Abstract
A previous study of European Caucasian patients with sporadic amyotrophic lateral sclerosis demonstrated that a polymorphism in the microtubule-associated protein Tau (MAPT) gene was significantly associated with sporadic amyotrophic lateral sclerosis pathogenesis. Here, we tested this association in 107 sporadic amyotrophic lateral sclerosis patients and 100 healthy controls from the Chinese Han population. We screened the mutation-susceptible regions of MAPT – the 3' and 5' untranslated regions as well as introns 9, 10, 11, and 12 – by direct sequencing, and identified 33 genetic variations. Two of these, 105788 A > G in intron 9 and 123972 T > A in intron 11, were not present in the control group. The age of onset in patients with the 105788 A > G and/or the 123972 T > A variant was younger than that in patients without either genetic variation. Moreover, the pa-tients with a genetic variation were more prone to bulbar palsy and breathing difficulties than those with the wild-type genotype. This led to a shorter survival period in patients with a MAPT genetic variant. Our study suggests that the MAPT gene is a potential risk gene for sporadic amyotrophic lateral sclerosis in the Chinese Han population.
Keywords: neural regeneration, sporadic amyotrophic lateral sclerosis, microtubule-associated protein Tau gene, MAPT, Chinese Han population, genotype, neuroregeneration
Research Highlights
-
(1)
We explored the association of the microtubule-associated protein Tau (MAPT) gene with spo-radic amyotrophic lateral sclerosis in the Chinese Han population.
-
(2)
Two genetic variations in MAPT (105788 A > G in intron 9 and 123972 T > A in intron 11) were detected in sporadic amyotrophic lateral sclerosis patients but not controls.
-
(3)
MAPT might be a predisposing gene for sporadic amyotrophic lateral sclerosis in the Chinese Han population.
INTRODUCTION
Sporadic amyotrophic lateral sclerosis is a fatal neurodegenerative disorder, in which loss of motor neurons in the spinal cord, brainstem, and cerebral cortex leads to progressive paralysis[1,2]. Symptoms selectively involve the upper and lower motor neurons, and many patients die within 3 to 5 years after onset[1,3]. However, the etiology of sporadic amyotrophic lateral sclerosis remains obscure. Many mechanisms, including viral infection, autoimmune reactions, excitotoxicity, metabolic/mitochondrial toxicity, abnormal apoptotic processes, protein misfolding, altered axonal transport, and gene mutations, have been proposed to explain the underlying pathogenesis[4,5]. However, there is no conclusive evidence that any of these mechanisms is responsible for even a small fraction of sporadic amyotrophic lateral sclerosis cases.
Various genes that cause familial amyotrophic lateral sclerosis have been identified; in contrast, the genetic aspects of sporadic amyotrophic lateral sclerosis are poorly understood[6]. Putative susceptibility genes for sporadic amyotrophic lateral sclerosis include vascular endothelial growth factor[7], angiogenin[8], apurinic endonuclease[9], hemochromatosis[10], survival motor neuron (SMN1, SMN2)[11,12], and the paraoxonase cluster on chromosome 7q (PON1, PON2, PON3)[13]. However, to date, no gene has been conclusively determined to be responsible for the pathogenesis of sporadic amyotrophic lateral sclerosis[14]. There is growing evidence of the importance of variants in the microtubule-associated protein Tau (MAPT) gene as a potential risk factor for degenerative diseases of motor neurons[15,16,17,18]. In addition, several neurodegenerative diseases are strongly associated with MAPT genetic variations, especially in the mutation-susceptible regions of the 3′ and 5′ untranslated regions (UTRs) and introns 9, 10, 11, and 12[17,18,19]. The Tau proteins, which stabilize microtubules, are the products of alternative splicing from the MAPT gene. When Tau proteins are defective, and no longer stabilize microtubules properly, they can result in dementia or neurodegenerative disease[20,21]. Drugs to modify neuronal microtubules might be a therapeutic target in the survival of apoptotic motor neurons during early stages of disease progression. Therefore, it is important to investigate the genetic background of sporadic amyotrophic lateral sclerosis, to determine the neurodegenerative and potential neuroregenerative processes[22].
We previously investigated the association of the dinucleotide polymorphism A0 in the MAPT gene with disease in 416 European Caucasian patients with amyotrophic lateral sclerosis and 242 controls. We demonstrated that the A0 polymorphism was highly prevalent in sporadic amyotrophic lateral sclerosis[17]. To examine this in a different population, here we investigated the association of the MAPT gene with sporadic amyotrophic lateral sclerosis in the Chinese Han population.
RESULTS
Clinical assessment of sporadic amyotrophic lateral sclerosis patients
A total of 107 patients with sporadic amyotrophic lateral sclerosis, consisting of 72 men and 35 women, were included in this study. These patients comprised 49 definite cases, 35 probable cases, and 23 possible cases, according to the El Escorial diagnostic criteria[23]. The average age of symptom onset was 52.7 ± 12.5 years (range 34–73 years). The time to affirmative diagnosis ranged from 1 month to 33 months, averaging 5.2 ± 6.4 months. The onset symptoms were limb weakness in 44 patients (44/107, 41.1%), muscle twitching in 29 patients (29/107, 27.1%), muscle wasting in the hands in 23 patients (23/107, 21.5%), and bulbar palsy in 11 patients (11/107, 10.3%). Upon development of the disease, 105 patients had limb weakness, 103 patients presented with muscle wasting, 89 patients complained of muscle twitching, 46 patients suffered from difficulty in swallowing, and 31 patients endured breathing difficulty during the different stages of disease. Physical examination revealed that the limb weakness presented asymmetrically. Among the 105 patients with limb weakness, 10 experienced single upper limb weakness, 15 had muscle weakness in both lower limbs, 11 had muscle weakness in both upper limbs, 5 had single lower limb weakness, 3 had unilateral limb weakness, and 61 had muscle weakness in all limbs. Proximal and distal muscle weakness was present in 91 patients, of whom 21 presented with more proximal weakness, 40 with more distal weakness, and 30 with equal proximal and distal weakness. There were 10 patients with isolated proximal weakness and 4 patients with isolated distal weakness. The intrinsic muscles of the hand were prominently atrophied in 103 patients, especially involving the thenar and hypothenar muscles and the first interosseous muscle. Muscle wasting distributed in the upper limbs (74/103), lower limbs (58/103), tongue (45/03), sternocleidomastoid (32/103), cervical muscles (27/103), and chest muscles (2/103). There were 89 patients with muscle twitching, which occurred in the hands (75/89), thighs (49/89), proximal muscles of the upper limbs (42/89), tongue (45/89), distal muscles of the lower limbs (32/89), and sternocleidomastoid (30/89). Eighty-eight patients were identified with pyramidal signs; 69 patients manifested with different extents of hyperreflexia. All patients had normal sensory function.
Electromyography was performed in all 107 patients. All patients revealed diffuse neurogenic lesions with prolonged duration, high amplitude, fibrillation potential, and giant potential. Motor conduction velocity and distal motor latency were normal or slightly prolonged. All patients underwent electromyography in the sternocleidomastoid, and 79 of them showed neurogenic involvement. Sixty-seven patients underwent electromyography in the paraspinal muscles, and 63 of them presented with a neurogenic pattern. The serum creatine kinase level was normal in 54 patients, elevated to 280–760 U/L in 32 patients, and was not determined in 21 patients. Cerebral and cervical magnetic resonance imaging was performed in 32 and 58 patients respectively; and no obvious changes were identified.
Follow-up assessment was carried out in all patients. The follow-up period ranged from 1.5 to 65 months (average 15.2 ± 17.3 months). By the last follow-up (February 2012), 67 patients had died, 19 patients had lost the ability to move, and the remaining 21 patients had progressive disease. The time from disease onset to death ranged from 3 months to 83 months (average 20.2 ± 15.7 months). The main causes of death were pneumonia (47/67), breathing difficulties (12/67), asphyxia (5/67), and injury (3/67). Forty-three patients were administered riluzole; however, there was no obvious efficacy.
Genetic analysis
We identified 33 nucleotide polymorphisms in the MAPT gene in the 107 sporadic amyotrophic lateral sclerosis cases (Table 1). A 105788 A > G heterozygous variant in intron 9 was found in 16 sporadic amyotrophic lateral sclerosis patients, and was absent from the controls (Figure 1). The A and G allele frequencies were 92.06% and 7.94% in sporadic amyotrophic lateral sclerosis patients, respectively, versus 100% and 0% in controls (P < 0.05). The frequency of the AA, GA, and GG genotypes was 85.05%, 14.02%, and 0.93% in sporadic amyotrophic lateral sclerosis patients, respectively, and 100%, 0%, and 0% in controls (P < 0.05). A 123972 T > A heterozygous variant in intron 11 was identified in 15 sporadic amyotrophic lateral sclerosis patients, and was absent from the controls (Figure 2). The T and A allele frequencies were 92.99% and 7.01% in sporadic amyotrophic lateral sclerosis patients, respectively, and 100% and 0% in controls (P < 0.05). The frequency of the TT, TA, and AA genotypes was 85.98%, 14.02%, and 0% in sporadic amyotrophic lateral sclerosis patients, respectively, and 100%, 0%, and 0% in controls (P < 0.05). Only one patient had both the 105788 A > G and 123972 T > A variants.
Table 1.
Genetic variations in the microtubule-associated protein Tau (MAPT) gene identified in Chinese Han sporadic amyotrophic lateral sclerosis patients

Figure 1.

Chromatograms showing the A > G variant at position 105788 in intron 9 of the MAPT gene.
(A) AA genotype; (B) AG genotype; (C) GG genotype. The position of the variant is indicated by arrows.
Figure 2.
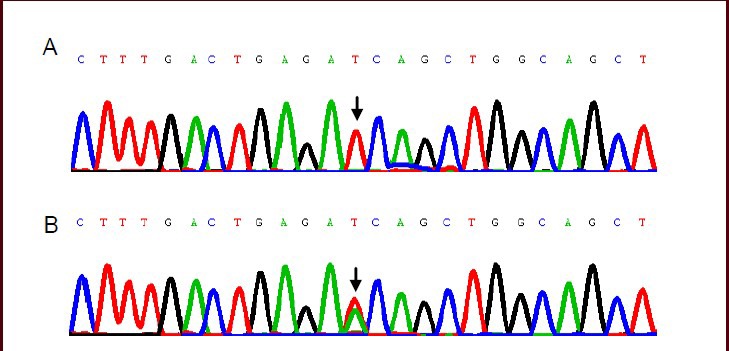
Chromatograms showing the T > A variant at position 123972 in intron 11 of the MAPT gene.
(A) TT genotype; (B) TA genotype. The position of the variant is indicated by arrows.
Genotype-phenotype correlations
We divided the sporadic amyotrophic lateral sclerosis patients into a “genetic wild-type” group and “genetic variant” group according to their two MAPT genotypes. We then compared the groups for the following variables: age, sex, duration of symptoms before diagnosis, limb weakness, muscle wasting, muscle twitching, bulbar palsy, breathing difficulty, pyramidal signs, hyperreflexia, sternocleidomastoid electromyography, serum creatine kinase, survival time, and pneumonia rate.
In the 105788 A > G variant group, the onset age was 34 to 67 years (average 47.5 ± 11.3 years), which was significantly younger than that in the 105788 A > G wild-type group (P < 0.05). Moreover, the 105788 A > G variant patients were more likely to have bulbar palsy and breathing difficulties than were the 105788 A > G wild- type patients (P < 0.01). There were no other significant differences between the two groups (Table 2).
Table 2.
Clinical variable analysis of Chinese Han patients with sporadic amyotrophic lateral sclerosis according to the ge-notype of the MAPT 105788 A > G variant.

A similar relationship between the clinical features and genotype was observed in the 123972 T > A variant group (Table 3). In the 123972 T > A variant group, the age of onset was from 37 to 72 years old (average 46.7 ± 10.3 years); this was significantly younger than that in the 123972 T > A wild-type group (P < 0.05). Moreover, the 123972 T > A variant patients had a higher frequency of bulbar palsy and breathing difficulties than did the 123972 T > A wild-type patients (P < 0.05). Thus, their survival time was shorter (P < 0.01). There were no other significant differences between the groups.
Table 3.
Clinical variable analysis of patients with sporadic amyotrophic lateral sclerosis according to the genotype of the MAPT 123972 T > A variant.

DISCUSSION
The sporadic amyotrophic lateral sclerosis patients in this study underwent follow-up since the time of definite diagnosis. Most patients first presented with asymmetrical limb weakness or muscle wasting. An increasing area of involvement and declining muscle strength were progressive, and most patients died from breathing difficulties or asphyxia. All patients revealed diffused neurogenic lesions with normal motor conduction velocity. Therefore, all patients were clinically definite cases, although there initially existed probable and possible cases according to the El Escorial criteria[23].
The MAPT gene spans 133.9 kb from the 5′ UTR to the end of the 3′ UTR and contains 16 exons. Exons 1 and 14 are transcribed but not translated[20,24]. The formation of MAPT mRNA is tissue-specific and developmentally regulated by alternative splicing. In the human brain, exons 4A, 6, and 8 are not present in mRNA. Three coding exons (2, 3, and 10) are alternatively spliced and are specific to adult brain[17,25]. Among the many identified MAPT mutations, the majority are missense, deletion, or silent mutations in the coding region, or intronic mutations close to the splice-donor sites following exons 2, 3, or 10[20]. MAPT mutations are categorized according to whether they directly affect Tau protein function and/or RNA splicing. Mutations in exon 9, 11, 12, and 13 disrupt the interactions between Tau and microtubules, thus reducing the ability of Tau to promote microtubule assembly. Conversely, mutations affecting alternative splicing result in a change to the Tau isoform ratios[21]. Mutations affecting splicing include both missense and silent mutations in exon 10 and intron mutations in the sequence closely flanking this exon. The regions described above are called the mutation-susceptible regions of the MAPT gene. These regions are associated with an increased risk of neurodegenerative disease[18,20].
The precise etiology of sporadic amyotrophic lateral sclerosis remains unknown; however, increasing evidence suggests that both genetic and environmental factors are involved, and that inheritance is especially important[1,26].
The MAPT gene has been previously suggested to be a susceptibility gene for sporadic amyotrophic lateral sclerosis[15,16,17,18]. There is now considerable evidence that it is involved in many neurodegenerative diseases, including Alzheimer's disease[22], frontotemporal dementia[27], supranuclear palsy[28], frontotemporal dementia with parkinsonism linked to chromosome 17[29], corticobasal degeneration[30], Parkinson's disease, and Pick's disease[31]. Although most sporadic amyotrophic lateral sclerosis cases are not classified as a tauopathy, sporadic amyotrophic lateral sclerosis can present with neuropathological findings that overlap with those of tauopathies, and have similar clinical features[32]. A susceptibility risk gene does not have to cause gross pathological effects in a neurodegenerative disease; for example, the APOE gene in Alzheimer's disease and the saitohin gene in Parkinson's disease do not directly cause pathogenesis[33]. It should be noted, however, that several previous reports have not supported the pathogenicity of MAPT gene mutations in sporadic amyotrophic lateral sclerosis. These include the recent report of Taes et al[34], in which the association between amyotrophic lateral sclerosis and the MAPT H1/H2 polymorphism was studied in 3540 European patients with amyotrophic lateral sclerosis and 8753 controls. The results indicated that the H1/H2 polymorphism did not influence amyotrophic lateral sclerosis susceptibility and did not affect the clinical phenotype[34]. Thus, it remains uncertain whether MAPT is a risk gene in sporadic amyotrophic lateral sclerosis. To our knowledge, there is a paucity of information regarding the possible association between MAPT variations and sporadic amyotrophic lateral sclerosis in the Chinese Han population. We sought to address that deficiency here.
In the present study, we analyzed the MAPT 3′ and 5′ UTRs and introns 9, 10, 11, and 12. We chose these regions because many nucleotide changes have been found in these regions or nearby[15,16,17,18]. We identified two variants, 105788 A > G in intron 9 and 123972 T > A in intron 11, which were highly prevalent in Chinese Han sporadic amyotrophic lateral sclerosis patients but were not found in controls. These variants might be associated with sporadic amyotrophic lateral sclerosis disease risk in the Chinese Han population. Both variants are located near exon 10 and may be involved in the regulation of alternative splicing, affecting the Tau isoform ratios.
In our study, patients with either variant displayed a younger age of onset than patients without a variant. Similarly, patients with a variant were more prone to bulbar palsy and breathing difficulties than patients without a MAPT variant, which ultimately led to a shorter survival period in patients with a genetic variant. Apoptosis might be promoted by the abnormal Tau isoform ratios that these variants might cause. In short, patients with 105788 A > G or 123972 T > A not only had a greater susceptibility to sporadic amyotrophic lateral sclerosis than did patients without a variant, but also tended to have worse clinical progression and outcome. Our findings suggest that genetic variation in MAPT is a risk factor for sporadic amyotrophic lateral sclerosis in the Chinese Han population. However, because of the relatively small sample size of the present study, we cannot draw firm conclusions. Further studies with larger groups are necessary.
SUBJECTS AND METHODS
Design
Contemporary, non-randomized, controlled, retrospective analysis.
Time and setting
A total of 107 sporadic amyotrophic lateral sclerosis patients were recruited at the First Affiliated Hospital of Nanchang University, China, from July 1998 to January 2012. One hundred controls were collected from the Health Examination Centre of the First Affiliated Hospital of Nanchang University, China, during 2011.
Subjects
All recruited sporadic amyotrophic lateral sclerosis patients were from the Chinese Han population, with both parents also of Chinese Han origin. Methods reported by Serre et al[35] were employed to rule out population stratification within these samples besides relying on self-reported ethnicity. The sporadic amyotrophic lateral sclerosis patients were diagnosed according to the El Escorial criteria of the World Federation of Neurology[23].
Patients were identified in the course of routine clinical ascertainment of movement disorders in the Neurology Department. All patients underwent brain and spinal magnetic resonance imaging to exclude other neurological diseases that mimic the clinical picture of amyotrophic lateral sclerosis, such as tumors, demyelinating disorders, hydrocephalus, and cervical myelopathy.
A total of 100 non-neurological Chinese Han individuals were used as control subjects. None had a medical history of amyotrophic lateral sclerosis, Alzheimer's disease, ataxia, autism, bipolar disorder, brain aneurysm, dementia, dystonia, or Parkinson's disease, and none had any first-degree relative with a known primary neurological disorder. Control individuals were matched to the sporadic amyotrophic lateral sclerosis patients by age and sex and comprised 47 men and 23 women; the mean age at sample collection was 51.9 ± 10.2 years (range 40–75 years).
All patients and controls gave written consent in accordance with the Administrative Regulations for Medical Institutions issued by the State Council of China, as well as the Declaration of Helsinki[36].
Methods
Molecular analysis of the MAPT gene
Peripheral blood samples (elbow, 4 mL) were drawn, and DNA was isolated using a Fastgene-DNAfast 5000 kit (Fastgene Co., Ltd., Shanghai, China). Primers were designed using Primer 3 software (http://primer3.wi.mit.edu/; Table 4). Primer sequences were located in the 3′ and 5′ UTRs plus introns 9, 10, 11, and 12 of the MAPT gene. These comprise the mutation-susceptible regions of this gene.
Table 4.
Primer sequences

PCR reactions were carried out in a final volume of 50 μL using Taq DNA polymerase (Takara, Dalian, China). The cycling conditions included a hot start at 94°C for 5 minutes, followed by 30 cycles at 94°C for 30 seconds, 57–59°C for 30 seconds (depending on the primer pair), and 72°C for 45 seconds followed by a 7-minute extension step at 72°C.
The specificity of the PCR was assessed by electrophoresis of the products on 1.5% agarose gels. PCR products were purified and sequenced using an ABI 3730xl automated sequencer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions.
All identified nucleotide alterations were confirmed by additional sequencing in the opposite direction. Sequences were compared with those in the National Center for Biotechnology Information GenBank database (chr17: 41326624–41462546, MIM:157140, GeneID: 4137, NG_007398.1, GI:167830493) and analyzed using DNAMAN software, v5.2.2 (Lynnon Corp., Berkeley, CA, USA).
Statistical analysis
The association of the different clinical variables was analyzed by univariate analysis. Statistical assessment was conducted using the SAS statistical software package (SAS Institute Inc., Cary, NC, USA). Continuous variables were expressed as mean ± SD and were analyzed using Student's t-test. Categorical variables were expressed as absolute numbers and proportion of cases and were compared using the chi-square test. Allele frequency was determined via direct counting. Difference in genotype distribution between the groups was obtained using the chi-square test. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We are grateful to the sporadic amyotrophic lateral sclerosis patients who generously contributed their time and a blood sample for this research.
Footnotes
Funding: This project was funded by the National Natural Science Foundation of China, No. 30560042 and 81260194; Jiangxi Provincial Health Bureau of Science and Technology Program, No. 20111028.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Bioethics Committee of the First Affiliated Hospital of Nanchang University, China.
REFERENCES
- [1].Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- [2].Costa J, Swash M, de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis: a systematic review. Arch Neurol. 2012 doi: 10.1001/archneurol.2012.254. [DOI] [PubMed] [Google Scholar]
- [3].Inghilleri M, Iacovelli E. Clinical neurophysiology in ALS. Arch Ital Biol. 2011;149(1):57–63. doi: 10.4449/aib.v149i1.1264. [DOI] [PubMed] [Google Scholar]
- [4].De Chiara G, Marcocci ME, Sgarbanti R, et al. Infectious agents and neurodegeneration. Mol Neurobiol. 2012;46(3):614–638. doi: 10.1007/s12035-012-8320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7(9):710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- [6].Cooper-Knock J, Kirby J, Ferraiuolo L, et al. Gene expression profiling in human neurodegenerative disease. Nat Rev Neurol. 2012;8(9):518–530. doi: 10.1038/nrneurol.2012.156. [DOI] [PubMed] [Google Scholar]
- [7].Lambrechts D, Storkebaum E, Morimoto M, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34(4):383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- [8].Bloem BR, Scheffer H, van Nuenen BF, et al. Angiogenin variants in Parkinson disease and amyotrophic lateral sclerosis. Ann Neurol. 2011;70(6):964–973. doi: 10.1002/ana.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Greenway MJ, Alexander MD, Ennis S, et al. A novel candidate region for ALS on chromosome 14q11.2. Neurology. 2004;63(10):1936–1938. doi: 10.1212/01.wnl.0000144344.39103.f6. [DOI] [PubMed] [Google Scholar]
- [10].Sutedja NA, Sinke RJ, Van Vught PW, et al. The association between H63D mutations in HFE and amyotrophic lateral sclerosis in a Dutch population. Arch Neurol. 2007;64(1):63–67. doi: 10.1001/archneur.64.1.63. [DOI] [PubMed] [Google Scholar]
- [11].Veldink JH, Kalmijn S, Van der Hout AH, et al. SMN genotypes producing less SMN protein increase susceptibility to and severity of sporadic ALS. Neurology. 2005;65(6):820–825. doi: 10.1212/01.wnl.0000174472.03292.dd. [DOI] [PubMed] [Google Scholar]
- [12].Corcia P, Camu W, Halimi JM, et al. SMN1 gene, but not SMN2, is a risk factor for sporadic ALS. Neurology. 2006;67(7):1147–1150. doi: 10.1212/01.wnl.0000233830.85206.1e. [DOI] [PubMed] [Google Scholar]
- [13].Slowik A, Tomik B, Wolkow PP, et al. Paraoxonase gene polymorphisms and sporadic ALS. Neurology. 2006;67(5):766–770. doi: 10.1212/01.wnl.0000219565.32247.11. [DOI] [PubMed] [Google Scholar]
- [14].Ince PG, Highley JR, Kirby J, et al. Molecular pathology and genetic advances in amyotrophic lateral sclerosis: an emerging molecular pathway and the significance of glial pathology. Acta Neuropathol. 2011;122(6):657–671. doi: 10.1007/s00401-011-0913-0. [DOI] [PubMed] [Google Scholar]
- [15].Yang W, Strong MJ. Widespread neuronal and glial hyperphosphorylated Tau deposition in ALS with cognitive impairment. Amyotroph Lateral Scler. 2012;13(2):178–193. doi: 10.3109/17482968.2011.622405. [DOI] [PubMed] [Google Scholar]
- [16].Sundar PD, Yu CE, Sieh W, et al. Two sites in the MAPT region confer genetic risk for Guam ALS/PDC and dementia. Hum Mol Genet. 2007;16(3):295–306. doi: 10.1093/hmg/ddl463. [DOI] [PubMed] [Google Scholar]
- [17].Münch C, Prechter F, Xu R, et al. Frequency of a Tau genotype in amyotrophic lateral sclerosis. J Neurol Sci. 2005;236(1-2):13–16. doi: 10.1016/j.jns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- [18].Schraen-Maschke S, Dhaenens CM, Delacourte A, et al. Microtubule-associated protein Tau gene: a risk factor in human neurodegenerative diseases. Neurobiol Dis. 2004;15(3):449–460. doi: 10.1016/j.nbd.2003.12.009. [DOI] [PubMed] [Google Scholar]
- [19].Guerreiro RJ, Washecka N, Hardy J, et al. A thorough assessment of benign genetic variability in GRN and MAPT. Hum Mutat. 2010;31(2):E1126–1140. doi: 10.1002/humu.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Caffrey TM, Wade-Martins R. The role of MAPT sequence variation in mechanisms of disease susceptibility. Biochem Soc Trans. 2012;40(4):687–692. doi: 10.1042/BST20120063. [DOI] [PubMed] [Google Scholar]
- [21].Zhong Q, Congdon EE, Nagaraja HN, et al. Tau isoform composition influences rate and extent of filament formation. J Biol Chem. 2012;287(24):20711–20719. doi: 10.1074/jbc.M112.364067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Crespo-Biel N, Theunis C, Van Leuven F. Protein Tau: prime cause of synaptic and neuronal degeneration in Alzheimer's disease. Int J Alzheimers Dis 2012. 2012 doi: 10.1155/2012/251426. 251426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- [24].Trabzuni D, Wray S, Vandrovcova J, et al. MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum Mol Genet. 2012;21(18):4094–103. doi: 10.1093/hmg/dds238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Galimberti D, Scarpini E. Genetics of frontotemporal lobar degeneration. Front Neurol. 2012;3:52. doi: 10.3389/fneur.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schmidt S, Allen KD, Loiacono VT, et al. Genes and environmental exposures in veterans with amyotrophic lateral sclerosis: the GENEVA study. Rationale, study design and demographic characteristics. Neuroepidemiology. 2008;30(3):191–204. doi: 10.1159/000126911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Premi E, Padovani A, Borroni B. Frontotemporal Lobar Degeneration. Adv Exp Med Biol. 2012;724:114–127. doi: 10.1007/978-1-4614-0653-2_9. [DOI] [PubMed] [Google Scholar]
- [28].Choumert A, Poisson A, Honnorat J, et al. G303V Tau mutation presenting with progressive supranuclear palsy-like features. Mov Disord. 2012;27(4):581–583. doi: 10.1002/mds.24060. [DOI] [PubMed] [Google Scholar]
- [29].Charlesworth G, Gandhi S, Bras JM, et al. Tau acts as an independent genetic risk factor in pathologically proven PD. Neurobiol Aging. 2012;33(4):838.e7–11. doi: 10.1016/j.neurobiolaging.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jung HH, Bremer J, Streffer J, et al. Phenotypic variation of autosomal-dominant corticobasal degeneration. Eur Neurol. 2012;67(3):142–150. doi: 10.1159/000334731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van Eersel J, Bi M, Ke YD, et al. Phosphorylation of soluble Tau differs in Pick's disease and Alzheimer's disease brains. J Neural Transm. 2009;116(10):1243–1251. doi: 10.1007/s00702-009-0293-y. [DOI] [PubMed] [Google Scholar]
- [32].Gozes I. Tau pathology and future therapeutics. Curr Alzheimer Res. 2010;7(8):685–696. doi: 10.2174/156720510793611628. [DOI] [PubMed] [Google Scholar]
- [33].Namboori PK, Vineeth KV, Rohith V, et al. The ApoE gene of Alzheimer's disease (AD) Funct Integr Genomics. 2011;11(4):519–522. doi: 10.1007/s10142-011-0238-z. [DOI] [PubMed] [Google Scholar]
- [34].Taes I, Goris A, Lemmens R, et al. Tau levels do not influence human ALS or motor neuron degeneration in the SOD1G93A mouse. Neurology. 2010;74(21):1687–1693. doi: 10.1212/WNL.0b013e3181e042f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Serre D, Montpetit A, Paré G, et al. Correction of population stratification in large multi-ethnic association studies. PLoS One. 2008;3(1):e1382. doi: 10.1371/journal.pone.0001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994 Sep 01; [Google Scholar]


