Abstract
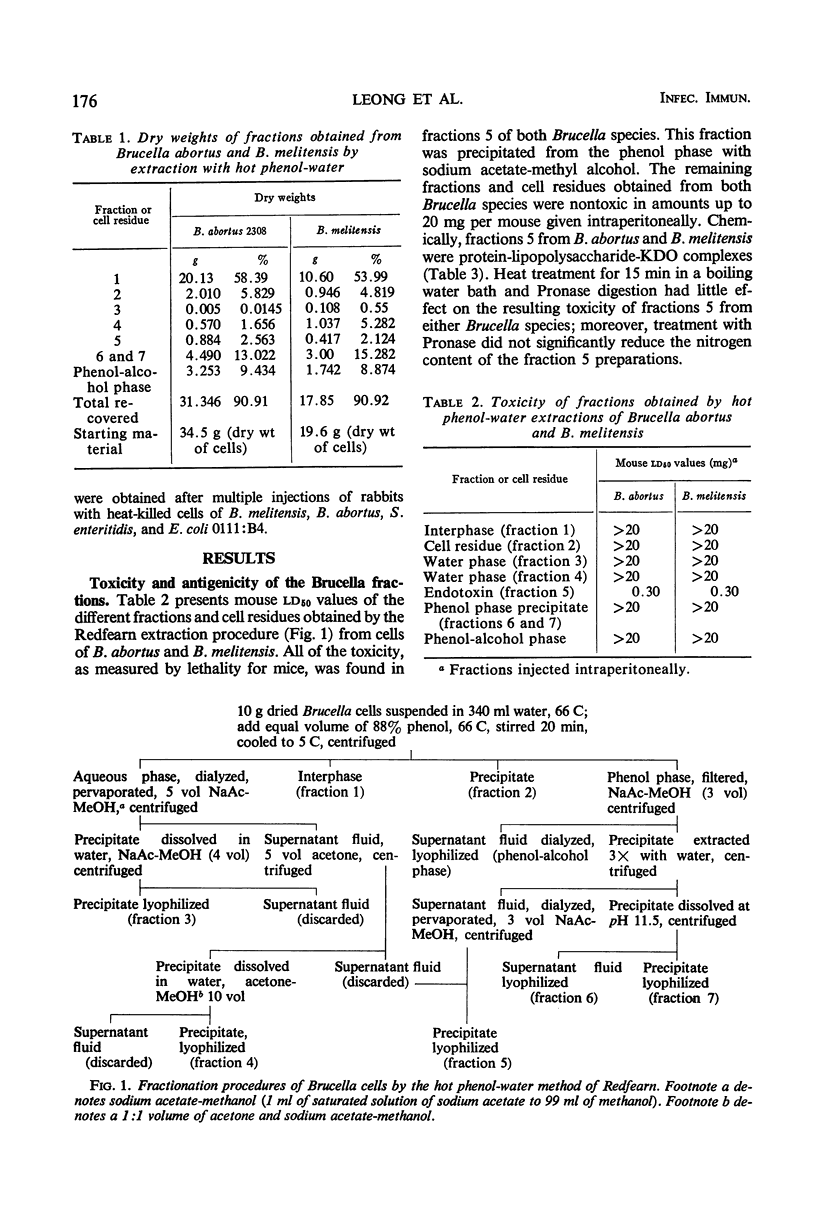
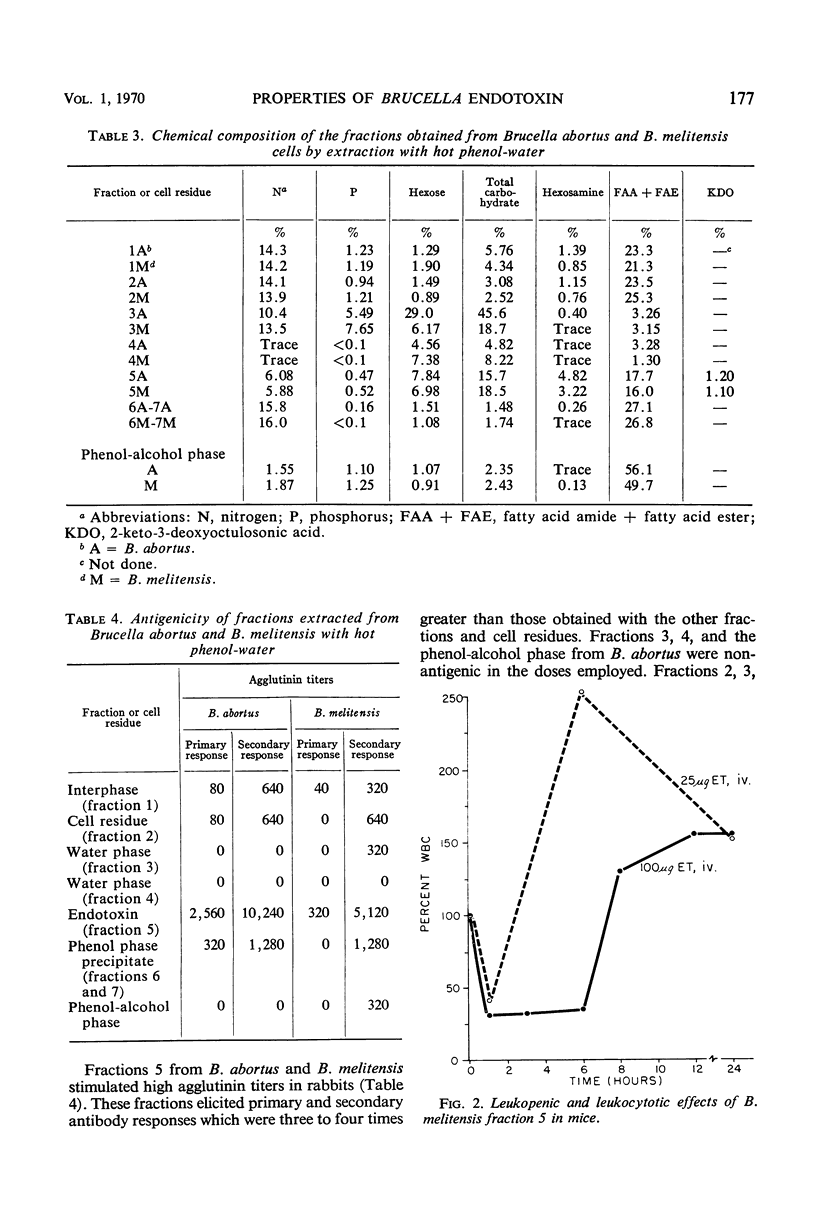
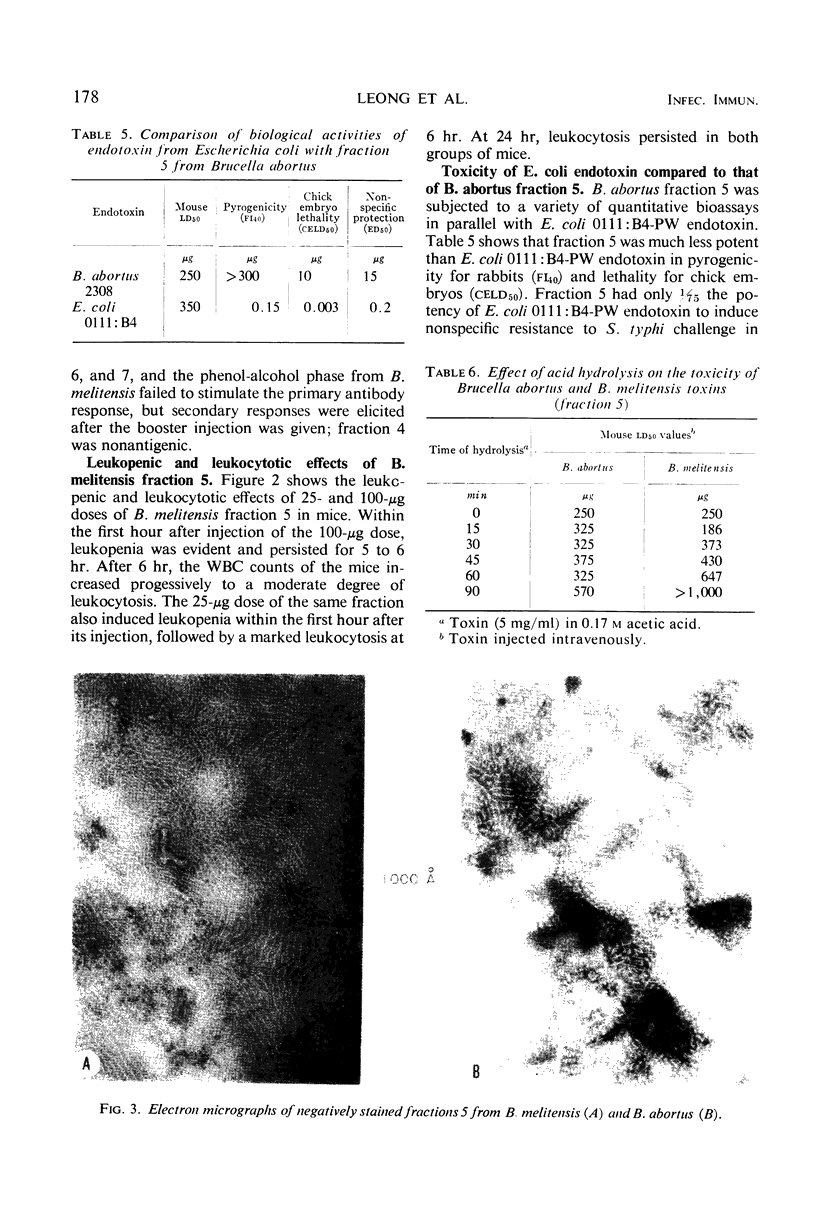
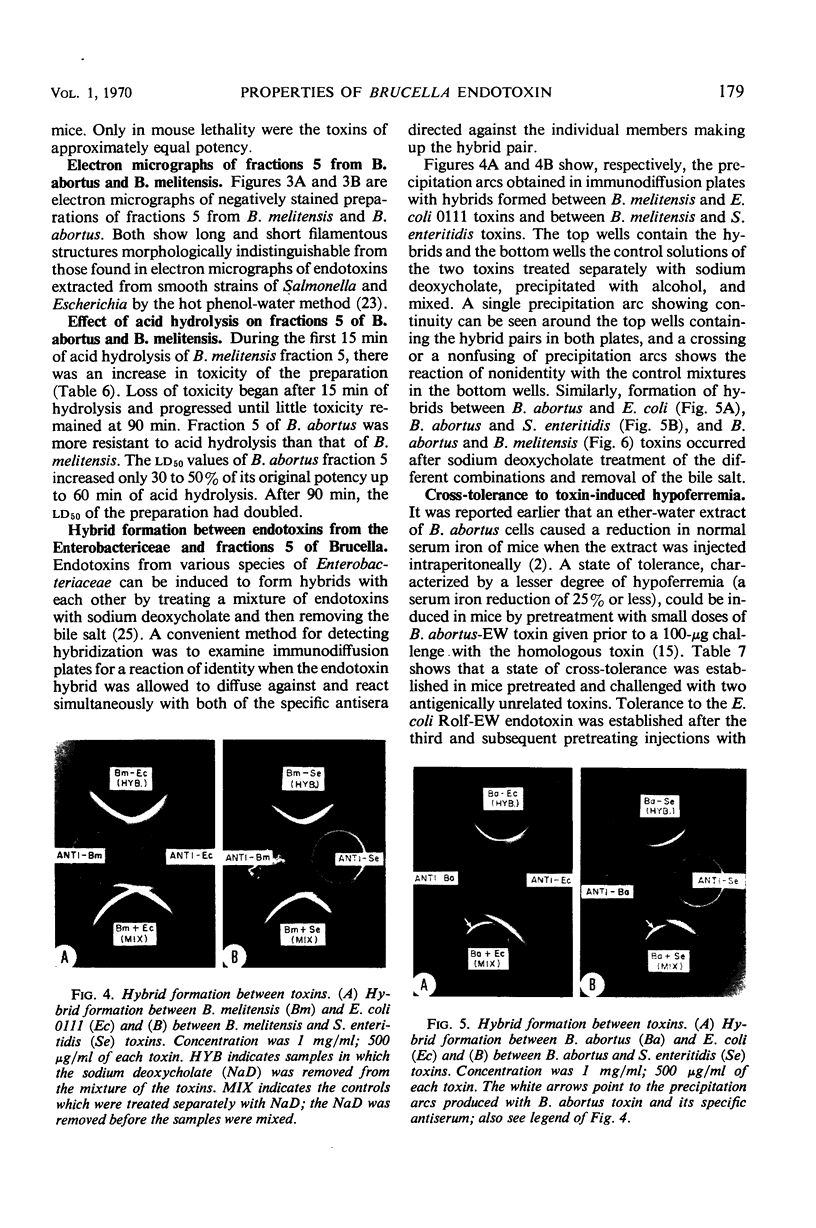
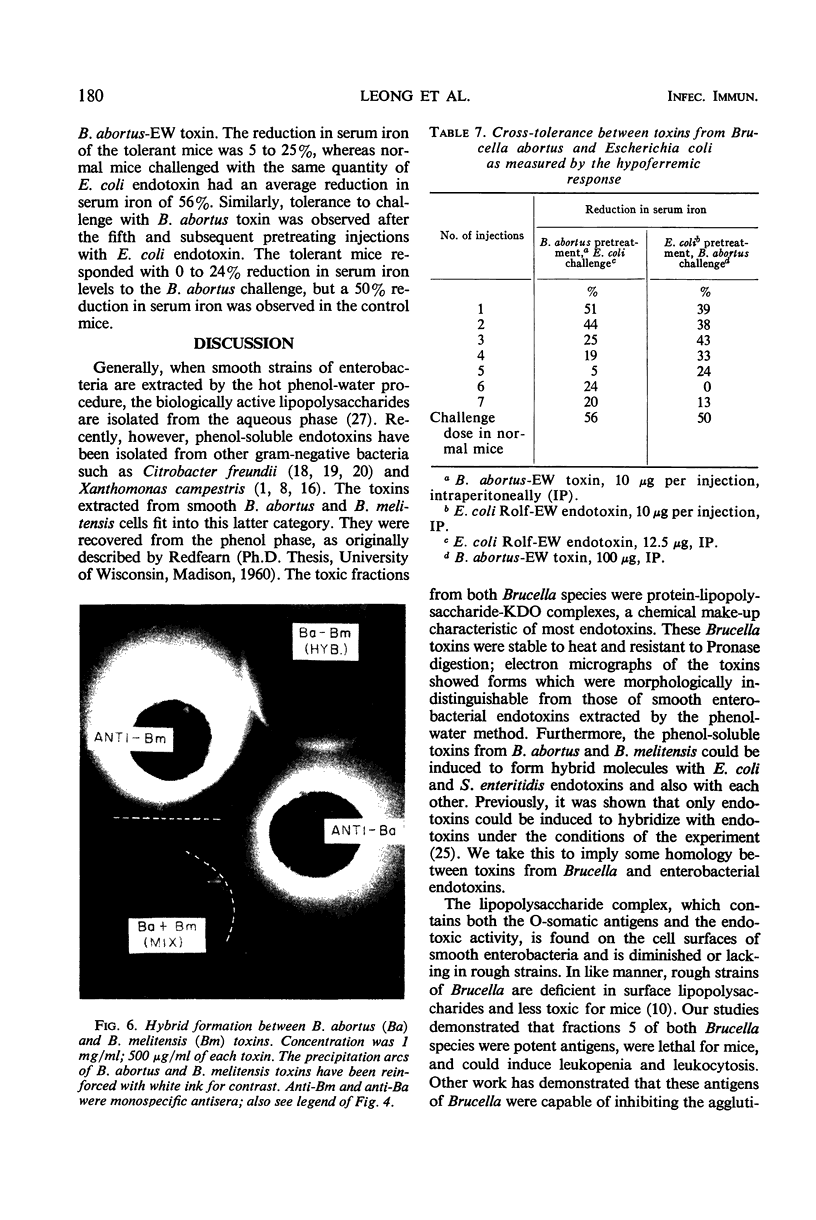
Hot phenol-water extraction of smooth Brucella abortus and B. melitensis cells yielded a toxic fraction which was recovered from the phenol phase (fraction 5). Chemically, fractions 5 from both Brucella species were lipid-carbohydrate-protein-2 keto-3-deoxyoctulosonic acid complexes which were stable to heat and resistant to Pronase digestion. Electron micrographs of the Brucella toxins were morphologically indistinguishable from those of enterobacterial endotoxins. Biologically, Brucella toxins were lethal for mice and immunogenic for rabbits. An intravenous injection of Brucella toxin induced severe leukopenia with subsequent leukocytosis in mice. Cross-tolerance experiments with mice demonstrated that pretreatment with B. abortus toxin lessened the hypoferremia produced by challenge with Escherichia coli endotoxin. Furthermore, fractions 5 from B. abortus and B. melitensis were able to form hybrids with E. coli and Salmonella enteritidis endotoxins and also with each other. Although Brucella toxins possess many structural and biological properties in common with endotoxins from the Enterobacteriaceae, some quantitative differences in their biological potencies were observed. Brucella toxins were relatively innocuous in tests for pyrogenicity in rabbits and lethality for chick embryos. In nonspecific protection tests, Brucella toxin had only 1/75 the potency of E. coli endotoxin in protecting mice against challenge with virulent S. typhi. However, on the basis of the data presented and on the work done previously, we concluded that the heat-stable toxins of B. abortus and B. melitensis were endotoxins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Volk W. A. Isolation and identification of N-acetyl-3-amino-3,6-dideoxy-D-galactose, a cell wall constituent of Xanthomonas campestris. J Biol Chem. 1965 Dec;240(12):4549–4555. [PubMed] [Google Scholar]
- Baker P. J., Wilson J. B. Chemical composition and biological properties of the endotoxin of Brucella abortus. J Bacteriol. 1965 Oct;90(4):895–902. doi: 10.1128/jb.90.4.895-902.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Wilson J. B. Hypoferremia in mice and its application to the bioassay of endotoxin. J Bacteriol. 1965 Oct;90(4):903–910. doi: 10.1128/jb.90.4.903-910.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Leong D., Wilson J. B. Surface antigens of smooth brucellae. J Bacteriol. 1968 Oct;96(4):893–901. doi: 10.1128/jb.96.4.893-901.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Wilson J. B. Antigenic relationship of Brucella ovis and Brucella melitensis. J Bacteriol. 1967 Apr;93(4):1262–1268. doi: 10.1128/jb.93.4.1262-1268.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUSHI K., ANACKER R. L., HASKINS W. T., LANDY M., MILNER K. C., RIBI E. EXTRACTION AND PURIFICATION OF ENDOTOXIN FROM ENTEROBACTERIACEAE: A COMPARISON OF SELECTED METHODS AND SOURCES. J Bacteriol. 1964 Feb;87:391–400. doi: 10.1128/jb.87.2.391-400.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASKINS W. T., LANDY M., MILNER K. C., RIBI E. Biological properties of parent endotoxins and lipoid fractions, with a kinetic study of acid-hydrolyzed endotoxin. J Exp Med. 1961 Nov 1;114:665–684. doi: 10.1084/jem.114.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J., Ashwell G. Isolation of a bacterial lipopolysaccharide from Xanthomonas campestris containing 3-acetamido-3,6-dideoxy-D-galactose and D-rhamnose. J Biol Chem. 1966 Mar 25;241(6):1424–1428. [PubMed] [Google Scholar]
- JONES L. M. A recommended method for the preparation of monospecific Brucella sera. Bull World Health Organ. 1958;19(1):177–186. [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Zanardi M., Leong D., Wilson J. B. Taxonomic position in the genus Brucella of the causative agent of canine abortion. J Bacteriol. 1968 Feb;95(2):625–630. doi: 10.1128/jb.95.2.625-630.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMPSCHMIDT R. F., UPCHURCH H. F. Effects of bacteria endotoxin on plasma iron. Proc Soc Exp Biol Med. 1962 May;110:191–193. doi: 10.3181/00379727-110-27463. [DOI] [PubMed] [Google Scholar]
- Leong D., Diaz R., Wilson J. B. Identification of the toxic component of Brucella abortus endotoxin and its labeling with radioactive chromate. J Bacteriol. 1968 Feb;95(2):612–617. doi: 10.1128/jb.95.2.612-617.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D., Wilson J. B. Distribution of chromate-labeled Brucella abortus endoxin in tolerant mice. J Bacteriol. 1969 Jan;97(1):32–41. doi: 10.1128/jb.97.1.32-41.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Ruschmann E., Westphal O., Raff R., Wheat R. Occurrence of 3-amino-3,6-dideoxyhexoses in Salmonella and related bacteria. J Bacteriol. 1967 May;93(5):1681–1687. doi: 10.1128/jb.93.5.1681-1687.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner K. C., Finkelstein R. A. Bioassay of endotoxin: correlation between pyrogenicity for rabbits and lethality for chick embryos. J Infect Dis. 1966 Dec;116(5):529–536. doi: 10.1093/infdis/116.5.529. [DOI] [PubMed] [Google Scholar]
- RIBI E., HASKINS W. T., LANDY M., MILNER K. C. Preparation and host-reactive properties of endotoxin with low content of nitrogen and lipid. J Exp Med. 1961 Nov 1;114:647–663. doi: 10.1084/jem.114.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIBI E., MILNER K. C., PERRINE T. D. Endotoxic and antigenic fractions from the cell wall of Salmonella enteritidis; methods for separation and some biologic activities. J Immunol. 1959 Jan;82(1):75–84. [PubMed] [Google Scholar]
- Raff R. A., Wheat R. W. Carbohydrate composition of the phenol-soluble lipopolysaccharides of Citrobacter freundii. J Bacteriol. 1968 Jun;95(6):2035–2043. doi: 10.1128/jb.95.6.2035-2043.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff R. A., Wheat R. W. Characterization of 3-amino-3,6-dideoxy-D-glucose from a bacterial lipopolysaccharide. J Biol Chem. 1967 Oct 25;242(20):4610–4613. [PubMed] [Google Scholar]
- Raff R. A., Wheat R. W. Occurrence of a 3-amino sugar in the cell wall of Citrobacter freundii 8090. Biochim Biophys Acta. 1966 Sep 26;127(1):271–273. doi: 10.1016/0304-4165(66)90507-1. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brown R., Haskins W. T., Malmgren B., Milner K. C., Rudbach J. A. Reaction of endotoxin and surfactants. I. Physical and biological properties of endotoxin treated with sodium deoxycholate. J Bacteriol. 1966 Nov;92(5):1493–1509. doi: 10.1128/jb.92.5.1493-1509.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A., Milner K. C., Ribi E. Hybrid formation between bacterial endotoxins. J Exp Med. 1967 Jul 1;126(1):63–79. doi: 10.1084/jem.126.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]







