Abstract
Medical-grade synthetic poly(lactic-co-glycolic acid) polymer can be used as a biomaterial for nerve repair because of its good biocompatibility, biodegradability and adjustable degradation rate. The stress relaxation and creep properties of peripheral nerve can be greatly improved by repair with poly(lactic-co-glycolic acid) tubes. Ten sciatic nerve specimens were harvested from fresh corpses within 24 hours of death, and were prepared into sciatic nerve injury models by creating a 10 mm defect in each specimen. Defects were repaired by anastomosis with nerve autografts and poly(lactic-co-glycolic acid) tubes. Stress relaxation and creep testing showed that at 7 200 seconds, the sciatic nerve anastomosed by poly(lactic-co-glycolic acid) tubes exhibited a greater decrease in stress and increase in strain than those anastomosed by nerve autografts. These findings suggest that poly(lactic-co-glycolic acid) exhibits good viscoelasticity to meet the biomechanical require-ments for a biomaterial used to repair sciatic nerve injury.
Keywords: neural regeneration, peripheral nerve injury, sciatic nerve injury model, nerve autograft, poly(lactic-co-glycolic acid), transplantation, repair, stress relaxation, creep, biomaterial, neuroregeneration
Research Highlights
-
(1)
This study made innovative use of stress relaxation and creep as indices of the anastomotic ef-fects of nerve autografts compared with poly(lactic-co-glycolic acid) tubes used to treat a model of sciatic nerve injury.
-
(2)
A better understanding of the stress relaxation and creep properties of nerve autografts and poly (lactic-co-glycolic acid) tubes will help to demonstrate the feasibility of poly(lactic-co-glycolic acid) tubes for the repair of sciatic nerve injury.
INTRODUCTION
In many previous studies, nerve autografts are most frequently used to repair sciatic nerve injury[1,2,3,4,5,6,7,8,9,10,11]. Recently, owing to developments in tissue engineering technology and the emergence of new biomaterials, synthetic polymers have been synthesized to meet demand. Degradable synthetic polymers can be absorbed in vivo. Poly (lactic-co-glycolic acid) can be used as a scaffold material for tissue engineering owing to its good biocompatibility and biodegradation properties[12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Hou et al[26] used poly(lactic-co-glycolic acid) tubes loaded withmesenchymal stem cells or nerve autographs to bridge 10 mm sciatic nerve gaps. They found similar outcomes for these groups, including the density of myelinated nerve fibers, the average diameter of nerve fibers and the myelin sheath thickness of proximal sciatic nerve. Pan et al[27] transplanted dextran/poly(lactic-co-glycolic acid) scaffolds into mouse subcutaneous tissue. The transplanted scaffolds were reduced to 50% the original size after 3 days, 75% of them by size were absorbed after 3 weeks and the surrounding tissue was completely recovered. Liu et al[28] concluded that poly(lactic-co- glycolic acid) tubes exhibit some reparative effects on sciatic nerve injury.
The biomechanical properties of poly(lactic-co-glycolic acid) have been widely studied. Yu et al[29] reported that poly(lactic-co-glycolic acid) scaffolds were safe and non-toxic, degrading in solutions at pH 6.68–7.33. During degradation, the elastic modulus first increased and then decreased. They also found that the degradation of embedded poly(lactic-co-glycolic acid) scaffolds was synchronous to the in-growth of newly generated tissue, with the number of macrophages surrounding the poly(lactic- co-glycolic acid) scaffold increasing at first and then decreasing. Zhao et al[30] confirmed that the tensile strength of poly(lactic-co-glycolic acid) scaffolds increased with an increasing proportion of polylactic acid. These previous studies focused on peripheral nerve injury repair by poly(lactic-co-glycolic acid) from the perspectives of biocompatibility, degradation and preparation of tissue-engineered scaffolds. However, the viscoelasticity of injured sciatic nerve is important in the repair of sciatic nerve injury. For this reason, this study prepared sciatic nerve injury models and investigated the stress relaxation and creep of these models after anastomosis with nerve autografts or poly(lactic-co-glycolic acid) tubes.
RESULTS
Stress changes of sciatic nerve injury models after anastomosis with nerve autografts or poly(lactic-co-glycolic acid) tubes
In both the nerve autograft and poly(lactic-co-glycolic acid) tube groups, the stress in sciatic nerve injury specimens decreased rapidly in the initial 600 seconds. Thereafter, the rate of decrease slowed, and tended towards stability at 7 200 seconds. The stress relaxation curves of both groups decreased exponentially. In the nerve autograft group, the stress was 0.500 MPa at 0 seconds and 0.424 MPa at 7 200 seconds (decreased by 0.076 MPa). In the poly(lactic-co-glycolic acid) tube group, the stress was 0.50 MPa at 0 seconds and 0.419 MPa at 7 200 seconds (decreased by 0.081 MPa). The decrease of stress at 7 200 seconds was similar in both groups (P > 0.05, Figure 1).
Figure 1.
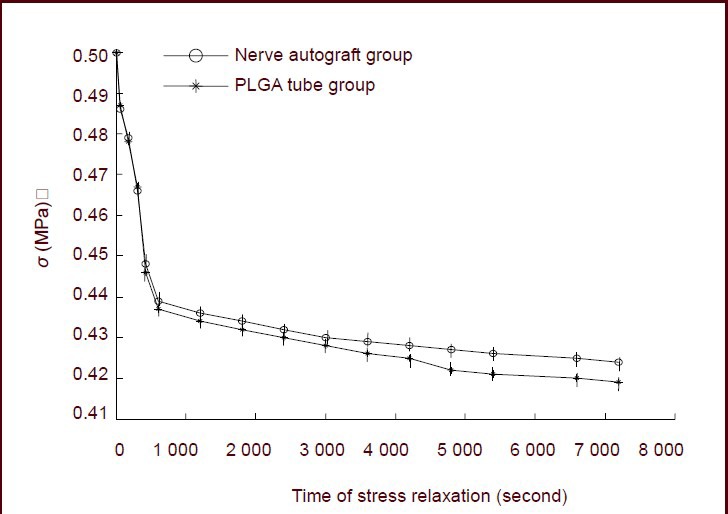
Stress relaxation curves for the nerve autograft and poly(lactic-co-glycolic acid) (PLGA) groups.
Data are expressed as mean ± SD of 10 sciatic nerve specimens for each group and were analyzed using paired t-tests. σ represents the stress value.
The stress values in sciatic nerve specimens from both groups were normalized. The functions describing the normalized stress relaxation values in each group at 0 seconds were 1. At 7 200 seconds, the normalized stress relaxation value functions were 0.85 in the nerve autograft group and 0.84 in the poly(lactic-co-glycolic acid) tube group (Figure 2).
Figure 2.
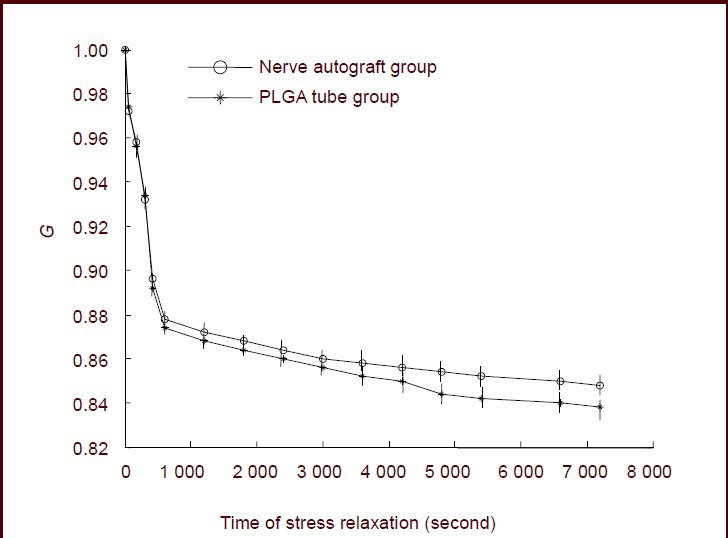
Normalized stress relaxation functions for the nerve autograft and poly(lactic-co-glycolic acid) (PLGA) groups.
Data are expressed as mean ± SD of 10 sciatic nerve specimens for each group and were analyzed using paired t-tests. G represents the normalized, dimensionless, stress relaxation functions.
Normalized stress relaxation value functions for sciatic nerve in the nerve autograft and poly(lactic-co-glycolic acid) tube groups
Following previously described methods[31], normalized stress relaxation functions were established for sciatic nerve specimens from the two groups. Because Figure 1 demonstrated that the stress relaxation curves decreased exponentially, the functions took the form:
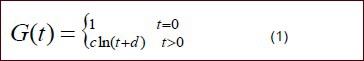
In this equation, c, d are undetermined coefficients.
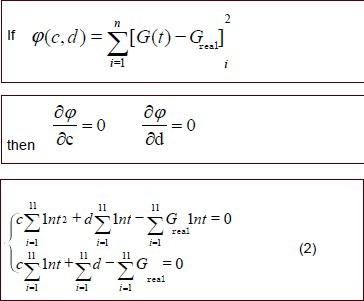
In this equation, c, d are undetermined coefficients.
Experimental data were fit to equation (2) and parameters c and d were calculated for the nerve autograft and poly(lactic-co-glycolic acid) tube groups. The calculated values of c and d were introduced into equation (1) to generate the normalized stress relaxation functions for the two groups.
Nerve autograft group:

Poly(lactic-co-glycolic acid) tube group:

Strain changes in sciatic nerve injury models after anastomosis with nerve autografts or poly(lactic-co- glycolic acid) tubes
In both the nerve autograft and poly(lactic-co-glycolic acid) groups, the strain increased rapidly in the first 600 seconds. The rate of increase slowed down thereafter and tended towards stability at 7 200 seconds. The creep curves of the two groups increased exponentially. In the nerve autograft group, the strain was 9.28% at 0 seconds and 12.48% at 7 200 seconds (increased by 3.20%). In the poly(lactic-co-glycolic acid) tube group, the stress was 9.50% at 0 seconds and 13.75% at 7 200 seconds (increased by 4.25%). Paired t-tests showed that the increase in strain at 7 200 seconds in the poly(lactic- co-glycolic acid) tube group was significantly greater than in the nerve autograft group (P < 0.05; Figure 3).
Figure 3.
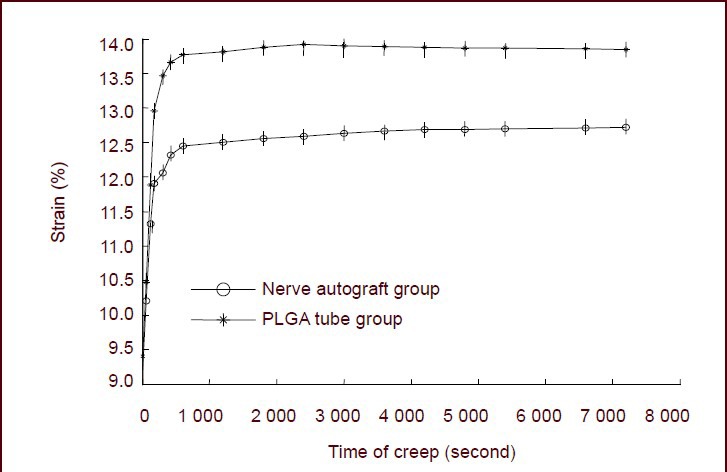
Creep curves of sciatic nerve specimens for the nerve autograft and poly(lactic-co-glycolic acid) (PLGA) groups.
The increase in strain at 7 200 seconds in the PLGA group was significantly greater than in the nerve autograft group (P < 0.05). Data are expressed as mean ± SD of 10 sciatic nerve specimens from each group and were analyzed using paired t-tests.
The strain values in the sciatic nerve specimens from the two groups were normalized. The normalized strain value functions in both groups were 1 at 0 seconds. At 7 200 seconds, the normalized strain value was 1.128 in the nerve autograft group and 1.138 in the poly(lactic-co- glycolic acid) tube group. The creep function value at 7 200 seconds in the poly(lactic-co-glycolic acid) group was significantly greater than in the nerve autograft group (P < 0.05; Figure 4).
Figure 4.
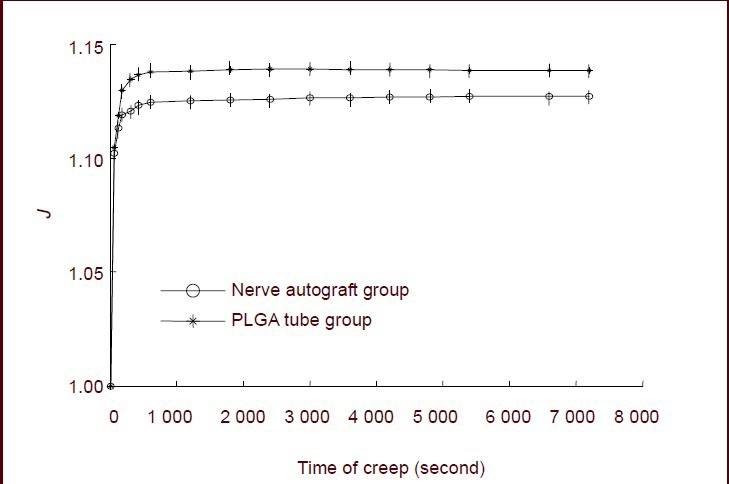
Normalized creep function curves of sciatic nerve specimens for the nerve autograft and poly(lactic-co-glycolic acid) (PLGA) groups.
Creep at 7 200 seconds in the PLGA group was significantly greater than in the nerve autograft group (P < 0.05). Data are expressed as mean ± SD of 10 sciatic nerve specimens from each group and were analyzed using paired t-tests. J represents the normalized, dimensionless, creep functions.
Normalized creep functions of sciatic nerve specimens in the nerve autograft and poly(lactic-co- glycolic acid) tube groups
Following previously described methods[31], normalized creep functions for sciatic nerve specimens from the two groups were established.
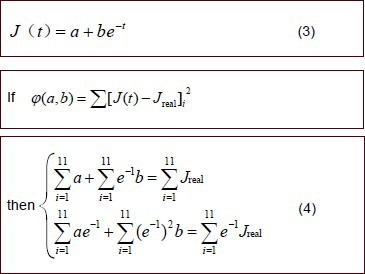
Creep test data from both groups were introduced into equation (4) and parameters a and b were determined for both groups. These values of a and b were introduced into equation (3) to generate the normalized creep functions for the sciatic nerve specimens of the two groups:
Nerve autograft group:

Poly(lactic-co-glycolic acid) tube group:
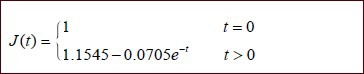
Characteristics of sciatic nerve structure after stress relaxation and creep tests
By scanning electron microscopy, sciatic nerve specimens in the nerve autograft and poly(lactic-co-glycolic acid) groups after stress relaxation and creep testing exhibited poor arrangement of nerve fibers and morphological changes to the nerve fiber surface connective tissue, endoneurium, myelin sheath and axons, as well as occlusion of vessel of the basilar membrane by fragmented axonal and myelin sheath tissues. There were no obvious differences between these two groups (Figure 5).
Figure 5.
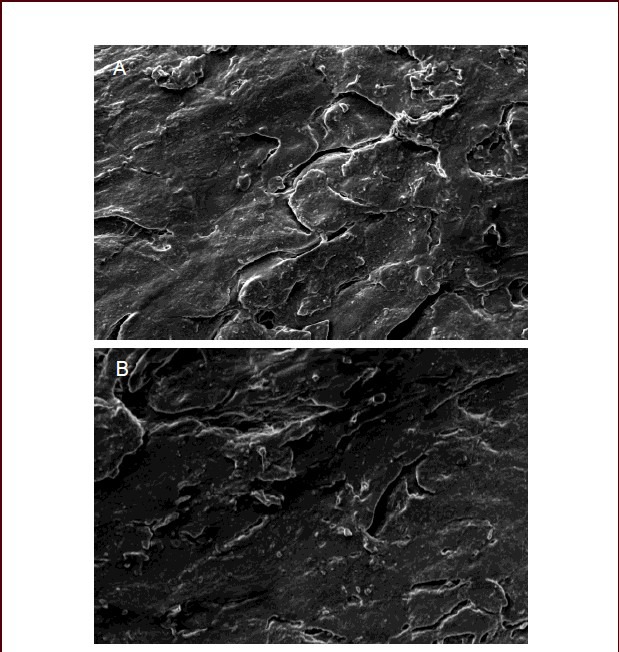
Morphological changes of sciatic nerve specimens in the nerve autograft and poly(lactic-co-glycolic acid) tube groups (scanning electron microscope, × 2 000).
In the nerve autograft group (A) and poly(lactic-co- glycolic acid) tube group (B), nerve fibers were poorly arranged, morphological changes to the nerve fiber surface connective tissue, endoneurium, myelin sheath and axons were observed, and vessels of the basilar membrane were occluded by fragmented axonal and myelin sheath tissue.
DISCUSSION
The synthetic polymer poly(lactic-co-glycolic acid), which is approved by US Food and Drug Administration, exhibits good biocompatibility and biodegradability, as well as an adjustable degradation rate. It has been used as an alternative biomaterial for repair of defected hard tissues and shows great promise in the reconstruction of soft tissues, such as tendon and ligament[32,33]. Poly(lactic-co- glycolic acid) tubes are porous, which increases nutrient uptake and metabolite excretion during the process of neurogenesis, facilitates the in-growth of vessels, and provides sufficient nutrients for seeded Schwann cells, accelerating neurogenesis. The limited pore diameters exclude lymphocytes and fibroblasts, effectively avoiding an inflammatory reaction and fibrous scar tissue formation[34]. Poly(lactic-co-glycolic acid) tubes and sciatic nerves are viscoelastic materials. A quantitative analysis of stress relaxation and creep characteristics of injured sciatic nerve specimens after anastomosis with nerve autografts or poly(lactic-co-glycolic acid) tubes is important for characterizing the clinical treatment of peripheral nerve injury by poly(lactic-co-glycolic acid) tubes. Strong evidence exists that poly(lactic-co-glycolic acid) has good physiochemical and biological properties[35,36,37,38,39]. Taken together, these data suggest that the viscoelasticity of poly(lactic-co-glycolic acid) is superior to that of human sciatic nerve. Results from this study have confirmed, as expected, that poly(lactic-co-glycolic acid) exhibited good viscoelasticity.
The viscoelasticity of natural and artificial biomaterials can be suitable for human physiological function by matching stress relaxation and creep properties. Stress relaxation refers to the adaptive response of biomaterials to a constant applied strain, and creep refers to the adaptive response of biomaterials to a constant applied stress. Under the condition of constant tensile strain, poly(lactic-co-glycolic acid) tubes exhibit similar stress relaxation to nerve autografts, which may help to alleviate the tension at the anastomotic stoma after transplantation of poly(lactic-co-glycolic acid) tubes to bridge sciatic nerve gaps. Under constant physiological loads, the poly(lactic-co-glycolic acid) tubes transplanted into the injured sciatic nerve exhibit sufficient length to limit the gap in the nerve, prevent shifts of the anastomotic stoma, and facilitate regeneration and functional reconstruction of the sciatic nerve. In previous studies of the biomechanical properties of poly(lactic-co-glycolic acid) scaffolds, Yu et al[29] only measured their elastic modulus and Zhao et al[30] only tested their tension. These traditional methods can help to briefly describe the biomechanical behaviors of poly(lactic-co-glycolic acid) scaffolds, but cannot describe the rheological and viscoelastic properties of poly(lactic-co-glycolic acid). This study made innovative use of stress relaxation and creep as indices of the anastomotic effects of nerve autografts compared with poly(lactic-co-glycolic acid) tubes used to treat a model of sciatic nerve injury.
Poly(lactic-co-glycolic acid) exhibits similar viscoelasticity in stress relaxation and creep testing to human sciatic nerves, and therefore is suitable for use in the repair of injured sciatic nerve. The present findings support that poly(lactic-co-glycolic acid) tubes were suitable for repair of injured peripheral nerves, such as the sciatic nerve. Owing to individual factors, for example human donor age and health, the experimental data show a little variation, but still provide reasonable clinical reference values.
MATERIALS AND METHODS
Design
A biomechanical contrast observation study.
Time and setting
This study was performed at the Center for Mechanical Experiments, Jilin University, China from December 2010 to August 2011.
Materials
Ten sciatic nerve specimens were harvested from five fresh Chinese adult male cadaveric donors within 24 hours of death; these were aged 21–28 years and provided by the Department of Medical Anatomy, Jilin University, China. These specimens were preserved at 4°C in physiological saline before use.
Methods
Preparation of poly(lactic-co-glycolic acid) tubes
Poly(lactic-co-glycolic acid) (polylactic acid: glycolic acid of 70:30, Changchun SinoBiomaterials Co., Ltd., Changchun, Jilin Province, China) was diluted to 10% in solution with trichloromethane. The poly(lactic-co-glycolic acid) solution was injected into pre-fabricated molds and left at room temperature for the trichloromethane to naturally volatize over 48 hours. Twenty-five poly(lactic- co-glycolic acid) tubes (10 mm in length, 12 mm in external diameter, 9.5 mm in internal diameter) were created. After 24 hours of treatment in the solvent extractor (20 L/h; −0.1 MPa), these poly(lactic-co-glycolic acid) tubes were preserved in a vacuum until use.
Preparation of sciatic nerve injury models
Forty sciatic nerve specimens (25 mm in length) were harvested from fresh cadaveric donors, within 24 hours of death, by an experienced microsurgery expert. Following previously reported methods[20], these sciatic nerve specimens were prepared into sciatic nerve injury models by creating a 10-mm-long gap on each specimen and then dividing them into two groups: a nerve autograft group (n = 20) and a poly(lactic-co-glycolic acid) tube group (n = 20). In the nerve autograft group, a piece of nerve autograft was sutured into the gap with 7-0 nylon suture (Qingdao Naike Medical Materials Co., Ltd., Qingdao, Shandong Province, China). In the poly(lactic- co-glycolic acid) tube group, a similar procedure was performed using a poly(lactic-co-glycolic acid) tube rather than the nerve autograft. Eight sutures were required for each specimen. Specimens not meeting the experimental requirements were rejected.
Stress relaxation testing
A universal testing machine (Shimadzu Corporation, Tokyo Prefecture, Japan) was used. The machine has the ability to apply constant stress or strain while keeping time and collecting data. The machine is equipped with an incubator (−30–250°C that can maintain constant temperatures. Following previously reported methods[40], the length (25 mm), external diameter (11.9–12.1 mm) and internal diameter (9.4–9.8 mm) of the sciatic nerve specimens were measured using a reading microscope (Changchun Third Optical Instrument Factory, Changchun, Jilin Province, China).
Following previously reported methods[31], under the condition of tensile stress, the specimens were loaded and unloaded 30 times for pre-loading and then tested. At normal body temperature (36.5 ± 0.5°C), 10 specimens from each group were subjected to strain stimulation at a velocity of 60%/minute. When the stress reached 0.5 MPa, the strains were 8.63% in the nerve autograft and 9.68% in the poly(lactic-co-glycolic acid) tubes groups. The strain was kept constant and the experimental time was 7 200 seconds. After the designated time, stress relaxation data were automatically output by the computer connected to the universal testing machine. During the experiment, the specimens were kep hydrated with physiological saline (Figure 6). Using the formula G(t)= σ(t)/σ(0), the normalized stress relaxation function G(t) was calculated. In the equation, σ(0) indicates the stress value at 0 seconds and σ(t) is the stress value at t seconds.
Figure 6.

Experimental process of the stress relaxation test.
The tested specimens were subjected to strain stimulation at a velocity of 60%/minute. When the stress reached 0.5 MPa, the strain was 8.63% in the nerve autograft (A) and 9.68% in the poly(lactic-co-glycolic acid) tube groups (B). The strain was kept constant and the experimental time was 7 200 seconds.
Creep testing
The clamping method, experimental temperature, and pre-loading used in creep testing were similar to those of stress relaxation testing. Ten specimens from each group were subjected to stress stimulation at a velocity of 0.5 GPa/minute. When the strain reached 8.63% in each group, the stress was 0.4 MPa in the nerve autograph and 0.31 MPa in the poly(lactic-co-glycolic acid) tube groups. The stress was kept constant and the experimental time was 7 200 seconds. After the designated time, creep data were automatically output by the computer connected to the universal testing machine Figure 7. Using the formula J(t)=L(t)/L(0), the normalized creep function J(t) was calculated. In the equation, L(0) indicates the length of the tested specimens and L(t) is the length of specimens at t seconds.
Figure 7.

Experimental process of the creep test.
The specimens were subjected to stress stimulation at a velocity of 0.5 GPa/min. When the strain reached 8.63% in each group, the stress was 0.4 MPa in the nerve autograft (A) and 0.31 MPa in the poly(lactic-co-glycolic acid) tube groups (B). The stress was kept constant and the experimental time was 7 200 seconds.
Observation of sciatic nerve cross-section ultrastructure after stress relaxation and creep testing under a scanning electron microscope
After stress relaxation and creep testing, one sciatic nerve randomly selected from each group was pre-fixed with 4% glutaraldehyde, post-fixed with 1% osmic acid, hydrated by a gradient of acetone, dried at the critical point and gold-coated in the vacuum. Finally, the structure of nerve cells, myelin sheath, axons and basement membrane on the nerve cross-sections was observed under a scanning electron microscope (Carl Zeiss, Jana, Germany).
Statistical analysis
Data were expressed as mean ± SD and statistically analyzed using SPSS 11.0 software (SPSS, Chicago, IL, USA). Paired t-tests were used to compare experimental data between the nerve autograft and poly(lactic-co- glycolic acid) tube groups. A level of P < 0.05 was considered statistically significant. The stress relaxation and creep data from sciatic nerve specimens were normalized and equations of normalized stress relaxation and creep data were established.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study received full approval from the Animal Ethics Committee, China-Japan Union Hospital of Jilin University in China.
REFERENCES
- [1].Meng H, Li M, You F, et al. Assessment of processed human amniotic membrane as a protective barrier in rat model of sciatic nerve injury. Neurosci Lett. 2011;496(1):48–53. doi: 10.1016/j.neulet.2011.03.090. [DOI] [PubMed] [Google Scholar]
- [2].Henry FP, Goyal NA, David WS, et al. Improving electrophysiologic and histologic outcomes by photochemically sealing amnion to the peripheral nerve repair site. Surgery. 2009;145(3):313–321. doi: 10.1016/j.surg.2008.11.005. [DOI] [PubMed] [Google Scholar]
- [3].O’Neill AC, Randolph MA, Bujold KE, et al. Photochemical sealing improves outcome following peripheral neurorrhaphy. J Surg Res. 2009;151(1):33–39. doi: 10.1016/j.jss.2008.01.025. [DOI] [PubMed] [Google Scholar]
- [4].Kadam SS, Sudhakar M, Nair PD, et al. Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy. 2010;12(8):982–991. doi: 10.3109/14653249.2010.509546. [DOI] [PubMed] [Google Scholar]
- [5].Karcher DM, Fleming-Waddell JN, Applegate TJ. Developmental changes in insulin-like growth factor (IGF)-I and -II mRNA abundance in extra-embryonic membranes and small intestine of avian embryos. Growth Horm IGF Res. 2009;19(1):31–42. doi: 10.1016/j.ghir.2008.05.003. [DOI] [PubMed] [Google Scholar]
- [6].Kong XY, Cai Z, Pan L, et al. Transplantation of human amniotic cells exerts neuroprotection in MPTP-induced Parkinson disease mice. Brain Res. 2008;1205:108–115. doi: 10.1016/j.brainres.2008.02.040. [DOI] [PubMed] [Google Scholar]
- [7].Cheng FC, Tai MH, Sheu ML, et al. Enhancement of regeneration with glia cell line-derived neurotrophic factor- transduced human amniotic fluid mesenchymal stem cells after sciatic nerve crush injury. J Neurosurg. 2010;112(4):868–879. doi: 10.3171/2009.8.JNS09850. [DOI] [PubMed] [Google Scholar]
- [8].Elgazzar RF, Mutabagani MA, Abdelaal SE, et al. Platelet rich plasma may enhance peripheral nerve regeneration after cyanoacrylate reanastomosis: a controlled blind study on rats. Int J Oral Maxillofac Surg. 2008;37(8):748–755. doi: 10.1016/j.ijom.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [9].Chen Z, Lu XC, Shear DA, et al. Synergism of human amnion-derived multipotent progenitor (AMP) cells and a collagen scaffold in promoting brain wound recovery: pre-clinical studies in an experimental model of penetrating ballistic-like brain injury. Brain Res. 2011;1368:71–81. doi: 10.1016/j.brainres.2010.10.028. [DOI] [PubMed] [Google Scholar]
- [10].Dong W, Chen H, Yang X, et al. Treatment of intracerebral haemorrhage in rats with intraventricular transplantation of human amniotic epithelial cells. Cell Biol Int. 2010;34(6):573–577. doi: 10.1042/CBI20090248. [DOI] [PubMed] [Google Scholar]
- [11].Wolford LM, Rodrigues DB. Autogenous grafts/allografts/ conduits for bridging peripheral trigeminal nerve gaps. Atlas Oral Maxillofac Surg Clin North Am. 2011;19(1):91–107. doi: 10.1016/j.cxom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- [12].Heath CA, Rutkowski GE. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 1998;16(4):163–168. doi: 10.1016/s0167-7799(97)01165-7. [DOI] [PubMed] [Google Scholar]
- [13].Li XK, Cai SX, Liu B, et al. Characteristics of PLGA-gelatin complex as potential artificial nerve scaffold. Colloids Surf B Biointerfaces. 2007;57(2):198–203. doi: 10.1016/j.colsurfb.2007.02.010. [DOI] [PubMed] [Google Scholar]
- [14].Hu X, Shen H, Yang F, et al. Preparation and cell affinity of microtubular orientation-structured PLGA(70/30) blood vessel scaffold. Biomaterials. 2008;29(21):3128–3136. doi: 10.1016/j.biomaterials.2008.04.010. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Y, Reddy VJ, Wong SY, et al. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/ chitosan. Tissue Eng Part A. 2010;16(6):1949–1960. doi: 10.1089/ten.TEA.2009.0221. [DOI] [PubMed] [Google Scholar]
- [16].Madurantakam PA, Cost CP, Simpson DG, et al. Science of nanofibrous scaffold fabrication: strategies for next generation tissue-engineering scaffolds. Nanomedicine (Lond) 2009;4(2):193–206. doi: 10.2217/17435889.4.2.193. [DOI] [PubMed] [Google Scholar]
- [17].Zhang P, Hong Z, Yu T, et al. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide- co-glycolide) and hydroxyapatite surface-grafted with poly(L-lactide) Biomaterials. 2009;30(1):58–70. doi: 10.1016/j.biomaterials.2008.08.041. [DOI] [PubMed] [Google Scholar]
- [18].Zhang HA, Zhang CJ, Yi JY. Degradation characteristics of PLGA/HA bone scaffold material. Cailiao Daobao. 2010;24(7):46–47. [Google Scholar]
- [19].Blackwood KA, McKean R, Canton I, et al. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials. 2008;29(21):3091–3104. doi: 10.1016/j.biomaterials.2008.03.037. [DOI] [PubMed] [Google Scholar]
- [20].Stitzel J, Liu J, Lee SJ, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27(7):1088–1094. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- [21].Liao S, Murugan R, Chan CK, et al. Processing nanoengineered scaffolds through electrospinning and mineralization suitable for biomimetic bone tissue engineering. J Mech Behav Biomed Mater. 2008;1(3):252–260. doi: 10.1016/j.jmbbm.2008.01.007. [DOI] [PubMed] [Google Scholar]
- [22].Shen H, Hu X, Yang F, et al. Combining oxygen plasma treatment with anchorage of cationized gelatin for enhancing cell affinity of poly(lactide-co-glycolide) Biomaterials. 2007;28(29):4219–4230. doi: 10.1016/j.biomaterials.2007.06.004. [DOI] [PubMed] [Google Scholar]
- [23].Prasad BR, Brook MA, Smith T, et al. Controlling cellular activity by manipulating silicone surface roughness. Colloids Surf B Biointerfaces. 2010;78(2):237–242. doi: 10.1016/j.colsurfb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- [24].Petrie Aronin CE, Sadik KW, Lay AL, et al. Comparative effects of scaffold pore size, pore volume, and total void volume on cranial bone healing patterns using microsphere-based scaffolds. J Biomed Mater Res A. 2009;89(3):632–641. doi: 10.1002/jbm.a.32015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hu J, Feng K, Liu X, et al. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30(28):5061–5067. doi: 10.1016/j.biomaterials.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hou SY, Zhang HY, Quan DP, et al. Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience. 2006;140(1):101–110. doi: 10.1016/j.neuroscience.2006.01.066. [DOI] [PubMed] [Google Scholar]
- [27].Pan H, Jiang H, Chen W. The biodegradability of electrospun Dextran/PLGA scaffold in a fibroblast/macroph- age co-culture. Biomaterials. 2008;29(11):1583–1592. doi: 10.1016/j.biomaterials.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu XF, Zheng LF, Xie LS, et al. PLGA tube repairs sciatic nerve defect in rats. Disan Junyi Daxue Xuebao. 2008;30(15):1462–1465. [Google Scholar]
- [29].Yu WJ, Wang XG, Hu XL, et al. Preliminary evaluation of the biological properties of poly-lactide-co-glycolic acid (PLGA) knitted mesh. Shengwu Yixue Gongcheng Xue Zazhi. 2011;28(1):163–169. [PubMed] [Google Scholar]
- [30].Zhao L, He CG, Gao YJ, et al. Study on influence of copolymer compositions of PLGA on properties of scaffolds. Zhongguo Shengwu Gongcheng Zazhi. 2008;28(5):22–28. [Google Scholar]
- [31].Ma HS, Zhang ZJ, Li XH. Experimental study on viscoelasticity of the upper branch of fetal brachial plexus. Zhongguo Shengwu Yixue Gongcheng Xuebao. 2004;23(3):274–278. [Google Scholar]
- [32].Urita Y, Komuro H, Chen G, et al. Evaluation of diaphragmatic hernia repair using PLGA mesh-collagen sponge hybrid scaffold: an experimental study in a rat model. Pediatr Surg Int. 2008;24(9):1041–1045. doi: 10.1007/s00383-008-2212-y. [DOI] [PubMed] [Google Scholar]
- [33].Jung Y, Park MS, Lee JW, et al. Cartilage regeneration with highly-elastic three-dimensional scaffolds prepared from biodegradable poly(L-lactide-co-epsilon-caprolactone) Biomaterials. 2008;29(35):4630–4636. doi: 10.1016/j.biomaterials.2008.08.031. [DOI] [PubMed] [Google Scholar]
- [34].Zhang YJ, Jin Y, Nie X, et al. Application study on repairation of sciatic nerve defects with peripheral nerve by tissue engineering. Zhonghua Shenjing Waike Jibing Yanjiu Zazhi. 2004;3(2):141–144. [Google Scholar]
- [35].Esmaeili F, Ghahremani MH, Esmaeili B, et al. PLGA nanoparticles of different surface properties: preparation and evaluation of their body distribution. Int J Pharm. 2008;349(1-2):249–255. doi: 10.1016/j.ijpharm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- [36].Basarkar A, Devineni D, Palaniappan R, et al. Preparation, characterization, cytotoxicity and transfection efficiency of poly(DL-lactide-co-glycolide) and poly(DL-lactic acid) cationic nanoparticles for controlled delivery of plasmid DNA. Int J Pharm. 2007;343(1-2):247–254. doi: 10.1016/j.ijpharm.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Murakami H, Kobayashi M, Takeuchi H, et al. Utilization of poly(DL-lactide-co-glycolide) nanoparticles for preparation of mini-depot tablets by direct compression. J Control Release. 2000;67(1):29–36. doi: 10.1016/s0168-3659(99)00288-6. [DOI] [PubMed] [Google Scholar]
- [38].Chung TW, Wang SS, Tsai WJ. Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials. 2008;29(2):228–237. doi: 10.1016/j.biomaterials.2007.09.027. [DOI] [PubMed] [Google Scholar]
- [39].Zhang HL, Chen ZQ, Li YM, et al. Study on cell compatibility of electrospun PLGA/HA composite scaffolds. Harbin Yiyao. 2011;31(5):321–322. [Google Scholar]
- [40].Liu GY, Zhang Q, Jin Y, et al. Autologous nerve anastomosis versus human amniotic membrane anastomosis A rheological comparison following simulated sciatic nerve injury. Neural Regen Res. 2011;6(31):2424–2428. [Google Scholar]


