Abstract
Akt (protein kinase B) and mammalian target of rapamycin (mTOR) have been implicated in the pathogenesis of cell death and cognitive outcome after cerebral contusion in mice; however, a role for Akt/mTOR in concussive brain injury has not been well characterized. In a mouse closed head injury (CHI) concussion traumatic brain injury (TBI) model, phosphorylation of Akt (p-Akt), mTOR (p-mTOR), and S6RP (p-S6RP) was increased by 24 hours in cortical and hippocampal brain homogenates (P<0.05 versus sham for each), and p-S6RP was robustly induced in IBA-1+ microglia and glial fibrillary acidic protein-positive (GFAP+) astrocytes. Pretreatment with inhibitors of Akt or mTOR individually by the intracerebroventricular route reduced phosphorylation of their respective direct substrates FOXO1 (P<0.05) or S6RP (P<0.05) after CHI, confirming the activity of inhibitors. Rapamycin pretreatment significantly worsened hidden platform (P<0.01) and probe trial (P<0.05) performance in CHI mice. Intracerebroventricular administration of necrostatin-1 (Nec-1) before CHI increased hippocampal Akt and S6RP phosphorylation and improved place learning (probe trials, P<0.001 versus vehicle), whereas co-administration of rapamycin or Akt inhibitor with Nec-1 eliminated improved probe trial performance. These data suggest a beneficial role for Akt/mTOR signaling after concussion TBI independent of cell death that may contribute to improved outcome by Nec-1.
Keywords: Akt, concussion, mice, necrostatin-1, rapamycin, traumatic brain injury
Introduction
Concussion traumatic brain injury (TBI) is a significant public health problem affecting millions of civilians with sports injuries and thousands of war fighters with blast TBI.1, 2 Even a single concussion can cause prolonged neurologic deficits and accumulation of beta-amyloid plaques in brain, whereas repeated concussions may result in permanent cognitive dysfunction, psychiatric disorders, and pathologic changes in the brain, known as chronic traumatic encephalopathy.3, 4 No specific therapy exists to treat or prevent neurologic sequelae of concussion, in part because of incomplete understanding of the specific mechanisms that lead to neurologic dysfunction.4, 5
Akt (protein kinase B) and mammalian target of rapamycin (mTOR) are protein kinases involved in translation, transcription, metabolism, and other cellular homeostatic functions.6 mTOR exists in two distinct multi-protein complexes, mTOR complex 1 (mTORC1; inhibited by rapamycin) and mTORC2 (rapamycin insensitive).6 Akt is activated by phosphorylation of Thr 308 by phosphoinositide-dependent kinase-1 and of Ser 473 by mTOR Complex 2 (mTORC2), whereas mTORC1 is activated in part by Akt as well as Akt-independent mechanisms. Direct substrates of Akt include Ser9 of glycogen synthase kinase-3 beta and Thr24, Ser256, and Ser319 of FoxO1 transcription factor,7 whereas the Ser235/236 site in S6 kinase is a direct target of p70S6K, a substrate of mTORC1. mTOR regulates translation at the synapse and plays an important role in the physiology of normal learning and memory;8 dysregulation of mTOR activity is associated with synaptic dysfunction and impaired cognition in mice and humans with fragile X syndrome and other neurodevelopmental disorders.9, 10, 11, 12 However, phosphorylation of Akt and mTOR and their direct substrates occurs in contused mouse brain, and pharmacological inhibition of Akt and mTOR together improves post-injury cognitive outcome after controlled cortical impact (CCI) in mice.13
The aforementioned observations, along with other studies showing beneficial effects of rapamycin, suggest a detrimental role for acute induction of Akt and mTOR in cerebral contusion models.13, 14, 15 However, whether the same is true for concussion TBI is unknown. In a prior study of cerebral concussion using a weight drop model, Rubovitch et al.16 showed increased phosphorylation of Akt in the hippocampus, but no effect of indirect inhibition of Akt phosphorylation on cognitive outcome.16
Necrostatin-1 (Nec-1) is a small-molecule inhibitor of receptor-interacting protein kinase-1, a serine/threonine kinase that mediates programmed necrosis and inflammation.17 We previously showed that in a cell death TBI model Nec-1 inhibits microglial inflammation and improves cognitive outcome.18 Receptor interacting protein kinase-1 (RIPK1) has also been reported to link Akt activation with Toll-like receptor 4 signaling in non-neural cell types independent of cell death,19 and Akt activation acts downstream of RIPK1 and is required for tumor necrosis factor alpha production during programmed necrosis in non-neural cells.20 These studies suggest a linkage between RIPK1 and Akt. As our closed head injury (CHI) model is devoid of neuronal cell death and features microgliosis as well as tumor necrosis factor induction,21 this model presents a unique opportunity to examine a possible non-cell death role for Nec-1 in the pathogenesis of concussion TBI.
The purpose of the current study was to determine whether Akt and mTOR activation occurs after concussive TBI in a model devoid of acute cell death,21 and whether these pathways might contribute to postinjury cognitive outcome. In addition, we sought to examine whether Nec-1 may have beneficial effects on outcomes related to Akt activation. We hypothesized that concussive TBI would lead to phosphorylation of Akt and mTOR and their respective downstream substrates, and that pharmacological inhibition of Akt and mTOR would improve cognitive outcome as previously reported after CCI in mice.13
Materials and methods
Animals
All experimental protocols were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and conducted according to the National Institutes of Health guide for the care and use of laboratory animals. Studies were performed using adult (8–12 weeks, 25–30 g) male C57/BL6 mice (Jackson Laboratories, Bar Harbor, ME, USA). Mice were housed in a pathogen-free environment with 12-hour day night cycles. For all experiments, mice were randomly allocated to experimental groups before CHI, and experimenters were masked to treatment groups during production of CHI and behavioral testing.
Mouse Closed Head Injury Concussion Model
Closed head injury simulating concussion TBI was induced as previously reported.21 Mice were anesthetized with 4% isoflurane (Anaquest, Memphis, TN, USA) for 45 seconds in a 70% N2O–30% O2 Fluotec 3 vaporizer (Colonial Medical, Amherst, NH, USA). Mice were placed on a Kimwipe (Kimberly-Clark, Irving, TX, USA) and grasped by the tail. The head was positioned under a hollow tube (length 66 inches; diameter 10 mm). A 54 g metal bolt was dropped on the head between the coronal and lambdoid sutures, whereupon the mouse head readily penetrated the Kimwipe and rotated downward in the anterior–posterior plane. The control group consisted of age-matched sham-injured mice (anesthesia but no weight drop). Injured mice were recovered in room air in their cages. The model does not produce hypoxia or hypotension, edema, blood–brain barrier damage, or acute and chronic cell death/tissue loss, and mice regain consciousness typically within 2–7 minutes after injury.21
Western Blotting
After CHI or sham injury, brains were removed and hippocampi and cortices were carefully dissected and frozen in liquid nitrogen. Brain tissue was homogenized in RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS, Boston BioProducts, Ashland, MA, USA) plus protease inhibitor (Roche, Indianapolis, IN, USA) and phosphatase inhibitor (Roche) immediately before use. Samples were centrifuged at 10,000 g for 10 minutes and supernatants obtained. Protein concentration was measured using the DCTM protein assay (Bio-Rad, Richmond, CA, USA). Samples were run in precast 3–8% Tris-acetate or 10% Tris-HCl gels (Bio-Rad) and semi-dry transferred onto polyvinylidene fluoride membranes. After blocking in 5% non-fat milk for 1 hour, membranes were incubated overnight at 4 °C with primary antibodies (Cell Signaling, Beverly, MA, USA) against phospho (ser235/236)-S6RP (1:1000), phospho (Ser473)-Akt (1:750), phospho (Ser2448)-mTOR (1:1000), phospho (Ser256)-FoxO1 (1:1000), S6RP (1:1500), Akt (1:1000), or mTOR (1:1000). Horseradish peroxidase-conjugated secondary antibody was used for ECL-plus (GE Healthcare, Woburn, MA, USA) detection. The results were normalized to β-actin (1:5000, Abcam, Cambridge, MA, USA). Relative optical densities for protein bands were determined using image analysis (Kodak Image Station, Rochester, NY, USA). For all experiments, a minimum of n=3 mice/condition were studied.
Preparation of Brain Tissue for Immunohistochemistry
Mice were killed under deep anesthesia by transcardial perfusion with 4% paraformaldehyde in phosphate-buffered saline pH 7.4. Brains were post-fixed in 4% paraformaldehyde for 24 hours and cryoprotected in 25% sucrose for 24 hours at room temperature. Brains were then frozen and sectioned on a cryostat (12 μm).
Immunohistochemistry
Sections were boiled in antigen unmasking solution (Vector Laboratories, Burlingame, CA, USA) for 15 minutes, rinsed in phosphate-buffered saline, and blocked with 10% normal donkey serum for 2 hours at room temperature. Sections were incubated overnight at 4 °C with antibody against phospho (ser245/236)-S6RP (1:250), followed by Cy3-conjugated anti-rabbit secondary antibody. For double staining, sections were reacted with mouse anti-mouse NeuN-FITC (1:400, Millipore, Danvers, MA, USA) or mouse anti-IBA-1 (1:300, Abcam), followed by incubation with fluorescence-conjugated secondary antibodies (Cy2-conjugated anti-mouse IgG; 1:300, Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Mouse monoclonal anti-glial fibrillary acidic protein antibody (1:2000; Sigma, St Louis, MO, USA) was used to detect astrocytes. Images were photographed using a Nikon Eclipse Ti-S fluorescence microscope.
Administration of Rapamycin, Akt inhibitor, and Necrostatin-1
Mice were anesthetized and administered rapamycin (1.25 or 12.5 μM; EMD Millipore, Billerica, MA, USA), Akt inhibitor viii (1.25 or 12.5 μM, EMD Millipore, a selective Akt 1 and Akt 2 isoform inhibitor), or 7-Cl-O-Nec-1 (4 mM, a kind gift of Greg Cuny18), or an equal volume of vehicle (4 μL) in each cerebral ventricle immediately before sham or CHI as previously described.22 The rationale for administering Nec-1 or Akt/mTOR inhibitors directly into the brain was to better ascertain their effects on brain Akt/mTOR signaling and cognitive function while avoiding potential systemic toxicity and possible limitations of drug delivery across the blood–brain barrier. Pre-injury administration was used to establish proof of concept that Akt/mTOR signaling plays a role in recovery after concussion TBI without having to deal with therapeutic window considerations at the early stages of investigation in the CHI model.
Evaluation of Spatial Learning and Memory
The Morris water maze (MWM) was used to evaluate spatial learning and memory as previously described.21 Testing was started 72 hours post injury, and completed by 4 days after injury. The apparatus consisted of a white pool 83 cm in diameter and 60 cm deep, filled with water to a depth of 29 cm. Highly visible cues were positioned on the walls of the tank and around the room. Water temperature was maintained at 25°C. A clear plexiglass goal platform 10 cm in diameter was positioned 0.5 cm below the surface of the water ∼15 cm from the southwest wall. Each mouse was subjected to a series of 1–2 trials per day. For each trial, mice were randomized to one of four starting locations (North, South, East, and West) and placed in the pool facing the wall. The maximum time allotted to find the submerged platform was 90 seconds. If the mouse failed to reach the platform within this time it was placed on the platform by the experimenter and allowed to remain there for 10 seconds. Two sets of visible platform trials were performed, with the goal platform raised 0.5 cm above the water and clearly marked with a red tape. For probe trials, mice were placed in the tank opposite the target quadrant and the time spent in the target quadrant was quantified (60 seconds maximum). Mice were subjected to two hidden platform trials on test days 1 and 2, and one hidden platform trial on test day 3. On test day 3, a probe trial was done 2–3 hours after the fifth hidden platform trial. Both visible platform trials were done on test day 3 or 4.
Statistical Analyses
Data are mean±s.e.m.. Analyses were performed using Graphpad Prism 6 (San Diego, CA, USA) or SAS software (Cary, NC, USA). To assess the effects of CHI versus sham injury on MWM performance, a repeated-measures analysis of variance (RM ANOVA) mixed model accounting for sham/CHI and the three treatment groups (vehicle, Akt inhibitor viii, or rapamycin), without interaction, was performed in SAS. Within the sham injury or CHI groups, two-factor RM ANOVA (group × time) with Dunnett's test for multiple comparisons was used to analyze hidden and visible platform data with vehicle-treated mice as controls. Probe trial data were assessed by ANOVA and Dunnett's test, again with vehicle-treated mice as controls. Because sham-injured mice and CHI groups were not run concomitantly in MWM experiments evaluating the effects of Nec-1, sham-injured groups and CHI groups were analyzed separately by RM ANOVA and Dunnett's test. Probe trial data were analyzed by ANOVA and Sidak's multiple comparison test. Western blot densitometry data were analyzed by t-test. For all analyses, P<0.05 was considered significant.
Results
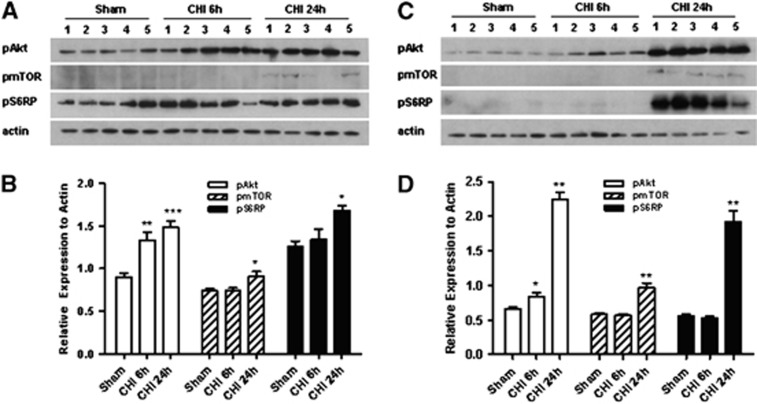
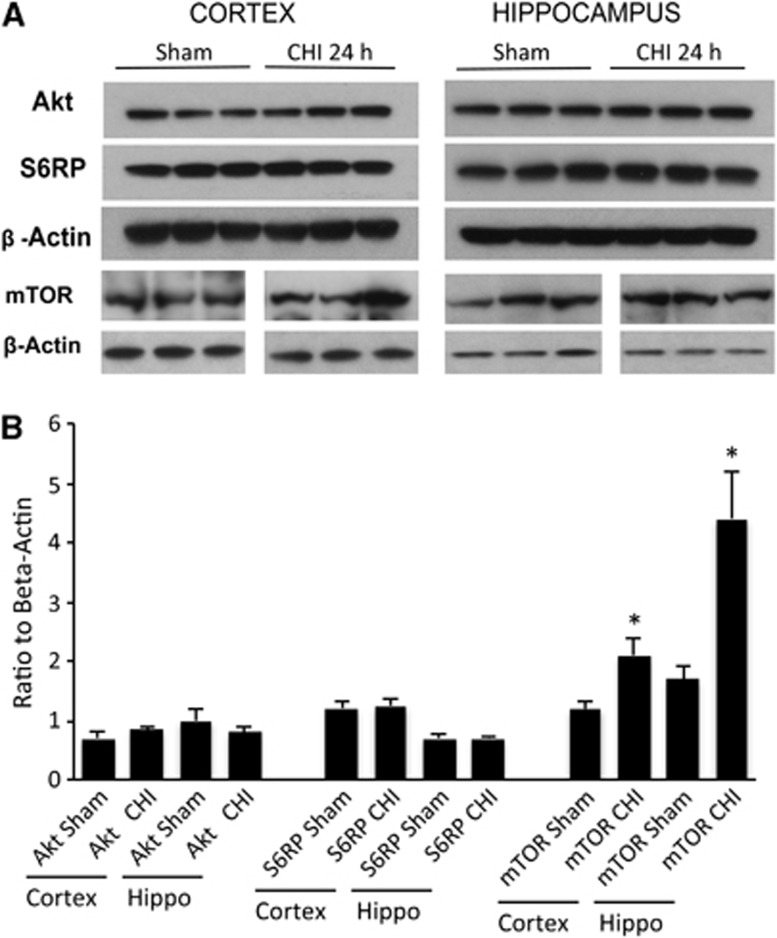
All mice survived CHI and the experimental period. Figure 1 shows expression of phosphorylated Akt, mTOR, and S6RP in the injured cortex and hippocampus after CHI. Compared with sham injury, p-Akt was induced in the injured cortex at 6 and 24 hours, whereas p-S6RP was induced at 24 hours (Figures 1A and 1C). In the injured hippocampus, p-Akt was modestly induced at 6 hours and robustly expressed at 24 hours, whereas marked induction of p-mTOR and p-S6RP also occurred at 24 hours (Figures 1C and 1D). No changes in total Akt or S6RP were observed between sham injured and CHI mice at 24 hours in the cortex or hippocampus; however, total mTOR was increased in the cortex (P<0.05) and hippocampus (P<0.02) at 24 hours after CHI (Figure 2).
Figure 1.
Expression of phosphorylated Akt (protein kinase B) (p-Akt), mammalian target of rapamycin (mTOR) (p-mTOR), and S6RP (p-S6RP) after sham injury or closed head injury (CHI). (A) Representative western blots of cortical brain homogenates at 6 or 24 hours after CHI. (B) Densitometric quantitation of phosphorylated proteins from western blots in panel A. *P<0.05 versus sham. **P<0.005 versus Sham. ***P<0.0005 versus sham. (C) Representative western blots of phosphoproteins in the hippocampus at 6 or 24 hours after CHI. (D) Densitometric quantitation of phosphorylated proteins from western blots in panel C. *P<0.05 versus sham injured; **P<0.001 versus sham injured (n=5/group).
Figure 2.
(A) Representative western blots and (B) densitometry of total Akt (protein kinase B), mammalian target of rapamycin (mTOR), and S6RP in sham and injured mice. No significant differences in expression of Akt (protein kinase B) or S6 were observed in the cortex or hippocampus between sham injured and CHI groups (P>0.25, n=3–6/group). Compared with sham injured mice, total mTOR was increased in the cortex and hippocampus at 24 hours after CHI. *P<0.05 versus sham, n=4–5/group).
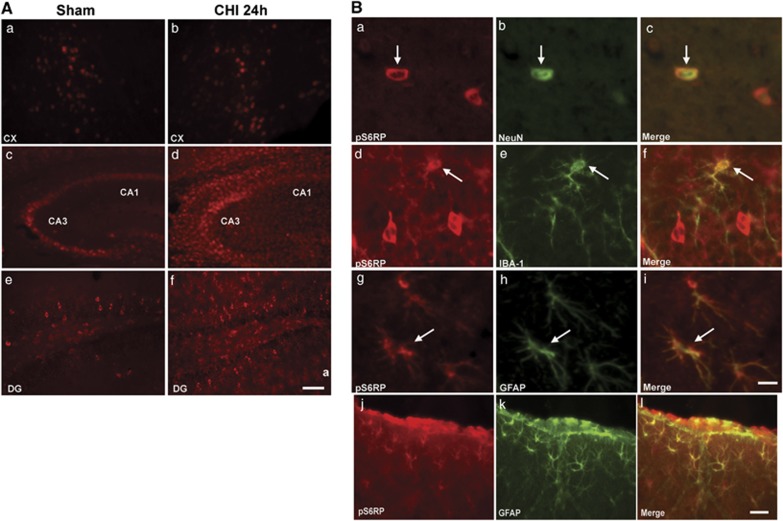
In sham-injured mice, p-S6RP was detected in cells with morphology of neurons in the cortex, CA3, and dentate gyrus, and was induced in cells with morphology of glia in CA3 and the dentate gyrus of injured mice (Figure 3A). Co-localization experiments confirmed expression of phospho-S6RP in NeuN+ neurons, IBA-1+ microglia, and glial fibrillary acidic protein-positive (GFAP+) astrocytes in the injured hippocampus and cortex (Figure 2B and data not shown). Thus, glial cells and neurons in the cortex and hippocampus were a prominent source of p-S6RP after CHI.
Figure 3.
Representative photomicrographs of p-S6RP expression in the cortex and hippocampal brain regions CA3, CA1, and dentate gyrus (DG) in sham injured and closed head injured mice (n=5/group) at 24 hours. (A) Only minor changes in p-S6RP were observed after injury in the cortex (a, b), whereas p-S6RP was robustly induced in cells with morphology of microglia and astrocytes in CA3/CA1 (c, d) and DG (e, f) regions. Scale bar, 50 μm (a, b, e, f), 100 μm (c, d). (B) Identification of cell types in the hippocampus (a–i) and cortex (j–l) expressing p-S6RP 24 hours after closed head injury (CHI). p-S6RP colocalized with NeuN+ neurons (a–c), IBA-1+ microglia (d–f), and glial fibrillary acidic protein-positive (GFAP+) astrocytes (g–i) in the hippocampus and with GFAP+ astrocytes in the cortex (j–l) after CHI. Scale bar a–i, 10 μm; scale bar j–l, 50 μm.
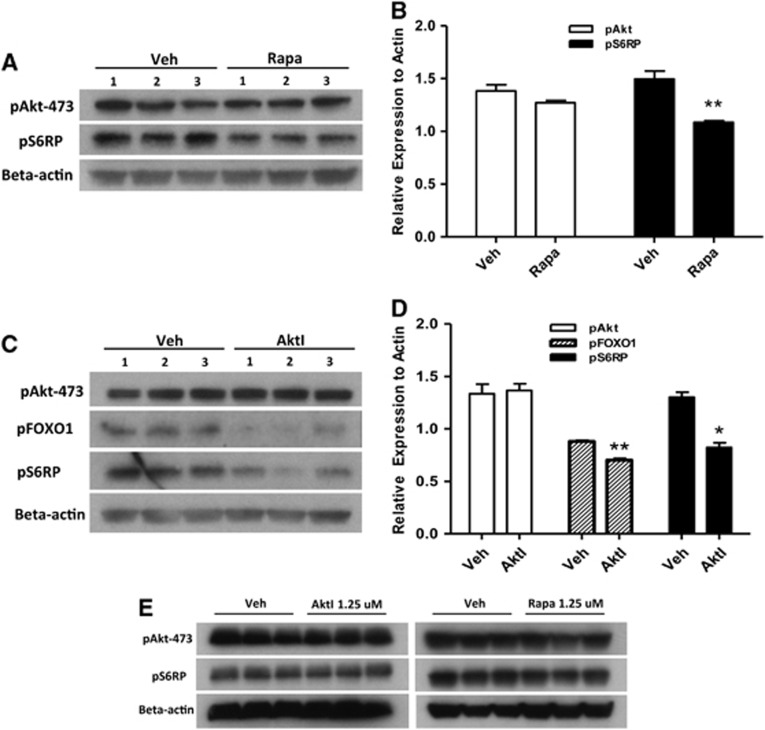
To determine a possible functional role for Akt and mTOR signaling after CHI, we administered rapamycin, an inhibitor of mTORC1,6 Akt inhibitor viii, or vehicle to mice before CHI or sham injury and performed MWM testing (days 3–6) or western blot analyses (day 1) of Akt and mTOR substrates in the hippocampi. We chose doses of inhibitors based on our prior experience in a CCI model13 and their ability to inhibit substrate phosphorylation at 24 hours after CHI (Figure 4). As expected, treatment with rapamycin (12.5 μmol/L) reduced phosphorylation of S6RP but not Akt (Figures 4A and 4B), whereas treatment with Akt inhibitor viii significantly reduced phosphorylation of the direct Akt substrate FOXO-1 as well as that of S6RP (Figures 4C and 4D). Neither rapamycin nor Akt inhibitor viii administered at 1.25 μmol/L had any effect on phosphorylation of their respective substrates (Figure 4E and densitometry data not shown), confirming the dose-dependent in vivo activity of rapamycin and Akt inhibitor viii.
Figure 4.
Effect of rapamycin and Akt (protein kinase B) inhibitor viii administration on downstream substrate phosphorylation in the hippocampus after closed head injury (CHI). (A, B) Compared with vehicle (Veh)-treated mice, mice administered rapamycin (12.5 μmol/L) had significantly reduced expression of pS6RP (**P<0.001 versus vehicle, n=3/group). (C, D) Intracerebroventricular administration of Akt viii (12.5 μmol/L) had no effect on Akt phosphorylation (Ser-473) but significantly reduced expression of p-FOXO1 and p-S6RP assessed by densitometry (**P<0.001, *P<0.05 versus Veh, n=3/group). (E) Lower concentrations of inhibitors (1.25 μmol/L) had no apparent effect on Akt or S6 phosphorylation after CHI.
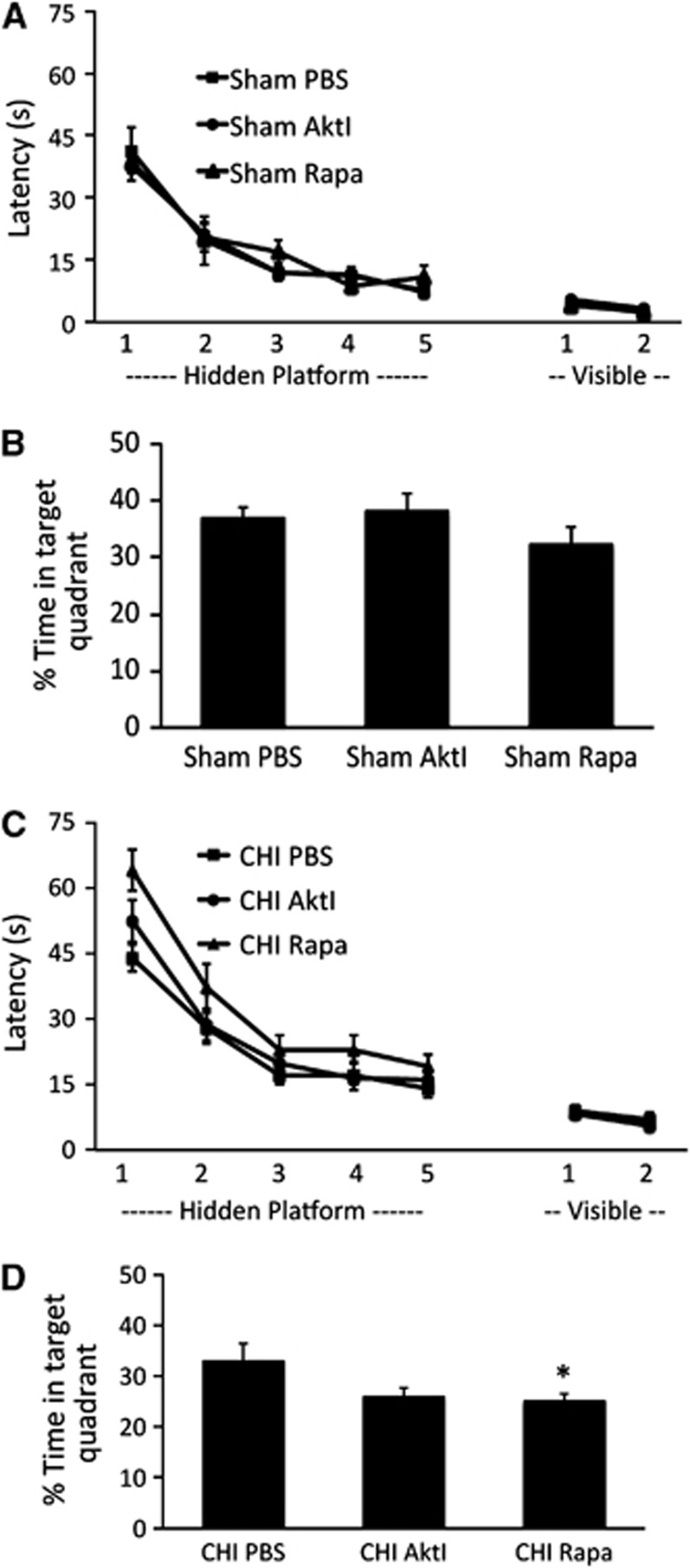
We next examined MWM performance in sham-injured and CHI mice treated with vehicle, rapamycin (12.5 μmol/L), or Akt inhibitor viii (12.5 μmol/L). Using a mixed-model RM ANOVA accounting for injury status (sham versus CHI) and three treatment groups (Akt viii, rapamycin, or vehicle) without interaction, a significant effect of CHI on hidden platform trials was observed (P<0.01 for CHI versus sham injury). In sham-injured mice, MWM performance (hidden or visible platform trials) did not differ among vehicle-, Akt inhibitor viii-, or rapamycin-treated groups (Figure 5A). In CHI mice, treatment groups were significantly different (P=0.0031 RM ANOVA) but only rapamycin administration significantly worsened the performance in hidden platform (P<0.01 versus vehicle, Dunnett's test) and probe trials (P<0.05 versus vehicle, ANOVA and Dunnett's test)(Figure 5B). In contrast, treatment with Akt inhibitor viii had no effect on hidden or visible platform or probe trials (P=0.06) in CHI mice (Figure 5B).
Figure 5.
Effect of Akt (protein kinase B) inhibitor viii and rapamycin on cognitive outcome after sham or closed head injury (CHI). (A, B) Sham injured mice (n=10/group) were administered Akt inhibitor viii (Akt viii), rapamycin (Rap), or vehicle (Veh) intracerebroventricularly and Morris water maze testing was performed 3 days later. Although all sham-injured groups learned the task (P<0.001 for time, hidden and visible platform trials), no differences in hidden (P=0.37 for group, repeated-measures ANOVA (RM ANOVA)) or visible platform (P=0.16 for group, RM ANOVA), or probe trials (P=0.4 ANOVA, B) were observed. (C, D) Of the CHI groups (n=18/group), all mice learned the hidden platform paradigms (P<0.001 for time in each group). In the RM ANOVA used to evaluate treatment effects there was a significant group effect (P=0.0031). In post hoc analyses, rapamycin-treated mice performed significantly worse than vehicle-treated mice in hidden platform (P<0.01 Dunnett's test) and probe trials (P<0.05 ANOVA and *P<0.05 Dunnett's test, D). No differences in visible platform performance were noted among CHI groups (P=0.71 for group) and no differences in hidden, visible platform, or probe trials were noted between CHI-VEH and CHI-Akt viii groups.
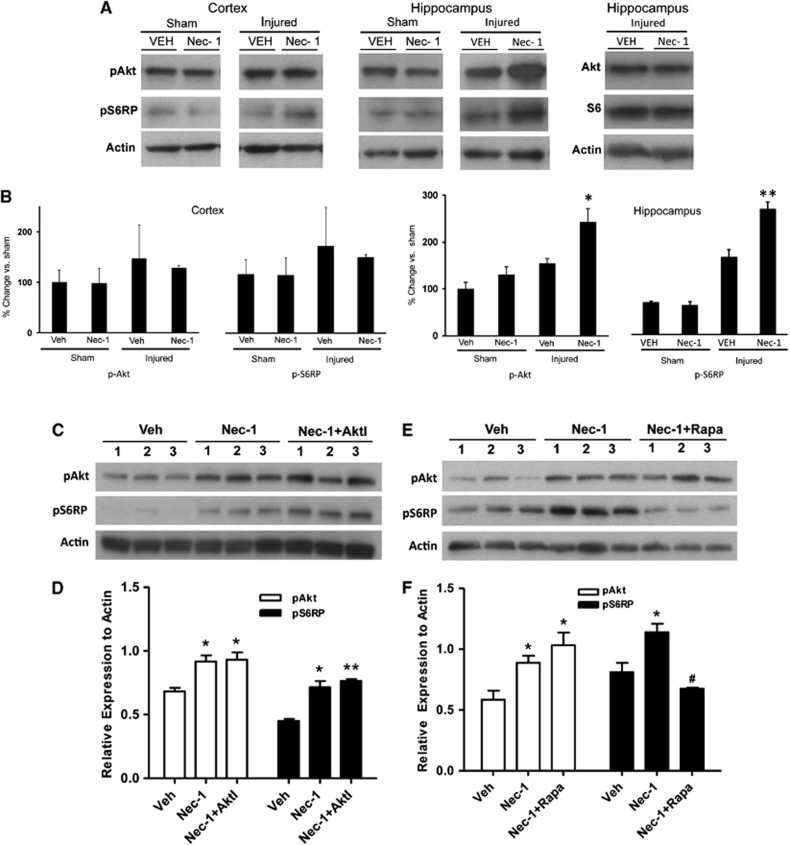
Previous studies from our laboratory showed a beneficial effect of Nec-1 on cognitive outcome after experimental contusion in mice.18 To evaluate Nec-1 in our concussion model, we administered Nec-1 to mice before sham or CHI and assessed Akt/S6RP phosphorylation and MWM performance. In sham-injured mice, Nec-1 treatment had no effect on pAkt or pS6RP expression in the cortex or hippocampus (Figures 6A–C). In CHI mice, treatment with Nec-1 increased phosphorylation of Akt and S6RP compared with vehicle in the hippocampus, but not cortex. Necrostatin-1 treatment did not affect total Akt or S6 protein expression in the hippocampus after CHI (Figure 6A) (densitometry S6RP: CHI Nec-1, 86+17% CHI Veh, 92+26% beta actin, P=0.83, n=5–6/group; densitometry Akt: CHI Nec-1, 74+11% CHI Veh, 75+8% beta actin, P=0.96, n=3/group). In CHI mice, co-administration of Akt inhibitor viii with Nec-1 did not affect the Nec-1-mediated increase in hippocampal Akt and S6RP phosphorylation (Figures 6C and 6D). However, co-administration of rapamycin with Nec-1 inhibited the Nec-1-induced phosphorylation of S6RP, but not Akt, in injured hippocampus (Figures 6E and 6F).
Figure 6.
Necrostatin-1 administration increases Akt (protein kinase B) and S6 phosphorylation after closed head injury (CHI). (A) Administration of necrostatin-1 (Nec-1) before CHI had no effect on Akt or S6 phosphorylation in the cortex in sham or CHI mice, but pretreatment with Nec-1 increased phosphorylation of Akt-473 and S6RP in hippocampal brain homogenates at 24 hours after CHI. After CHI, Nec-1 administration did not increase total Akt (P=0.96, n=3/group) or total S6 (P=0.83 versus Veh, n=5–6/group) in the hippocampus. (B) Densitometric analyses of western blot data of the phosphoproteins shown in A. *P<0.01 versus injured, Vehicle (Veh; n=4/group); **P<0.005 versus injured, Veh (n=4/group). (C) Co-administration of Nec-1 and Akt inhibitor viii (AktI) did not prevent the increase in hippocampal p-Akt 473 or p-S6RP observed with Nec-1 alone. (D) Within-group densitometric analyses of the western blot data in C (*P<0.05 versus Veh; **P<0.01 versus Veh, n=3/group). (E) Compared with vehicle, treatment with Nec-1 increased phosphorylation of hippocampal Akt-473 and S6RP, whereas co-administration of Nec-1 and Rapa prevented the increase in p-S6RP observed with Nec-1 alone. (F) Densitometric analyses of western blot data in E. *P<0.05 versus Veh; #P<0.05 versus Nec-1 treatment (n=3/group).
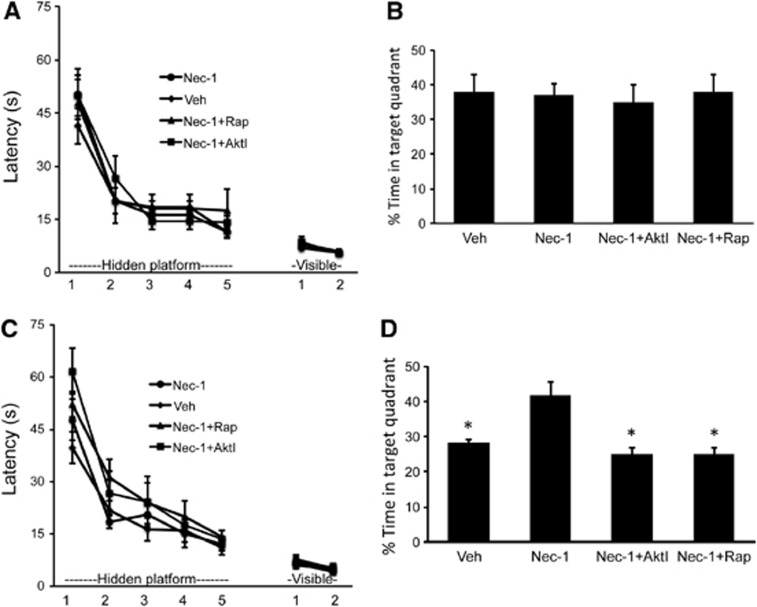
To assess a functional role for Nec-1 in recovery after CHI, sham-injured and CHI mice were administered Nec-1, vehicle, or combinations of Nec-1/Akt inhibitor viii or Nec-1/rapamycin and subjected to MWM testing 3 days later. In sham-injured mice, no effect of Nec-1 or combined inhibitors with Nec-1 was observed in hidden, visible platform, or probe trials compared with vehicle-treated shams (Figure 7A). In CHI mice, treatment with Nec-1 alone had no effect on hidden or visible platform performance, but significantly improved post-injury probe trial scores compared with vehicle controls (Figures 7C and 7D). However, probe trial performance was reduced to chance levels in CHI mice treated with Nec-1+Akt inhibitor viii or Nec-1+rapamycin (Figure 7D). Taken together, the results indicate a beneficial effect of Nec-1, possibly mediated through Akt/mTOR activity, on spatial memory after concussive TBI.
Figure 7.
Effect of necrostatin-1 and Akt (protein kinase B)/mammalian target of rapamycin (mTOR) inhibitor treatment on cognitive outcome after closed head injury (CHI). (A) In sham-injured mice (n=8/group), all groups demonstrated improvement over time (P<0.001 for time); however, there was no effect of group (necrostatin-1 (Nec-1, 4 mM), Nec-1 plus rapamycin (Rapa, 12.5 μmol/L), Nec-1 plus Akt viii, or vehicle (VEH)) on performance in hidden (P=0.9 repeated-measures ANOVA (RM ANOVA)) or visible platform (P=0.7 RM ANOVA) or (B) probe trials (P=0.97 ANOVA). (C) In closed head injury (CHI) mice (n=8–13/group), all groups improved their performance (P<0.001 for time), but no differences were observed among groups in hidden (P=0.17 for group, RM ANOVA) or visible (P=0.88 for group, RM ANOVA) platform trials. (D) Regarding probe trials, ANOVA revealed a highly significant difference among the four groups (P<0.0001). In post hoc analyses, probe trial latencies were significantly lower in vehicle-treated, Nec-1/Akt viii, and Nec-1/Rapamycin co-treatment groups compared with Nec-1 treatment (*P<0.001 for each comparison with Nec-1, Sidak's multiple comparison test). No differences were observed between Veh, Nec-1/Akt, and Nec-1/Rap groups.
Discussion
Despite a rapidly growing awareness of the importance of concussion TBI as a public health problem,23 specific molecular mechanisms leading to concussion-induced neurological dysfunction remain to be identified.4, 5 We found that CHI induced phosphorylation of Akt and mTOR and their direct downstream substrates FOXO1 and S6RP, respectively, in cortical and hippocampal brain homogenates. Expression of p-S6RP was upregulated in hippocampal Iba-1-reactive microglia and GFAP-reactive astrocytes and was detected in neurons in both brain regions. Pharmacological inhibitors of Akt and mTOR reduced phosphorylation of their intended targets, and rapamycin impaired cognitive function after CHI. The beneficial effects of Nec-1 on post-injury cognitive recovery were associated with increased phosphorylation of Akt and S6RP, and were abrogated by co-administration of Akt inhibitor viii or rapamycin. In striking contrast to our previous findings in a CCI model,13 the data suggest a protective role for Akt and mTOR pathways after concussive TBI.
Closed head injury used herein models specific features of human concussion, including impact and rotational acceleration–deceleration forces, loss of consciousness, neuroinflammation, and cognitive deficits;24, 25 however, this model does not induce detectable acute cell death or chronic brain tissue loss.21 Although Akt and mTOR activation have been noted in a number of TBI models,13, 15, 16 the current study is the first to our knowledge to examine Akt/mTOR in a concussion model devoid of neuronal cell death. Akt activation in the brain following TBI is mainly considered in the context of limiting apoptosis.26 The current study demonstrates that Akt/mTOR activation may be a beneficial response to concussion (impact and inertial forces) independent of focal injury and acute cell death.
Similar to our previous report in a mouse CCI model,13 phosphorylation of Akt-473 and S6RP was induced after CHI in the cortex and hippocampus. p-Akt-473 was modestly but significantly increased in the cortex at 6 hours, whereas p-S6RP was strongly induced at 24 hours in both regions (Figure 1). These findings, along with the observation that Akt inhibitor viii blocked CHI-induced phosphorylation of S6RP (Figures 4C and 4D), are consistent with activation of AKT and mTOR in the CHI model. In contrast, both Akt and mTOR pathways were strongly induced by 4 hours after CCI.13 In two other CHI models, Akt phosphorylation was induced in the hippocampus between 1 and 24 hours in mice,16 but only transiently at 1 hour after diffuse brain injury in rats,27 whereas mTOR activation was reported from 30 minutes to 24 hours after fluid percussion in rats.28 Thus, activation of Akt and mTOR is a generalized feature of TBI,29 but their exact temporal course is model-specific.
Rapamycin (but not Akt inhibitor viii) impaired cognitive function after CHI, with rapamycin-treated mice performing worse in hidden platform and probe trials. These data suggest that mTOR more so than Akt might be critical for recovery of cognitive function after CHI. In contrast, in a CCI model, AKT/mTOR inhibitors improved the outcome only when administered together, but had no effect when given alone.13 Because probe trials are a measure of hippocampus-dependent spatial memory function, we focused our biochemical analyses of Akt and mTOR pathways in this brain region. As expected, administration of rapamycin inhibited CHI-induced phosphorylation of S6RP, a direct substrate of mTOR, but not Akt-473, which is phosphorylated by the rapamycin-insensitive mTORC2.6 Administration of Akt inhibitor viii reduced phosphorylation of FOXO1 (a direct Akt substrate), and S6RP, consistent with mTORC1 as a substrate for Akt.6 Thus, intracerebroventricular administration of Akt and mTOR inhibitors in doses similar to those previously reported by us13 inhibited phosphorylation of their respective targets with activity at 12.5 but not 1.25 μmol/L (Figure 4).
One obvious difference between CCI and CHI is the presence of cell death in CCI. It is possible that our data may be revealing differential roles of Akt pathways in brain function versus cell death. In TBI accompanied by significant necrosis, inhibition of cell death by targeting Akt/mTOR may result in overall benefit.20 In the case of no cell death (CHI), Akt/mTOR may promote recovery of brain function and inhibition may be detrimental.
How might Akt and mTOR contribute to recovery after concussive TBI? In neurons, inhibition of Akt/mTOR might be expected to impair synaptic protein synthesis needed for normal learning and memory.30 Regulated Akt and mTOR signaling is critical for synaptic plasticity mechanisms,30 and several genetic disorders with impaired cognition feature dysregulated Akt/mTOR activation.31 Transient activation of Akt/mTOR by NMDA and TrkB receptors is required for long-term potentiation of synaptic transmission, however constitutive overexpression of Akt in CA1 is detrimental to learning in rats, possibly because of loss of temporal regulation of Akt.30, 32 Akt can also modulate NMDA/TrkB receptor signaling leading to increased calcium influx after NMDA and BDNF stimulation.33 Akt and/or mTOR might also facilitate normal learning through NMDA, AMPA, and other receptor trafficking or phosphorylation.34, 35, 36 Thus, from a neuron-centric view, inhibition of Akt/mTOR after CHI, and even in sham-injured animals, might be expected to have detrimental effects on learning and memory. However, we did not observe deficits in sham-injured mice, perhaps because inhibitors were no longer present in the brain by the time MWM testing was begun (72 hours after ICV injection). Rather, our findings suggest that rapamycin interacts with CHI to produce detrimental effects on cognition in the recovery phase, perhaps through neuronal mechanisms or others.
The finding that Akt/mTOR pathways are activated after CHI in the hippocampus (Figures 1 and 2), a site for significant morphological activation of astrocytes and microglia,21 and that administration of rapamycin worsens hidden platform and probe trial performance (Figure 5), suggests that glia-specific protein synthesis (mediated by Akt/mTOR) may also contribute to cognitive outcome after CHI. In normal (uninjured) brain, microglia and astrocytes contribute in distinct ways to synaptic function, learning, and memory. Microglia continuously survey adult neuronal synapses37 and release BDNF to regulate synaptic function and learning,38 whereas astrocytes play an active role in neuronal plasticity by releasing neurotransmitters that modulate synaptic activity and function.39 Microglia are hypothesized to mediate cognitive dysfunction after TBI,40 but how glial cells respond to concussive TBI is not well understood, and it is possible that glial Akt/mTOR activation is a reactive beneficial response to limit cognitive synaptic dysfunction after CHI. The data presented herein using rapamycin (which inhibited mTOR activation) support this possibility. However, whether glia versus neuronal mechanisms predominate in this response remains to be determined.
A novel finding of the current study is that Nec-1, a specific inhibitor of RIPK1,17 improved probe trial performance after CHI (Figure 7). This finding presents a possible new direction in therapy for concussion TBI. Necrostatin-1 prevents RIPK1-mediated necrosis in susceptible cell lines and reduces cognitive deficits after CCI;17, 18 however, Nec-1 has not been reported in a non-cell death TBI model. The current study suggests a possible link between the beneficial effects of Nec-1 on cognitive function and augmentation of Akt/mTOR signaling after CHI, a finding that lends support to the possibility that Akt/mTOR signaling is a protective response to CHI. Of note, in the presence of Nec-1, Akt inhibitor viii impaired Nec-1-mediated place memory gains after CHI but did not inhibit increased phosphorylation of S6RP (compare Figures 4 and 6), suggesting that increased mTOR activity alone is not sufficient for the beneficial effects of Nec-1 in the CHI model, and that another Akt substrate might be more important in this regard. Alternatively, Nec-1 inhibition of RIPK1, a serine/threonine kinase, might affect phosphorylation and function of glutamatergic receptors, although direct RIPK1 substrates are largely unknown.
Traumatic brain injury is a heterogeneous disease with pathoanatomic features that are injury mechanism-dependent, and it is not surprising that certain molecular pathways may be detrimental in one type of injury (e.g., Akt/mTOR in focal contusion) and beneficial in another (e.g., concussive TBI). The current findings have potential treatment implications for TBI patients and design of clinical trials: Akt and mTOR inhibitors might improve outcome in patients with cerebral contusion,13 but their use could be detrimental in patients with concussive or ‘diffuse' TBI.21 In patients with both concussive and contusion injury subtypes, the net result of Akt/mTOR inhibition on postinjury cognitive function may be unpredictable. The current study underscores the importance of testing small-molecule inhibitors in diverse preclinical models with different pathoanatomic subtypes to help inform clinical trials for patients with TBI.
This study has a number of important limitations. The pharmacological agents used herein may have off-target effects; thus we cannot prove a functional role for RIPK1, Akt, and/or mTOR with pharmacological means alone. We did not perform detailed dose–response studies of inhibitors beyond the two doses reported; therefore we may have missed the optimal inhibiting dose of Akt inhibitor viii or rapamycin. A genetic strategy allowing for cell-specific, temporally controlled gene knockdown or inhibition is needed to validate the functional roles of these molecules in concussion. Second, the clinical translation of findings herein is limited by intracerebroventricular administration of inhibitors before injury. Thus, it is impossible to know whether systemic and/or post-treatment administration would be able to produce the same effects. Third, the CHI model used produces a mild cognitive phenotype, making it difficult to show the positive effects of pharmacological agents. A more severe model would be more suitable to show a beneficial effect of treatments on hidden platform trials. Nonetheless, the current study is the first that we know of to provide a detailed analysis of Akt/mTOR pathway activation independent of cell death in a concussion TBI model. Future studies using systemic administration of RIPK1 inhibitors pre- or post-injury will set the stage for development of therapeutics designed to limit cognitive deficits after concussion TBI, for which no specific therapy currently exists.
The authors declare no conflict of interest.
Footnotes
This work was conducted with support from Harvard Catalyst and financial contributions from Harvard University and its affiliated academic health care centers, grant number 1UL1 TR001102-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health. This work was supported by a grant from NINDS 5RO1NS047447 (MJW), a grant from the National Football League Players Association, and Harvard Catalyst (1UL1 TR001102-01). Authors have no disclosures or conflict of interest to report.
References
- Centers for Disease C, Prevention Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged </=19 years—United States, 2001-2009. MMWR. 2011;60:1337–1342. [PubMed] [Google Scholar]
- Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci Biobehav Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury: football, warfare, and long-term effects. Minnesota Med. 2010;93:46–47. [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC.The molecular pathophysiology of concussive brain injury Clin Sports Med 20113033–48.vii-iii. [DOI] [PubMed] [Google Scholar]
- Shaw NA. The neurophysiology of concussion. Progr Neurobiol. 2002;67:281–344. doi: 10.1016/s0301-0082(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Sanchez E, Hagerman RJ, Mu Y, Nguyen DV, Wong H, et al. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav. 2012;11:332–341. doi: 10.1111/j.1601-183X.2012.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troca-Marin JA, Alves-Sampaio A, Montesinos ML. Deregulated mTOR-mediated translation in intellectual disability. Prog Neurobiol. 2012;96:268–282. doi: 10.1016/j.pneurobio.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson TT, Johnston MV. Plasticity and mTOR: towards restoration of impaired synaptic plasticity in mTOR-related neurogenetic disorders. Neural Plasticity. 2012;2012:486402. doi: 10.1155/2012/486402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zhang J, Qiu J, Zhu X, Degterev A, Lo EH, et al. Combination therapy targeting Akt and mammalian target of rapamycin improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2012;32:330–340. doi: 10.1038/jcbfm.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don AS, Tsang CK, Kazdoba TM, D'Arcangelo G, Young W, Zheng XF. Targeting mTOR as a novel therapeutic strategy for traumatic CNS injuries. Drug Discov Today. 2012;17:861–868. doi: 10.1016/j.drudis.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Rubovitch V, Edut S, Sarfstein R, Werner H, Pick CG. The intricate involvement of the insulin-like growth factor receptor signaling in mild traumatic brain injury in mice. Neurobiol Dis. 2010;38:299–303. doi: 10.1016/j.nbd.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nature Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD, et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivarelli MS, McDonald D, Miller M, Cusson N, Kelliher M, Geha RS. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp Med. 2004;200:399–404. doi: 10.1084/jem.20040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Ahuja R, Osafo-Addo AD, Barrows D, Kettenbach A, Skidan I, et al. Akt regulates TNFalpha synthesis downstream of RIP1 kinase activation during necroptosis. PLoS One. 2013;8:e56576. doi: 10.1371/journal.pone.0056576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuman J, Meehan WP, 3rd, Zhu X, Qiu J, Hoffmann U, Zhang J, et al. Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J Cereb Blood Flow Metab. 2011;31:778–789. doi: 10.1038/jcbfm.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl D, You Z, Lo EH, Kim HH, Whalen MJ. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2007;27:1806–1818. doi: 10.1038/sj.jcbfm.9600487. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury—football, warfare, and long-term effects. N Engl J Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci. 2012;6:58. doi: 10.3389/fncel.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Aubry M, Cantu RC, Dvorak J, Echemendia RJ, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport, Zurich, November 2012. J Athl Train. 2013;48:554–575. doi: 10.4085/1062-6050-48.4.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshita N, Lewen A, Sugawara T, Chan PH. Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol Dis. 2002;9:294–304. doi: 10.1006/nbdi.2002.0482. [DOI] [PubMed] [Google Scholar]
- Valable S, Francony G, Bouzat P, Fevre MC, Mahious N, Bouet V, et al. The impact of erythropoietin on short-term changes in phosphorylation of brain protein kinases in a rat model of traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:361–369. doi: 10.1038/jcbfm.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Atkins CM, Liu CL, Alonso OF, Dietrich WD, Hu BR. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2007;27:939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Ikonomovic MD, Nathaniel PD, Kochanek PM, Marion DW, et al. Increased phosphorylation of protein kinase B and related substrates after traumatic brain injury in humans and rats. J Cereb Blood Flow Metab. 2006;26:915–926. doi: 10.1038/sj.jcbfm.9600238. [DOI] [PubMed] [Google Scholar]
- Graber TE, McCamphill PK, Sossin WS. A recollection of mTOR signaling in learning and memory. Learn Memory. 2013;20:518–530. doi: 10.1101/lm.027664.112. [DOI] [PubMed] [Google Scholar]
- Troca-Marin JA, Casanas JJ, Benito I, Montesinos ML. The Akt-mTOR pathway in Down's syndrome: the potential use of rapamycin/rapalogs for treating cognitive deficits. CNS Neurol Disord Drug Targets. 2014;13:34–40. doi: 10.2174/18715273113126660184. [DOI] [PubMed] [Google Scholar]
- Chao CC, Ma YL, Lee EH. Protein kinase CK2 impairs spatial memory formation through differential cross talk with PI-3 kinase signaling: activation of Akt and inactivation of SGK1. J Neurosci. 2007;27:6243–6248. doi: 10.1523/JNEUROSCI.1531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Perez AM, Llansola M, Felipo V. Modulation of NMDA receptors by AKT kinase. Neurochem Int. 2006;49:351–358. doi: 10.1016/j.neuint.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, et al. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Miyauchi J, Tsirka SE. Microglia: an active player in the regulation of synaptic activity. Neural Plasticity. 2013;2013:627325. doi: 10.1155/2013/627325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Cali C, Bezzi P. Gliotransmission and the tripartite synapse. Adv Exp Med Biol. 2012;970:307–331. doi: 10.1007/978-3-7091-0932-8_14. [DOI] [PubMed] [Google Scholar]
- Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]