Abstract
The neuropeptides, substance P and calcitonin gene-related peptide, have been shown to be involved in pain transmission and repair of sciatic nerve injury. A model of sciatic nerve defect was prepared by dissecting the sciatic nerve at the middle, left femur in female Sprague Dawley rats. The two ends of the nerve were encased in a silica gel tube. L5 dorsal root ganglia were harvested 7, 14 and 28 days post sciatic nerve injury for immunohistochemical staining. Results showed that substance P and citonin gene-related peptide expression increased significantly in dorsal root ganglion of rats with sciatic nerve injury. This increase peaked at 7 days, declined at 14 days, and reduced to normal levels by 28 days post injury. The findings indicate that the neuropeptides, substance P and calcitonin gene- related peptide, mainly increased in the early stages after sciatic nerve injury.
Keywords: neural regeneration, peripheral nerve injury, sciatic nerve, dorsal root ganglion, spinal cord, neuropeptides, calcitonin gene-related peptide, substance P, pain, neuroprotection, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
Substance P and calcitonin gene-related peptide expression in dorsal root ganglia were altered over time in rats with sciatic nerve injury.
-
(2)
Results showed that the expression of the neuropeptides, substance P and calcitonin gene-related peptide, mainly increased in the early stages after sciatic nerve injury. These neuro-peptides may possibly serve as an index for evaluating early peripheral nerve injury.
-
(3)
Substance P and calcitonin gene-related peptide are involved in transmitting pain signals and play a role in repair following sciatic nerve injury.
INTRODUCTION
Several methods have been utilized to repair peripheral nerve injury[1,2,3,4], but results have not been favorable. Thus, it is important to understand the mechanism of peripheral nerve injury. Neuropeptides are a recently discovered neurotransmitter, with a wide range of bioac-tivities[2]. A large number of clinical and experimental observations have demonstrated that peripheral nerves participate in the repair of nerve injury by secreting various neuropeptides from peripheral nerve endings[2,3,4]. In particular, increasing attention has been paid to neu- ropeptide substance P and calcitonin gene- related peptide[5].
Substance P and calcitonin gene-related peptide are the main neuropeptides in peripheral nerve ganglia. They can transmit nociception and function as excitatory transmitters in primary sensory neurons. Furthermore, they anterogradely transmit nociceptive information to the central nervous system and are retrogradely released in local tissues, leading to hyperalgesia[6].
In addition, substance P and calcitonin gene- related peptide are local regulators for mitosis of mesenchymal cells, including bone marrow-derived mesenchymal stem cells, and/or functions of nerve cells[5]. They can regulate microcirculation in bone, and bidirectionally mediate nociceptive transmission and nerve tissues[6]. Many studies have emphasized the roles of substance P and calcitonin gene-related peptide in nerve injury, but their expression at different time points remains unclear.
The present study established a rat model of sciatic nerve injury and assessed substance P and calcitonin gene-related peptide expression in dorsal root ganglia at different time points.
RESULTS
Quantitative analysis of experimental animals
A total of 40 rats were obtained, with 30 being used to establish a model of sciatic nerve defect by cutting the middle of the femur. Ten model rats were used for the 7, 14, and 28 day time points. The remaining 10 rats were used as normal controls and sampled at 7 and 14 days following model establishment. All 40 rats were included in the final analysis.
Expression of substance P and calcitonin gene-related peptide in L5 dorsal root ganglia from rats with sciatic nerve injury
Immunohistochemistry revealed substance P-positive products in dorsal root ganglion of normal rats stained lightly yellow or dark yellow, and were mainly distributed in the cytoplasm. After model establishment for 7 days, substance P-positive products were stained brown and seen mainly in intercellular substance. Staining at this time point gradually darkened. At 14 days, the dark yellow substance P-positive products mainly distributed in loose connective tissue. By 28 days, the expression of substance P-positive products was similar to levels seen in normal rats (Figure 1).
Figure 1.
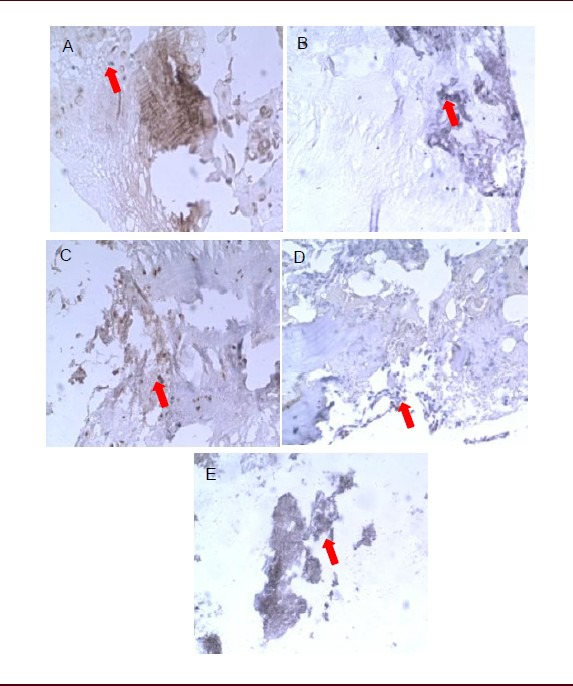
Effects of sciatic nerve defects on substance P expression in L5 dorsal root ganglia of rats (immunohistochemical staining, streptavidin-biotin complex, × 400).
Light microscopy showed that substance P-positive products (arrows) stained dark yellow or brown, mainly distributing in fibrous tissue.
(A) Substance P-positive products were dense and darkly stained in the dorsal root ganglion of normal rats at 7 days. No significant difference was found in staining or distribution of substance P-positive products between 14 (B) and 7 days (A) in the dorsal root ganglion of normal rats.
(C) Substance P-positive products stained darker in rats with sciatic nerve injury than in normal rats and expression was significantly increased at 7 days.
Expression of substance P was reduced and staining became lighter in rats with sciatic nerve injury at 14 days (D) compared with 7 days (C), but was still higher than in normal rats.
(E) Expression of substance P in rats with sciatic nerve injury was similar to that in normal rats at 28 days.
Changes in calcitonin gene-related peptide expression were similar to those seen for substance P. More specifically, in normal rats, calcitonin gene-related peptide-positive products stained darkly yellow in dorsal root ganglion. At 7 days after model establishment, positive products stained brown and were mainly distributed intercellularly, at a higher density than substance P-positive products. At 14 days, calcitonin gene-related peptide-positive products were mainly distributed in loose connective tissue and were stained dark yellow or brown. By 28 days, the expression of calcitonin gene-related peptide products was similar to levels seen in normal rats (Figure 2).
Figure 2.
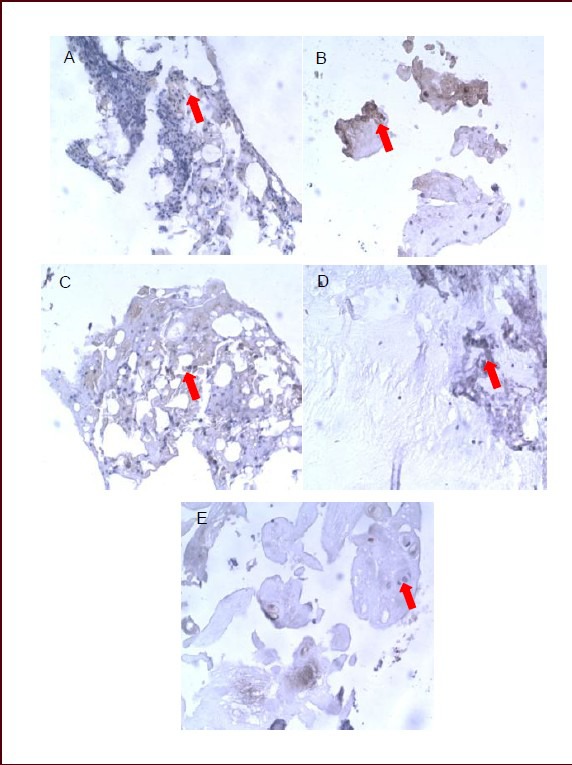
Effects of sciatic nerve defect on calcitonin gene-related peptide expression in L5 dorsal root ganglia of rats (immunohistochemical staining, streptavidin-biotin complex, × 400).
Light microscopy showed that calcitonin gene-related peptide-positive products (arrows) stained dark yellow or brown, mainly distributing in fibrous tissue.
(A) Calcitonin gene-related peptide-positive products were dense and darkly stained in the dorsal root ganglion of normal rats at 7 days.
No significant difference was found in staining or distribution of calcitonin gene-related peptide-positive products between 14 (B) and 7 days (A) in the dorsal root ganglion of normal rats.
(C) Calcitonin gene-related peptide-positive products stained darker and significantly increased in rats with sciatic nerve injury than in normal rats at 7 days.
Expression of calcitonin gene-related peptide was reduced and the staining became lighter in rats with sciatic nerve injury at 14 days (D) compared with 7 days (C), but remained higher than in normal rats.
(E) Expression of calcitonin gene-related peptide in rats with sciatic nerve injury was similar to that in normal rats at 28 days.
Changes in intensity of substance P and calcitonin gene-related peptide expression in L5 dorsal root ganglia from rats with sciatic nerve injury
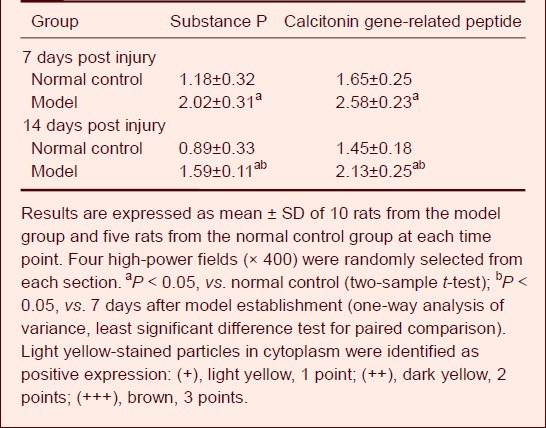
Semi-quantitative analysis using a medical image analysis system showed that the intensity of substance P and calcitonin gene- related peptide expression remained unchanged in the dorsal root ganglia of normal rats at 7 and 14 days after model establishment. Compared with the normal control group, substance P and calcitonin gene-related peptide expression was significantly increased in the model group at 7 and 14 days (P < 0.05). In addition, the intensity of substance P and calcitonin gene-related peptide expression was significantly higher at 7 days compared with 14 days in the model group (P < 0.05), but no significant difference was found between 7 and 14 days in the normal control group (P > 0.05; Table 1).
Table 1.
Comparison of the intensity of substance P and calcitonin gene-related peptide expression between groups
Changes in absorbance of substance P and calcitonin gene-related peptide staining in L5 dorsal root ganglia from rats with sciatic nerve injury
Quantitative analysis of immunohistochem-istry using the JEDA-801D Morphology Image Analysis System showed that the absorbance of substance P and calcitonin gene-related peptide in dorsal root ganglia reached a peak value in rats with sciatic nerve injury at 7 days post injury, slightly declined at 14 days, and was restored to normal level by 28 days (Figure 3).
Figure 3.
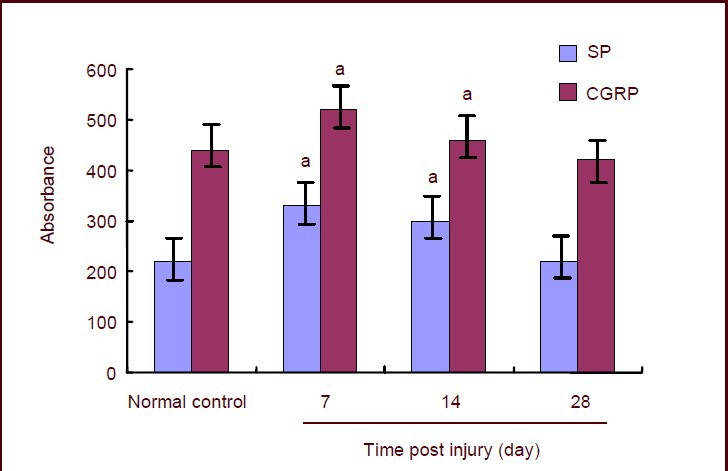
Absorbance of substance P (SP) and calcitonin gene-related peptide (CGRP) in L5 dorsal root ganglia of rats with sciatic nerve injury.
Data are expressed as mean ± SD of 10 rats from the normal control and model group at each time point (mean value of all rats at 7 and 14 days in normal control group). Immunohistochemistry results were quantitatively analyzed using the JEDA-801D Morphology Image Analysis System. aP < 0.05, vs. normal control group (two-sample t-test).
DISCUSSION
Role of the neuropeptides substance P and calcitonin gene-related peptide in the early stages of peripheral nerve injury
Peripheral nerve injury is axonal injury of nerve cells, and restoration depends on axonal regeneration. Post peripheral nerve injury, Wallerian degeneration occurs in distal neurites, axoplasmic transport is blocked, and local inflammatory reactions result in proliferation of various types of cells to form a tissue barrier. These processes are accompanied by neuronal apoptosis in dorsal root ganglia. Thus, successful regeneration of injured peripheral nerves mainly depends on central motor neuron viability and the microenvironment that allows nerve growth[7].
After peripheral nerve injury, motor end-plates and sensory corpuscles disappear due to degeneration and denervation, hindering peripheral nerve regeneration[8]. Recent evidence indicates that motor end-plates and sensory corpuscles can regenerate[9,10]. However, little is known about neuropeptide changes in this environment. Substance P and calcitonin gene-related peptide are known to mediate nociception[5]. Alterations in substance P and calcitonin gene-related peptide activity reflect the function of the posterior horn of the spinal cord, therefore neural regeneration post peripheral nerve injury represents restoration of posterior horn function[11]. If this regeneration does not occur, the function of the posterior horn may not be completely recovered[12]. The present study assessed the changes in substance P and calcitonin gene-related peptide in dorsal root ganglia of rats with left sciatic nerve defects. Results showed that positive substance P and calcitonin gene-related peptide expression in dorsal root ganglion gradually increased at 7 days post injury. As substance P and calcitonin gene-related peptide can transmit nociceptive information in an anterograde fashion to the central nervous system[5], increased release of substance P and calcitonin gene-related peptide provides a microenvironment for neural regeneration. Thus, broken nerves can heal well at this time[6]. In the present study, expression of substance P and calcitonin gene-related peptide was higher at 14 days post sciatic nerve injury compared with the normal control group, consistent with previous results[13,14]. By 28 days, expression of substance P and calcitonin gene-related peptide gradually decreased and returned to normal level, supporting previous clinical denervation studies[3]. Thus, we conclude that substance P and calcitonin gene-related peptide play an important role in the early stages of neural regeneration in dorsal root ganglia. The protective effects of substance P and calcitonin gene-related peptide after peripheral nerve injury may be associated with injured neurons, autoregulation in the central nervous system and sprouting of lateral buds[14,15,16].
Role of substance P and calcitonin gene-related peptide in the metabolism of the nervous system
Substance P is a member of the tachykinin family, produced from a polyprotein precursor after differential splicing of the preprotachykinin A gene, and consists of 11 amino acids linked by 10 peptide bonds[16]. Tachykinin receptors include the G-protein-coupled neurokinin-1, neurokinin A (NK2) and neurokinin B (NK3), with neurokinin-1 being the most sensitive to substance P[16]. When the receptor binds ligand it promotes hydrolysis of phosphinositides, a protein coupling agent, producing inositol 1,4,5-trisphosphate, which increases intracellular Ca2+ concentration, alters membrane potential, and results in neurophysiologic effects[16]. Substance P has high affinity for the neurokinin-1 receptor, and therefore can increase viability and proliferation of nerve cells. Calcitonin gene-related peptide is extensively distributed in the nervous system, and copied from the same gene as calcitonin. It is located at 11p15.4, is 7.6 kb, and produces calcitonin gene-related peptide mRNA after transcription. Calcitonin gene-related peptide is known as an effective vasodilator, and induces local ischemia/ necrosis of nerve tissues with inflammatory factors. In addition, it plays a role in neural regeneration[17]. Calcitonin gene-related peptide can bind receptors on nerve cells to promote cyclic adenosine monophosphate formation, insulin-like growth factor mRNA expression, and synthesis of insulin-like growth factor polypeptide to improve nerve cell activity. Calcitonin gene-related peptide can also inhibit differentiation of mouse bone marrow-derived mesenchymal stem cells into nerve cells[18]. Vasodilation caused by substance P and calcitonin gene-related peptide is mainly based on non-endothelium-dependent vasodilation of vascular smooth muscle[19,20]. Substance P directly acts at vascular endothelial cells, resulting in rapid and transient vasodilation, in addition to mast cell degranulation, histamine release and inflammatory reaction regulation[19]. Calcitonin gene- related peptide can promote angiogenesis and increase blood flow to local nerve tissues[5].
Significance of substance P and calcitonin gene-related peptide expression in dorsal root ganglia of rats with sciatic nerve injury
Although the mechanism surrounding peripheral nerve injury remains uncertain, some conclusions have been formulated[7]. Gleichmann et al[21] found that after peripheral nerve lesion a transient increase in stromal cell-derived factor-1beta mRNA expression was observed, indicating that stromal cell-derived factor-1beta may play a role in neural regeneration. An early study established a bilateral sciatic nerve injury model in rats and injected Hoechst-labeled bone marrow stromal stem cells into L4 dorsal root ganglion[22]. Results showed that the bone marrow stromal stem cells only migrated to L3, L5, and L6 ganglion that innervated injured sciatic nerves. This observation indicates that stem cells could promote neural regeneration. In another model of peripheral neuropathy, Schwann cell secreted erythropoietin was the most effective endogenous mechanism of preventing axonal degeneration[23]. Furthermore, erythropoietin can protect cortical neurons from HIV-1/gp120-induced apoptosis[24]. In the present study, we established a left sciatic nerve model in rats and observed substance P and calcitonin gene-related peptide expression in dorsal root ganglia. Results indicated that substance P and calcitonin gene-related peptide expression significantly increased at 7 and 14 days post injury, demonstrating that these neuropeptides are involved in the metabolism of injured nerves[16,17,18]. In addition, substance P and calcitonin gene-related peptide expression reached peak levels at 7 days post injury, gradually declined at 14 days and returned to normal levels by 28 days post injury. This suggests that substance P and calcitonin gene-related peptide are mainly expressed in the early stages after injury, and may be useful as a sensitive index to estimate the early stages of peripheral nerve injury. Furthermore, Substance P and calcitonin gene-related peptide are derived from a sensory neuropeptide. They can protect nerves against further injury and potentiate normal growth or reconstruction of nerves as well as biomechanical alignment[25,26].
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
Experiments were performed at the Animal Experimental Center of Anhui Medical University, China from November 2011 to June 2012.
Materials
A total of 40 clean grade, healthy, female, Sprague Dawley rats, aged 4 weeks, weighting 180–230 g, were purchased from the Animal Center of Anhui Medical University [No. SCXK (Wan) 2011-001]. Animal protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China in 2006[27].
Methods
Establishment of sciatic nerve defect model
Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (3 mL/kg), and an oblique incision was made at the posterior femur to expose the left sciatic nerve. The sciatic nerve was dissected out, and cut off at the mid femur. Both broken ends were encased in sterilized 10-mm silica gel tubes (Supratec, Attendorn, Germany) to a depth of 1.5 mm to create a 7-mm defect. The distal and proximal ends were anastomosed with the tube using 10-0 microsuture line[28]. The incision was then sutured closed.
Sampling of L5 dorsal root ganglion
Samples of dorsal root ganglion were harvested at 7, 14 and 28 days post injury (7 and 14 days for the normal control group). Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate and placed in a supine position. The left sciatic nerve was exposed, and L5 dorsal root ganglia connected with the vertebral canal were harvested, fixed in 4% paraformaldehyde, dehydrated, cleared, embedded in wax, and sectioned (40 μm) using a RM201644820 histotome (Leica, Solms, Germany).
Immunohistochemical staining
Paraffin sections were baked at 60°C for 2 hours, dewaxed, hydrated, and mixed with freshly prepared 3% H2O2 for 10 minutes. Sections were then washed three times with distilled water, and antigens were retrieved using a microwave. Sections were subsequently cooled, washed twice with PBS and incubated with normal goat serum blocking solution at room temperature for 20 minutes. Following this blocking step, sections were incubated with primary antibody (rabbit anti-substance P and calcitonin gene-related peptide polyclonal antibody; 1:200; Abcam, Cambridge, UK) at room temperature for 2 hours, and biotinlabeled mouse anti-rabbit IgG (1:100; Abcam) at room temperature for a further 2 hours. Three washes with PBS were conducted between each step. Sections were then incubated with streptavidin-biotin complex (Abcam) at room temperature for 20 minutes, washed four times with PBS and then visualized with diaminobenzidine (Abcam). Staining was finally observed using a light microscope [Motic B5; Motic (Xiamen) Electric Group Co., Ltd., Xiamen, Fujian Province, China].
Image analysis
Images were loaded into a Medical Image Analysis System (Cambridge Instrument, Cambridge, UK) using Motic B5 microscopic imaging system [Motic (Xiamen) Electric Group Co., Ltd.]. Four high-power fields (× 400) were randomly selected from each section. Immunohistochemical staining results were identified[29]: (+), light yellow, 1 point; (++), dark yellow, 2 points; (+++), brown, 3 points. Images were analyzed using the JEDA-801D Morphology Image Analysis System (Cambridge Instrument) and mean absorbance was calculated.
Statistical analysis
Data were analyzed using SPSS version 13.0 (SPSS, Chicago, IL, USA) and were expressed as mean ± SD. Intergroup differences were compared utilizing two- sample t-test, and intragroup differences at different time points were compared using one-way analysis of variance. Between-group comparison was conducted using least significant difference test. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the Major Program of Science and Technology of Ministry of Education, No. 207049.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Ethics Committee of Animal Experimental Center of Anhui Medical University, China.
REFERENCES
- [1].Lago N, Rodríguez FJ, Guzmán MS, et al. Effects of motor and sensory nerve transplants on amount and specificity of sciatic nerve regeneration. J Neurosci Res. 2007;85(12):2800–2812. doi: 10.1002/jnr.21286. [DOI] [PubMed] [Google Scholar]
- [2].Yamada C, Yamada Y, Tsukiyama K, et al. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149(2):574–579. doi: 10.1210/en.2007-1292. [DOI] [PubMed] [Google Scholar]
- [3].Liu Y, Pei GX. The factor of nervus peripheralis to bone and tissue-engineered bone. Guoji Gukexue Zazhi. 2006;27(1):12–15. [Google Scholar]
- [4].Schlomer JJ, Storey BB, Ciornei RT, et al. Calcitonin gene-related peptide inhibits early B cell development in vivo. J Leukoc Biol. 2007;81(3):802–808. doi: 10.1189/jlb.0306229. [DOI] [PubMed] [Google Scholar]
- [5].Wu ZM, Ni JJ, Ling SC. Substance P and/or calcitonin gene-related peptide immunoreactive neurons in dorsal root ganglia possibly involved in the transmission of nociception in rat penile frenulum. Zhonghua Nankexue Zazhi. 2007;13(12):1068–1071. [PubMed] [Google Scholar]
- [6].Huebner AK, Keller J, Catala-Lehnen P, et al. The role of calcitonin and alpha-calcitonin gene-related peptide in bone formation. Arch Biochem Biophys. 2008;473(2):210–217. doi: 10.1016/j.abb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- [7].de Ruiter GC, Malessy MJ, Alaid AO, et al. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211(2):339–350. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lykissas MG, Batistatou AK, Charalabopoulos KA, et al. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res. 2007;4(2):143–151. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- [9].Furuya T, Hashimoto M, Koda M, et al. Treatment of rat spinal cord injury with a Rho-kinase inhibitor and bone marrow stromal cell transplantation. Brain Res. 2009;1295:192–202. doi: 10.1016/j.brainres.2009.07.087. [DOI] [PubMed] [Google Scholar]
- [10].Fandel TM, Albersen M, Lin G, et al. Recruitment of intra-cavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61(1):201–210. doi: 10.1016/j.eururo.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhu JY, Zhu QS, Huang YT. Quantitative analysis of SP and CGRP in the dorsal horn of rabbit spinal cord following delayed repair of peripheral nerve injury. Zhongguo Linchuang Kangfu. 2002;6(22):3332–3333. [Google Scholar]
- [12].Qi T, Christopoulos G, Bailey RJ, et al. Identification of N-terminal receptor activity-modifying protein residues important for calcitonin gene-related peptide, adrenomedullin, and amylin receptor function. Mol Pharmacol. 2008;74(4):1059–1071. doi: 10.1124/mol.108.047142. [DOI] [PubMed] [Google Scholar]
- [13].Mikic B, Zhang M, Webster E, et al. Effect of y2 receptor deletion on whole bone structural behavior in mice. Anat Rec (Hoboken) 2008;291(1):14–18. doi: 10.1002/ar.20624. [DOI] [PubMed] [Google Scholar]
- [14].Wang L, Wang KZ, Zhang L, et al. Effect of calcitonin gene related peptide on avascular necrosis of the femoral head. Zhongguo Xiufu Chongjian Waike Zazhi. 2010;24(9):1078–1081. [PubMed] [Google Scholar]
- [15].Christoforakis JJ, Sanchez-Ballester J, Hunt N, et al. Synovial shelves of the knee: association with chondral lesions. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1292–1298. doi: 10.1007/s00167-006-0085-y. [DOI] [PubMed] [Google Scholar]
- [16].Villa I, Mrak E, Rubinacci A, et al. CGRP inhibits osteo-protegerin production in human osteoblast-like cells via cAMP/PKA-dependent pathway. Am J Physiol Cell Physiol. 2006;291(3):C529–537. doi: 10.1152/ajpcell.00354.2005. [DOI] [PubMed] [Google Scholar]
- [17].Guo Q, Zhang L. Effect of brain injury on expression of bone morphogenetic protein 2 in fracture healing process. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(10):1040–1044. [PubMed] [Google Scholar]
- [18].Li Y, Tan Y, Zhang G, et al. Effect of calcitonin gene-related peptide on the expression and activity of nitric axide synthase during man dibular bone healing in rabbtis: a experimental study. J Oral Maxillofac Surg. 2009;67(2):273–279. doi: 10.1016/j.joms.2008.06.077. [DOI] [PubMed] [Google Scholar]
- [19].Granholm S, Lundberg P, Lerner UH. Expression of the calcitonin receptor, calcitonin receptor-like receptor, and receptor activity modifying proteins during osteoclast differentiation. J Cell Biochem. 2008;104(3):920–933. doi: 10.1002/jcb.21674. [DOI] [PubMed] [Google Scholar]
- [20].Naot D, Comish J. The role of peptides and receptors of the Calcitonin family in the regulation of bone metabolism. Bone. 2008;43(5):813–818. doi: 10.1016/j.bone.2008.07.003. [DOI] [PubMed] [Google Scholar]
- [21].Gleichmann M, Gillen C, Czardybon M, et al. Cloning and characterization of SDF-1gamma, a novel SDF-1 chemokine transcript with developmentally regulated expression in the nervous system. Eur J Neurosci. 2000;12(6):1857–1866. doi: 10.1046/j.1460-9568.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- [22].Patel JR, McCandless EE, Dorsey D, et al. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci U S A. 2010;107(24):11062–11067. doi: 10.1073/pnas.1006301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Feeley BT, Gallo RA, Sherman S, et al. Management of osteoarthritis of the knee in the active patient. J Am Acad Orthop Surg. 2010;18(7):406–416. doi: 10.5435/00124635-201007000-00003. [DOI] [PubMed] [Google Scholar]
- [24].Michael JW, Schluter-Brust KU, Eysel P. The epidemiology, etiology, diagnose, and trement of osteoarthritis of the knee . Dtsch Arztebl Int. 2010;107(9):152–162. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li J, Kreicbergs A, Bergström J, et al. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: a study in rat angulated tibia. J Orthop Res. 2007;25(9):1204–1212. doi: 10.1002/jor.20406. [DOI] [PubMed] [Google Scholar]
- [26].Li ZH, Zhou ZH, Xie JY, et al. Effect of neuropeptide Y on bone mineral density after bilateral sciatic and femoral denervation and immobilization. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2009;13(33):6428–6432. [Google Scholar]
- [27].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [28].Chen J, Yin ZS, Zhang H, et al. Effect of erythropoietin on Bcl-2 and Bax expression of dorsal root ganglion after sciatic nerve injury in rats. Anhui Yike Daxue Xuebao. 2011;46(9):846–849. [Google Scholar]
- [29].Li X, Wang M, Xiao Y, et al. Expression of substance P and calcitonin gene-related peptide in adenomyosis. Zhongguo Fuchanke Linchuang Zazhi. 2008;9(3):214–216. [Google Scholar]


