Abstract
Aims/hypothesis
We aimed to study the relationship between measures of adiposity, insulin sensitivity and N-terminal pro-B-type natriuretic peptide (NT-proBNP) in the Diabetes Prevention Program (DPP).
Methods
The DPP is a completed clinical trial. Using stored samples from this resource, we measured BMI, waist circumference (WC), an insulin sensitivity index (ISI; [1/HOMA-IR]) and NT-proBNP at baseline and at 2 years of follow-up in participants randomised to placebo (n=692), intensive lifestyle intervention (n=832) or metformin (n=887).
Results
At baseline, log NT-proBNP did not differ between treatment arms and was correlated with baseline log ISI (p<0.0001) and WC (p=0.0003) but not with BMI (p=0.39). After 2 years of treatment, BMI decreased in the lifestyle and metformin groups (both p<0.0001); WC decreased in all three groups (p<0.05 for all); and log ISI increased in the lifestyle and metformin groups (both p<0.001). The change in log NT-proBNP did not differ in the lifestyle or metformin group vs the placebo group (p>0.05 for both). In regression models, the change in log NT-proBNP was positively associated with the change in log ISI (p<0.005) in all three study groups after adjusting for changes in BMI and WC, but was not associated with the change in BMI or WC after adjusting for changes in log ISI.
Conclusion/interpretation
Circulating NT-proBNP was associated with a measure of insulin sensitivity before and during preventive interventions for type 2 diabetes in the DPP. This relationship persisted after adjustment for measures of adiposity and was consistent regardless of whether a participant was treated with placebo, intensive lifestyle intervention or metformin.
Keywords: Diabetes prevention, Insulin sensitivity, Natriuretic peptides, NT-proBNP, Obesity
Introduction
Natriuretic peptides are cardiac-derived hormones that influence ventricular remodelling, salt and water homeostasis, and vascular tone [1]. Multiple epidemiological studies have demonstrated low concentrations of atrial natriuretic peptide and B-type natriuretic peptide (BNP) in obese [1, 2] and insulin-resistant individuals [3], as well as in individuals who later developed type 2 diabetes [4, 5]. Understanding whether natriuretic peptides are primarily associated with insulin resistance or obesity in individuals at high risk of type 2 diabetes may elucidate further the metabolic role of these biomarkers in the development of the disease.
The Diabetes Prevention Program (DPP) was a randomised clinical trial of lifestyle and pharmacological interventions to prevent diabetes in high-risk, overweight or obese individuals [6]. The main results of the DPP, published in 2002, were that both an intensive lifestyle intervention and metformin lowered body weight, insulin resistance and diabetes incidence relative to placebo [7]. The availability of stored samples from the DPP and random allocation to placebo or clinical interventions provided an opportunity to examine the association between N-terminal pro-B-type natriuretic peptide (NT-proBNP; as a representative natriuretic peptide), obesity and insulin sensitivity prior to and during preventive therapies in a population at high risk of developing type 2 diabetes.
Methods
The DPP Research Group conducted a randomised clinical trial from 1996 to 2001 at 27 centres in the United States; full inclusion and exclusion criteria and study design issues have been published [6]. The institutional review board at each centre approved the protocol, and all participants gave written informed consent.
Measurements
Measurements were performed at baseline and at 2 years of follow-up. Previously aliquoted serum samples stored in the DPP repository at −80°C since collection during the DPP trial were thawed, spun and centrifuged for 5 min at 1500 rpm. NT-proBNP concentrations were measured using a cobas e601 immunoanalyser and proBNP II assay (both Roche Diagnostics, Indianapolis, IN, USA) in the Biomarker Research Laboratory/TIMI Clinical Trials Laboratory at the Brigham and Women’s Hospital. NT-proBNP concentrations measured at less than the limit of detection (5 pg/ml, affecting 187 samples at baseline, 12 samples at year 1 and 146 samples at year 2) were assigned a random number between 0 and 5 pg/ml. NT-proBNP values were loge-transformed (log NT-proBNP) to approximate a normal distribution.
Laboratory measurements for fasting plasma glucose and fasting insulin were performed by glucokinase and radioimmunoassay methods, respectively, during the DPP study [6, 8]. An insulin sensitivity index (ISI) was derived by the reciprocal of HOMA-IR: (1/HOMA-IR = 22.5/[fasting glucose (mmol/l) × fasting insulin (pmol/L)]). The ISI was calculated at baseline and follow-up, and was loge-transformed (log ISI) to approximate a normal distribution.
Statistical analysis
Due to prior sample use for other biomarker studies in the DPP, the percentage of stored samples missing for the NT-proBNP measurement varied across the treatment groups (for baseline samples: placebo 30%, intensive lifestyle 15%, metformin 11%). Thus, to correct for potential biases introduced by the non-random missingness of samples, an inverse probability weighting (IPW) method was used in appropriate analyses [9]. Statistics with IPW are weighted statistics with the weight being the inverse of the stratum-specific sampling fraction. A combination of age categories (24–44 years, 45–60 years and >60 years), sex, diabetic status at the end of the DPP, and treatment group were used as sampling strata for the baseline and change analysis. Due to a different number of samples at baseline and follow-up, two sets of stratum-specific weights were used to provide estimates of NT-proBNP at the two time points. The primary study results of treatment effect were restored after using the IPW, demonstrating the effectiveness of the method. In the data analyses of this manuscript, results with or without the IPW are not drastically different.
General linear models were used to examine the association between change in log NT-proBNP and change in both log ISI and a measure of adiposity (BMI or WC). These multivariate models were adjusted for age, sex, self-reported race and ethnicity, and for baseline log NT-proBNP, baseline log ISI, and baseline BMI or baseline WC.
All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA). Two-sided p values are reported and statistical significance was set at α=0.05.
Results
Baseline characteristics of the 3,234 participants who enrolled in the DPP study are given in Table 1. NT-proBNP at baseline was similar for directly measured and estimated values using IPW for each study arm, respectively (median [interquartile range] in pg/ml): placebo (33.4 [17.1–62.3] vs 32.8 [16.7–59.1]); intensive lifestyle intervention (30.9 [14.0–59.9] vs 30.4 [13.9–57.5]); metformin (29.7 [14.0–54.2] vs 29.5 [14.1–53.6]). Baseline log NT-proBNP was correlated with baseline log ISI (+0.17, p<0.0001) and WC (−0.063, p=0.0003) but not with baseline BMI (−0.015, p=0.39).
Table 1.
Baseline characteristics of the DPP participants
| Characteristic | Placebo | Intensive lifestyle intervention | Metformin |
|---|---|---|---|
| n | 1,082 | 1,079 | 1,073 |
| Age, years | 49.5 (43.1–57.3) | 49.7 (42.2–58.5) | 50.4 (43.9–57.3) |
| Female, n (%) | 747 (69.0) | 734 (68.0) | 710 (66.2) |
| Ethnicity, n (%) | |||
| White | 586 (54.2) | 580 (53.8) | 602 (56.1) |
| African-American | 220 (20.3) | 204 (18.9) | 221 (20.6) |
| Hispanic | 168 (15.5) | 178 (16.5) | 162 (15.1) |
| American Indian | 59 (5.5) | 60 (5.6) | 52 (4.9) |
| Asian | 49 (4.5) | 57 (5.3) | 36 (3.4) |
| BMI, kg/m2 | 33.2 (28.9–37.7) | 32.6 (28.9–37.2) | 32.8 (29.1–37.3) |
| WC, cm | 104.0 (94.8–114.0) | 103.3 (94.8–113.4) | 104.0 (94.5–113.3) |
| ISI | 0.16 (0.11–0.24) | 0.17 (0.11–0.24) | 0.16 ( 0.11–0.24) |
| NT-proBNP, pg/mL | 33.4 (17.1–62.3)a | 30.9 (14.0–59.9)b | 29.7 (14.0–54.2)c |
Numbers are median (interquartile range) for continuous measures and n (%) for categorical measures
Baseline NT-proBNP measured in 762 participants
Baseline NT-proBNP measured in 916 participants
Baseline NT-proBNP measured in 959 participants
Baseline NT-proBNP measured in 583 participants
All other numbers are from the original DPP randomisation
BMI (in kg/m2) decreased in the intensive lifestyle and metformin groups and did not change in the placebo group (−1.93 ± 0.09, p<0.0001; −0.74 ± 0.07, p<0.0001; −0.00 ± 0.06, p=1.00, respectively). WC (in cm) decreased in the intensive lifestyle, metformin and placebo groups (−5.55 ± 0.23; −2.12 ± 0.22; −0.79 ± 0.21; all p<0.0001, respectively). Log ISI (in log units) increased from baseline in the intensive lifestyle and metformin groups but not in the placebo group (+0.21 ± 0.02, p<0.0001; 0.11 ± 0.02, p<0.0001; −0.041 ± 0.02, p=0.51, respectively). The changes in log ISI, BMI and WC were all significantly different in the intensive lifestyle or metformin group vs the placebo group (all comparisons p<0.001).
Follow-up clinical information was available for 3,015 DPP participants. The median change in NT-proBNP for each treatment group was similar whether measured directly or estimated using IPW, respectively: intensive lifestyle intervention (3.19 pg/ml, n=832; 3.06 pg/ml, n=1079); metformin (1.45 pg/ml, n=887; 1.50 pg/ml, n=1073); placebo (1.33 pg/ml, n=642; 1.16 pg/ml, n=1082). These changes in NT-proBNP, using IPW, represented increases of 16.1%, 6.9% and 7.3% from baseline in the intensive lifestyle, metformin and placebo groups, respectively. However, the change in log NT-proBNP in the intensive lifestyle or metformin group did not differ from the change in the placebo group (p=0.16 and p=0.46, respectively).
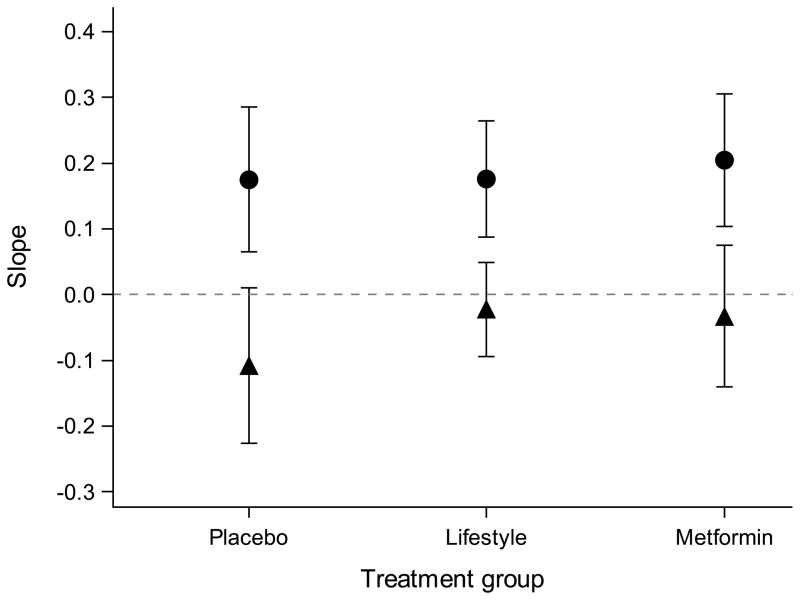
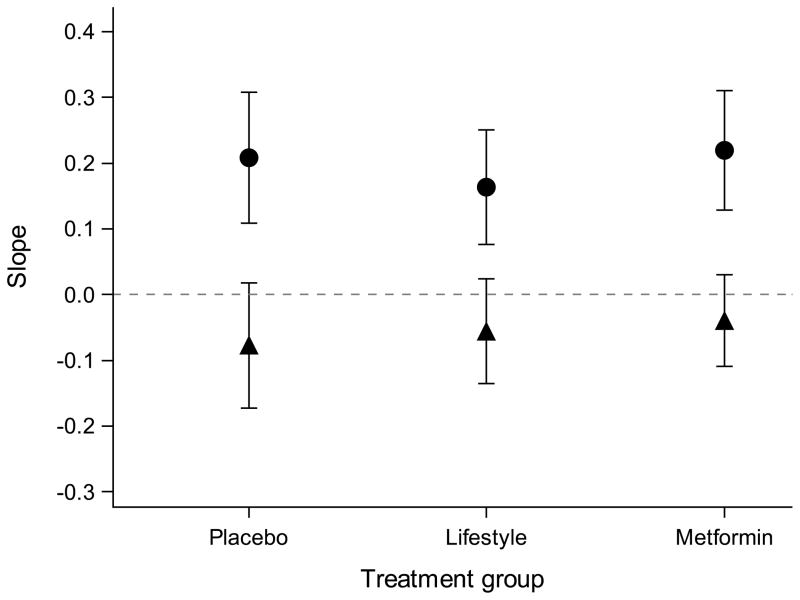
In statistical models adjusted for baseline traits and change in BMI or WC at follow-up, the change in log NT-proBNP was associated with the change in log ISI for the placebo, intensive lifestyle and metformin groups (p<0.005 for all three groups; Fig. 1). In the same models adjusted for change in log ISI, the change in log NT-proBNP was not associated with the change in BMI or WC in any of the treatment groups (p>0.05 for all; Fig. 1). The point estimate of the relationship between log NT-proBNP and log ISI, BMI or WC from multivariate models was similar across all study arms and did not differ between the placebo group and the intensive lifestyle or metformin group (p>0.05 for all).
Fig. 1.
Modelling change in log of NT-proBNP from baseline to follow-up by change in log of ISI and change in BMI or change in WC. (a) The relationship (linear regression slopes and 95% CI) between change in log NT-proBNP and change in BMI (black circles) or log ISI (black triangles). (b) The relationship (linear regression slopes and 95% CI) between change in log NT-proBNP and change in WC (black circles) or log ISI (black triangles). The point estimates are similar across all treatment groups, suggesting that the relationship between change in log NT-proBNP and change in BMI, WC or log ISI are consistent regardless of the intervention. The 95% CIs for the relationship between change in NT-proBNP and BMI or WC crosses 0 in all treatment groups, demonstrating no statistically significant relationship. Predictors (change in log ISI, BMI and WC) are scaled by their SDs in all regression models. All models were adjusted for age; sex; ethnicity; and baseline log NT-proBNP, log ISI and BMI or WC
Discussion
The present study is the first investigation of the natriuretic peptide system performed in the context of a large-scale diabetes prevention trial. While change in NT-proBNP was not a pre-specified endpoint of the DPP, we were able to use stored samples for our analyses. Our principal finding is that NT-proBNP was associated with insulin sensitivity at baseline and during preventive interventions for type 2 diabetes. This relationship persisted after adjustment for measures of obesity and was consistent regardless of whether a participant was treated with placebo, intensive lifestyle intervention or metformin. Together, these results suggest that NT-proBNP is a robust marker of insulin sensitivity and is independent of measures of obesity or preventive interventions for type 2 diabetes. Strengths of this study include the large sample size; the well-phenotyped, multi-ethnic cohort; the randomised allocation of lifestyle and pharmacological interventions; the inclusion of a placebo group; and the standardised ascertainment of NT-proBNP on stored samples at baseline and follow-up using a well-validated assay.
Limitations of our study also deserve comment. First, we did not measure natriuretic peptides other than NT-proBNP in the DPP samples. We chose to measure NT-proBNP because it is more stable in plasma and less influenced by acute haemodynamic changes compared with mature BNP. Second, NT-proBNP was not a pre-specified endpoint of the DPP and measurements were made on available samples, which themselves had non-random proportions of missingness per intervention arm. Thus, statistical methods were needed to estimate NT-proBNP within the intended randomised clinical trial design of the DPP. Third, we did not have longitudinal measures of cardiac ejection fraction or other haemodynamic measures. While rates of congestive heart failure and oedema were not increased in the active treatment groups [10], this does not specifically exclude subclinical changes in cardiac function or fluid retention nor other variables unaccounted for in our models that may have influenced NT-proBNP concentrations.
In summary, NT-proBNP was associated with measures of insulin sensitivity before and during preventive interventions for type 2 diabetes in the DPP. Higher concentrations of NT-proBNP occurred in the context of greater insulin sensitivity. Given the potentially favourable effects of natriuretic peptide signalling on metabolism, prospective studies are warranted to assess whether directly altering BNP concentrations might influence metabolic risk.
Supplementary Material
Acknowledgments
The investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) provided funding to the clinical centres and the coordinating centre for the design and conduct of the study, and for the collection, management, analysis and interpretation of the data. The Southwestern American Indian centres were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources supported data collection at many of the clinical centres. Funding for data collection and participant support was also provided by the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, the Office of Research on Women’s Health, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the Intramural Research Program of the NIDDK. LifeScan, Health O Meter, Hoechst Marion Roussel, Merck-Medco Managed Care, Merck, Nike Sports Marketing, Slim Fast Foods and Quaker Oats donated materials, equipment or medicines for concomitant conditions. McKesson BioServices, Matthews Media Group and the Henry M. Jackson Foundation provided support services under subcontract with the coordinating centre.
The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. A complete list of centres, investigators and staff can be found in the ESM.
Funding
GAW received support from NIH training grant DK007028, from the Scholars in Clinical Science Program of Harvard Catalyst—The Harvard Clinical and Translational Science Center (award no. UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic healthcare centres), and the American Diabetes Association Merck Clinical and Translational Post-doctoral Fellowship. R01-HL-08675 (TJW) supported the natriuretic peptide measurements. JCF is supported by R01 DK072041 from NIDDK.
Abbreviations
- BNP
B-type natriuretic peptide
- DPP
Diabetes Prevention Program
- ISI
Insulin sensitivity index
- IPW
Inverse probability weighting
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- WC
Waist circumference
Footnotes
Contribution statement
All authors made substantial contributions to the manuscript and gave final approval of the version to be published. GAW, YM, JCF and TJW conceived the design, interpreted the data, drafted and revised the manuscript. CAC, RG, PJ, EH, KM, EB-C and JD interpreted the data and reviewed and revised the article for critical content. GAW is responsible for the integrity of the work as a whole.
Duality of interest
TJW has received assay or research support from Diasorin, Singulex, Siemens, Brahms and Critical Diagnostics, as well as consulting fees or honoraria from Diasorin, Singulex and Roche Diagnostics. PJ has received Grant/Research Support from Abbott, AstraZeneca, Beckman Coulter, Daiichi Sankyo, GlaxoSmithKline, Merck, Roche Diagnostics, Takeda, and Waters Technologies and Salary/Consulting fees from T2 Biosystems and Quanterix. All other authors declare that they have no duality of interest in relation to the study.
References
- 1.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 2.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–1353. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 4.Magnusson M, Jujic A, Hedblad B, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmö Diet and Cancer Study. The Journal of Clinical Endocrinology and Metabolism. 2012;97:638–645. doi: 10.1210/jc.2011-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazo M, Young JH, Brancati FL, et al. NH2-terminal pro-brain natriuretic peptide and risk of diabetes. Diabetes. 2013;62:3189–3193. doi: 10.2337/db13-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care. 2000;23:1619–1629. doi: 10.2337/diacare.23.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller WA. Regression analysis for sample survey. Sankhyā. 1975;37:117–132. [Google Scholar]
- 10.The Diabetes Prevention Program Research Group. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–1156. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.