Abstract
Transactivation response DNA-binding protein 43 (TDP-43) proteinopathy has recently been reported in chronic traumatic encephalopathy, a neurodegenerative condition linked to prior history of traumatic brain injury (TBI). While TDP-43 appears to be vulnerable to proteolytic modifications under neurodegenerative conditions, the mechanism underlying the contribution of TDP-43 to the pathogenesis of TBI remains unknown. In this study, we first mapped out the calpain or caspase-3 TDP-43 fragmentation patterns by in vitro protease digestion. Concurrently, in cultured cerebrocortical neurons subjected to cell death challenges, we identified distinct TDP-43 breakdown products (BDPs) of 35, 33, and 12 kDa that were indicative of dual calpain/caspase attack. Cerebrocortical culture incubated with calpain and caspase-fragmented TDP-43 resulted in neuronal injury. Furthermore, increased TDP-43 BDPs as well as redistributed TDP-43 from the nucleus to the cytoplasm were observed in the mouse cortex in two TBI models: controlled cortical impact injury and overpressure blast-wave-induced brain injury. Finally, TDP-43 and its 35 kDa fragment levels were also elevated in the cerebrospinal fluid (CSF) of severe TBI patients. This is the first evidence that TDP-43 might be involved in acute neuroinjury and TBI pathology, and that TDP-43 and its fragments may have biomarker utilities in TBI patients.
Keywords: calpain, caspase, neurodegeneration, TDP-43, traumatic brain injury
Introduction
Transactivation response DNA-binding protein 43 (TDP-43) is a highly conserved 414-amino-acid protein with an apparent molecular weight of ∼43 kDa. Under normal physiologic conditions, TDP-43 predominantly resides in the nucleus, but in disease, it redistributes to the cytoplasm and is sequestered into inclusions where it can be phosphorylated, ubiquitinated, and proteolytically cleaved to generate C-terminal fragments (CTFs).1 TDP-43 was identified as a primary component in ubiquitin-positive, tau-negative inclusions of frontotemporal lobar degeneration and amyotrophic lateral sclerosis (ALS).2 Pathologic TDP-43 inclusions are also present in other neurodegenerative diseases such as Alzheimer's disease (AD),3 Lewy body-related diseases, Pick's disease, and Huntington's disease.4, 5 For this common pathology, these diseases were grouped together as a new entity of neurodegenerative disorders, classified as TDP-43 proteinopathies.6
As the identification of 25 kDa truncated CTFs of TDP-43 in brain extracts from frontotemporal lobar degeneration and ALS patients, much effort has been directed toward ascertaining the molecular properties of those TDP fragments. In a report by Zhang et al,7 the authors eloquently demonstrated a role for executioner caspases, including caspase-3 and caspase-7, in cleaving TDP-43. Further evidence that caspase cleavage of TDP-43 may occur in vivo was obtained in postmortem brains affected by AD, Pick's disease, and Parkinson's disease.8, 9 Current understanding suggests that proteolytic processing of TDP-43 is caspase dependent and several caspase-dependent cleavage sites have previously been suggested.7, 10, 11 However, a new study has provided strong evidence of calpain-dependent TDP-43 fragments in the spinal cord and brain of ALS patients and a high vulnerability of ALS-linked mutant TDP-43 to cleavage by calpain.12 Based on these results, TDP-43 is susceptible to be the substrate of both calpain and caspase-3. Although extensive studies have focused on this protein, it remains unknown whether the proteolysis of TDP-43 by caspase or calpain contributes to generation of pathologic diversity in TDP-43 proteinopathies. Similarly, the toxicity of calpain-dependent TDP-43 fragments has yet to be identified31613 In addition, it has been recently demonstrated that TDP-43 pathology may feature in syndromes of cognitive impairment associated with repetitive traumatic brain injury (TBI), including in former boxers with dementia pugilistica and in retired American football players with chronic traumatic encephalopathy.14, 15 However, it remains unclear how biochemical TDP-43 alternation might contribute to inclusion formation or disease pathogenesis. Calpain and caspase-3 cysteine proteases are important mediators of cell death and dysfunction in numerous central nervous system diseases and injuries, including TBI.16, 17 In addition, inhibitors of both calpain and caspase-3 can confer neuroprotection after TBI in animal models.18, 19 As TDP-43 is vulnerable to both of these proteases in vitro and in other neurodegenerative conditions7, 12 we thus hypothesize that calpain and/or caspase-3-cleaved TDP-43 might be a pathologic feature of TBI.
To identify potential distinct TDBP-43 cleavage products in this present study, in vitro-purified TDP-43 protein and mouse cortex lysates were digested with calpain and caspase-3 enzymes, to establish the pattern of TDP-43 fragmentation by immunoblotting probed with C-terminal, N-terminal, and internal TDP-43 antibodies. We also tested the hypothesis that TDP-43 protein might be differentially susceptible to proteolytic attack by calpain and caspase-3, respectively, depending on the type of neurotoxic or neurodegenerative conditions after in vitro neurotoxin treatments and in vivo TBI in mice. In addition, the release pattern of TDP-43 was monitored in cerebrospinal fluid (CSF) from TBI patients to assess CSF levels of TDP-43 and its potential breakdown products (BDPs).
Methods
All animal protocols conform to the National Institutes of Health guidelines on the use of laboratory animals and are approved by the University of Florida Institutional Animal Care and Use Committee (#201207692, 201207683, 201207558).
In Vitro Calpain-1 or Caspase-3 Digestion of Rat/Mouse Cortex Lysate or Purified Transactivation Response DNA-Binding Protein 43
Rat or mouse cortex were isolated from the brain and lysed for 90 minutes at 4°C with lysis buffer containing 50 mmol/L Tris–HCl (pH 7.4), 5 mmol/L EDTA, 5 mmol/L EGTA, 1% Triton X-100, and 1 mmol/L DTT. In vitro digestion of cortex lysate (100 μg) or purified recombinant human TDP-43 protein (3 μg, OriGene, MD, USA) was performed with human erythrocyte calpain-1 (Calbiochem/EMD Bioscience, Billerica, MA, USA) and human recombinant caspase-3 (BD Pharmingen, San Jose, CA, USA), in a buffer containing 100 mmol/L Tris–HCl (pH 7.4), 20 mmol/L DTT, with or without 1 mmol/L CaCl2, at room temperature for 30 minutes (calpain-1) or 4 hours (caspase-3). Protease reactions were stopped by the addition of a protease inhibitor cocktail solution (Roche Biochemicals, Indianapolis, IN, USA).
Primary Cortical Neuronal Culture and Treatment
Primary cortical neurons were prepared from embryonic day 18 Sprague–Dawley rat fetuses and plated on poly-L-lysine coated 12-well plates (Erie Scientific, Portsmouth, NH, USA). Cells were allowed to grow in an atmosphere of 10% CO2 at 37°C for 3 days and then treated with 1 μmol/L 4-amino-6-hydrazino-7-D-ribofuranosyl-7H-pyrrolo (2,3-D)-pyrimidine-5-carboxamide (ARC) for 2 days. The ARC was removed, and fresh 10% PDHS in DMEM was added, after which the cells were grown for an additional 10 to 14 days. For neurotoxic challenges, in addition to untreated controls, the following conditions were used: calcium ionophore A23187 (25 μmol/L; Sigma, St Louis, MO, USA) as a calpain-dominated pro-necrosis challenge for 24 hours; staurosporine (STS; 10 μmol/L; Sigma) as apoptotic inducer for 24 hours. For pharmacologic intervention, cultures were pretreated 1 hour before the A23187 or STS challenge with either the calpain inhibitor SNJ1945 (Senju Pharmaceuticals, Kobe, Japan) or the pan-caspase inhibitor Z-VAD (OMe)-FMK (R&D, Minneapolis, MN, USA).
Cell Lysate Collection and Preparation
Primary neuronal cells were collected and lysed for 90 minutes at 4°C with a lysis buffer containing 50 mmol/L Tris (pH 7.4), 5 mmol/L EDTA, 1% (v/v) Triton X-100, 1 mmol/L DTT, and a Mini-Complete protease inhibitor cocktail tablet (Roche Biochemicals). The lysates were centrifuged at 10,000 g for 5 minutes at 4°C to remove insoluble debris, and then were snap-frozen and stored at −80°C until use.
Lactate Dehydrogenase Release Assay of Cell Death
A lactate dehydrogenase (LDH) release assay (Cyto- Tox One Reagent, Promega, Madison, WI, USA) was performed to assess cell death. Primary cortical neurons were cultured as described above, and then treated with digested recombinant TDP-43 protein by either calpain or caspase-3. Culture media were collected at 24 hours after treatment and assayed for LDH release by following the manufacturer's instructions. Three replicates were assayed.
Propidium Iodide Staining
Cell culture and TDP-43 fragments treatment are the same as above described. Forty-eight (48) hours after treatment, 1 μmol/L propidium iodide (PI) was added to the cell culture for a final concentration of 2.5 μmol/L as previous study described.20 Then the cell cultures were gently stirred and incubated at 37°C in the dark for 5 minutes. The absorbance at 530 nm was monitored using an EVOS FL digital fluorescent microscope. After incubation, fluorescence imaging was immediately captured with a × 20 objective. Three replicates were assayed.
In Vivo Models of Traumatic Brain Injury
Controlled cortical impact
A controlled cortical impact (CCI) device was used to model TBI.21 CB57BL/6 mice (male, 3 to 4 months old, Charles River Laboratories, Raleigh, NC, USA.) were anesthetized with 4% isoflurane in oxygen as a carrier gas for 4 minutes followed by maintenance anesthesia of 2% to 3% isoflurane. After reaching a deep plane of anesthesia, mice were mounted in a stereotactic frame in a prone position, and secured by ear and incisor bars. A midline cranial incision was made and a unilateral (ipsilateral) craniotomy (3 mm diameter) was performed adjacent to the central suture, midway between the bregma and the lambda. The dura mater was kept intact over the cortex. Brain trauma will be induced using a PSI TBI-0310 Impactor (Precision Systems and Instrumentation, LLC, Natick, MA, USA) by impacting the right cortex (ipsilateral cortex) with 2 mm diameter impactor tip at a velocity of 3.5 m/second, 1.5 mm compression depth, and a 200 ms dwell time (compression duration). Sham-injured control animals underwent identical surgical procedures but did not receive an impact injury.
Overpressure blast-wave-induced brain injury
Mouse blast injury was modeled after Cernak, et al22 After reaching a deep plane of anesthesia, the mouse was mounted in prone position to the animal holder. The head was laid on a flexible mesh surface to diminish surface reflection of blast waves and decrease the formation of secondary waves that would potentially exacerbate the injury. The neck, head, torso, and abdomen of the animal were fixed to the animal holder in an effort to avoid any movement, and thus tertiary blast effects. The design features a 4-inch inner diameter shock tube that is ∼7 ft in length and the fixture positioned the specimen at 53 cm (20.87′) upstream from the driven section opening. Control over burst and incident pressure is achieved by adjusting the thickness of Mylar membranes. The mice were tested at an average incident overpressure of 30 psi, with Mylar membranes thicknesses of 0.03 inch. Mice were subjected to a blast wave directed at the animal lying below, while those exposed to sham blast were exposed to the sound of blast, but not directly to the blast wave.
Brain Tissue Collection and Preparation
At the different post-TBI time points (24 hours, 3 and 7 days), the animals were anesthetized and killed by decapitation. In the CCI animals, the most injured parts were the central part of the cerebral cortex while the most injury areas were the frontal cortex in overpressure blast-wave-induced brain injury (OBI) mice. Injured cortical tissues in TBI animals and cortex in similar areas from naïve animals were collected. For western blot analysis, the tissue samples were pulverized to a fine powder with a small mortar and pestle set over dry ice. The pulverized brain tissue was then lysed for 90 minutes at 4°C with lysis buffer as described above. Tissue lysates were then centrifuged at 10,000 g for 10 minutes at 4°C. The supernatants were snap-frozen and stored at −80°C until use.
Human Cerebrospinal Fluid Samples
The control samples CSF (n=15) were purchased from Bioreclaimation (Westbury, NY, USA). Archived de-identified CSF samples (n=21) from a severe TBI study were collected from consented adult subjects presenting to the Emergency Department of Ben Taub General Hospital, Baylor College of Medicine, (Houston, TX, USA). The study protocol was approved by the Baylor College of Medicine IRB. For subjects after sustaining blunt trauma to the head with a Glasgow coma scale <8, CSF samples were collected for up to 10 days or until an intraventriculostomy was no longer clinically indicated. Cerebrospinal fluid was sampled from the buretrol of the CSF drainage system by a qualified and trained hospital employee according to the hospital's standard procedures. Alternatively, timed CSF samples (10 mL) with a total collection time not exceeding 1 hour were diverted to 15-mL conical polypropylene centrifuge tubes (BD Falcon, San Jose, CA, USA). The CSF samples (5 to 10 mL) were then centrifuged at 4,000 g with a tabletop centrifuge at room temperature for 5 to 7 minutes to remove loose cells and debris. A volume of 1 mL aliquots of cleared CSF (supernatant) was pipetted into 2 mL cryogenic tube and snap-frozen and stored at −80°C ultralow freezer until use. For this study, timed CSF samples collected within 24 hours from injury were used. Cerebrospinal fluid samples were 5:1 concentrated by using Amicon Ultra centrifugal filters (Millipore, Billerica, MA, USA). A volume of 10 μl concentrated CSF samples was used for western blotting.
Immunocytochemistry/Immunohistochemistry
Immunocytochemistry analysis was performed on primary neuron cultures. Cells were fixed with 4% paraformaldehyde for 10 minutes, washed with PBS, and permeabilized with 0.1% Triton X-100 for 5 minutes. A routine staining was performed after a 1-hour blocking step in 10% goat serum. Immunohistochemistry analysis was performed on paraffin-embedded 4 to 6 μm brain sections. After deparafinization, the slides were incubated for 10 minutes at 95°C in Trilogy solution (Cell Marque, Hot Springs, AK, USA) for antigen retrieval and blocked for endogenous peroxides with 3% hydrogen peroxide. Next, they were blocked with 2% normal goat serum, followed by a routine staining procedure. Polyclonal rabbit-anti-TDP-43 (Proteintech Group, Chicago, IL, USA) at a dilution of 1:200 was used. Alexa 488-conjugated goat–anti-rabbit secondary antibody (Molecular Probes, Eugene, OR, USA) was added at a dilution of 1:1,000. The cells/tissues were counterstained with 4,6-diamidine-2-phenylindole (Vector Laboratories, Burlingame, CA, USA). Fluorescent images were captured with a × 40 objective on the OLYMPUS DP71 fluorescent microscope (Olympus America Inc, Center Valley, PA, USA).
SDS–PAGE Electrotransfer and Immunoblot Analysis
Protein concentrations of cell or tissue lysates were determined via Bio-Rad DC Protein Assay (Bio-Rad, Hercules, CA, USA). Protein-balanced samples were prepared in a 2 × sample loading buffer containing 0.25 mol/L Tris (pH 6.8), 0.2 mol/L DTT, 8% SDS, 0.02% bromophenol blue, and 20% glycerol in distilled water. A quantity of 20 μg of protein per lane was loaded and then was routinely resolved by SDS–PAGE and separated proteins were laterally transferred to polyvinylidene fluoride membranes. The following antibodies were used: TDP-43-N (N-terminal) (1:1,000, #10782-2-AP) and TDP-43-C (C-terminal) (1:1,000, #12892-1-AP, ProteinTech Group); TDP-43-IN (internal sequence) (1:1,000, #4285, Prosci Incorporated, Poway, CA, USA) and alpha-fodrin (αII-spectrin) (1:1,000, BML-FG6090, ENZO Life Sciences, Farmingdale, NY, USA). Immunoreactive bands were detected by developing with biotin, avidin-conjugated alkaline phosphatase, nitro blue tetrazolium, and 5-bromo-4-chloro- 3-indolyl phosphate. A 250 K to 14 K rainbow molecular weight marker (RPN800E, GE Healthcare, Bio-Sciences, Pittsburgh, PA, USA) was used to identify the protein. Quantitative evaluation of protein levels was performed via computer-assisted densitometric scanning (NIH ImageJ, version 1.6 software).
Statistical Analysis
In vitro animal tissue lysate and protein digestion experiments were performed in triplicate per experiment and mean values are used, and the mean values from three independent experiments performed were used to create a group mean based on the total sample size. For experiments concerning in vivo TBI models, the minimum number of animals per group at each time point was five (n=5). For samples from humans, 15 control and 21 TBI subjects were included in the CSF analysis. Densitometric values represent the mean±s.e.m. Statistical significance was determined using a one-way analysis of variance test, with a significance level of P<0.05.
Results
TDP-43 Fragmentation Patterns by Calpain or Caspase-3
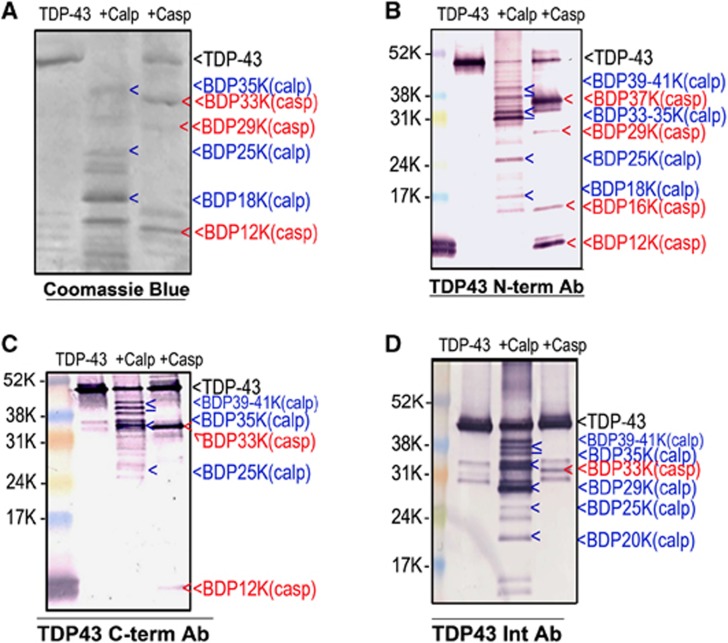
To identify the calpain or caspase-3-specific TDP-43 fragments, we subjected recombinant human TDP-43 to calpain-1 at 1:200 or caspase-3 at a 1:50 protease/substrate ratio digestion. We then probed untreated TDP-43 and digested TDP-43 samples by SDS–PAGE followed by immunoblotting with C-terminal, N-terminal, and internal TDP-43 antibodies. As we expected, calpain and caspase-3 digested TDP-43 into different immunoreactive fragments. Visualization of N-terminal TDP-43 fragmentations (NTFs) using N-terminal-targeted antibody on immunoblot revealed calpain-generated major TDP-43 BDPs at 39 to 41 kDa, 33 to 35 kDa (multiple bands) as well as a few minor, smaller fragments, while caspase-3 digestion produced major 37 kDa and 12 kDa fragments and minor fragments at 29 kDa and 16 kDa (Figure 1B). Examination of CTFs utilizing C-terminal-specific antibody revealed patterns complimentary to that of N-terminal visualization. Calpain digestion revealed TDP-43 BDPs at ∼35 kDa and 39 to 41 kDa in size. Interestingly, no 37 kDa fragment was detected when digested by caspase-3. Instead, fragments of 33 and 12 kDa in size were observed (Figure 1C). Similar calpain or caspase-3-mediated major fragments were visualized by Coomassie Brilliant Blue staining or by immunoblotting with internal TDP-43 antibody that can detect both CTFs and NTFs (Figures 1A and 1D). Both calpain and caspase-3-generated ∼25 kDa CTFs were reported by Zhang et al7 and Yamashita T et al.12 However, the 25 kDa band was found only by calpain digestion. We also observed a major 29 kDa fragment after calpain digestion using the internal TDP-43 antibody. (Figure 1D). There were several minor fragments that we did not label.
Figure 1.
Purified recombinant transactivation response DNA-binding protein 43 (TDP-43) fragmentation patterns by calpain or caspase-3. Recombinant human TDP-43 protein (3 μg) was digested in vitro by calpain-1 or caspase-3. Intact protein and TDP-43 fragments were resolved by SDS/PAGE (A–C), 4% to 20% gel, (D): 10% to 20% gel). Proteins were visualized by Coomassie Brilliant Blue staining (A) or immunoblotted with C-terminal, N-terminal, or internal TDP-43 antibodies, respectively (B–D). Calpain-1 (blue arrows) and caspase-3 (red arrows) generated major TDP-43 fragments of different size (>, single band, ⩾, multiple bands). We note that the full-length TDP-43 (in D) appears to migrate slightly lower than that in B or C—this is due to the longer electrophoresis run time in D, thus stretching out the protein bands vertically.
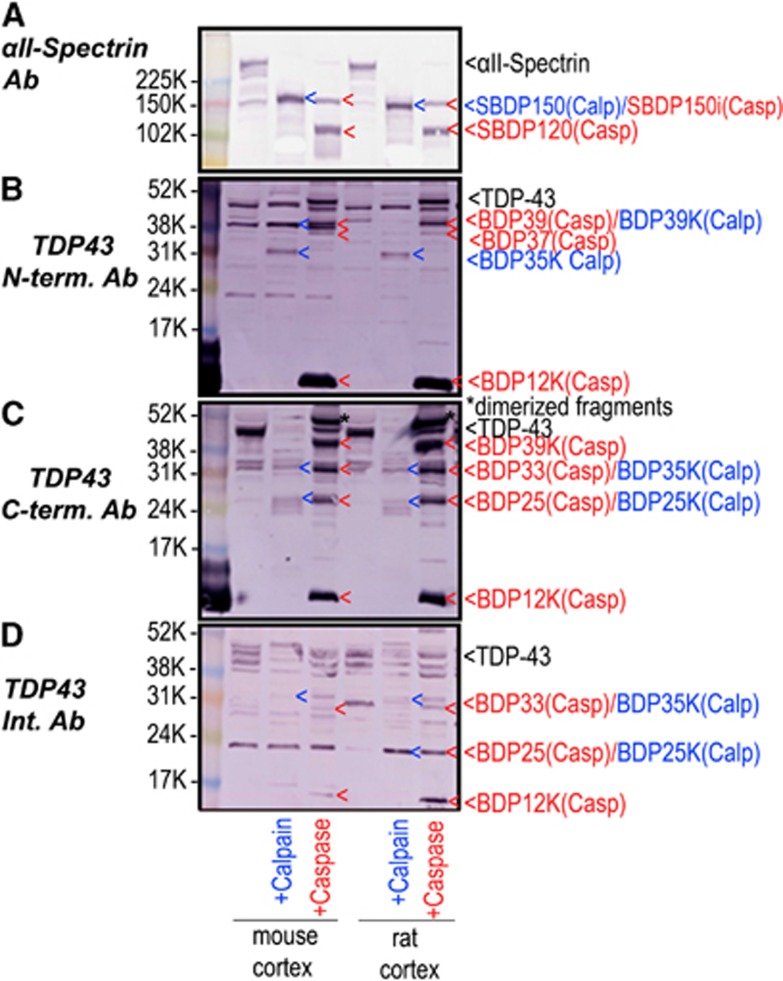
To further confirm the TDP-43 fragmentation patterns, naïve mouse and rat cortex lysates were digested by either calpain or caspase-3 in vitro. Figures 2B–D shows that both calpain and caspase-3 digested native TDP-43. αII-Spectrin was monitored in parallel as a marker of calpain and caspase activity as previously described.17 The BDPs of the protein αII-spectrin (SBDP) are as follows: SBDP150 fragment is generated by both calpain and caspase-3, while SBDP150i and SBDP120 are generated only by caspase-3 (Figure 2A). Overall, we note that there are more cleavages and BDPs of TDP-43 with in vitro digestion of recombinant TDP-43 protein (Figure 1) than with protease of native TDP-43 in brain lysate (Figure 2). This might be because recombinant protein is more vulnerable to protease cleavage.
Figure 2.
Transactivation response DNA-binding protein 43 (TDP-43) in mouse or rat brain lysate is sensitive to in vitro calpain and caspase-3 digestion. The lysate of naïve mouse or rat cortex was in vitro cleaved by calpain-1 and caspase-3: Calpain-1 (1:200 protease/substrate ratio) or caspase-3 digestion (1:50 protease/substrate ratio). The pattern of the TDP-43 fragmentation was monitored with C-terminal, N-terminal, or internal TDP-43 antibodies respectively (B, C, and D). αII-Spectrin was probed with apoptosis and necrosis markers (A). Similar results were obtained in duplicate experiments. (A–C: 4 to 10% gel was used. D: 10 to 20% gel was used)
We compared TDP-43 fragmentation patterns in mouse and rat cortex lysate digestion with those of the purified recombinant TDP-43 protein digestion (Figure 1). We note that C-terminal-targeting antibody gives the most robust immunostaining of TDP-43 in cell lysates (Figure 2). Digestion of native TDP-43 within rat/mouse brain lysate generated major 39/12 kDa caspase fragments, and to a lesser extent 33/25 kDa caspase fragments (Figure 2C). Similarly, recombinant TDP-43 protein yields 39/12 kDa major BDPs on caspase digestion (Figure 1C). However, calpain digestion of native TDP-43 in brain lysate produced 35/25 kDa fragments (Figure 2C). Calpain digestion of recombinant TDP-43 also produced 35 and 25 fragments, but also additional 39 to 41 kDa fragments (Figure 1C). We also noted a higher molecular weight species (* 46 kDa) on caspase digestion (Figure 2C). It might be a dimerized fragment, but the exact nature of it is still unknown.
With N-terminal-directed antibody, digestion of brain lysate produced 39/35 kDa calpain fragments and 39/37/12 kDa caspase fragments (Figure 2B). In comparison, recombinant TDP-43 protein digestion also generated 39/35 kDa calpain fragments and 37/12 kDa caspase fragments (Figure 1B). It differs in that there appears to be more minor fragments when recombinant TDP-43 protein was digested, probably owing to the non-native folding of the protein.
With internal sequence-directed antibody, digestion of brain lysate produced 35/25 kDa calpain fragments and 33/25/12 kDa caspase fragments, respectively (Figure 2D), while recombinant TDP-43 protein digestion produced additional 39 to 41/35/29/25/20 kDa calpain fragments and mainly 33 kDa caspase fragments, respectively (Figure 1D).
Taken together, the above results demonstrated that the overall naïve TDP-43 proteolysis patterns matched with that of in vitro digestion of purified recombinant TDP-43, although with some differences, as discussed.
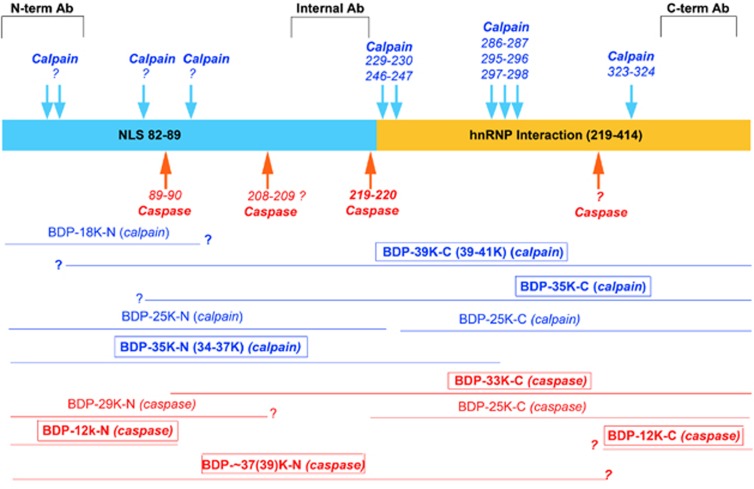
With this new data, combined with information from previous reports, we mapped out the various cleavage sites and the corresponding TDP-43 BDPs that are produced (Figure 3). Both N-terminal and CTFs were mapped in this model. Blue indicates the calpain-cleaved BDPs and red represents caspase-3-specific fragments. This multiple cleavage breakdown pattern is generally consistent with previous studies on calpain or caspase-3 proteolysis of TDP-43. In the schematic (Figure 3), the common and major fragments are highlighted with rectangles. The major CTFs are identified as BDP-39K-C and BDP-35K-C by calpain and BDP-33K-C and BDP-12K-C by caspase. Also, major NTFs are found as BDP-35K-N by calpain, BDP-∼37(39)K and BDP-12K by caspase (boxed, Figure 3). However, in this cleavage site map, it is also clear that there are some calpain or caspase-3 cleavage sites that are still unknown. In addition, other fragments (Figures 1 and 2) can be observed, but they are minor fragments or are generated by over-digestion in vitro.
Figure 3.
Schematic of transactivation response DNA-binding protein 43 (TDP-43) proteolysis by calpain and caspase-3 pathways. In this model, caspase cleaves at the N-terminal (Asp89 ↓Ala90, Asp219 ↓Val220, based on Zhang YJ, et al7) producing caspase-dependent CTFs at 33 and 2 5 kDa and unknown C-terminal cleavage sites generate fragments of 39 and 12 kDa. However, calpain cleaves at least six sites (Phe229↓Ala230; Leu243↓Cys244; Gln286↓Gly287; Gly295↓Gly296; Ala297↓Gly298; Met323↓Ala324, based on Yamashita T, et al12) producing multiple fragments. But the cleavage sites generating major calpain-specific C-terminal fragments (CTFs) of 39 and 35 kDa remain unknown.
Pathologic TDP-43 Fragments Distinguish Cell Death Pathways
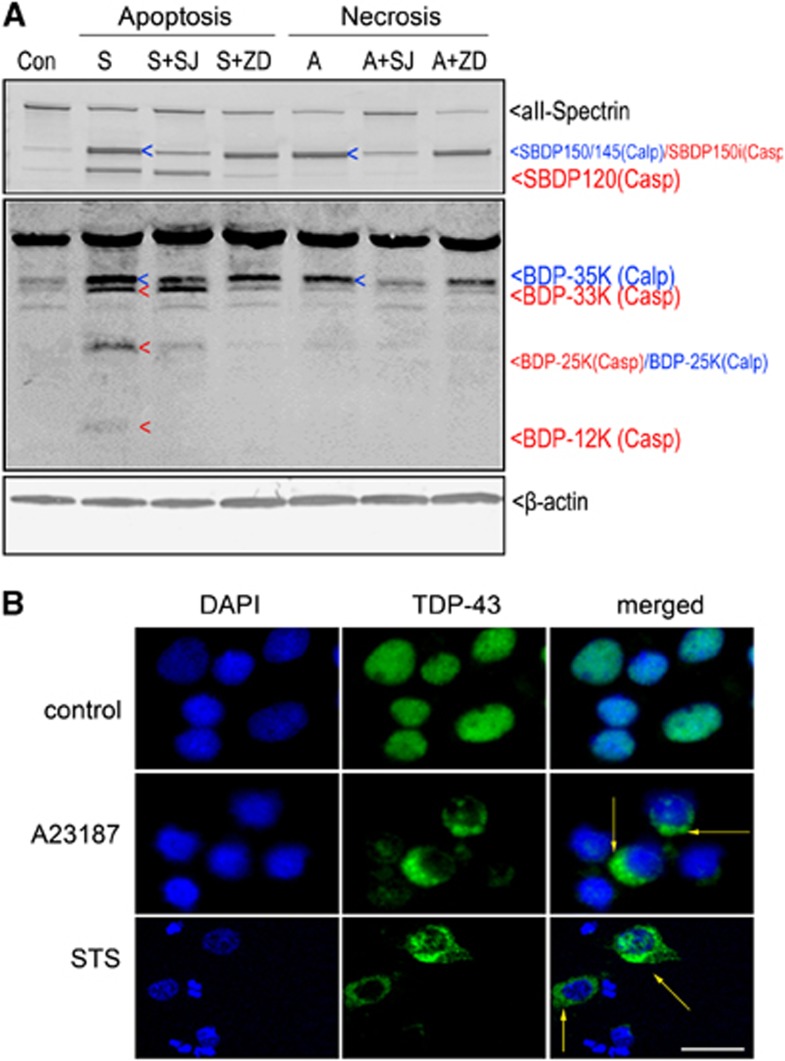
In our previous studies, calpain is activated by calcium ionophore A23187, which increases intracellular Ca2+ levels in intact cells, and by the apoptosis inducer staurosporine, which was used to mimic neurodegenerative conditions producing caspase (and to a lesser extent calpain) activation.23 With this method, the integrity of TDP-43 after neurotoxin induction was examined. In this study, no expected reduction in the intact TDP-43 was observed after either A23187 (20 μmol/L) or staurosporine (STS, 1 μmol/L) treatment. However, it appeared the 35, 33, 25, and 12 kDa fragments were likely TDP-43 BDPs (Figure 4A, middle panel). To distinguish calpain- or caspase-3-specific TDP-43 proteolysis during protein extraction, we looked for matching TDP-43 degradation in cortical cultures pretreated with either the specific calpain inhibitor SNJ1945 or the pan-caspase inhibitor Z-VAD before neurotoxin treatment. The results showed that the formation of 35 kDa fragment (blue arrows) in A23187 treatment was blocked by SNJ1945, but not by Z-VAD. Therefore, calpain appears to be responsible for proteolysis of the intact 43 kDa TDP-43 protein into the 35 kDa BDP. In contrast, STS-mediated generation of the 33 kDa TDP BDPs was effectively blocked by Z-VAD whereas SNJ1945 offered no protection. 25K and 12K BDP were strongly inhibited by caspase inhibitor Z-VAD, but only partially blocked by calpain inhibitor SNJ1945 suggesting these fragments might be generated by both calpase-3 or alternately by calpain. Interestingly, STS also led to 35 kDa TDP-43 fragments, which were again blocked by SNJ195, indicating STS could induce calpain and generate the calpain-cleaved fragment. The calpain inhibitor blocked mainly the SBDP145, but not the SBDP120, while the caspase inhibitor blocked the SBDP120, but not SBDP150/145. Perhaps most importantly, we identified that 35 kDa TDP-43 BDP strikingly paralleled the production of the 150/145 kDa αII-spectrin BDPs, which were monitored as markers for calpain activity in A23187 and STS treatment, while 120 kDa SBDP paralleled the caspase-3-mediated 33 kDa TDP-43 fragment induced by STS. (Figure 4A top panel). Taken together, pro-apoptotic STS treatment produces a neurodegenerative paradigm where there is a dual and balanced contribution of both calpain and caspase in TDP-43 fragmentation. Meanwhile, pro-necrotic A23187 treatment is responsible for the calpain-mediated TDP-43 fragmentation in this system.
Figure 4.
Transactivation response DNA-binding protein 43 (TDP-43) fragments distinguish cell death pathways. (A) Primary cortical neurons were pretreated with calpain (30 μmol/L SNJ1945, SJ) and caspase-3 (20 μmol/L Z-VAD, ZD) inhibitors for 1 hour or with serum-free medium alone, and then exposed to staurosporine (STS; S, 1 μmol/L) or calcium ionophore A23197 (A, 20 μmol/L) for 24 hours. Immunoblots of cell extracts with rabbit C-terminal anti-TDP-43 antibody revealed the caspase-specific 33, 25 and 12 kDa bands and calpain-specific 35 kDa band (middle). Calpain-specific TDP-43 breakdown products (BDPs) are indicated by the blue ‘<', while caspase-specific BDPs are indicated by the red ‘<'. In addition to probing C-terminal TDP-43 antibodies, αII-spectrin was also probed as control cell death markers (top). β-Actin was used as a loading control (bottom). (B) Immunofluorescent staining for endogenous TDP-43 in primary cortical neurons shows normal nuclear localization but no cytoplasmic labeling. Meanwhile, necrotic cells induced by A23187 or apoptotic cells induced by STS show a notable increase in cytoplasmic TDP-43 staining (yellow arrow heads). Magnification, × 400, scale bar, 5 μm.
A key feature of TDP-43 proteinopathy is the presence of modified TDP-43 located predominantly within the cytoplasm of neurons. Next, we examined the intracellular distribution of TDP-43 after treatment with A23187 or STS as shown above. In contrast to untreated cells, which mostly exhibited nuclear localization of TDP-43 immunoreactivity (Figure 4B, top), treatment with either A23187 (middle) or STS (bottom) exhibited a notable increase in cytoplasmic TDP-43 staining. However, no pathologic TDP-43 aggregate was observed under STS-induced apoptosis or A23187-mediated necrosis condition, perhaps reflecting a relatively acute onset of neurotoxicity, thus preventing the TDP-43 aggregate formation over time.
Truncated TDP-43 Fragments by Both Calpain and Caspase-3 are Neurotoxic
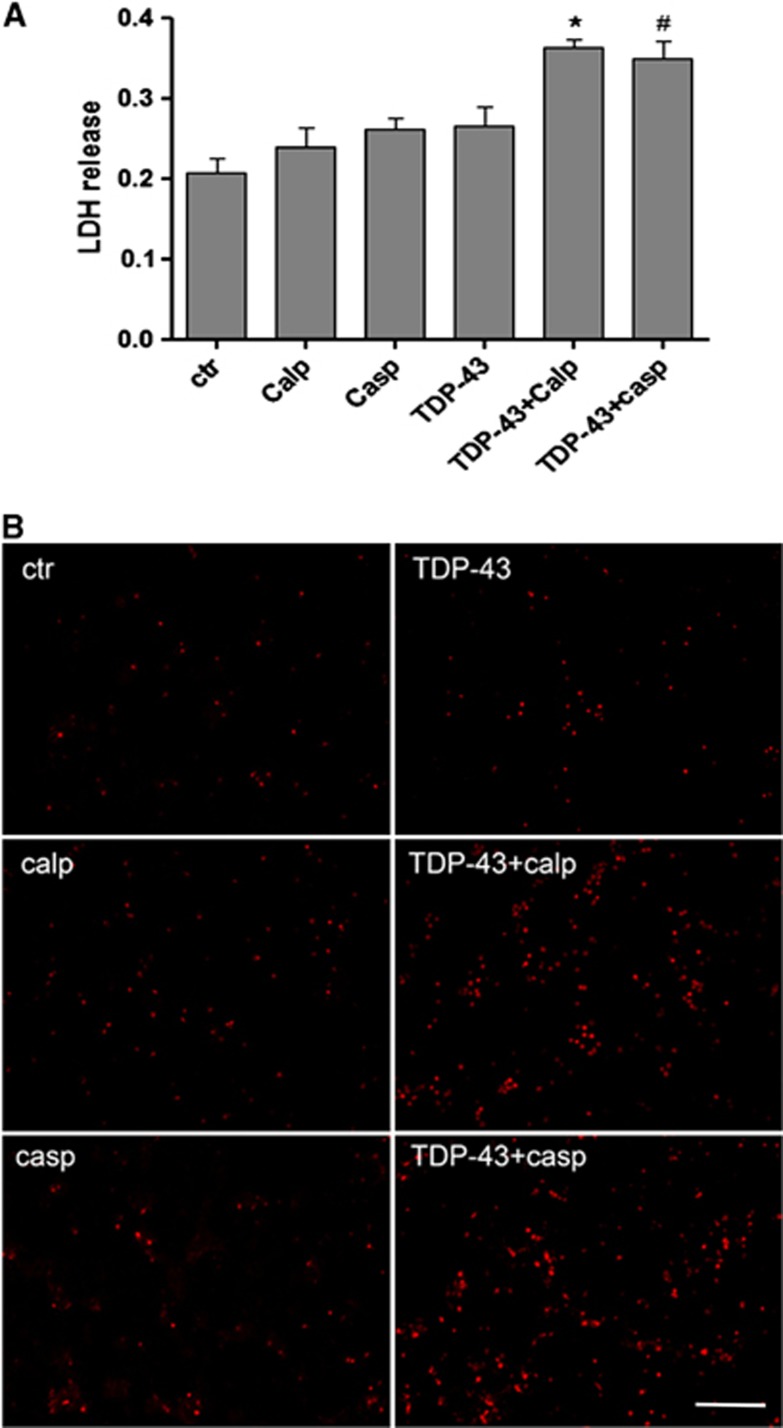
To examine the toxicity of TDP-43 fragments, LDH release assays were performed to investigate the role of calpain and caspase-mediated TDP-43 fragments in neuronal cell injury. In our design, rat cerebrocortical cultures were treated with recombinant TDP-43 fragments generated by calpain or caspase-3 digestion. Untreated cells and control solutions (calpain or caspase alone) were used as three control groups. We found that these control solutions did not induce neuronal death. Compared with LDH levels in control solution-treated cells, LDH levels were no different in full-length TDP-43 protein-treated cells but were significantly increased in the cells treated by calpain- and caspase-3-generated TDP-43 fragments. Calpain-induced TDP-43 fragments showed more cellular toxicity than caspase-3-mediated TDP-43 fragments (Figure 5A).
Figure 5.
Transactivation response DNA-binding protein 43 (TDP-43) fragments enhance cellular toxicity. (A) The release of lactate dehydrogenase (LDH) into media was used as an indicator of cell toxicity. Lactate dehydrogenase levels were measured 48 hours after cells were treated with digested TDP-43. Data from three separate experiments. Values represent means± s.e.m., n=6. A difference was considered to be statistically significant when *P<0.05 compared with calpain control solution, #P<0.05 compared with caspase control solution. (B) Cellular toxicity detected by propidium iodine (PI) nuclear staining. Cells treated with TDP-43, digested TDP-43 by either caspase-3 or calpain for 24 hours. Magnification × 200, scale bar, 50 μm.
To further confirm whether the calpain- or caspase-3-mediated TDP-43 fragments were associated with cellular toxicity, we performed PI staining in non-fixed cells 48 hours after treatment. In living cells, PI would not penetrate the nuclear membrane; however, when cells are bound to die, their nuclear membrane becomes more porous, thus allowing PI to enter and stain the DNA. Compared with the control solution group, treatment with TDP-43 fragments induced by either caspase-3 or calpain showed increased PI-positive cells, which corresponded with the results from the LDH assay (Figure 5B). In addition, on phase contrast microscopy, we found that it is the neuronal population that are dying with TDP-43 fragment treatment, while glial cells are resistant to such challenges (results not shown), consistent with the previous report on neurotoxicity of caspase-mediated TDP-43 CTF.13, 24
TDP-43 Integrity after Experimental Traumatic Brain Injury in Mice
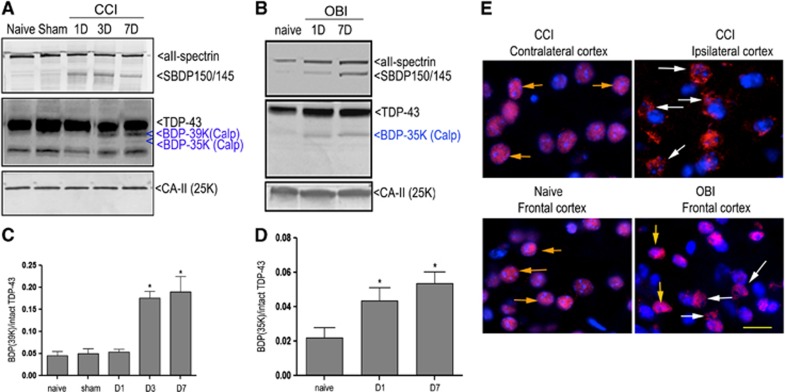
Next, we examined whether TDP-43 was proteolyzed in vivo after TBI. We examined the cortex tissues harvested 1, 3, and 7 days post TBI. Immunoblot analysis was performed in these experiments, and major TDP-43 BDPs were quantified as a ratio of TDP-43 BDP/intact TDP-43. Our results show notable decrease in intact TDP-43 observed in the ipsilateral cortex from mice subjected to severe focal TBI (CCI) from 3 days post injury, while at the same time, the 39 kDa TDP-43 BDPs were observed in days 3 and 7 post injury, and a weak 35 kDa TDP-43 fragment was found to appear on day 7 (P<0.05, Figures 6A and 6C). In another TBI mouse model, examination of the cortex from mice subjected to mild blast-wave overpressure brain injury (OBI) (for setup see Supplementary Figure 1) was done. Our results showed that there was also an increase of 35 kDa CTFs even at 24 hours post injury, and that these BDPs last at least 7 days (Figures 6B and 6D). Furthermore, αII-spectrin immunoblots showed that the formation of the calpain-dependent SBDP150/145 paralleled the formation of the 35-kDa TDP-43 BDP. These results demonstrate that TDP-43 proteolysis correlated well with calpain activation over time after TBI.
Figure 6.
Transactivation response DNA-binding protein 43 (TDP-43) proteolysis in mouse cortex after traumatic brain injury (TBI). The brain samples were collected from naïve, sham, ipsilateral cortex of the controlled cortical impacted (CCI) mice (1.5 mm impact depth) (A) and the frontal cortex of overpressure blast-wave-induced brain injury (OBI) mice (30 psi peak pressure) (B) at 1, 3 days after injury. Brain lysates were subjected to immunoblotting and probed with either C-terminal or internal TDP-43, αII-spectrin or carbonic anhydrase II (CA-II) antibodies. Quantitative analysis of the ratio of breakdown product to total TDP-43 in CCI mouse cortex (C) and OBI mouse cortex (D) was done by densitometry analysis. Values represent means±s.e.m., n=5. *P<0.05 compared with naïve (one-way analysis of variance). (E) Shows redistribution of nuclear TDP-43 in the mouse cortex after TBI. Immunohistochemistry for naïve mice or contralateral cortex of CCI mice indicated that TDP-43 resides mainly in the nucleus of cortical neurons (orange arrowheads). However, some neurons in the ipsilateral cortex from CCI mice and frontal cortex from OBI mice showed diffuse immunoreactivity in the cytoplasm 24 hours post injury (white arrowheads) consistent with cytosolic translocation of TDP-43. Magnification × 400, scale bar, 20 μm.
In neurodegenerative diseases, TDP-43 is suggested to redistribute to the cytoplasm. We thus sought to examine this possibility after TBI in vivo. Using C-terminal TDP-43 antibody, the primary localization of TDP-43 was observed within neuronal nuclei of the contralateral cortex of CCI mice, as was the case for endogenous TDP-43 in naïve mice (Figure 6E, CCI). Interestingly, a small subset of cortical neurons in the ipsilateral cortex of mice after CCI showed diffuse cytoplasmic TDP-43 staining (yellow arrows) outside of the nucleus. Such diffuse cytoplasmic TDP-43 staining was also present in OBI mice after OBI (Figure 6, OBI, yellow arrows).
Activated Calpain Cleaves TDP-43 in Severe Traumatic Brain Injury Patients and Releases it into Biofluid
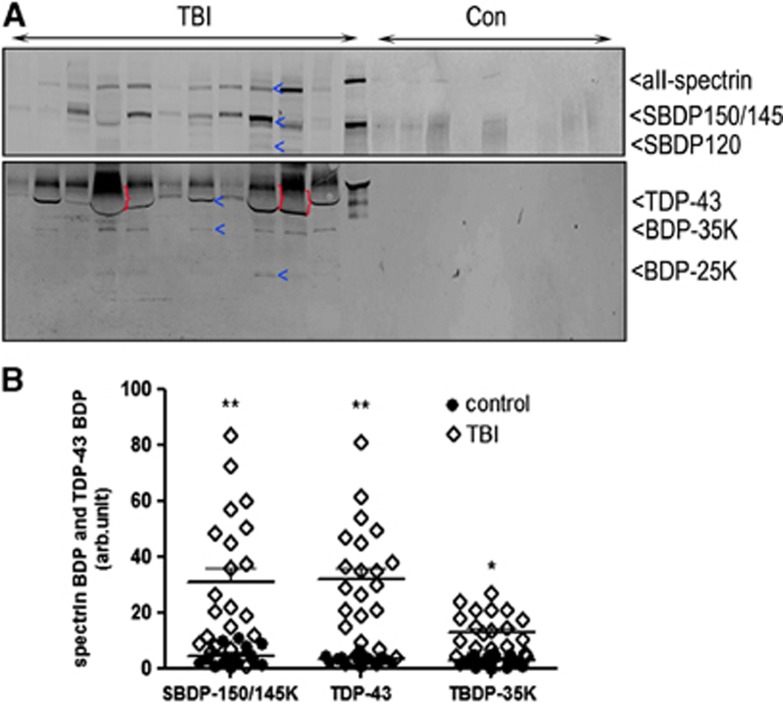
We conclude our translational study by examining the integrity of TDP-43 after severe human TBI and the possible release of intact TDP-43 and/or its fragments into CSF. The study included 21 severe TBI patients and 15 controls. The patient demographic and injury information are presented in Supplementary Table 1. A comparison of the levels of intact TDP-43 and its BDPs as well as SBDPs in patients with TBI versus controls is shown in Figure 7. Interestingly, within the first 24 hours after TBI, intact TDP-43 was detected in the CSF from patients with severe TBI but not in the controls. Meanwhile, αII-spectrin breakdown product 150/145 levels, but not SBDP120 levels, were significantly elevated in injury subjects over normal controls (P<0.01), demonstrating that necrotic cell death mechanisms are activated in humans within 24 hours after severe TBI25 (Figure 7B). This result was consistent with the observed calpain-mediated 35 kDa TDP-43 fragment (CTF) in the CSF from 17 of 21 patients (81%). The mean levels of TDP-43 and BDP-35K are significantly higher in TBI over normal controls (P<0.01 for TDP-43; P<0.05 for TDP-43-BDP-35K) (Figure 7B). Beside this, a 25 kDa fragment (BDP-25K CTF) was also observed in TBI CSF where robust SBDP150 signals were also observed (Figure 7A, lane 4, 5, 9, 10).
Figure 7.
Transactivation response DNA-binding protein 43 (TDP-43) and breakdown products (BDPs) released into human cerebrospinal fluid (CSF) after severe traumatic brain injury (TBI). (A) For the determination of TDP-43 levels, 10 μL of fivefold concentrated CSF samples from either controls or TBI patients in the first 24 hours post TBI were used. Released TDP-43 was detected with C-terminal TDP-43 antibody. αII-Spectrin was also probed as a control biofluid marker. Each lane represents an individual subject. For western, the high level of albumin (66K) (indicted with ‘}'), pushes down and distort the band of intact TDP-43. (B) Scatterplots for SBDP150/145K, TDP-43, and BDP-35K C-terminal fragments levels in human CSF. Each dot represents data from one subject (control=10, TBI=14). Horizontal lines: means±s.e.m. Black circles represent controls while open diamonds indicate TBI patients. *P<0.05 and **P<0.01 compared with control (one-way one-way analysis of variance).
Discussion
TDP-43 has two highly conserved RNA recognition motifs flanked by the N-terminal and the C-terminal tail.26 The most common underlying biochemical mechanism of TDP-43 cleavage is caspase-dependent producing 35 and 25 kDa fragments have been identified.7, 27 In a recent report, six calpain cleavages of TDP-43 have also been putatively reported by Yamashita T, et al.12 In the present work, we compared TDP-43 fragments generated by caspase-3 and calpain TDP-43 cleavages (Figure 3). Our multiple TDP-43 cleavage patterns is generally consistent with previous studies on calpain or caspase-3 proteolysis of TDP-43. The minor differences in the number of fragments or fragment sizes might be because of slight differences in protease digestion conditions. Importantly, our results confirmed the multiple calpain-dependent fragments between 34 to 41 kDa that correspond to the multiple calpain cleavage sites reported by Yamashita et al12 By comparing major CTFs, we found that both calpain and caspase-3 can in fact generate ∼25 kDa fragments correlating with the previous reports.12, 28 Furthermore, the calpain-specific CTFs are at 35 kDa, while caspase-3-specific CTFs are at 33 and 12 kDa as identified by our in vitro experiments (Figure 4A). When comparing major NTFs, we observed that calpain generates 35 kDa N-terminal fragments; in contrast, caspase-3 generated 39 and 12 kDa NTFs (Figures 1 and 2). We also noted that there were more cleavages and BDPs of TDP-43 with in vitro digestion of recombinant TDP-43 protein (Figure 1) than with protease of native TDP-43 in brain lysate (Figure 2). We tried different incubation time and concentrations with recombinant protein. However, there still are many fragments with recombinant protein digestion—this might be because recombinant protein might or might not be natively folded, thus more vulnerable to protease cleavage. In addition, for in vitro digestion experiments, the protease inhibitor cocktail could not be added to tissue lysis buffer. Under this condition, we found that TDP-43 is highly vulnerable to postmortem endogenous proteolysis. Thus, some background fragments are generated in the control samples (Figure 2). In Figure 6, we were trying to observe in vivo cleavages after brain injury, so protease inhibitor cocktail was used when preparing tissue lysate. Because of this, fewer BDPs are observed. In addition, whether multiple smaller fragments generated by calpain can also be found in injured neurons after TBI, or that they are simply in vitro BDPs owing to the exaggerated calpain activity that further cleaves the large fragments into smaller ones need further studies.
Abnormal TDP-43 fragments are one of the key features contributing to several neurodegenerative diseases. However, few studies have addressed whether TDP-43 fragments confer a gain or loss of function. One group found that caspase-3 mediated 25 kDa TDP-43 CTFs impair neurite growth during differentiation of cultured rodent neurons, and that full-length TDP-43 rescues this phenotype, suggesting that TDP-43 CTFs act by a dominant-negative mechanism.28 Zhang et al reported that an ∼25 kDa TDP-43 fragment corresponding to the C-terminal truncation product of caspase-cleaved TDP-43 leads to the formation of toxic, insoluble, cytoplasmic inclusions.28 However, another study reported that expression of full-length TDP-43 in Drosophila melanogaster was toxic to various neuronal cell types, whereas expression of TDP-43 CTFs did not result in neurotoxicity.29 In our study, LDH release assays and PI staining results show that calpain and caspase-3-cleaved TDP-43 fragments induced cellular toxicity (Figure 5). Additional mechanistic studies might be required to determine the effect of full-length TDP-43 versus TDP-43 fragments in neurotoxicity.
TDP-43 pathology is considered as a secondary feature in several other neurodegenerative diseases including AD.30, 31 Caspase-cleaved TDP-43 was found in Hirano bodies, tangles, reactive astrocytes, and neuritic plaques of the AD brain as noted by Rohn.8 Again, the other study demonstrates that TDP-43 aggregates are found in AD patients and most of these depositions are cytoplasmic inclusions.32 In our study, activated calpain-cleaved TDP-43 into small fragments in two acute TBI animal models including CCI and OBI (Figures 6A and 6C). Although fragment-specific antibodies to detect calpain or caspase-cleaved TDP-43 BDPs were not developed and used in this study, the established calpain/caspase dual-substrate αII-spectrin was probed. It was shown in the case of TBI that prominent calpain-mediated SBDP150/SBDP145 was detected, with no observable bands of caspase-3-mediated SBDP120. These results argue that calpain might be the major protease cleaving TDP-43 after acute TBI. This is in contrast to chronic neurodegenerative paradigms where TDP-43 is cleaved prominently by caspase-3. Meanwhile, immunohistochemistry showed that 24 hours post injury, TDP-43 redistributed to the cytoplasm in the injured cortex of these TBI mice (Figure 6). Although no TDP-43 aggregates were found in this study, overexpression of truncated TDP-43 CTFs can potentially yield numerous cytoplasmic inclusions, presumably through a ‘seeding' reaction,11 similar to that recently observed for tau and α-synuclein.33 Consistent with this data is the recent identification of a C-terminal prion-like domain in TDP-43.34 This prion-like domain has been implicated in the aggregation of TDP-43 in cultured cells.35 Thus, it is possible that TDP-43 fragmentation in acute TBI might facilitate TDP-43 aggregate formation over time.
Predicting the severity and outcome of TBI is difficult given the lack of objective, laboratory-based biomarkers.36, 37, 38, 39, 40 Here we demonstrated for the first time that intact TBP-43 and 35-kDa TDP-43 CTF are released into the CSF compartment in severe TBI patients (Figure 7). For this pilot study of archived CSF samples, both TDP-43 and αII-spectrin were determined. Within the first 24 hours after TBI, intact TDP-43 as well as its 35-kDa BDP were detected in 17 of 21 patients but not in the controls. TDP-43 is a nuclear protein and redistributes to cytoplasm in pathologic condition followed by disruption of the nuclear membrane and extracellular releasing into the medium. In parallel, evidence of the presence of phosphorylated-TDP-43 protein in plasma samples from ALS patients is reported by Foulds et al.31 Thus, our findings illustrate the release of TDP-43 and its BDPs may provide valuable information about diagnosis and outcome prediction after TBI.
Taken together, our studies have demonstrated that TDP-43 is dually vulnerable to calpain and caspase-3 proteolysis after acute neurotoxic and TBI conditions. Also, our results indicate protease-generated TDP-43 fragments may gain neurotoxic function and induce secondary injury. We also show that TDP-43 and its BDPs can be used as potential protein biomarkers for TBI. However, it will be important to confirm the presence of these potential TBP-43 biomarkers in blood samples using more sensitive methods, such as enzyme-linked immunosorbent assay. Finally, further study should be considered to characterize the neurotoxicity of TDP-43 and its major BDPs in vivo.
Acknowledgments
The authors thank Dr Zhiqun Zhang for helpful discussion.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study is in part supported by BSCIRTF fund from the State of Florida.
Supplementary Material
References
- Lee EB, Lee VMY, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP-43-mediated neurodegeneration. Nat Rev Neurosci. 2011;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann MM, Sampathu DMD, Kwong LKL, Truax ACA, Micsenyi MCM, Chou TTT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Dugger BN, Dickson DW, Wang DS. TDP-43 in aging and Alzheimer's disease—a review. Int J Clin Exp Pathol. 2011;4:147. [PMC free article] [PubMed] [Google Scholar]
- Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008;67:1159–1165. doi: 10.1097/NEN.0b013e31818e8951. [DOI] [PubMed] [Google Scholar]
- Yokota O, Tsuchiya K, Arai T, Yagishita S, Matsubara O, Mochizuki A, et al. Clinicopathological characterization of Pick's disease versus frontotemporal lobar degeneration with ubiquitin/TDP-43-positive inclusions. Acta Neuropathol. 2009;117:429–444. doi: 10.1007/s00401-009-0493-4. [DOI] [PubMed] [Google Scholar]
- Forman MS, Trojanowski JQ, Lee VMY. TDP-43: a novel neurodegenerative proteinopathy. Curr Opin Neurobiol. 2007;17:548–555. doi: 10.1016/j.conb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-J, Xu Y-F, Dickey CA, Buratti E, Baralle F, Bailey R, et al. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530–10534. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn TT. Caspase-cleaved TAR DNA-binding protein-43 is a major pathological finding in Alzheimer's disease. Brain Res. 2008;1228:189–198. doi: 10.1016/j.brainres.2008.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, Kokoulina P. Caspase-cleaved TAR DNA-binding protein-43 in Pick's disease. Int J Physiol Pathophysiol Pharmacol. 2009;1:25–32. [PMC free article] [PubMed] [Google Scholar]
- Dormann D, Capell A, Carlson AM, Shankaran SS, Rodde R, Neumann M, et al. Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J Neurochem. 2009;110:1082–1094. doi: 10.1111/j.1471-4159.2009.06211.x. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Kametani F, Arai T, Akiyama H, Hasegawa M. Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum Mol Genet. 2009;18:3353–3364. doi: 10.1093/hmg/ddp275. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Hideyama T, Hachiga K, Teramoto S, Takano J, Iwata N, et al. A role for calpain-dependent cleavage of TDP-43 in amyotrophic lateral sclerosis pathology. Nat Commun. 2012;3:1307. doi: 10.1038/ncomms2303. [DOI] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Xu Y, Truax AC, Uryu K. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Trojanowski JQ, Smith DH. Acute and chronically increased immunoreactivity to phosphorylation-independent but not pathological TDP-43 after a single traumatic brain injury in humans. Acta Neuropathol. 2011;122:715–726. doi: 10.1007/s00401-011-0909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Elliott PJ, Hayward NJ, Dean RL, Harbeson S, Straub JA, et al. Calpain as a novel target for treating acute neurodegenerative disorders. Neurol Res. 1995;17:249–258. doi: 10.1080/01616412.1995.11740322. [DOI] [PubMed] [Google Scholar]
- Wang KK. Calpain and caspase: can you tell the difference. Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. NURT. 2010;7:31–42. doi: 10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Abai B, Grosvenor A, Vorwerk CK, Smith DH, Meaney DF. Traumatic axonal injury results in biphasic calpain activation and retrograde transport impairment in mice. J Cereb Blood Flow Metab. 2003;23:34–42. doi: 10.1097/01.WCB.0000035040.10031.B0. [DOI] [PubMed] [Google Scholar]
- Klimowicz-Bodys MD, Batkowski F, Ochrem AS, Savič MA. Comparison of assessment of pigeon sperm viability by contrast-phase microscope (eosin-nigrosin staining) and flow cytometry (SYBR-14/propidium iodide (PI) staining) [evaluation of pigeon sperm viability] Theriogenology. 2012;77:628–635. doi: 10.1016/j.theriogenology.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Hook G, Yu J, Sipes N, Pierschbacher M, Hook V, Kindy MS. The cysteine protease cathepsin B is a key drug target and cysteine protease inhibitors are potential therapeutics for traumatic brain injury. J Neurotrauma. 2013;31:515–529. doi: 10.1089/neu.2013.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Merkle AC, Koliatsos VE, Bilik JM, Luong QT, Mahota TM, et al. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol Dis. 2011;41:538–551. doi: 10.1016/j.nbd.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Larner SF, Liu MC, Zheng W, Hayes RL, Wang KKW. Multiple alphaII-spectrin breakdown products distinguish calpain and caspase dominated necrotic and apoptotic cell death pathways. Apoptosis. 2009;14:1289–1298. doi: 10.1007/s10495-009-0405-z. [DOI] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Lee EB, Chen-Plotkin A, Swanson E, Unger T, et al. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J Clin Invest. 2011;121:726–738. doi: 10.1172/JCI44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JA, Lewis SB, Valadka AB, Papa L, Hannay HJ, Heaton SC, et al. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Doudeva LG, Wang YT, Shen CKJ, Yuan HS. Structural insights into TDP-43 in nucleic-acid binding and domain interactions. Nucleic Acids Res. 2009;37:1799–1808. doi: 10.1093/nar/gkp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-J, Xu Y-F, Cook C, Gendron TF, Roettges P, Link CD, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci USA. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Tan W, Whittle C, Qiu L, Cao L, Akbarian S, et al. The C-terminal TDP-43 fragments have a high aggregation propensity and harm neurons by a dominant-negative mechanism. PLoS One. 2010;5:e15878. doi: 10.1371/journal.pone.0015878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Y, Ray P, Rao EJ, Shi C, Guo W, Chen X, et al. A Drosophilas model for TDP-43 proteinopathy. Proc Natl Acad Sci USA. 2010;107:3169–3174. doi: 10.1073/pnas.0913602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, et al. Traumatic brain injury and time to onset of Alzheimer's disease: a population-based study. Am J Epidemiol. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- Foulds P, McAuley E, Gibbons L, Davidson Y, Pickering-Brown SM, Neary D, et al. TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer's disease and frontotemporal lobar degeneration. Acta Neuropathol. 2008;116:141–146. doi: 10.1007/s00401-008-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology. 2009;29:566–573. doi: 10.1111/j.1440-1789.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- Guo JL, Lee VMY. Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286:15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman M, Johnson BS, King OD, Gitler AD, Shorter J. Prion-like disorders: blurring the divide between transmissibility and infectivity. J Cell Sci. 2010;123:1191–1201. doi: 10.1242/jcs.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Zetterberg H, Minthon L, Blennow K, Londos E. The cognitive profile and CSF biomarkers in dementia with Lewy bodies and Parkinson's disease dementia. Int J Geriatr Psychiatry. 2011;26:100–105. doi: 10.1002/gps.2496. [DOI] [PubMed] [Google Scholar]
- Tabaraud FF, Leman JPJ, Milor AMA, Roussie JMJ, Barrière GG, Tartary MM, et al. Alzheimer CSF biomarkers in routine clinical setting. Acta Neurol Scand. 2012;125:416–423. doi: 10.1111/j.1600-0404.2011.01592.x. [DOI] [PubMed] [Google Scholar]
- Tsitsopoulos PP, Marklund N. Amyloid-β peptides and tau protein as biomarkers in cerebrospinal and interstitial fluid following traumatic brain injury: a review of experimental and clinical studies. Front Neurol. 2013;4:79–79. doi: 10.3389/fneur.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergenroeder GW, Redell JB, Moore AN, Dash PK. Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol Diagn Ther. 2008;12:345–358. doi: 10.1007/BF03256301. [DOI] [PubMed] [Google Scholar]
- Wang KKW, Moghieb A, Yang Z, Zhang Z.Systems biomarkers as acute diagnostics and chronic monitoring tools for traumatic brain injuryIn: Southern ŠO, (eds). SPIE; 201387230O–87230O–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.