoflurane (0.4%) anesthesia.3 This stimulation model has potential to serve as a functional imaging marker for striatal functional integrity for various types of preclinical applications in rodents. Nevertheless, a stable model for longitudinal imaging of striatal function is missing. Alpha-chloralose is not an ideal anesthetic for survival study because of its gastrointestinal inflammatory effect,11 while the sedative effect of medetomidine is time dependent and is not ideal for studies with longer experimental time.12 In contrast, soflurane is the most widely used anesthetics for small animal imaging. Robust and repeatable fMRI responses have been shown in the primary somatosensory cortex (S1) in well-ventilated rats under isoflurane anesthesia.13, 14, 15 Whether the aforementioned negative striatal fMRI response can be observed under isoflurane anesthesia remain unknown. The ability to do so could have long-term applications to assess striatal function during disease progression as well as monitor treatment effects in vivo.
To these ends, we used a well-established CBV fMRI technique in mechanically ventilated rats under isoflurane anesthesia. Nine forepaw stimulus pulse widths (0.1, 0.3, 0.5, 1, 3, 5, 7, 10, and 30 milliseconds) were studied, with fixed 10 mA amplitude known to generate optimal CBV decreases2, 4, 6 and 12 Hz stimuli known to evoke robust CBF and LFP responses in the primary somatosensory cortex (S1) under 1% to 1.2% isoflurane anesthesia.13, 14, 16 Local field potentials were recorded at the ipsilateral S1 (iS1), contralateral S1 (cS1), and ipsilateral striatum (iCPu) in separate animals under identical experimental conditions to corroborate the fMRI findings. Contralateral CPu (cCPu) response was not recorded because of our hardware limitation and the fact that no difference was detected between the two hemispheres in this model.2, 3, 4, 5, 6 To investigate whether the striatal negative fMRI response can serve as a marker for striatal functional integrity, we used stimulation of 10 mA, 3 milliseconds pulse width, and 12 Hz in two groups of rats underwent 20- and 45-minute middle cerebral artery occlusion (MCAO) and followed the fMRI responses up to 28 days.
Materials and methods
Subjects
A total of 33 adult male Sprague-Dawley rats (weighing 250 to 300 g, 8 to 10 weeks old; Charles River Laboratories) were studied. All experimental procedures were approved by the Institutional Animal Care and Utilization Committee, UT Health Science Center at San Antonio and were performed under ARRIVE guidelines. Animals were housed in the vivarium (12:12-hour light–dark cycle, controlled humidity and temperature) with free access to food and water. In the first study (imaging striatal function), 11 normal rats were used to determine the optimal CBV fMRI responses with varied pulse widths under isoflurane anesthesia and 5 normal rats were used to investigate the corresponding LFP responses under the same experimental conditions. In the second study (imaging striatal functional recovery), two groups of 5 rats each were included to longitudinally study the striatal function and functional recovery with different occlusion time before and after stroke up to 28 days. Of note, 3 additional rats in the second study died before the last imaging time point (day 28) and were excluded from the sample size pool. In addition, a group of 4 rats were used to verify whether the striatal functional recovery has a neural origin and an additional group of 5 rats were used to study the effect of varying pulse width on mean arterial blood pressure (MABP) and heart rate.
Animal Preparation
Rats were initially anesthetized with 3% isoflurane and orotracheally intubated for mechanical ventilation (Model 683, Harvard Apparatus, South Natick, MA, USA). After the animal was secured in an MRI-compatible rat stereotactic headset, isoflurane was reduced to 1% to 1.2%. End-tidal CO2 was continuously monitored via a capnometer (Surgivet, Smith Medical, Waukesha, WI, USA) and kept between 3% and 3.5%. Noninvasive end-tidal CO2 values were calibrated against invasive blood-gas samplings under identical baseline conditions, resulting an arterial pCO2 of 37.6±4.7 mm Hg (mean±s.d., n=8).17 Rectal temperature was maintained at 37.0°C±0.5°C with warm-water circulating pad. Heart rate and blood oxygen saturation level were continuously monitored by a MouseOx system (STARR Life Science Corp., Oakmont, PA, USA). The heart rate was kept between 350 and 400 bpm and oxygen saturation level was maintained above 97%. An established CBV fMRI technique was employed18 by using high-dose 30-mg/kg monocrystalline iron oxide nanoparticles to eliminate potential BOLD contamination.
Stimulation
In the first study, two needle electrodes were inserted under the skin of the right forepaw: one between the first and second digits and the other between the third and fourth digits. These electrodes were then fixed with surgical tape and the stimulation was confirmed by observing digit twitching. Nine forepaw stimulus pulse widths (0.1, 0.3, 0.5, 1, 3, 5, 7, 10, and 30 milliseconds) were studied in a pseudo-random manner, with fixed 10 mA amplitude generating optimal CBV decreases2, 4, 5 and 12-Hz square wave providing robust CBF and LFP responses under 1% to 1.2% isoflurane anesthesia.13, 14, 16 This stimulation amplitude is known to induce pain and therefore striatal responses.3, 6 Strong digit and forelimb twitching was observed during noxious stimulation. However, no or noticeable motion artifact was detected. In addition, the subjects showed no physical damage in the ear canal at the end of the experiment, indicating the animal head was secured and stabilized by the ear bars during imaging, and the anesthesia level used in this study should be sufficient to suppress major motion. Two to five repeated trials were made for each pulse width. In the second study (imaging striatal functional recovery), needle electrodes were inserted under the skin of the left and right forepaw, such that both forepaws were stimulated simultaneously in series at 10 mA, 12 Hz, and 3 milliseconds pulse width. To evaluate the effect of varying stimulus pulse width, MABP and heart rate were measured via a PE-50 tubing cannulated to femoral artery. For all stimulations, at least 3 minutes break was given between trials.
Magnetic Resonance Imaging Experiments
In the first study (imaging striatal function) on normal rats (n=11), MR images were acquired on a 7 T, 30-cm bore magnet and a 40 G/cm gradient (Bruker, Billerica, MA, USA). A custom-made circular surface coil (ID ∼2 cm) was placed on the rat head. Magnetic field homogeneity was optimized using standard FASTMAP shimming with first-order shims on an isotropic voxel of 7 × 7 × 7 mm encompassing the imaging slices. A T2-weighted pilot image was taken in the midsagittal plane to localize the anatomic position by identifying the anterior commissure (bregma −0.8 mm).19 Cerebral blood volume fMRI was acquired with single-shot gradient echo-planar imaging sequence using spectral width=300 kHz, repetition time/echo time=1,000/13.8 milliseconds, partial Fourier acquisition=5/8, field of view=2.56 × 2.56 cm2, slice thickness=1.5 mm, number of slice=7, and matrix=96 × 96. Functional magnetic resonance imaging paradigm included 60 sets of echo-planar images before stimulation, 30 sets during stimulation, and 60 sets after stimulation.
In the second study (imaging striatal functional recovery), rats were subjected to 20-minute (n=5) and 45-minute (n=5) MCAO. Reperfusion was performed by withdrawing the occluder into the external carotid artery, restoring blood flow to the brain. To achieve 20-minute MCAO, we unfortunately could not perform CBF MRI before reperfusion. For the 20-minute MCAO group, MR images were longitudinally acquired at control time point, (3 to 5 days before MCAO), on day 0 (immediately after MCAO and reperfusion), day 7, and day 28 after MCAO (total of 20 MRI experiments). Imaging time points were essentially the same for the 45-minute MCAO group except the control (pre-MCAO) data were not acquired (total of 15 MRI experiments).
In addition to the fMRI, quantitative CBF and apparent diffusion coefficient (ADC) were measured before monocrystalline iron oxide nanoparticle injection and fMRI runs. CBF was acquired by continuous arterial spin-labeling technique with a separate neck coil for labeling using single-shot, gradient echo-planar imaging, spectral width=300 kHz, repetition time/echo time=3,000/10.3 milliseconds, partial Fourier acquisition=5/8, labeling duration=2.46 seconds, postlabeling delay=0.25 seconds, field of view=2.56 × 2.56 cm2, slice thickness=1.5 mm, and acquisition matrix=96 × 96. Sixty pairs of images were acquired for signal averaging. Diffusion-weighted images were acquired with single-shot, spin-echo, echo-planar imaging along 30 diffusion encoding directions with b value=0 and 1.2 milliseconds/μm2 with same localization and resolution as CBF. Other imaging parameters were: δ/Δ=4/16 milliseconds, repetition time=3,000 milliseconds, and echo time=35 milliseconds. Acquisitions were repeated four times to increase signal-to-noise ratio.
Electrophysiology Recording
In the first study (imaging striatal function), electrophysiology was performed in a separate group of rats (n=5). The procedure was essentially the same as that described previously.20 A stereotaxic frame (Model 900, David Kopf Instruments, Tujunga, CA, USA) was used to position the head. The animal physiologic conditions were kept the same as the MRI experiment, except craniotomies were performed to implant recording electrodes. A stainless steel screw was placed 2-mm caudal to lambda as a reference electrode. After the dura was removed, custom designed polyimide-based 16-channel microelectrode arrays21 with inter-contact spacing of 74 μm were implanted to cS1, iS1 (coordinates from bregma: anterior-posterior +0.5 mm; medial-lateral ±4 mm; and ventral-dorsal −2 mm; approached with a coronal angle of 22.5°), and CPu (anterior-posterior −0.8 mm; medial-lateral +4 mm; and ventral-dorsal −6.5 mm). Data were sampled from the contact lead located at cortical layer V (∼VD −1.4 mm) or the dorsal striatum (∼VD −5.9 mm), and filtered on a preamplifier between 0.3 and 500 Hz and sampled at 1 kHz. Data were acquired using a Cerebus multichannel data acquisition system (Blackrock Microsystems, Salt Lake City, UT, USA). At the end of the experiment, a 30-μA direct current was delivered to the deepest contact lead for 10 seconds to label the recording site. T2-weighted MRI scans were performed to confirm the recording site. This method is comparable to histology as it is sufficient to confirm the electrode tip in the target area. In the second study (imaging striatal functional recovery), LFP was measured in a separate group of rats (n=4), underwent 20-minute MCAO to verify whether neuronal recovery can be observed. The recording parameters were identical to the first study, but with only one electrode implanted into the iCPu and stimulation of 10 mA, 12 Hz, and 3-millisecond pulse width was used. Recording was performed longitudinally at four time points identical to fMRI studies (control, day 0, day 7, and day 28).
Data Analysis
Image analysis was performed using a custom-written program22 in Matlab (Math-Works, Natick, MA, USA) and statistical parametric mapping. Automatic coregistration using statistical parametric mapping codes were applied to realign time-series data to the first time point within subjects using and then again across subjects. Correlation coefficient analysis was performed on a pixel-by-pixel basis to correlate MR signal changes with electrical stimulation paradigm with a significant threshold of P<0.05 (Bonferroni corrected). Spatial averaging across subjects was performed to generate averaged correlation coefficient map after atlas-based coregistration as described previously,22 where CBV increases and decreases are indicated by red–yellow and blue–green colors, respectively. The stimulus-evoked ΔR2* value, which varies linearly with the stimulus-evoked CBV fraction, was calculated as follows after monocrystalline iron oxide nanoparticles injection: 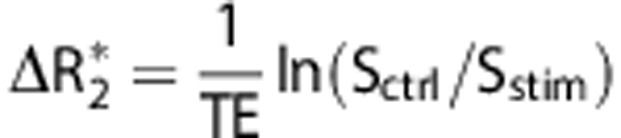 , where Sctrl and Sstim are the MR signal intensities before and during stimulation, respectively. CBF was calculated as:
, where Sctrl and Sstim are the MR signal intensities before and during stimulation, respectively. CBF was calculated as:  , where SC and SL are the MR signal intensities from the control and labeled images, respectively; λ is the water brain–blood partition coefficient, T1 is that of tissue, and α is the arterial spin-labeling efficiency. The values of λ, T1, and α were 0.9, 1.8 seconds, and 0.7, respectively. Apparent diffusion coefficient maps were calculated by using: ADC=In(S0/S1)/(b1−b0), where S1 is the signal intensity obtained with b1 (1.2 milliseconds/μm2) and S0 is the signal intensity obtained with b0 (0 milliseconds/μm2). Regions of interest (ROIs) were placed on the cS1, iS1 (both 8 × 8 pixels) and the iCPu (13 × 8 pixels) to extract fMRI time-course data after the images are coregistered. No apparent spatial shift was observed at different stimulus pulse widths. Therefore data were analyzed with the same ROIs across trials. Slice at bregma −0.8 mm was selected for subsequent analysis because this is the primary area being affected by MCAO and strong cortical and striatal forepaw fMRI responses were also observed at this location.
, where SC and SL are the MR signal intensities from the control and labeled images, respectively; λ is the water brain–blood partition coefficient, T1 is that of tissue, and α is the arterial spin-labeling efficiency. The values of λ, T1, and α were 0.9, 1.8 seconds, and 0.7, respectively. Apparent diffusion coefficient maps were calculated by using: ADC=In(S0/S1)/(b1−b0), where S1 is the signal intensity obtained with b1 (1.2 milliseconds/μm2) and S0 is the signal intensity obtained with b0 (0 milliseconds/μm2). Regions of interest (ROIs) were placed on the cS1, iS1 (both 8 × 8 pixels) and the iCPu (13 × 8 pixels) to extract fMRI time-course data after the images are coregistered. No apparent spatial shift was observed at different stimulus pulse widths. Therefore data were analyzed with the same ROIs across trials. Slice at bregma −0.8 mm was selected for subsequent analysis because this is the primary area being affected by MCAO and strong cortical and striatal forepaw fMRI responses were also observed at this location.
Electrophysiology data were analyzed by using a custom-written program in Matlab. The 30-second raw LFP data were split into 360 sweeps of 83 milliseconds poststimulus periods based on the stimulus time stamps (12 Hz). The averaged evoked LFP was summated during the 83 milliseconds poststimulus period, denoted as ΣLFP. Data were then averaged across subjects to provide group-averaged responses.
Statistical analysis was performed by SPSS software (IBM, Armonk, NY, USA). Paired t-test was used to compare the responses between hemispheres. Repeated-measures analysis of variance with Fisher's post hoc test was used to compare stimulus-evoked changes in ΔR2* and ΣLFP values in the first study, and the ΔR2*, ADC, CBF, and ΣLFP values at different time points in the second study. The significance level was set at P<0.05.
Ethics Statement
All studies reported followed the guidelines of the OLAW (Office of Laboratory Animal Welfare) Guide for the Care and Use of Laboratory Animals as well as the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. The experiments were approved by the University of Texas Health Science Center IACUC. The manuscript was written in accordance with the ARRIVE guidelines.
Results
Striatal and Cortical Neurovascular Function in Normal Subjects
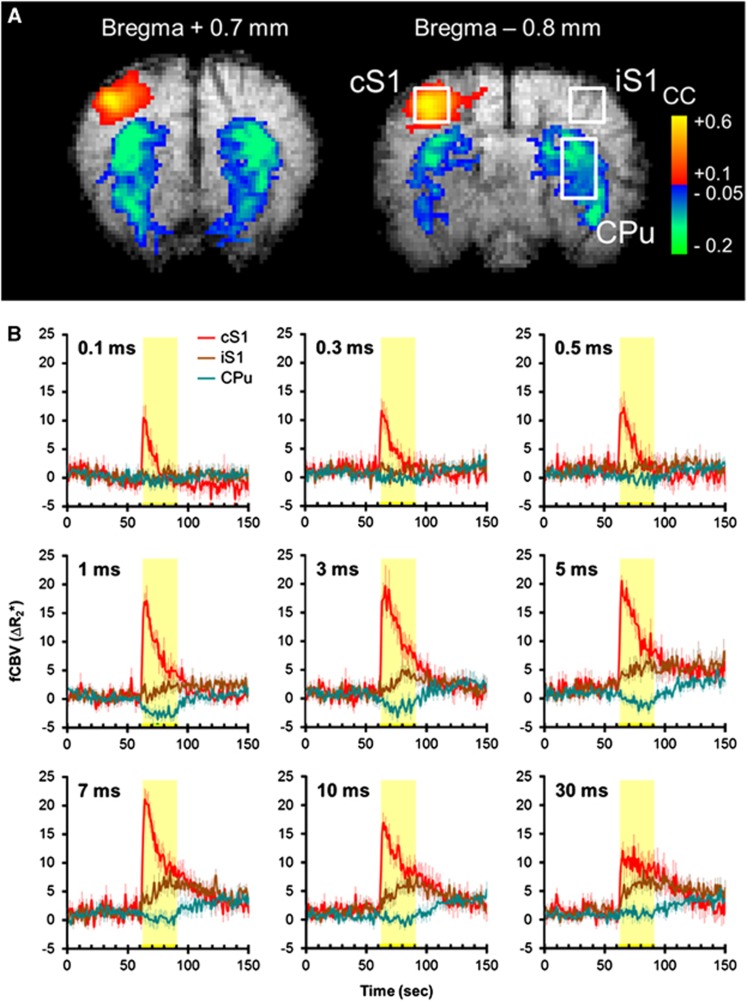
Table 1 shows the effect of graded forepaw stimulation on MABP and heart rate. Representative CBV fMRI images at bregma +0.7 and −0.8 mm are shown in Figure 1A. Stimulation at right forepaw with 10 mA, 12 Hz, and 3 milliseconds pulse width resulted in significant CBV increases in the cS1 and CBV decreases in bilateral CPu. Figure 1B shows group-averaged functional CBV time-course data under nine different pulse widths (0.1 to 30 milliseconds; n=11). Our previous and current findings showed no significant side-to-side difference of striatal signals between both hemispheres under this stimulation model and therefore the CPu time-course data were only discussed on the ipsilateral side. Cerebral blood volume in the cS1 responded immediately to the stimulation, showing an overshoot at the stimulus onset across all stimulation conditions that gradually decreased over time. In contrast, CBV in the CPu responded comparatively slower to the stimulation and was only apparent at 1 to 5 milliseconds pulses.
Table 1. Effect of stimulus pulse width on blood pressure and heart rate (mean±s.e.m.).
| Pulse width |
MABP (mm Hg) |
HR (beats per minute) |
||
|---|---|---|---|---|
| Baseline | Stimulation | Baseline | Stimulation | |
| 0.1 | 122.3±3.8 | 125.4±3.2 | 406±6.8 | 408.7±6.1 |
| 0.3 | 118.2±3.6 | 122.8±5.1 | 409.6±7.6 | 418.7±9.6 |
| 0.5 | 120.6±5.1 | 129.3±4.3 | 417.9±6.4 | 424.7±7.3 |
| 1 | 116.4±5.4 | 128.4±3.4 | 416.5±7.4 | 432.4±8.6 |
| 3 | 116.4±5.9 | 129.6±4.2 | 406.4±10.3 | 423.6±11.9 |
| 5 | 120.7±5.3 | 137.4±2.5a | 407.2±10.1 | 426.6±13.4 |
| 7 | 119.4±4.4 | 134.6±1.8a | 414.5±8.7 | 434.1±9.1 |
| 10 | 117±4.6 | 134.9±3.3a | 418.5±6.3 | 437.7±8.8 |
| 30 | 122.6±4.4 | 136.7±8.9a | 409.4±9.9 | 439±14.6 |
Abbreviations: MABP, mean arterial blood pressure; HR, heart rate.
denotes P<0.05.
Figure 1.
Cerebral blood volume functional magnetic resonance imaging (CBV fMRI) of right forepaw stimulation under isoflurane anesthesia. (A) Representative fMRI responses at two adjacent slices. Stimulation was 10 mA, 12 Hz, and 3 milliseconds. Stimulation-evoked CBV elevation was evident in the contralateral S1 (cS1), whereas CBV reduction was evident in the CPu of both hemispheres. (B) Group-averaged CBV fMRI time courses in the cS1, ipsilateral S1 (iS1), and ipsilateral striatum (CPu; n=11). Regions of interest are shown in A. The yellow-shaded regions indicate stimulation period. Stimuli fixed at 10 mA and 12 Hz. The intensity of the time-course data (ΔR2*) is linearly related to CBV fraction, in a unit of per second. Error bars are s.e.m. values.
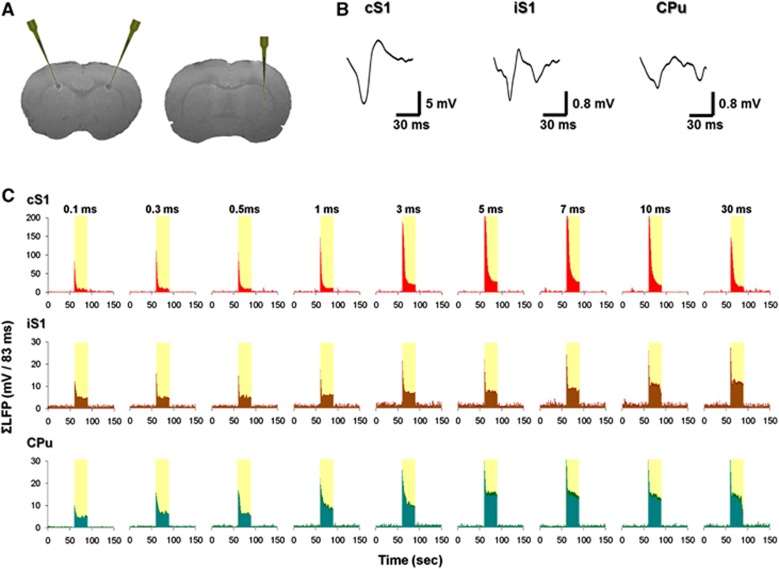
Under identical experimental condition, LFPs were recorded at the cS1, iS1, and CPu (Figure 2A). Typical LFP waveforms are shown in Figure 2B. In the cS1, LFP peaked at 50 milliseconds after stimulus onset. This early negative peak was also observed in the iS1 and CPu. Around 100 milliseconds after stimuli onset, a late negative peak was also recorded in the iS1 as well as in the CPu with further delay.23 For all the nine pulse widths tested in this study, averaged ΣLFP time courses showed a clear overshoot at the stimulus onset and decreased over time in the cS1, iS1, and CPu (Figure 2C). Increased ΣLFPs were observed in the CPu, which does not match to the trend of CBV changes.
Figure 2.
Local field potential (LFP) recording of right forepaw stimulation under isoflurane anesthesia. (A) T2-weighted magnetic resonance imaging scans showing the recording site. At the end of the experiment, a 30-μA direct current was delivered to the deepest contact lead for 10 seconds for each electrode. Data were recorded at cortical layer V, ∼0.6 mm above the lesion site. (B) Typical averaged LFP waveforms in the contralateral S1 (cS1), ipsilateral S1 (iS1), and ipsilateral striatum (CPu). (C) Group-averaged ΣLFP time courses in the cS1, iS1, and CPu (n=5). Stimuli fixed at 10 mA and 12 Hz. Error bars (labeled in deeper colors) are s.e.m. values.
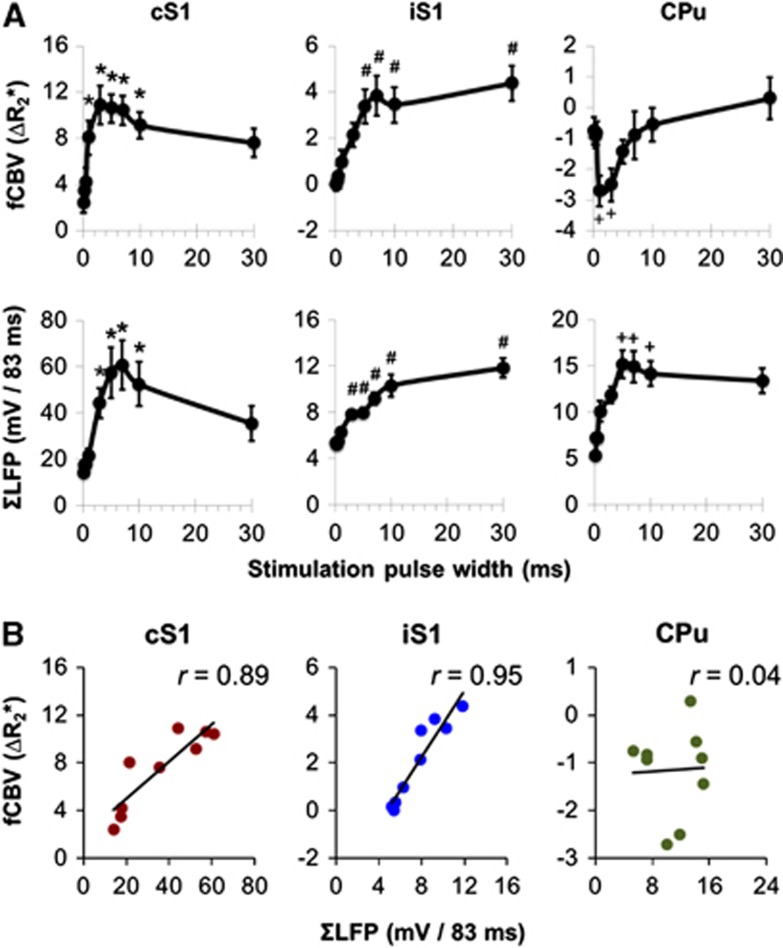
Figure 3A compared CBV fMRI and ΣLFP data in cS1, iS1, and CPu under nine stimulation pulse widths. In the cS1, both CBV and ΣLFP increased with pulse width (P<0.05), reaching a peak around 3 to 10 milliseconds, and then decreased at 30 milliseconds pulses (P<0.05). In the iS1, both CBV and ΣLFP increased with stimulus pulse width and reaching a plateau ∼3 to 5 milliseconds (P<0.05). The peak CBV and ΣLFP responses were ∼3 and 5 times lower compared with the responses in cS1, respectively. In the CPu, CBV responses were mostly negative and peaked ∼1 to 3 milliseconds (P<0.05), while the ΣLFP responses were mostly positive and peaked ∼5 to 10 milliseconds (P<0.05), similar to that in the cS1. Significant correlation was found between CBV and ΣLFP in the cS1 (r=0.89, P<0.05) and the iS1 (r=0.95, P<0.05), but not in the CPu (r=0.04, P>0.05; Figure 3B).
Figure 3.
Comparison of cerebral blood volume functional magnetic resonance imaging (CBV fMRI) and ΣLFP. (A) Pulse-width-dependent CBV fMRI and ΣLFP responses. P<0.05 indicates statistical significance. *different from 0.1 to 0.5 milliseconds; #different from 0.1 to 1 milliseconds; +different from 0.1 to 0.5 and 30 milliseconds. (B) Correlation analysis revealed significant neurovascular coupling in the contralateral S1 (cS1) and ipsilateral S1 (iS1; both P<0.05), but not in the ipsilateral striatum (CPu; P>0.05). LFP, local field potential.
Striatal and Cortical Apparent Diffusion Coefficient and Cerebral Blood Flow Changes after Stroke
The group-averaged ADC and CBF maps in the 20- and 45-minute MCAO groups (n=5 each) are shown in Figure 4 and their temporal evolution were analyzed for the cortical and striatal ROIs as shown in Figure 6.
Figure 4.
Group-averaged cerebral blood flow (CBF) and apparent diffusion coefficient (ADC) maps at different time points. Middle cerebral artery occlusion (MCAO) was performed to induce stroke on the right hemisphere; (A) 20-minute MCAO group (n=5) and (B) 45-minute MCAO group (n=5).
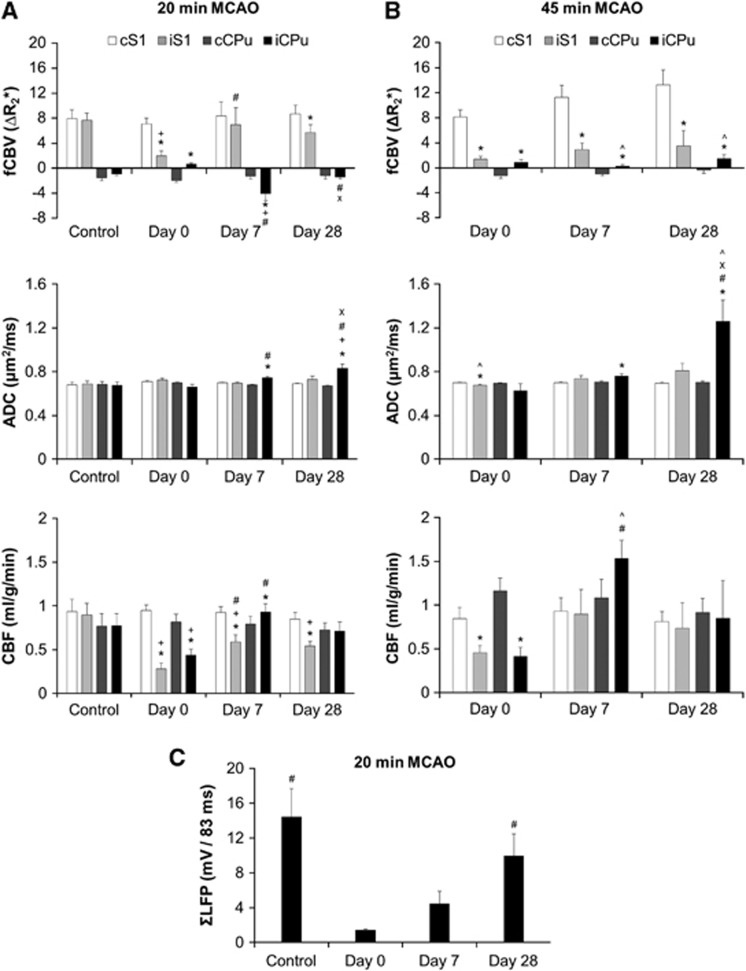
In the 20-minute MCAO group, reperfusion restored ADC values in the ROIs and showed no significant difference compared with the contralateral hemisphere on day 0 (P>0.05). This may be because the ischemic lesion core was only partially included in the iCPu ROI and primarily located at the external globus pallidus. Ipsilateral striatum showed higher ADC on day 7 and day 28 (P<0.05). Cerebral blood flow values in the iS1 and iCPu decreased significantly on day 0 compared with the contralateral side and the prestroke control (P<0.05). CBF in the iS1 increased on day 7 compared with day 0 (P<0.05), but was still significantly lower than cS1 (P<0.05). Cerebral blood flow in the iCPu increased on day 7 compared with day 0 (P<0.05) and was significantly higher than cCPu (P<0.05). On day 28, CBF in the iCPu was recovered and was not significantly different from cCPu (P>0.05), while the CBF in the iS1 was still significantly lower than the prestroke control and the cS1 (P<0.05).
In the 45-minute MCAO group, significant ADC decrease in the iS1 was found immediately after reperfusion on day 0 (P>0.05). Similar to the 20-minute MCAO group, iCPu also showed a slightly lower ADC compared with control, but was not significantly different from the cCPu (P>0.05). Ipsilateral striatum showed higher ADC on day 7 and day 28 (P<0.05). On day 28, the ADC was significantly higher than that in the 20-minute MCAO group (P<0.05). Cerebral blood flow values in both the iS1 and iCPu were significantly lower on day 0 compared with the contralateral side (P<0.05). On day 7, significant hyper-perfusion was found in the iCPu compared with day 0 as well as to the matched time point in the 20-minute MCAO group (P<0.05). Hyper-perfusion in the cortex was apparent on the lateral side of the brain and the boundary of the hyper-perfused area was close to iS1 ROI. No hyper-perfusion was found on day 28 (P>0.05).
Striatal and Cortical Functional Cerebral Blood Volume Changes after Stroke
In the 20-minute MCAO group (n=5), stimulation at 12 Hz, 10 mA, and 3 milliseconds pulse width was applied to both forepaws. At the control time point, bilateral CBV increases were shown in the S1 and bilateral CBV decreases was shown in the CPu (Figure 5A and Figure 6A). After stroke, the CBV fMRI data acquired immediately after reperfusion (day 0) showed intact functional responses in the area contralateral to the stroke side (cS1 and cCPu), while the stimulus-evoked CBV increases in the iS1 reduced significantly compared with the contralateral side and the iS1 response acquired at the prestroke control time point (P<0.05). The CBV decreases in the iCPu was completely eliminated on day 0 (P<0.05). Compared with day 0, the iS1 response recovered significantly on day 7 (P<0.05). No significant difference was found between cS1 and iS1 responses on day 7 (P>0.05), but only on day 28 (P<0.05). Interestingly, iCPu was hyper-reactive and showed stronger CBV decreases compared with the contralateral side, prestroke control data, and day 0 (P<0.05). On day 28, the iCPu response was weaker than that on day 7 (P<0.05) and showed no significant difference compared with control (P>0.05).
Figure 5.
Cerebral blood volume functional magnetic resonance imaging (CBV fMRI) responses of the (A) 20-minute middle cerebral artery occlusion (MCAO) group (n=5) and (B) 45-minute MCAO group (n=5). CBV fMR images are expressed as coregistered group-averaged maps of the correlation coefficient. Stimulation was applied simultaneously at two forepaws with 10 mA, 12 Hz, and 3 milliseconds pulse width. Control images were acquired 3 to 5 days before stroke. Images acquired immediately after MCAO and reperfusion was designated as day 0. Averaged CBV fMRI time courses at corresponding time points are shown below. Regions of interest are shown in inset. The yellow-shaded regions indicate stimulation period. The unit of y-axis is in per second. Error bars are s.e.m. values. cCPu, contralateral CPu; cS1, contralateral S1; iCPu, ipsilateral striatum; iS1, ipsilateral S1.
Figure 6.
CBV functional magnetic resonance imaging response, ADC, and CBF in (A) 20-minute MCAO (n=5) and (B) 45-minute MCAO group (n=5). The regions of interest are the same as shown in Figure 4B. (C) ΣLFP in 20-minute MCAO (n=4), where the recording electrode was implanted into the iCPu. P<0.05 indicates statistical significance. *different from contralateral side; +different from control; #different from day 0; Xdifferent from day 7; ^different from the matched time point in the 20-minute MCAO group. Error bars are s.e.m. values. ADC, apparent diffusion coefficient; CBF, cerebral blood flow; CBV, cerebral blood volume; cCPu, contralateral CPu; cS1, contralateral S1; fCBV, functional CBV; iCPu, ipsilateral striatum; iS1, ipsilateral S1; LFP, local field potential; MCAO, middle cerebral artery occlusion.
In the 45-minute MCAO group (n=5), identical stimulation was applied (Figure 5B and Figure 6B). Similar to the 20-minute MCAO group, the CBV fMRI data, acquired immediately after reperfusion (day 0), showed no apparent functional response in both iS1 and iCPu compared with the intact side (P<0.05). However, the iS1 and iCPu responses did not recover up to 28 days. Significant difference was found between the two hemispheres at all time points (P>0.05). Although, there is a tendency that the responses in the iS1 and cS1 gradually increased over time, no significant difference was detected. Compared with the 20-minute MCAO group, the CBV decreases in iCPu was significantly less on day 7 and day 28 (P<0.05).
Striatal Local Field Potential Changes after Stroke
To verify whether striatal neuronal function is recovered, LFPs were recorded at the iCPu in a separate group of animals, underwent 20-minute MCAO (n=4). Identical stimulation parameters were used. ΣLFP at the control time point showed no statistical difference compared with the normal control data in the first study (see Figure 3A, P>0.05). ΣLFP decreased significantly after stroke (day 0, P<0.05). On day 7, ΣLFP showed a trend of recovery, but did not reach a significant level (P=0.07). On day 28, significant functional recovery was observed compared with day 0 (P<0.05) and no significant difference was detected compared with prestroke control time point (Figure 6C).
Discussion
This study established a longitudinal fMRI protocol to study the rat striatum under isoflurane anesthesia. In normal rats, neurovascular function in the cortex and the striatum was evaluated by correlating CBV fMRI response with LFP results. This fMRI procedure was further applied to study striatal functional recovery in an ischemic stroke model with different occlusion time. The major findings were: (i) Cortical and striatal fMRI responses can be reliably and longitudinally obtained in rats under isoflurane anesthesia; (ii) the positive CBV responses in the S1 peaked ∼3 to 10 milliseconds pulse width when 12 Hz and 10 mA stimulation was applied, while the negative CBV responses in the CPu peaked ∼1 to 3 milliseconds pulse width; (iii) strong CBV–LFP correlations as a function of pulse width were observed in the cS1, iS1, but not in the CPu; (iv) this fMRI procedure provided additional information than ADC and CBF to characterize potential tissue abnormalities, offering a means to evaluate striatal dysfunction in stroke rats; (v) both fMRI and LFP responses were diminished immediately after stroke and recovered on day 28; and (vi) fMRI data showed that the 20-minute MCAO group had better recovery than 45-minute MCAO group.
Neurovascular Responses in the Striatum
The striatum involves in various aspects of brain signaling. We previously found that unilateral noxious electrical stimuli at the rat forepaw reliably evoked bilateral negative CBV responses in the striatum under alpha-chloralose anesthesia.6 These responses correlated with increased neuronal spike activity and increased Fos-immunoreactive cells. Consistent with the negative CBV responses, negative BOLD2, 3, 4, 7, 8 and CBF fMRI changes2 were also found in the striatum during stimulation. These data implied a unique control of the striatal circulation and the mechanism of action is potentially via direct control of local vasculature rather than conventional neurovascular coupling because striatal spike activity6 or metabolism2 were not reduced during stimulation. It has been shown that pain signaling may trigger this local vascular control. Morphine, a μ-opioid receptor agonist and a commonly used pain reliever, enhanced the stimulus-evoked negative striatal CBV responses and naloxone, a μ-opioid receptor antagonist, eliminated the evoked striatal fMRI responses.5 Antagonism of dopamine D2-like receptor markedly enhanced pain-related behavior.24 This was not surprising because opioid and dopamine D2 systems orchestrate closely. Acute upregulation of D2-like, but not D1-like receptors was found to occur after administration of μ-opioid receptor agonist.25 Positron-emission tomography studies using dopamine D2-like radioligands also support this notion by showing that administration of a μ-opioid receptor agonist significantly increased D2-like receptor binding in both the cortical and the striatal areas.26
Although, little is known about this unique hemodynamic regulation in the striatum, activation of D2-like receptors has been shown to induce striatal CBV decreases.27 Injection of D2-like receptor antagonist also reduced the striatal negative fMRI responses evoked by noxious forepaw stimuli.5, 6, 10 These results, in line with an earlier report,28 implied that dopamine and its receptors can regulate regional microcirculation. We recently showed that the striatal CBV decreases in this stimulation model is associated with the integrity of the dopaminergic innervation.10 The dopaminergic afferents in the striatum were selectively lesioned by injecting the 6-hydroxydopamine to the substantia nigra pars compacta, which sends long projecting dopaminergic efferent fibers to the striatum. Our data showed that the stimulus-evoked striatal CBV reduction was almost eliminated on the lesion side, where the cCPu still showed intact fMRI response. The remaining fMRI response showed very high spatial correlation with the residual dopaminergic fibers as revealed by tyrosine hydroxylase immunostaining.
In this study, we further investigated the striatal fMRI responses with modulation of nine different pulse widths and showed that the striatal CBV reduction is also evident under isoflurane anesthesia at certain pulse widths. Frequency, amplitude, and pulse width are critical parameters for electrical stimuli. It has been shown that 12-Hz forepaw stimuli evoked robust BOLD and CBF changes in the S1 under isoflurane anesthesia compared with other frequencies (3, 6, and 20 Hz).14 The striatal CBV reduction monotonically enhanced with the stimulus amplitude under different anesthetics/sedative.2, 4, 6 However, the stimulus pulse width that determines the amount of charge delivered has not been previously evaluated. It has been shown that at different stimulus pulse widths, the nerve fibers recruited by the stimuli were also different.29 A typical example is that peripheral sensory fibers are more effectively evoked with longer pulse width than motor fibers.30
Our fMRI data showed that the cS1 CBV response exhibited two phases during pulse-width modulation: in phase 1, the responses increased with pulse width and peaked ∼3 milliseconds. In phase 2, the responses decreased until 30 milliseconds (the longest pulse width measured in this study). The increasing response in phase 1 was because of a larger amount of charges being delivered to the forepaw and potentially recruited more sensory units sending action potentials to the S1 region. In contrast, the decreasing response in phase II may be because of effective refractory period since long pulse width has been shown to extend the recovery time course from neural refractoriness.31 This was also evident in our LFP measurements, showing reduced ΣLFP (less synaptic currents being generated) in the cS1 at 30 milliseconds pulse width. Similarly, the fMRI response in the iS1 increased with pulse width, but it did not decline up to 30 milliseconds pulse width. Cerebral blood volume response at the iS1 can be evoked by the pain pathway that projects bilaterally, subsequently evoked by the cS1 via callosal projections, or because of nonspecific cardiovascular effect.32, 33, 34 It has been shown that transient blood pressure changes could affect fMRI signals and the stimulus-evoked activation could overlap with MR signal changes because of MABP increases.33 Despite our electrophysiological data showed changes in LFP, and the fMRI responses are highly localized in the target area of interest. We cannot rule out the potential contribution of cardiovascular effects on all of the fMRI data presented herein. The ΣLFP peak response in the iS1 was several times lower than that the contralateral side and the responses were far from fully saturated, so the group neural refractoriness was not obvious. Both the fMRI pulse-width modulation curves in the cS1 and iS1 were highly correlated with the ΣLFP. These data from the cortex also served as within-subject controls for our measurement in the striatum.
Interestingly, the striatal CBV response showed a band-pass shape in pulse-width modulation and was only evident at 1 to 3 milliseconds pulse widths. Little or no CBV reduction was observed with extremely short (0.1 milliseconds) or long pulses (30 milliseconds). The mechanism leading reduced negative CBV response at long pulse width is not elusive and warrants further investigation. fMRI pulse-width modulation curve in the striatum did not correlate with the corresponding LFP measurement. During the modulation of pulse width, ΣLFP responses were positive, while the fMRI responses were mostly negative. The shape of the fMRI pulse-width modulation curve may be attributed to the nonspecific cardiovascular effect, whereby longer stimulus pulse width may evoke subsequent positive hemodynamic responses and eliminate striatal negative CBV changes. It is also plausible that the D2 signaling cascade in the basal ganglia circuit simply preferred 1 to 3 milliseconds pulse width under our experimental condition. We speculate that the negative fMRI response in the striatum is resulted from the process of the local vasculature that overrides the existing neurovascular coupling because fMRI responses were neither positively, nor negatively correlated with the ΣLFP. Given the striatum has dense and uniform distribution of the dopamine D2 receptors,35 this unique signal pattern therefore has the opportunity to serve as an imaging marker to evaluate striatal function and functional recovery in vivo. On a separate note, although CBV responses were consistently observed in the S1 and CPu under our stimulation protocol, CBV response in the thalamus was not detected. The S1 and striatum are both important regions in the pain network,22, 36, 37 in which the S1 is in the lateral pain system discriminating the location and intensity of the stimuli,38 whereas the striatum receives strong projections from the medial pain system.39 We did not detect thalamic activation, consistent with a prior study in rats.36 The absence of thalamic activity could be because of the effect of isoflurane on suppressing thalamic activity40 and/or regional dependence of neurovascular coupling.41
Striatal Functional Recovery after Stroke
Our data showed that forepaw electrical stimuli at 12 Hz, 10 mA, and 3 milliseconds pulse width generated robust striatal CBV reduction under isoflurane anesthesia. Three milliseconds pulse width also generated robust positive fMRI responses in the cortex, so the stimulus parameters do not need to be compromised for assessing the function of cortical or striatal region. This protocol is noninvasive and can be used longitudinally.
To show the feasibility of using this stimulus protocol for assessing striatal functional recovery in a longitudinal manner, two groups of rats underwent MCAO (20 and 45 minutes, respectively) followed by reperfusion were used. The stimulation protocol is essentially identical except both forepaws were stimulated simultaneously in series to evoke S1 activation at both hemispheres. Apparent diffusion coefficient and CBF showed similar trend as our previous reports.42 In the 45-minute MCAO group, postischemic hyper-perfusion was found on day 7 and striatal ADC was significantly higher on day 28 compared with the 45-minute MCAO group. As expected, there was a tendency that the 20-minute MCAO group (Figure 4) showed better functional activation and more robust fMRI time course in the iS1 at chronic time points (day 7 and day 28) compared with the 45-minute MCAO group (Figure 5), indicating a better functional recovery. Similarly, the striatal response also showed better recovery in the 20-minute MCAO group. The ipsilateral negative CBV responses in the striatum were quantitatively stronger on day 7 and day 28 in the 20-minute MCAO group. The between-group difference in the iS1 did not reach statistical significance. This may be because of the nature of the MCAO model that the boundary of the ischemic-reperfusion insult is usually at the forepaw area of S1 in our experimental condition.42 Functional recovery/reorganization of the somatosensory cortex after the stroke has been studied extensively42, 43, 44, 45, 46 and it was expected that shorter MCAO resulted in significantly better functional recovery.
In the 20-minute MCAO group, the ipsilateral striatal negative fMRI responses were diminished on day 0, hyper-reactive on day 7, and recovered on day 28. The abnormality of striatal fMRI responses after stroke could represent impairments of the vascular reactivity or neural dysfunction. Our data suggested that this abnormality could have a neural origin as the LFP response was also diminished on day 0 and recovered on day 28. However, a more thorough investigation on this topic is required to identify the cellular underpinnings of the negative fMRI signals in disease states. In the 45-minute MCAO group, the striatal negative fMRI signals did not recover on day 28, showing that these signals can be manipulated by disease severity. We suspect that the enhanced-negative fMRI response on day 7 in the 20-minute MCAO group is similar to our previous findings in normal subjects, because of the dopamine receptor function. Striatal D2-like receptor has been reported to upregulate in Parkinson's disease so as to compensate the reduced dopamine release in the striatum.47 In contrast, the D2 receptor has been found to downregulate when the animals were chronically exposed to quinpirole (a D2-like agonist) for 6 days.48 In a rat model of MCAO, newly generated striatal neurons expressing dopamine D2-like receptors was found to develop new efferent projections to the substantia nigra in the adult rat brain after stroke,49 indicating a potential involvement of the D2-expressing neurons in restoring striatal function. A potential limitation of the present study is that bilateral forepaws were stimulated simultaneously to minimize the scan time for stroke rats. This, however, precluded the opportunity to identify whether the striatal hypersensitivity in the 20-minute MCAO group on day 7 was in result of unilateral or interhemispheric reorganization.
Conclusions
This study established an fMRI procedure to longitudinally study striatal neurovascular function under isoflurane anesthesia and showed a novel fMRI application to investigate the striatal functional integrity after 20- or 45-minute MCAO. One advantage of this procedure is that no receptor-specific pharmaceutical ligand is involved and the response is evoked via peripheral stimuli reflecting endogenous brain signaling. High CBV–LFP correlation was found in the somatosensory cortex, but not in the striatum during stimulus pulse-width modulation. Our data unambiguously showed that negative CBV response could appear with positive LFP changes. We suspect that the negative fMRI response in the striatum originates from direct modulation of local vascular activity and overrides the activation-induced hyperemia. Our approach offers a means to evaluate striatal dysfunction and functional recovery in animal models. Future studies will combine optogenetic and pharmacogenetic techniques to dissect the contribution of different striatal neurons to the negative fMRI signals.
The authors declare no conflict of interest.
Footnotes
This work was supported in part by the American Heart Association (10POST4290091), Clinical Translational Science Awards (CTSA, parent grant UL1RR025767), and San Antonio Area Foundation to Dr Yen-Yu Ian Shih and the NIH/NINDS R01 NS45879 and VA MERIT to Dr Timothy Q Duong.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Shih YY, Wey HY, De La Garza BH, Duong TQ. Striatal and cortical BOLD, blood flow, blood volume, oxygen consumption, and glucose consumption changes in noxious forepaw electrical stimulation. J Cereb Blood Flow Metab. 2011;31:832–841. doi: 10.1038/jcbfm.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Welsh D, Williams M, Coimbra A, Urban MO, Hargreaves R, et al. fMRI of pain processing in the brain: a within-animal comparative study of BOLD vs. CBV and noxious electrical vs. noxious mechanical stimulation in rat. Neuroimage. 2012;59:1168–1179. doi: 10.1016/j.neuroimage.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage. 2008;39:248–260. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Chiang YC, Shyu BC, Jaw FS, Duong TQ, Chang C. Endogenous opioid-dopamine neurotransmission underlie negative CBV fMRI signals. Exp Neurol. 2012;234:382–388. doi: 10.1016/j.expneurol.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, et al. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. J Neurosci. 2009;29:3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, DeSalvo MN, et al. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci. 2011;31:15053–15064. doi: 10.1523/JNEUROSCI.0101-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Li R, Jones SR, Cho YR, et al. Interhemispheric neuroplasticity following limb deafferentation detected by resting-state functional connectivity magnetic resonance imaging (fcMRI) and functional magnetic resonance imaging (fMRI) Neuroimage. 2010;49:2467–2478. doi: 10.1016/j.neuroimage.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Chen CC, Shih YY, Chang C. Dopaminergic imaging of nonmotor manifestations in a rat model of Parkinson's disease by fMRI. Neurobiol Dis. 2012;49C:99–106. doi: 10.1016/j.nbd.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Silverman J, Muir WW., 3rd A review of laboratory animal anesthesia with chloral hydrate and chloralose. Lab Anim Sci. 1993;43:210–216. [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Schulte ML, Li R, Jones SR, et al. A protocol for use of medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. Neuroimage. 2009;46:1137–1147. doi: 10.1016/j.neuroimage.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Masamoto K, Fukuda M, Vazquez A, Kim SG. Frequency-dependent neural activity, CBF, and BOLD fMRI to somatosensory stimuli in isoflurane-anesthetized rats. Neuroimage. 2010;52:224–233. doi: 10.1016/j.neuroimage.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Fukuda M, Vazquez A, Kim SG. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci. 2009;30:242–250. doi: 10.1111/j.1460-9568.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Wang L, De La Garza BH, Li G, Cull G, Kiel JW, et al. Quantitative retinal and choroidal blood flow during light, dark adaptation and flicker light stimulation in rats using fluorescent microspheres. Curr Eye Res. 2013;38:292–298. doi: 10.3109/02713683.2012.756526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, et al. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego; 1998. [Google Scholar]
- Shih YY, Chen YY, Lai HY, Kao YC, Shyu BC, Duong TQ. Ultra high-resolution fMRI and electrophysiology of the rat primary somatosensory cortex. Neuroimage. 2013;73:113–120. doi: 10.1016/j.neuroimage.2013.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HY, Liao LD, Lin CT, Hsu JH, He X, Chen YY, et al. Design, simulation and experimental validation of a novel flexible neural probe for deep brain stimulation and multichannel recording. J Neural Eng. 2012;9:036001. doi: 10.1088/1741-2560/9/3/036001. [DOI] [PubMed] [Google Scholar]
- Shih YY, Chen YY, Chen CC, Chen JC, Chang C, Jaw FS. Whole-brain functional magnetic resonance imaging mapping of acute nociceptive responses induced by formalin in rats using atlas registration-based event-related analysis. J Neurosci Res. 2008;86:1801–1811. doi: 10.1002/jnr.21638. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Boeijinga PH, Lopes da Silva FH. Locally evoked potentials in slices of the rat nucleus accumbens: NMDA and non-NMDA receptor mediated components and modulation by GABA. Brain Res. 1990;529:30–41. doi: 10.1016/0006-8993(90)90808-o. [DOI] [PubMed] [Google Scholar]
- Magnusson JE, Fisher K. The involvement of dopamine in nociception: the role of D(1) and D(2) receptors in the dorsolateral striatum. Brain Res. 2000;855:260–266. doi: 10.1016/s0006-8993(99)02396-3. [DOI] [PubMed] [Google Scholar]
- Martin JR, Takemori AE. Further evidence that a single dose of an opiate can increase dopamine receptor sensitivity in mice. Eur J Pharmacol. 1987;135:203–209. doi: 10.1016/0014-2999(87)90612-1. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Aalto S, Kajander J, Oikonen V, Hinkka S, Nagren K, et al. Alfentanil increases cortical dopamine D2/D3 receptor binding in healthy subjects. Pain. 2004;109:86–93. doi: 10.1016/j.pain.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Muly EC, 3rd, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci. 1998;1:286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- Szlavik RB, de Bruin H. The effect of stimulus current pulse width on nerve fiber size recruitment patterns. Med Eng Phys. 1999;21:507–515. doi: 10.1016/s1350-4533(99)00074-0. [DOI] [PubMed] [Google Scholar]
- Tsui BC, Finucane B, Grau Th. Atlas of Ultrasound and Nerve Stimulation-Guided Regional Anesthesia. Springer: New York; 2007. [Google Scholar]
- Bielajew C, Jurgens S, Fouriezos G. The effect of pulse duration of refractory periods of neurons mediating brain-stimulation reward. Behav Brain Res. 1987;24:233–241. doi: 10.1016/0166-4328(87)90061-1. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Wang R, Foniok T, Wamsteeker JI, Qiao M, Tomanek B, Vivanco RA, et al. Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. Neuroimage. 2006;31:1–11. doi: 10.1016/j.neuroimage.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Tuor UI, McKenzie E, Tomanek B. Functional magnetic resonance imaging of tonic pain and vasopressor effects in rats. Magn Reson Imaging. 2002;20:707–712. doi: 10.1016/s0730-725x(02)00599-4. [DOI] [PubMed] [Google Scholar]
- Charuchinda C, Supavilai P, Karobath M, Palacios JM. Dopamine D2 receptors in the rat brain: autoradiographic visualization using a high-affinity selective agonist ligand. J Neurosci. 1987;7:1352–1360. doi: 10.1523/JNEUROSCI.07-05-01352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AS, Beech JS, Williams SC. Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation. Neuroimage. 2007;35:719–728. doi: 10.1016/j.neuroimage.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Shih YY, Chiang YC, Chen JC, Huang CH, Chen YY, Liu RS, et al. Brain nociceptive imaging in rats using (18)f-fluorodeoxyglucose small-animal positron emission tomography. Neuroscience. 2008;155:1221–1226. doi: 10.1016/j.neuroscience.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex. Proc Natl Acad Sci USA. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. The medial pain system: neural representations of the motivational aspect of pain. Brain Res Bull. 2002;59:163–180. doi: 10.1016/s0361-9230(02)00864-x. [DOI] [PubMed] [Google Scholar]
- Vahle-Hinz C, Detsch O, Siemers M, Kochs E. Contributions of GABAergic and glutamatergic mechanisms to isoflurane-induced suppression of thalamic somatosensory information transfer. Exp Brain Res. 2007;176:159–172. doi: 10.1007/s00221-006-0604-6. [DOI] [PubMed] [Google Scholar]
- Devonshire IM, Papadakis NG, Port M, Berwick J, Kennerley AJ, Mayhew JE, et al. Neurovascular coupling is brain region-dependent. Neuroimage. 2012;59:1997–2006. doi: 10.1016/j.neuroimage.2011.09.050. [DOI] [PubMed] [Google Scholar]
- Shen Q, Ren H, Cheng H, Fisher M, Duong TQ. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:1265–1279. doi: 10.1038/sj.jcbfm.9600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YR, Huang IJ, Lee SR, Tejima E, Mandeville JB, van Meer MP, et al. Measurements of BOLD/CBV ratio show altered fMRI hemodynamics during stroke recovery in rats. J Cereb Blood Flow Metab. 2005;25:820–829. doi: 10.1038/sj.jcbfm.9600084. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, et al. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci USA. 2001;98:12766–12771. doi: 10.1073/pnas.231235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Ramos-Cabrer P, Justicia C, Wiedermann D, Strecker C, Sprenger C, et al. Early prediction of functional recovery after experimental stroke: functional magnetic resonance imaging, electrophysiology, and behavioral testing in rats. J Neurosci. 2008;28:1022–1029. doi: 10.1523/JNEUROSCI.4147-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, et al. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Ibanez V, Sawle GV, Playford ED, Quinn N, Mathias CJ, et al. Striatal D2 receptor status in patients with Parkinson's disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11C-raclopride and positron emission tomography. Ann Neurol. 1992;31:184–192. doi: 10.1002/ana.410310209. [DOI] [PubMed] [Google Scholar]
- Chen JF, Aloyo VJ, Weiss B. Continuous treatment with the D2 dopamine receptor agonist quinpirole decreases D2 dopamine receptors, D2 dopamine receptor messenger RNA and proenkephalin messenger RNA, and increases mu opioid receptors in mouse striatum. Neuroscience. 1993;54:669–680. doi: 10.1016/0306-4522(93)90238-b. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang QW, Xu M, Guo JJ, Shen SW, Wang YQ, et al. New striatal neurons form projections to substantia nigra in adult rat brain after stroke. Neurobiol Dis. 2012;45:601–609. doi: 10.1016/j.nbd.2011.09.018. [DOI] [PubMed] [Google Scholar]