Abstract
IMPORTANCE
Human transmission of bovine spongiform encephalopathy causes the fatal neurodegenerative condition variant Creutzfeldt-Jakob disease (vCJD) and, based on recent human prevalence studies, significant subclinical prion infection of the UK population. To date, all clinical cases have been fatal, totaling 228 mostly young adults residing in the United Kingdom.
OBSERVATIONS
Here we describe the investigation and case history of a patient recently diagnosed as having vCJD in the United Kingdom. Although his presentation, imaging findings, cerebrospinal fluid investigation results, and clinical progression were typical of other cases, tonsillar biopsy and subsequent examination of multiple tissues at autopsy showed minimal deposition of disease-associated prion protein in peripheral lymphoreticular tissue. The result of a blood test for vCJD, the Direct Detection Assay for vCJD, was negative.
CONCLUSIONS AND RELEVANCE
These findings suggest that some patients with vCJD have very low peripheral prion colonization and therefore may not have detectable prion deposition in diagnostic tonsillar biopsy or markers of prion infection in blood. These results have implications for accurate interpretation of diagnostic tests and prevalence studies based on lymphoreticular tissue or blood.
Prion diseases are a group of fatal neurodegenerative disorders caused by the seeded polymerization of cellular prion protein (PrPC) into abnormal forms designated PrPSc.1 While most cases are sporadic or inherited by PrP gene mutation, the most striking epidemiological feature of these conditions is the occurrence of outbreaks or epidemics of acquired prion disease related to zoonoses or medical accidents. The transmission of the cattle prion disease, bovine spongiform encephalopathy, to humans as variant Creutzfeldt-Jakob disease (vCJD) is a recent example of this phenomenon, which devastated the British livestock industry and caused a public health crisis because the eventual size of the human epidemic was impossible to accurately predict.2
While the incidence of vCJD has thankfully been in decline for more than a decade, the United Kingdom and other European countries still face concerns about the disease for several reasons. First, incubation times to human prion disease can be remarkably long, more than 50 years for some patients with kuru, an acquired prion disease of the Papua New Guinean highlanders, and more than 35 years for some patients with disease acquired from contaminated cadaver-derived human growth hormone.3 Second, prions have been uniformly found in peripheral lymphoreticular tissue of patients with vCJD4-6 and vCJD prions may also be present in blood; the disease has now been transmitted by blood or blood products in at least 5 cases.7 Lymphoreticular deposition of PrP allows a pathological antemortem diagnosis of vCJD by tonsillar biopsy and, in our experience, has shown 100% sensitivity and specificity.4-7 Third, anonymous studies8 of discarded surgical lymphoreticular tissues have shown that the infection rate in the healthy UK population appears to be surprisingly high at approximately 1:2000. The proportion of such individuals who will go on to develop clinical vCJD, rather than remaining as asymptomatic carriers, is unknown, although studies of subclinical carrier states in animal models suggest that such carriers, where infecting bovine prions have induced human prion propagation, would pose a risk for transmitting a lethal infection to other humans if they were blood or organ donors or if their tissues contaminated surgical or medical instruments.9
The possibility of a screening blood test to help minimize such secondary transmissions came closer with the publication in 2011 of a prototype test with 71% sensitivity in a blinded study of vCJD.10 Sensitivity is an important issue if the test is to be used to detect subclinical infection for which no valid test samples are available. It remains possible that a subset of patients with vCJD has very low or undetectable prionaemia and poses little risk for public health and could not be detected by blood-based assays. Here we present a recent patient with a clinical diagnosis of vCJD who showed extremely low lymphoreticular deposition of prion protein, a finding that has altered our protocols for tonsillar biopsy at the National Prion Clinic and has implications for the interpretation of ongoing prevalence studies and interpretation of the sensitivity of experimental blood-based assays.
Report of a Case
A 36-year-old man lost his job owing to restructuring at his employer’s company. He developed low mood and did not make plans for alternative employment. He became abnormally repetitive in behavior and conversation. A month later, his walking became unsteady, and he developed choreiform arm movements. He was treated with antidepressants without effect. There was no history of treatment with growth hormone, corneal or dura mater grafting, or neurosurgery, and he had not required a blood transfusion or blood products. There was no family history of neuropsychiatric disease. Five months from onset of the mood disturbance, he scored only 10 of 30 on the Mini-Mental State Examination. On focal testing results, there were evident problems with episodic memory, orientation, comprehension, and frontal executive function. His speech was mildly slurred but fluent. On neurological examination, he had a choreoathetoid movement disorder. He was unable to sustain either finger grip or tongue protrusion. There was an upgaze paresis and abnormal saccadic movements. He was markedly dyspraxic. Muscle tone and power and tendon reflexes were normal. Coordination was very poor with gross finger nose ataxia, dysdiadochokinesis, and a very unsteady gait.
The patient’s parents provided written informed consent. Ethical approval was obtained from the National Hospital for Neurology and Neurosurgery Research Ethics Committee.
Brain magnetic resonance imaging was performed on 2 occasions and each showed the pulvinar sign consistent with vCJD. No mutations were detected on sequencing of PRNP; the codon 129 genotype was methionine homozygous (the genotype seen in all neuropathologically confirmed cases of vCJD). Electroencephalography and cerebrospinal fluid examinations did not suggest alternative diagnoses. The result of the Direct Detection Assay for vCJD infection in blood was negative. The clinical findings were compatible with vCJD and investigations were strongly supportive of this diagnosis; he met World Health Organization criteria for probable vCJD. After full discussion, the patient’s family wished for a definitive diagnosis during life and tonsillar biopsy was done.
For immunohistochemistry, tissues were fixed in 10% formalin for 24 hours (biopsy tissue) or 1 week (autopsy tissues), followed by 1-hour immersion into 98% formic acid and 24-hour postfixation in formalin and dehydration in graded alcohols and paraffin embedding. Paraffin sections of 5-μm thickness were prepared and sections were stained with anti-PrP monoclonal antibody KG9 (1:1000; IAH, TSE Resource Centre) and anti-PrP monoclonal antibody ICSM35 (1:1000 of 100 μg/mL; D-Gen), respectively. The tonsil biopsy was sectioned in levels of 25 μm to detect abnormal PrP.
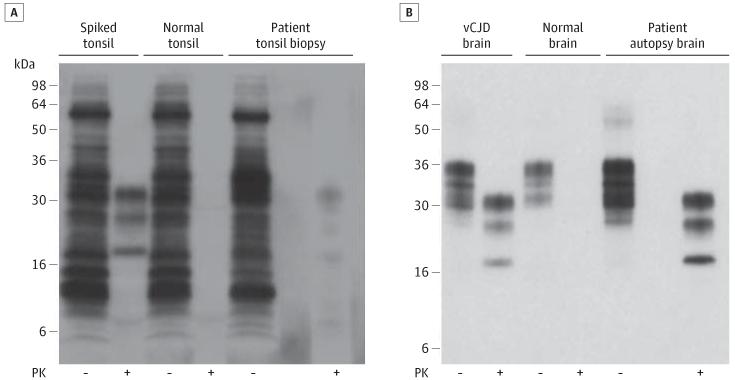
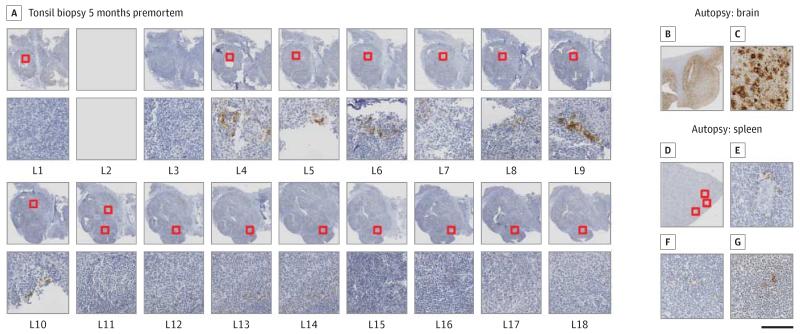
Frozen lymphoid tissue from the tonsil biopsy specimen was examined for the presence of disease-related prion protein by high sensitivity immunoblotting.6 This showed a weak type 4t PrPSc signal, indicative of vCJD,5,6 in 1 tissue homogenate (Figure 1), while another was negative (data not shown). No prion protein was detected by immunohistochemistry on the first 2 routine sections of the tonsil biopsy. We then prepared additional 16 levels from the remaining tissue block. Two separate small clusters of abnormal PrP were detected in levels 4 through 6 and 9. No other positive immunolabeling was detected (Figure 2).
Figure 1. Immunoblot Detection of Disease-Related Prion Protein in the Patient’s Tonsil and Brain.
A, 0.5-mL aliquots of 10% weight/volume (w/v) tonsil biopsy homogenate from the patient with suspected variant Creutzfeldt-Jakob disease (vCJD) or 10% (w/v) normal human tonsil homogenate, either lacking (normal tonsil) or containing a spike of 50-nL 10% (w/v) vCJD brain homogenate (spiked tonsil), were subjected to sodium phosphotungstic acid precipitation.6 20-μL aliquots of whole samples isolated prior to centrifugation were analyzed in the absence of proteinase K (PK) digestion (−) and compared with PK-digestion products derived from the entire sodium phosphotungstic acid pellets (+). B, Aliquots of 10% (w/v) autopsy brain homogenate from the patient, a normal human control case, and a vCJD case were analyzed before (−) or after (+) PK digestion. The immunoblots were developed with anti-prion protein monoclonal antibody 3F4 using high-sensitivity chemiluminescence.
Figure 2. Immunohistochemical Detection of Abnormal Prion Protein (PrP) in the Tonsil Biopsy (A) and the Autopsy Tissues (B-G).
A, Multiple levels of the tonsil were prepared and show abnormal PrP in 2 adjacent, but separate, follicles (levels 4-6 and 9). Both follicles show moderate mechanic artifacts. Stains show an overview (B) and details (C) of the autopsy specimen of the frontal cortex illustrating substantial deposits of abnormal PrP. D, Overview of the spleen section with the 3 follicles containing PrP highlighted. High-power magnification of the same follicles to show the scarcity of PrP deposition in follicular dendritic cells (E-G). The scale bar indicates 2 mm in A; 6 mm in B and D; and 200 μm in C and E-G.
The patient was prescribed doxycycline, 100 mg daily, an experimental therapeutic in prion disease,11 but this did not appear to alter his clinical course of steady deterioration in neurological and functional state. He died 12 months from clinical onset; an autopsy was conducted.
Neuropathological examination demonstrated spongiform vacuolation in all neocortical regions and in the cerebellum with frequent florid plaques and extensive deposition of abnormal PrP characteristic of vCJD. In stark contrast, 7 tissue blocks of the spleen were examined and showed only 3 follicles with abnormal PrP in follicular dendritic cells. Immunoblotting of the brain (frontal cortex) demonstrated the presence of type 4 PrPSc (Figure 2) pathognomic of vCJD.12 High sensitivity immunoblot analyses6 of single-tissue homogenates prepared from the tonsil, appendix, and spleen were scored negative for disease-related prion protein (data not shown).
Discussion
The negative result on first immunohistological examination of the tonsil was surprising and might have prompted the consideration of alternative diagnoses had highly sensitive immunoblotting for PrPSc not been done. Some patients with vCJD may have remarkably small amounts of peripheral PrPSc, a finding which has several implications. Previously, we reported 100% sensitivity and specificity for vCJD diagnosis based on tonsillar biopsy with examination of at least 20 tonsillar follicles.5,13 We now recommend that the entire biopsy specimen or several hundred follicles are assessed before reporting a confident negative finding.
Large prevalence studies in the United Kingdom have been conducted based on abnormal PrP immunohistology in discarded surgical appendix and tonsil tissues.8,13-15 The most recent and precise estimate8 of subclinical infection in the United Kingdom is 1 in 2000. This case report may contribute to the interpretation of these prevalence data because it highlights that even patients with end-stage vCJD may have minimal PrP deposition. Some of the immunohistological abnormalities seen in these studies were not as marked as is typically seen in terminal clinical vCJD cases, prompting concern that these findings may underestimate the prevalence of asymptomatic prion infection. It is reasonable to infer that subclinical infection may be similarly associated with minimal PrP deposition in appendiceal tissue because this is seen in autopsy after the terminal clinical disease.
Conclusions
The appendix prevalence data raise legitimate concerns about the prevalence of prionaemia because a large number of individuals with presumably subclinical vCJD prion infection are blood donors and are undergoing invasive dentistry and surgery. Based on observations of several mammalian species, abnormal PrP immunoreactivity in peripheral lymphoreticular tissue is likely to be an accurate surrogate for prionaemia.16-19 Although there is a clear and pressing need for a donor blood screening assay, a proportion of vCJD-infected individuals may pose lower risks for secondary transmission of prion infection. The Direct Detection Assay result was negative in this case. The published sensitivity of this assay in a blinded series of vCJD blood samples is 71%; therefore, a negative result in a single case is not surprising. Although this test is the only assay proven to detect vCJD infection from clinical cases, there are several other experimental blood tests for vCJD in different stages of development. Our data suggest that it may be impossible to achieve sensitivity approaching 100% using available validation samples because some patients with vCJD may have no or very little prionaemia. Apparently modest sensitivity results in blinded studies of test validity should be interpreted with caution.
Acknowledgments
Funding/Support: The clinical research work was funded by the Medical Research Council and Department of Health (England).
Role of the Sponsor: The Medical Research Council and Department of Health (England) had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Prof Collinge is a director and Drs Wadsworth and Jackson and Prof Collinge are shareholders and consultants of D-Gen Limited, an academic spinout company working in the field of prion disease diagnosis, decontamination, and therapeutics. D-Gen markets the ICSM35 antibody used in this study. Some of the work, including that undertaken as part of the National Prion Monitoring Cohort Study and by Prof Brandner, was funded by the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. No other disclosures were reported.
REFERENCES
- 1.Collinge J. Molecular neurology of prion disease. J Neurol Neurosurg Psychiatry. 2005;76(7):906–919. doi: 10.1136/jnnp.2004.048660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collinge J. Variant Creutzfeldt-Jakob disease. Lancet. 1999;354(9175):317–323. doi: 10.1016/S0140-6736(99)05128-4. [DOI] [PubMed] [Google Scholar]
- 3.Collinge J, Whitfield J, McKintosh E, et al. Kuru in the 21st century: an acquired human prion disease with very long incubation periods. Lancet. 2006;367(9528):2068–2074. doi: 10.1016/S0140-6736(06)68930-7. [DOI] [PubMed] [Google Scholar]
- 4.Hill AF, Zeidler M, Ironside J, Collinge J. Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet. 1997;349(9045):99–100. doi: 10.1016/S0140-6736(97)24002-X. [DOI] [PubMed] [Google Scholar]
- 5.Hill AF, Butterworth RJ, Joiner S, et al. Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet. 1999;353(9148):183–189. doi: 10.1016/s0140-6736(98)12075-5. [DOI] [PubMed] [Google Scholar]
- 6.Wadsworth JD, Joiner S, Hill AF, et al. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358(9277):171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 7.Wroe SJ, Pal S, Siddique D, et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet. 2006;368(9552):2061–2067. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- 8.Gill ON, Spencer Y, Richard-Loendt A, et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey [published online October 15, 2013] BMJ. doi: 10.1136/bmj.f5675. doi:10.1136/bmj.f5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill AF, Joiner S, Linehan J, Desbruslais M, Lantos PL, Collinge J. Species-barrier-independent prion replication in apparently resistant species. Proc Natl Acad Sci U S A. 2000;97(18):10248–10253. doi: 10.1073/pnas.97.18.10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgeworth JA, Farmer M, Sicilia A, et al. Detection of prion infection in variant Creutzfeldt-Jakob disease: a blood-based assay. Lancet. 2011;377(9764):487–493. doi: 10.1016/S0140-6736(10)62308-2. [DOI] [PubMed] [Google Scholar]
- 11.Trevitt CR, Collinge J. A systematic review of prion therapeutics in experimental models. Brain. 2006;129(pt 9):2241–2265. doi: 10.1093/brain/awl150. [DOI] [PubMed] [Google Scholar]
- 12.Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383(6602):685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 13.Frosh A, Smith LC, Jackson CJ, et al. Analysis of 2000 consecutive UK tonsillectomy specimens for disease-related prion protein. Lancet. 2004;364(9441):1260–1262. doi: 10.1016/S0140-6736(04)17143-2. [DOI] [PubMed] [Google Scholar]
- 14.Clewley JP, Kelly CM, Andrews N, et al. Prevalence of disease related prion protein in anonymous tonsil specimens in Britain: cross sectional opportunistic survey. BMJ. 2009;338:b1442. doi: 10.1136/bmj.b1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilton DA, Ghani AC, Conyers L, et al. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol. 2004;203(3):733–739. doi: 10.1002/path.1580. [DOI] [PubMed] [Google Scholar]
- 16.González L, Dagleish MP, Bellworthy SJ, et al. Postmortem diagnosis of preclinical and clinical scrapie in sheep by the detection of disease-associated PrP in their rectal mucosa. Vet Rec. 2006;158(10):325–331. doi: 10.1136/vr.158.10.325. 16531580. [DOI] [PubMed] [Google Scholar]
- 17.McCutcheon S, Alejo Blanco AR, Houston EF, et al. All clinically-relevant blood components transmit prion disease following a single blood transfusion: a sheep model of vCJD. PLoS One. 2011;6(8):e23169. doi: 10.1371/journal.pone.0023169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathiason CK, Powers JG, Dahmes SJ, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314(5796):133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 19.Bishop MT, Diack AB, Ritchie DL, Ironside JW, Will RG, Manson JC. Prion infectivity in the spleen of a PRNP heterozygous individual with subclinical variant Creutzfeldt-Jakob disease. Brain. 2013;136(pt 4):1139–1145. doi: 10.1093/brain/awt032. [DOI] [PMC free article] [PubMed] [Google Scholar]