Abstract
Background and study aims
The fanning technique for endoscopic ultrasound-guided fine-needle aspiration (EUS–FNA) involves sampling multiple areas within a lesion with each pass. The aim of this study was to compare the fanning and standard techniques for EUS–FNA of solid pancreatic masses.
Patients and methods
Consecutive patients with solid pancreatic mass lesions were randomized to undergo EUS–FNA using either the standard or the fanning technique. The main outcome measure was the median number of passes required to establish diagnosis. The secondary outcome measures were the diagnostic accuracy, technical failure, and complication rate of the two techniques.
Results
Of 54 patients, 26were randomized to the standard technique and 28 to the fanning technique. There was no difference in diagnostic accuracy (76.9% vs. 96.4 %; P=0.05), technical failure or complication rates (none in either cohort). There was a significant difference in both the number of passes required to establish diagnosis (median 1 [interquartile range 1–3] vs. 1 [1–1]; P=0.02) and the percentage of patients in whom a diagnosis was achieved on pass one (57.7% vs. 85.7 %; P = 0.02) between the standard and fanning groups, respectively.
Conclusions
The fanning technique of FNA was superior to the standard approach because fewer passes were required to establish the diagnosis. If these promising data are confirmed by other investigators, consideration should be given to incorporating the fanning technique into routine practice of EUS–FNA.
Introduction
Endoscopic ultrasound-guided fine-needle aspiration (EUS–FNA) is the current standard of care for establishing tissue diagnosis in patients with suspected pancreatic neoplasm [1]. The technique has a median sensitivity of 83%, specificity of 100%, and diagnostic accuracy of 88% [1– 5]. It also plays an important role in vascular staging of pancreatic tumors, which in turn has a major impact on patient management [1, 6].
EUS–FNA of pancreatic masses is considered to be technically more challenging than other lesions and requires a longer learning curve [7–9]. Consequently, the number of passes required to successfully diagnose a solid pancreatic mass varies, ranging from 2.5 to 7 [2–5, 10]. At busy tertiary centers with high procedure volumes, the ability to establish an accurate diagnosis with fewer EUS–FNA passes is important as it translates to shorter procedural duration, lower sedation requirement, improved patient safety, and ultimately, to improved efficiency of the endoscopy service. However, in the current EUS literature, there are no published studies regarding the best technique for performing EUS–FNA. A previous study that evaluated transabdominal ultrasound-guided FNA of pancreatic mass lesions reported that sampling the peripheral area of a mass resulted in an improvement in diagnostic accuracy [11]. In addition, a recent abstract by Wyse et al. reported that the sampling of multiple areas of a mass lesion improved both the aspirate quality and diagnostic sensitivity of EUS–FNA when compared with sampling only one area within a mass lesion [12]. Collectively, these observations suggest that the diagnostic utility of FNA can be enhanced by targeted, rather than random sampling.
The main objective of this randomized trial therefore was to determine whether sampling multiple areas within a mass lesion using the “fanning” technique would result in more rapid diagnosis requiring fewer FNA passes than the standard approach where only a single area within the mass is targeted. Furthermore, as the pancreas is the most challenging organ to sample during EUS, this study included only patients with solid pancreatic mass lesions.
Methods
Patients
A prospective study was undertaken of patients with solid pancreatic mass lesions who were referred for EUS. The study was approved by the Institutional Review Board at the University of Alabama at Birmingham, and written informed consent was obtained from all patients for participation in the study.
Procedural techniques
Patients were randomized to undergo EUS–FNA using either the standard or the fanning technique. At the time of the procedure, the endoscopy nurse opened an envelope containing computer-generated randomization assignments for the study patients. All procedures were performed by one endosonographer (S.V.) using a linear array echoendoscope (Olympus UCT140; Olympus, Tokyo, Japan). Procedures were performed under conscious sedation with patients in the left lateral decubitus position.
Pancreatic masses located in the head or uncinate process were sampled using a 25-G needle (Expect; Boston Scientific Corp., Natick, Massachusetts, USA) via the transduodenal route, and those in the pancreatic body or tail were sampled using a 22-G needle (Expect) via the transgastric route. The 25-G needles for transduodenal passes and 22-G needles for transgastric passes were based on the results of a previous study that demonstrated decreased needle dysfunction using this approach [13]. At FNA, suction was not applied in any of the cases, and after the first pass the stylet was not reintroduced into the needle assembly for subsequent FNAs.
Standard technique
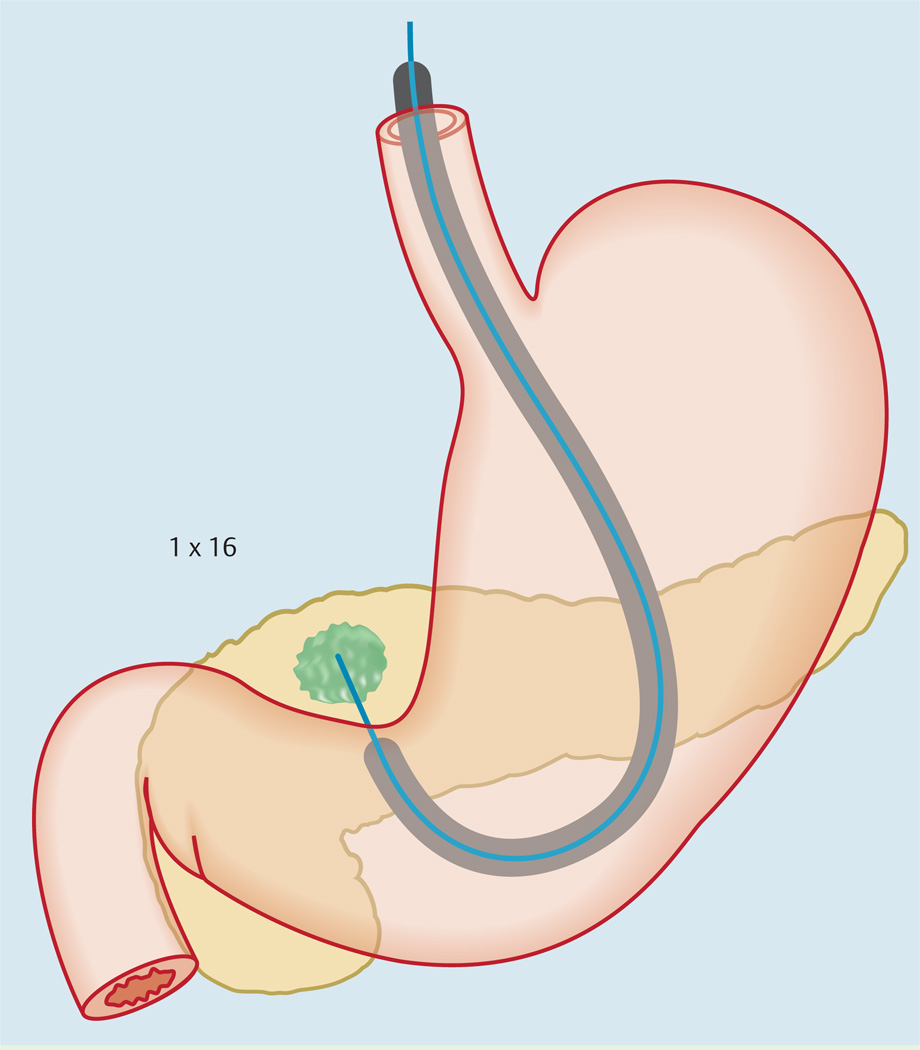
At individual passes, the needle tip was positioned at one location within the mass and then moved back and forth 16 times to procure tissue (1 × 16) (Fig.1). The area sampled was the periphery of the mass lesion in line with the natural trajectory of the FNA needle as it exited the echoendoscope (Video 1). For subsequent passes, a different margin of the mass was targeted but the needle movement was confined to the same area.
Fig.1.
Illustration showing the standard technique of endoscopic ultrasound-guided fine-needle aspiration.
Fanning technique
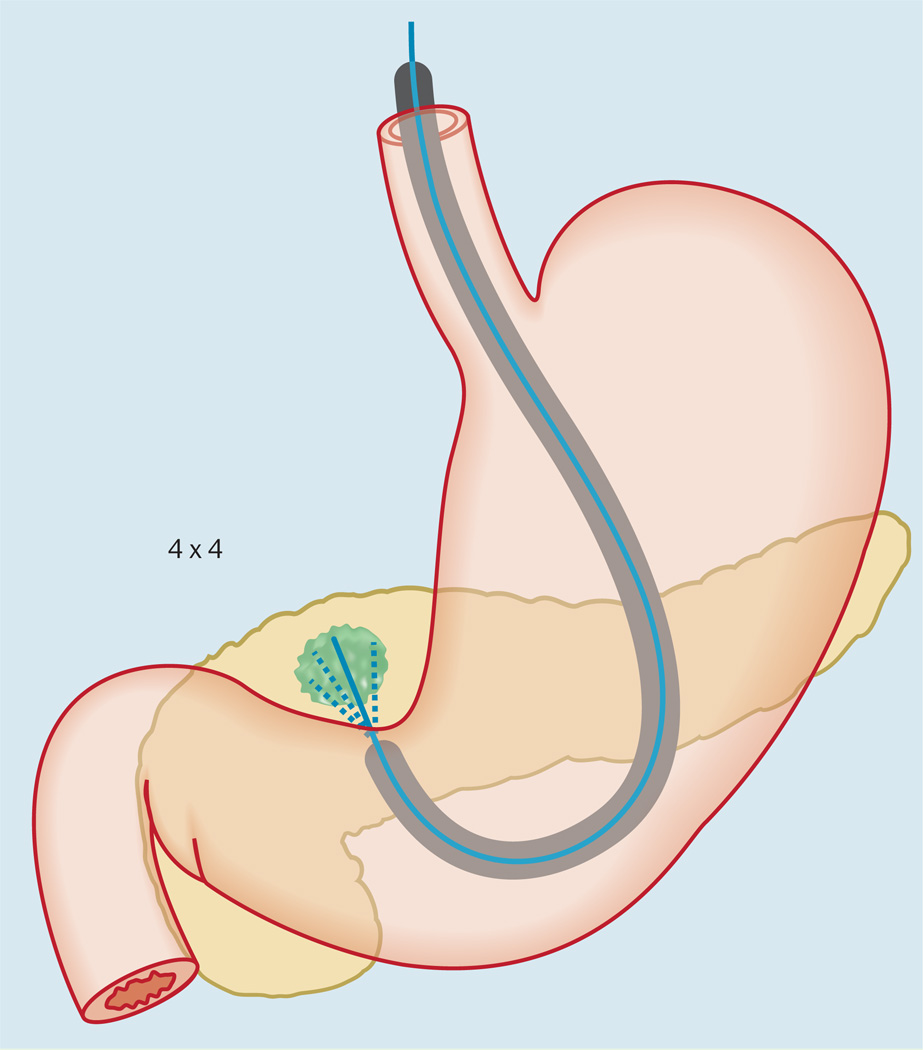
At individual passes, the needle was positioned at four different areas within the mass and then moved back and forth four times in each area to procure tissue (4 × 4). The needle was positioned at different areas within the mass by using the “up-down” dial of the echoendoscope and with minimal use of the elevator to avoid needle dysfunction. Aspiration was initiated at the left margin and then “fanned” (Video 2) until the right margin of the mass was sampled (Fig.2).
Fig.2.
Illustration showing the fanning technique of endoscopic ultrasound-guided fine-needle aspiration.
Following each pass, the procured material was placed onto slides for immediate interpretation by an on-site cytopathologist who was blinded to the procedural technique. Three maximum passes were performed with the initial technique until either the procured specimen was deemed to be of satisfactory diagnostic quality or technical failure occurred. Technical failure was defined as malfunction of the needle apparatus prior to establishing a diagnosis with the original sampling technique. If a definitive diagnosis was established within three passes, the procedure was terminated and the number of passes performed was documented. If no diagnosis was established after three passes (defined as diagnostic failure) or if technical failure occurred, the patient was crossed over and up to three further passes were performed using the alternative sampling method. If diagnostic or technical failure occurred again with the alternative technique, the procedure was aborted and a repeat EUS–FNA was arranged for a different day. The occurrence of any immediate complication was noted at the time of the procedure and late complications were documented with follow-up telephone calls 72 hours post-procedure.
Preparation of cytological specimens
After each pass, the procured specimen was air-dried on-site (air-dried smear) and stained with Diff-Quick stain (Dade Diagnostics, Miami, Florida, USA) to assess for sample adequacy. A provisional diagnosis was made on-site by an attending cytopathologist. Alcohol-stained smears were prepared using the Papanicolaou stain, and cell blocks were prepared for further analysis when deemed necessary.
Outcome measures
The primary outcome was the median number of passes required to establish a diagnosis using the standard and fanning sampling techniques. The secondary outcomes were the rates of diagnostic accuracy, technical failure, and complications for the two sampling techniques. Diagnostic accuracy was defined as the proportion of patients in whom successful on-site diagnosis was reached within three passes using the original EUS–FNA technique.
Statistical analysis
A two-tailed sample size calculation was performed with a type I error rate (α) of 0.05 and a power of 90% for detecting a one-pass difference between the two sampling techniques in reaching successful on-site diagnosis. The SD for the number of passes required was set at 1 for the fanning technique and 1.2 for the standard technique. This resulted in target sample sizes of 26 for the standard technique cohort and 26 for the fanning technique cohort. Baseline characteristics of the patient population, solid pancreatic mass lesions, and EUS–FNA procedure details were recorded. Continuous data were summarized as means (with SD) and medians (with interquartile range [IQR] and range) and compared using the Wilcoxon rank-sum test. Of note, for calculation of the summary statistics on the number of passes required for on-site diagnosis, the number of passes was designated as three passes in patients where diagnosis failed with the original technique. Categorical data were expressed as frequencies and proportions and compared using the chi-squared or Fisher’s exact test as indicated. The level of statistical significance was determined to be a P value of less than 0.05. All datasets were compiled using Microsoft Excel and analyzed with Stata 10.
Results
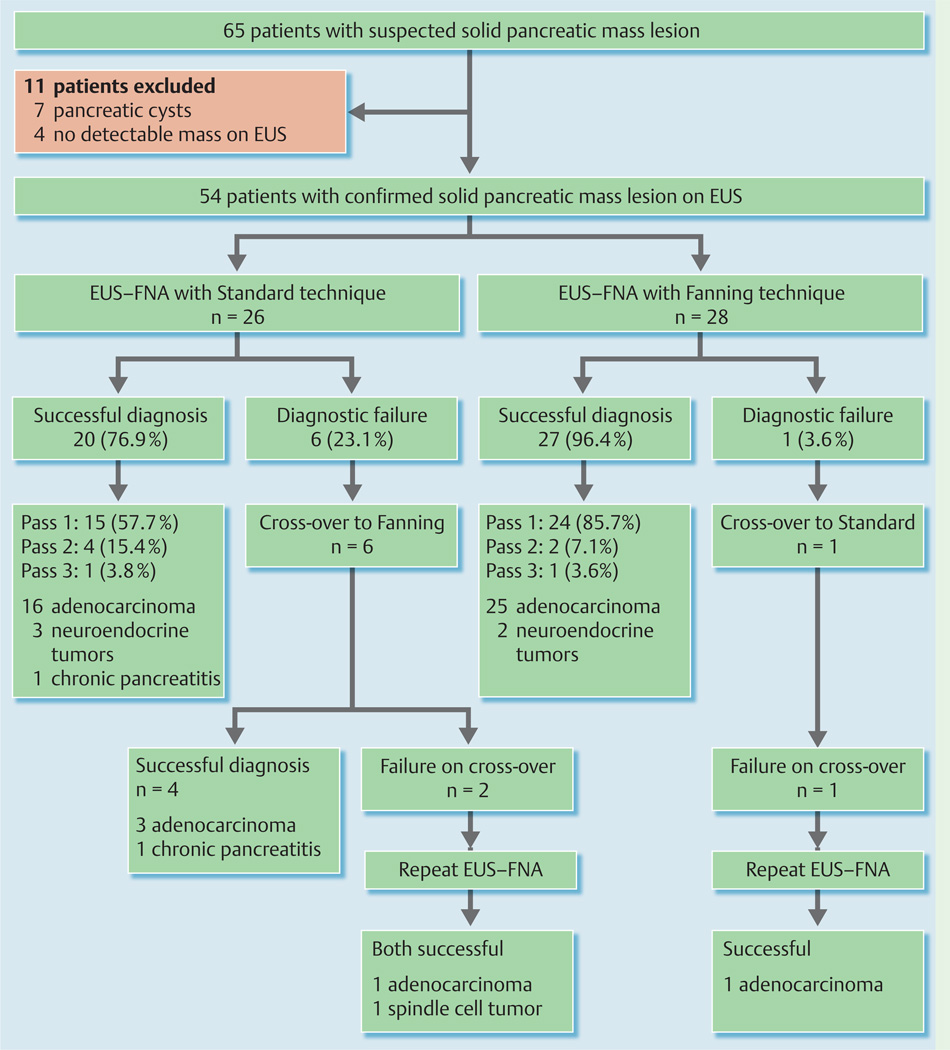
Between October and November 2011, 65 patients were screened and 11 were excluded because the pancreatic mass lesion was cystic in seven patients and no mass was identified at EUS in four (Fig.3). The remaining 54 patients were randomized to undergo EUS–FNA using the standard (n = 26) or the fanning technique (n = 28).
Fig.3.
Flow diagram summarizing the study results. EUS–FNA, endoscopic ultrasound-guided fine-needle aspiration.
None of these patients had undergone attempts at EUS–FNA at outside facilities. There was no significant difference in patient or tumor characteristics, tumor location, or FNA route between the two cohorts (Table1 and Table2). There was, however, a significant difference in the median mass size between the two groups (standard 40 mm vs. fanning 30 mm; P = 0.009).
Table 1.
Patient and pancreatic mass characteristics.
| Procedure technique | P value | ||
|---|---|---|---|
| Standard (n= 26) |
Fanning (n =28) |
||
| Age, years | |||
| Mean ± (SD) | 63.4 ± 10.8 | 64.4 ± 11.1 | |
| Median | 64 | 65.5 | 0.921 |
| IQR | 57 – 71 | 58 – 69.5 | |
| Range | 30 – 79 | 41 – 88 | |
| Sex, n (%) | |||
| Male | 13 (50) | 13 (46.4) | 0.792 |
| Female | 13 (50) | 15 (53.6) | |
| Race, n (%): | |||
| White | 19 (73.1) | 18 (64.3) | 0.492 |
| Black | 7 (26.9) | 10 (35.7) | |
| Prior EUS, n (%) | 0 | 3 (10.7) | 0.243 |
| Size of mass on EUS, mm | |||
| Mean ± SD | 38.1 ± 14.8 | 30.4 ± 7.2 | |
| Median | 40 | 30 | 0.0091 |
| IQR | 30 – 45 | 25 – 37.5 | |
| Range | 10 – 70 | 15 – 40 | |
| Tumor location, n (%) | |||
| Head/uncinate | 15 (57.7) | 21 (75.0) | 0.182 |
| Body/tail | 11 (42.3) | 7 (25.0) | |
| Final diagnosis, n (%) | |||
| Pancreatic tumor | 24 (92.3)4 | 28 (100)5 | 0.233 |
| Other | 2 (7.7)‡6 | 0 | |
ESU, endoscopic ultrasound; IQR, interquartile range.
Wilcoxon rank-sum test.
Chi-squared test.
Fisher’s exact test.
For the standard technique group, 20 of the tumors were pancreatic adenocarcinomas, three were pancreatic neuroendocrine tumors, and one was a pancreatic spindle cell tumor.
For the Fanning technique group, 26 of the tumors were pancreatic adenocarcinomas and two were pancreatic neuroendocrine tumors.
Both were chronic pancreatitis.
Table 2.
Technical details and outcomes of standard and fanning techniques of endoscopic ultrasound-guided fine-needle aspiration.
| Procedure technique | P value | ||
|---|---|---|---|
| Standard (n= 26) |
Fanning (n =28) |
||
| Access route, n (%) | |||
| Transduodenal | 15 (57.7) | 21 (75) | 0.181 |
| Transgastric | 11 (42.3) | 7 (25) | |
| Diagnostic accuracy, n (%) | 20 (76.9) | 27 (96.4) | 0.052 |
| No. of passes for diagnosis3 | |||
| Mean ± SD | 1.7 ± 0.88 | 1.2 ± 0.57 | |
| Median | 1 | 1 | 0.024 |
| IQR | 1 – 3 | 1 – 1 | |
| Range | 1 – 3 | 1 – 3 | |
| Pass one, n (%)5 | 15 (57.7) | 24 (85.7) | 0.021 |
| Pass two, n (%)6 | 4 (15.4) | 2 (7.1) | 0.412 |
| Pass three, n (%)6 | 1 (3.8) | 1 (3.6) | 0.992 |
| Diagnosis not reached, n (%)6, 7 | 6 (23.1) | 1 (3.6) | 0.052 |
| Repeat EUS – FNA, n (%) | 2 (7.7) | 1 (3.6) | 0.602 |
EUS, endoscopic ultrasound; IQR, interquartile range.
Chi-squared test.
Fisher’s exact test.
For calculation of the mean, median, IQR, and range for the number of passes required to establish a diagnosis, failure with the original technique was equated to three passes.
Wilcoxon rank-sum test.
Chi-squared test was used for comparison of diagnosis achieved on pass one between the two techniques as the expected frequency in all cells was at least 5.
Fisher’s exact test was used for comparison of diagnosis achieved on pass two and three and non-diagnostic passes between the two techniques as the expected frequency in at least one of the cells was below 5.
All cases of cross-over to the alternative technique occurred as a result of diagnostic failure. Technical failure was not observed in either cohort.
Primary outcome
Although both cohorts required a median of one pass to reach a diagnosis, there was a significant difference in the total number of passes required to establish the diagnosis between the standard and fanning cohorts (median 1 [IQR 1–3] vs. 1 [IQR 1–1]; P = 0.02), respectively. In addition, the proportion of patients in whom an on-site diagnosis was achieved on the first pass using the original technique was significantly greater in the fanning than the standard cohort (85.7% vs. 57.7 %; P = 0.02, respectively). Diagnosis was achieved on pass two and three in 15.4% and 3.8% of the standard cohort and in 7.1% and 3.6% of the fanning cohort, respectively (Table2).
Secondary outcomes
There was no significant difference in diagnostic accuracy between the fanning and standard techniques (96.4% and 76.9 %, respectively; P = 0.05). One patient in the fanning cohort was crossed over to the standard technique, which was also non-diagnostic. A repeat EUS–FNA was therefore performed after 1 week using the fanning technique, which was positive for adenocarcinoma. Of the six patients in the standard cohort who were crossed over to the fanning technique, a diagnosis was achieved in four patients, which included pancreatic adenocarcinoma in three and chronic pancreatitis in one. The two remaining patients with failed diagnosis using both techniques underwent repeat EUS–FNA (fanning technique) after 1 week that revealed pancreatic adenocarcinoma in one patient and spindle cell tumor in the other. No complications or technical failures (needle dysfunction) were encountered in either group.
At mean follow-up of 249 days, 39 of 46 patients with pancreatic adenocarcinoma received chemoradiation, of whom 18 died of progressive disease and 21 are currently receiving treatment. Three patients underwent surgical resection, and histopathology confirmed the diagnosis. One patient with pancreatic adenocarcinoma died prior to planned chemotherapy, and three patients who opted for palliative care died of disease progression. Four of the five patients with pancreatic neuroendocrine tumors and the one patient with pancreatic spindle cell tumor underwent surgery with confirmation of diagnosis by histopathology. Both patients diagnosed with chronic pancreatitis were doing well without clinical deterioration.
Discussion
In this randomized trial, the fanning technique was significantly superior to the standard approach for EUS–FNA of solid pancreatic mass lesions because fewer passes were required to establish a diagnosis, the majority of which were established on pass one. The center of a cancerous mass is considered to be more necrotic than the periphery and hence is more likely to yield non-diagnostic tissue when sampled at EUS–FNA [14]. In one study [15], the sensitivity of EUS–FNA was very low for diagnosing gastrointestinal stromal tumors larger than 10cm in size due to the presence of extensive necrosis in large tumors. Therefore to overcome this limitation, two studies have suggested that aspiration of lesions at the periphery or in multiple areas may improve the diagnostic accuracy [11, 12].
When the fanning technique was used for EUS–FNA, sampling was started at the left margin of the mass and the needle was “fanned” systematically until the right margin was reached. In our experience, this commonly yielded an aspirate that appeared “blood-tinged” when expressed onto a slide and had a high diagnostic accuracy even on the first pass. Conversely, when only a single area was targeted using the standard technique, the aspirate appeared cloudy and was predominantly necrotic on microscopy. Nonetheless, performing the fanning maneuver in lesions measuring less than 15 mm can be technically challenging. In these circumstances, the image needs to be magnified so that the lesion can be visualized better and the trajectory of the needle can be followed within the mass.
If the findings of the current study are confirmed by other investigators, we believe that the fanning technique should be incorporated into routine EUS–FNA practice, as it establishes a diagnosis with fewer passes and improves the efficiency of the practice. Even among patients who were crossed over to the fanning cohort following diagnostic failure with the standard approach, a definitive diagnosis was established in two-thirds of these patients. In others, the diagnosis was established only after repeating the EUS–FNA at a different session, probably because repeated sampling diminished the cellularity and increased the bloodiness of the aspirate.
There were some limitations to this study. First, only solid pancreatic mass lesions were sampled and hence the efficacy of the fanning technique on other lesion types is unknown. However, as pancreatic mass lesions are the most challenging to sample, we predict that the fanning technique would also be successful for sampling other lesion types. Secondly, it was not possible to blind the endoscopist to the technique used. However, as the cytopathologist was blinded to the sampling technique, the element of bias is likely to be minimal. Thirdly, we did not follow the patients longitudinally to assess the operating characteristics of the two techniques such as sensitivity for detecting malignancy. Fourthly, the size of the pancreatic mass in patients randomized to the standard technique was larger. As larger tumors are more necrotic, this could have contributed to the need for a greater number of passes being required to establish a diagnosis. Finally, the techniques adopted to perform FNA vary among endosonographers. At our center, we do not use suction or the stylet for performing FNA. Studies have shown that the use of stylet and suction increases the bloodiness of specimens but without improving the diagnostic yield (16,17). Therefore, in our practice, we never use suction except for cyst aspiration and rarely when an aspirate is scant (e. g. FNA of pancreatic masses in the setting of chronic pancreatitis).
In conclusion, the fanning technique of FNA is superior to the standard approach as fewer passes are required to establish a diagnosis. The results of this study suggest that further work is needed to confirm these findings in other lesions. If these promising data are validated by other investigators in their clinical practice, consideration should be given to incorporating the fanning technique into routine EUS–FNA practice.
Footnotes
Video 1: Demonstration of the standard technique of fine-needle aspiration in a patient with a pancreatic body mass.
online content including video sequences viewable at: www.thieme-connect.de/ejournals/abstract/endoscopy/ doi/10.1055/s-0032-1326268
Video 2: Demonstration of the fanning technique of fine-needle aspiration in a patient with a mass in the uncinate region of the pancreas.
online content including video sequences viewable at: www.thieme-connect.de/ejournals/abstract/endoscopy/ doi/10.1055/s-0032-1326268
Competing interests: S.Varadarajulu is a consultant for Boston Scientific Corp.
References
- 1.Dumonceau J-M, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2011;43:897–909. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 2.Eloubeidi MA, Jhala D, Chhieng DC, et al. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285–292. doi: 10.1002/cncr.11643. [DOI] [PubMed] [Google Scholar]
- 3.Savides TJ, Donohue M, Hunt G, et al. EUS-guided FNA diagnostic yield of malignancy in solid pancreatic masses: a benchmark for quality performance measurement. Gastrointest Endosc. 2007;66:277–282. doi: 10.1016/j.gie.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Fisher L, Segarajasingam DS, Stewart C, et al. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: performance and outcomes. J Gastroent Hepatol. 2009;24:90–96. doi: 10.1111/j.1440-1746.2008.05569.x. [DOI] [PubMed] [Google Scholar]
- 5.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc. 2010;71:91–98. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Hawes RH. The evolution of endoscopic ultrasound: improved imaging, higher accuracy for fine needle aspiration and the reality of endoscopic ultrasound-guided interventions. Curr Opin Gastroenterol. 2010;26:436–444. doi: 10.1097/MOG.0b013e32833d1799. [DOI] [PubMed] [Google Scholar]
- 7.Harewood GC, Wiersema LM, Halling AC, et al. Influence of EUS training and pathology interpretation on accuracy of EUS-guided fine needle aspiration of pancreatic masses. Gastrointest Endosc. 2002;55:669–673. doi: 10.1067/mge.2002.123419. [DOI] [PubMed] [Google Scholar]
- 8.Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33–37. doi: 10.1016/s0016-5107(03)02028-5. [DOI] [PubMed] [Google Scholar]
- 9.Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: a learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–708. doi: 10.1016/s0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 10.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–481. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 11.Ekberg O, Bergenfeldt M, Aspelin P, et al. Reliability of ultrasound-guided fine-needle biopsy of pancreatic masses. Acta Radiol. 1988;29:535–539. [PubMed] [Google Scholar]
- 12.Wyse JM, Paquin SC, Joseph L, et al. EUS–FNA without the stylet: the yield is comparable to that with the stylet and sampling of multiple sites during the same pass may improve sample quality and yield. Gastrointest Endosc. 2009;69:AB330–AB331. [Google Scholar]
- 13.Varadarajulu S, Blakely J, Latif S, et al. Quality assessment of current EUS–FNA assembly performance: adequate for use or opportunity for improvement? Gastrointest Endosc. 2011;73:AB174–AB175. [Google Scholar]
- 14.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 15.Sepe PS, Moparty B, Pitman MB, et al. EUS-guided FNA for the diagnosis of GI stromal tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254–261. doi: 10.1016/j.gie.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Sahai AV, Paquin SC, Gariépy G. A prospective comparison of endoscopic ultrasound-guided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010;42:900–903. doi: 10.1055/s-0030-1255676. [DOI] [PubMed] [Google Scholar]
- 17.Kundu S, Conway K, Gilbert K, et al. Suction or no suction? Interval results from an ongoing prospective, partially blinded, randomized trial of endoscopic ultrasound guided fine needle sampling of solid lesions. Gastrointest Endosc. 2009;69:S248–S249. [Google Scholar]