Abstract
Purpose
Many patients with left-sided breast cancer receive adjuvant radiotherapy during deep-inspiration breath hold (DIBH) to minimize radiation exposure to the heart. We measured the displacement of the left anterior descending artery (LAD) and heart due to cardiac motion during DIBH, relative to standard tangential fields for left breast cancer radiotherapy.
Materials and Methods
20 patients who had undergone CT-based coronary angiography with retrospective electrocardiographic gating were randomly selected for the study. Patients were scanned during DIBH to control the influence of respiration on cardiac motion. Standard medial and lateral tangential fields were placed and LADs contoured on systolic- and diastolic-phase CT datasets by clinicians. Displacement of the LAD during cardiac contractions was calculated in three directions: toward the posterior edge of the treatment fields (TPEF), left-right (LR), and anteroposterior (AP). Displacement of the entire heart was measured on maximum and minimum intensity projection CT images.
Results
The mean displacement of the LAD [average (range)] due to cardiac contraction without the influence of respiration for 20 patients was 2.3 mm (0.7–3.8 mm) TPEF, 2.6 mm (1.0–6.8 mm) LR, and 2.3 mm (0.6–6.5 mm) AP. At least 30% of the LAD volume was displaced more than 5 mm in any direction in 2 patients (10%), and less than 10% of the LAD volume was displaced more than 5 mm in 10 patients (50%). The extent of displacement of heart periphery during cardiac motion was negligible near the treatment fields.
Conclusions
Displacement of heart periphery near treatment fields was negligible during DIBH, but displacement of the LAD due to cardiac contraction varied substantially between and within patients. We recommend maintaining at least 5 mm distance between the LAD and the field edge for patients undergoing breast cancer radiotherapy during DIBH.
Keywords: systolic, diastolic, left anterior descending artery
Introduction
Radiotherapy (RT) is an effective treatment for breast cancer, reducing the risk of both local cancer recurrence and breast cancer death (1, 2). However, several studies have demonstrated increased risks of mortality from ischemic heart disease among patients undergoing RT for breast cancer (3–6). Once the adverse effects of RT on the heart were recognized, several groups evaluated the radiation dose to the heart during RT for breast cancer, especially for tumors of the left breast (7–11). Radiation guidelines have since been modified to minimize cardiac exposure by various means, including placement of RT fields to avoid cardiac exposure. Intensity modulation and various respiratory motion control techniques were also developed to reduce the dose received by the heart during breast RT.
Effective treatment techniques in terms of reducing heart dose depend on understanding the cardiac motion, both intrinsic motion, independent of respiration, and motion resulting from respiration. Deep-inspiration breath-hold (DIBH) technique (i.e. the patient takes a deep inspiration and holds his breath during radiation treatment, which could move heart away from the chest wall) has been used to minimize the dose to the heart during RT for cancer of left breast (12–15); however, its use requires accurate information about cardiac motion so that the safe proximity of the treatment field edge to the displaced heart can be determined and treatment plans can be optimized to minimize cardiac toxicity, particularly that arising from exposure of the left anterior descending artery (LAD). Although many studies have been done evaluating cardiac displacement during respiration (16), few studies have addressed the extent of displacement arising from intrinsic cardiac motion, i.e., movement from cardiac contraction that is independent of respiration. The purpose of this study was to determine the extent of displacement of the entire heart and the LAD resulting from the intrinsic motion of the heart, during DIBH, relative to typical tangential fields for left-sided breast cancer RT. We expect that the information obtained will be useful for further sparing the heart from radiation exposure during DIBH and for accurately estimating the cardiac doses delivered during breast RT using advanced techniques such as three-dimensional conformal RT, gated irradiation, and others.
Methods and Materials
The CT data used for this study were obtained from 20 randomly selected patients, who had undergone computed tomography (CT)–based coronary angiography with retrospective electrocardiographic (ECG) gating at MD Anderson Cancer Center. The study was approved by the institutional review board at MD Anderson. Two sets of CT scans from a GE LightSpeed 64-slice CT (GE Healthcare, Waukesha, WI) were obtained for each patient as per standard of care procedure for CT-based coronary angiography. One was an ECG-gated step-and-shoot scan of 2.5 mm per slice from the apex of the lungs to the adrenals during the diastolic phase of cardiac motion without injection of CT contrast agent; the other was a contrast-enhanced ECG-gated scan of 0.625 mm per slice from the carina to the bottom edge of the heart. The use of contrast facilitated delineation of the heart and LAD; a dual-injection system was used in which injection of 125 mL of Visipaque 320 (Nycomed, Switzerland) was followed by 50 mL of saline chase. Contrast was injected at 5 mL/s and the contrast-enhanced scan was conducted 30 s after the start of contrast injection. During both sets of scans, the patients were in DIBH with both arms raised above their shoulders in a position similar to that used during breast cancer RT. The contrast-enhanced scans were used to reconstruct 10 CT datasets over a single cardiac cycle for each patient.
Using images acquired during DIBH, the maximum (MIP) and the minimum (mIP) intensity projection CT images, which depict the largest and smallest extent of the heart motion, respectively, out of the 10 phases of each contrast-enhanced CT scan were derived (17) for each patient to assess the extent of heart motion without the influence of respiratory motion. The MIP and mIP CT images were superimposed and analyzed on a GE Advantage window workstation, with the measurements obtained on the largest cross-section of the heart on the coronal CT images. The largest vertical and horizontal displacements were measured to document the maximum motion of the heart in both the superior-inferior (SI) and the lateral (left-right, LR) directions. Figure 1 shows the superimposed MIP and mIP scans to illustrate heart displacement.
Figure 1.
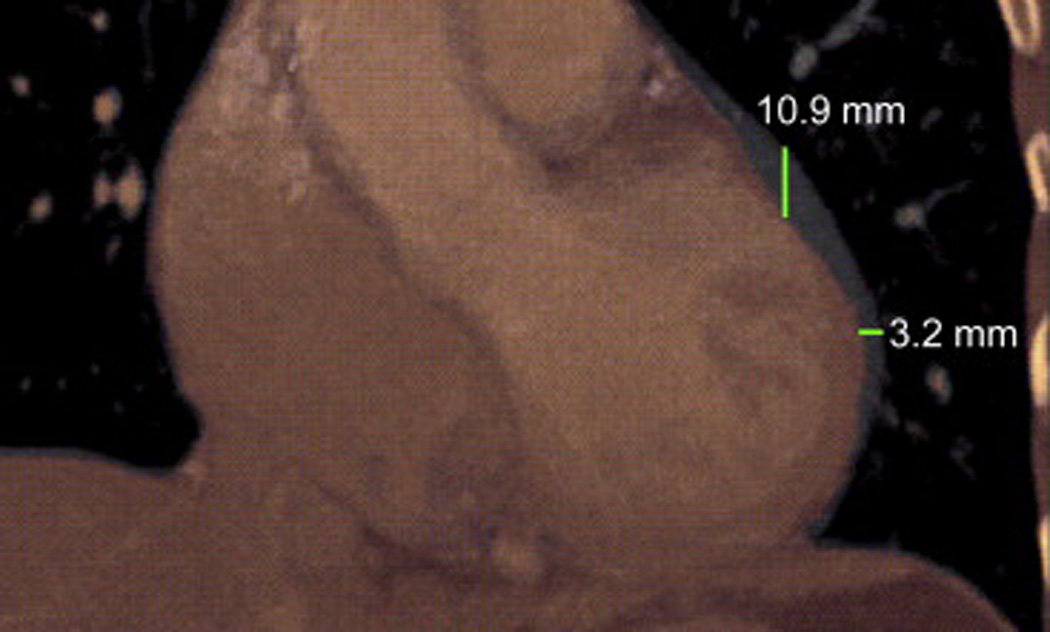
Superimposed minimum intensity projection (mIP, in thermal color) images and maximum intensity projection (MIP, in gray) images show the extent of cardiac motion in the SI and LR directions on a coronal-view slice of the CT images.
Standard medial and lateral tangential fields typically used for whole-breast irradiation of a left-sided tumor were placed on the non-contrast CT datasets (diastolic phase) for each patient. The treatment fields were designed to encompass the left breast tissue with the posterior edge of the fields less than 2 cm deep inside the lung as well. The LAD was delineated by clinicians using the contrast-enhanced CT datasets in both the systolic phase and the diastolic phase; the contrast-enhanced CT scans were registered to the non-contrast CT datasets. The displacement of the LAD was assessed in three directions: towards the posterior edge of the treatment fields (TPEF), in the LR direction, and in the anteroposterior (AP) direction and was calculated as displacement of the center of the LAD contours at each slice between the systolic-phase and diastolic-phase CT images. Figure 2 shows the placement of tangential fields, fused LAD contours for systolic phase and diastolic phase, and the axes illustrating TPEF, LR, and AP on an axial slice of a CT dataset. The labels a and b represent the shortest distance from the center of LAD to the posterior edge of the tangential field in systolic and diastolic phase CT images, respectively. The displacement of the LAD in the TPEF direction between the systolic and diastolic phases was defined as the absolute difference between a and b; displacements in the LR and AP directions between the systolic and diastolic phases were defined similarly.
Figure 2.
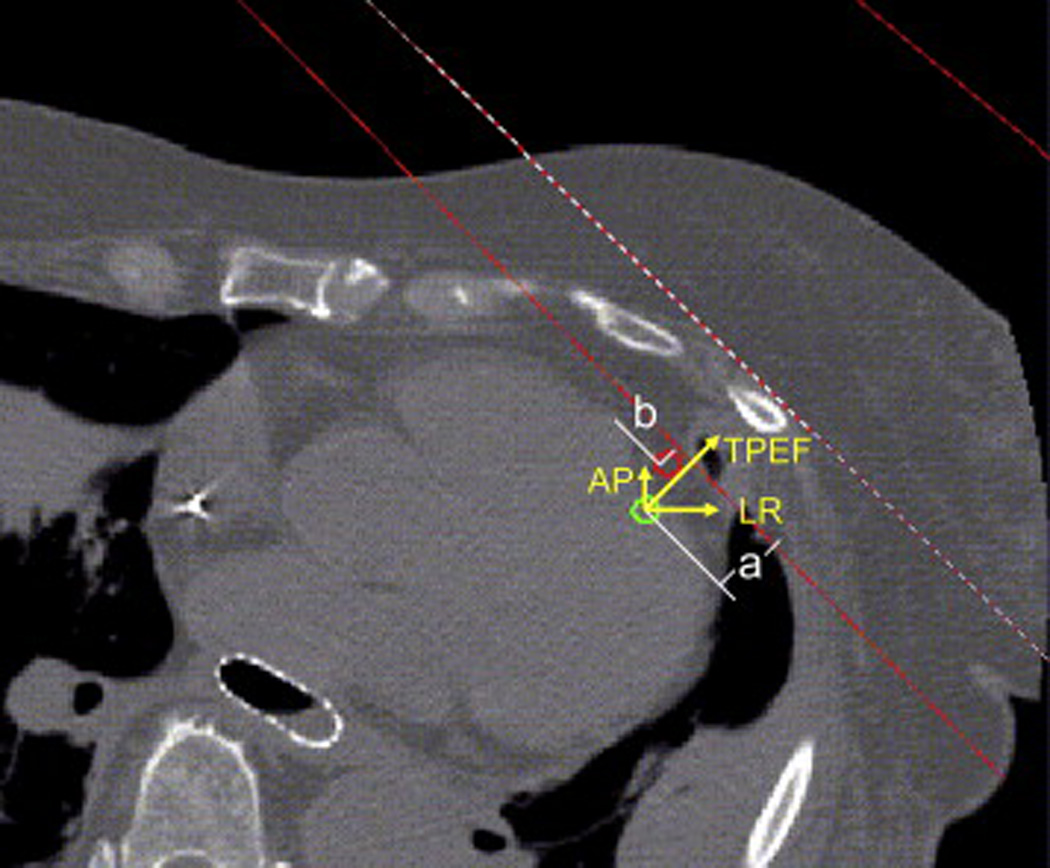
An axial slice of an averaged CT dataset from one patient illustrates the location of the tangential beams (red solid lines), fused LAD contours in the systolic phase (green contour) and the diastolic phase (red contour), and the three directional axes indicating anteroposterior (AP), left-right (LR), and toward the posterior edge of the treatment field (TPEF).
Statistical Analysis
Descriptive statistics (means and ranges) were calculated for the displacement of the entire heart in the SI and LR directions from the MIP and mIP CT images and for the displacement of the LAD in the TPEF, LR, and AP directions during systolic and diastolic motion for all the patients. LAD displacements in the three directions were compared by using two-tailed pairwise Wilcoxon signed-rank tests, with p values less than or equal to 0.05 considered to indicate statistically significant differences.
Results
The extent of heart displacement in the SI and LR directions, calculated from the MIP and mIP CT images from all the patients, is shown in Table 1. The mean displacement in the SI direction was 6.4 mm (range, 3.0–10.9 mm), and the mean displacement in the LR direction was 2.5 mm (range, 0–4.6 mm). The heart moved more than 5 mm in the SI direction in 16 of 20 patients, while movement in the LR direction tended to be less (none of the patients had movement that exceeded 5 mm in the LR direction).
Table 1.
Heart motion in superoinferior and left–right directions calculated from maximal and minimal intensity projection computed tomography images
| Pt. No. | SI (mm) | LR (mm) |
|---|---|---|
| 1 | 7.7 | 0.0 |
| 2 | 3.5 | 2.1 |
| 3 | 7.4 | 3.2 |
| 4 | 6.0 | 2.1 |
| 5 | 10.9 | 3.2 |
| 6 | 8.4 | 3.5 |
| 7 | 10.5 | 4.6 |
| 8 | 6.0 | 3.2 |
| 9 | 5.1 | 3.0 |
| 10 | 5.5 | 2.2 |
| 11 | 5.7 | 2.0 |
| 12 | 3.8 | 0.0 |
| 13 | 6.1 | 1.8 |
| 14 | 8.4 | 3.0 |
| 15 | 3.0 | 3.0 |
| 16 | 4.0 | 1.6 |
| 17 | 5.5 | 2.2 |
| 18 | 5.6 | 3.0 |
| 19 | 7.5 | 4.0 |
| 20 | 7.0 | 3.0 |
| Average | 6.4 | 2.5 |
| Maximum | 10.9 | 4.6 |
| Minimum | 3.0 | 0.0 |
| SD | 2.1 | 1.1 |
Abbreviations: Pt. No. = patient number; SI = superoinferior; LR = left–right; SD = standard deviation.
To further observe the extent of displacement of the cardiac periphery relative to the treatment fields as a function of cardiac motion during DIBH, we superimposed the systolic and diastolic CT images from all 20 patients and found that the major displacement of the periphery occurred in the posterior part of the heart, which is unlikely to affect the heart dose received from RT because of the anterior location of the RT fields. Displacement of the heart periphery on six sequential axial slices of CT from a representative patient, with systolic and diastolic CT images superimposed, is shown in Figure 3.
Figure 3.
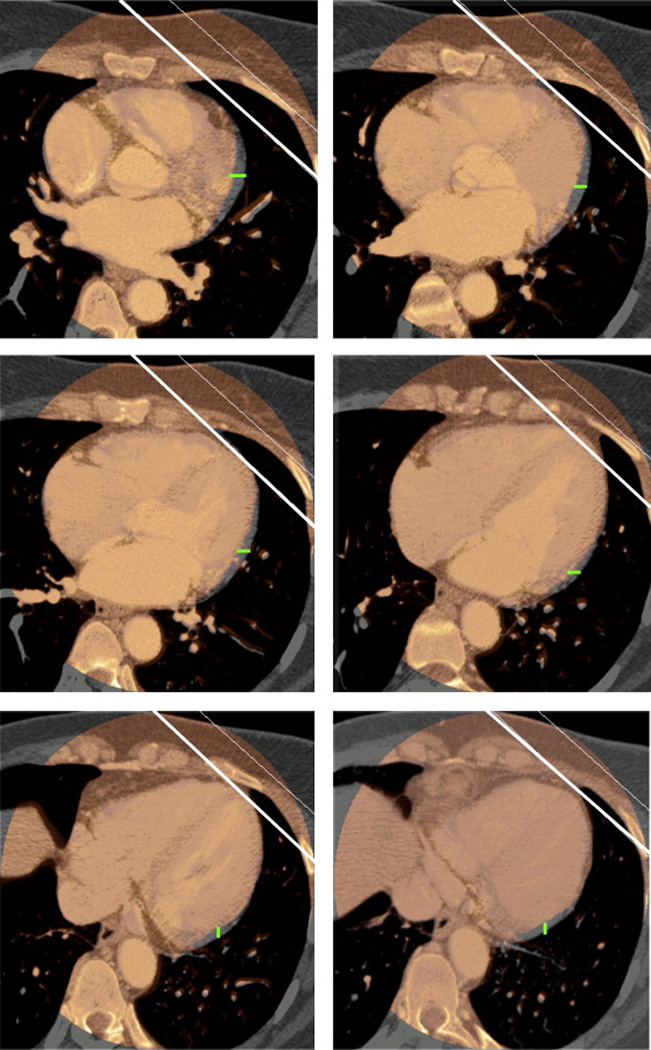
Displacement of the heart periphery relative to the RT treatment fields on six sequential axial CT slices, with superimposition of systolic (in thermal color) and diastolic (in gray) CT images. White lines illustrate the location of the tangential beams. Green lines illustrate the extent of displacement of the heart periphery between systole and diastole.
The extent of displacement of the LAD in three directions between systolic phase and diastolic phase for two patients is shown in Figure 4. Panel A illustrates large displacements in all three directions, with means of 3.8 mm (range 0.1–9 mm) in the TPEF, 4.4 mm (range, 0–10.1 mm) in the LR, and 1.7 mm (range, 0–10.9 mm) in the AP direction. Data from the other patient in panel B showed smaller displacements in all three directions, with means of 0.8 mm (range, 0–2.7 mm) in the TPEF, 1.0 mm (range, 0–3.6 mm) in the LR, and 0.6 mm (range, 0–2.9 mm) in the AP direction.
Figure 4.
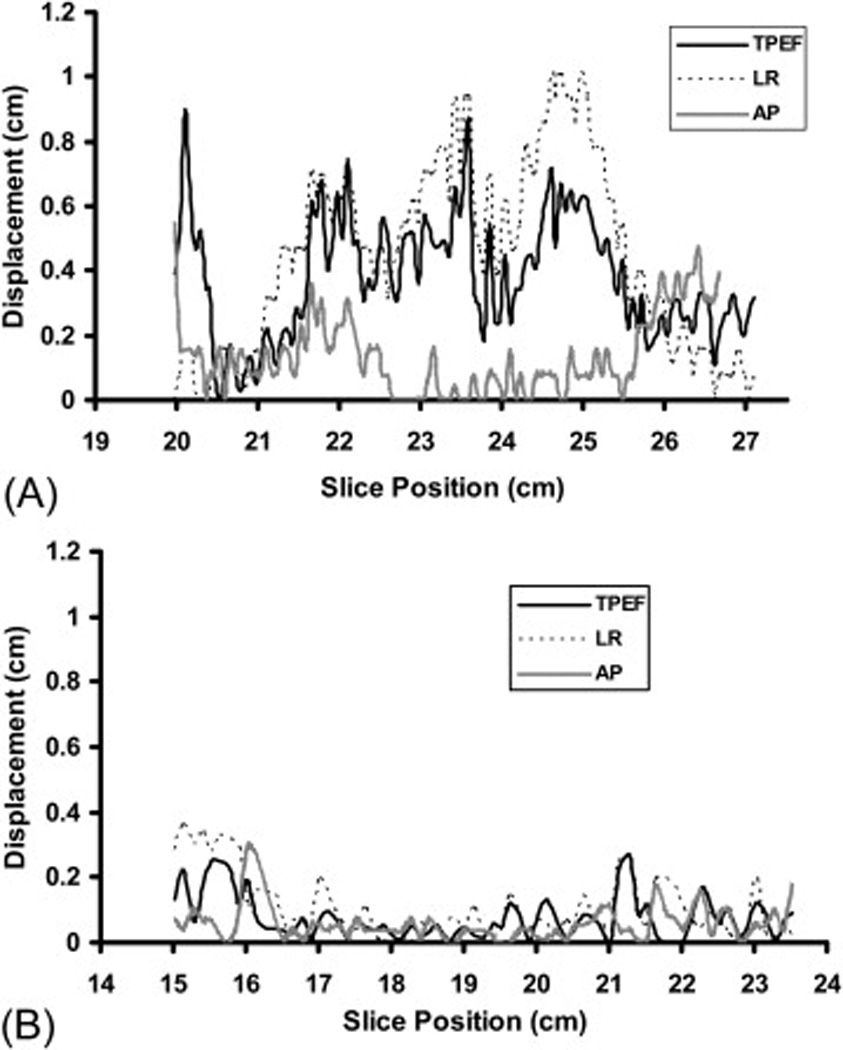
Displacement of LAD during systolic and dystolic phases of the cardiac cycle in three directions (toward the posterior edge of the treatment field [TPEF], left-right [LR], and anterposterior [AP]) on CT slices for a patient with large displacement (Panel A) and for patient with small displacement (Panel B). The × axis is the CT slice position (with smaller number indicating the superior heart and the larger number indicating inferior heart). The y axis is the displacement distance.
The percentages of LAD volume displaced that exceeded certain values (e.g., 2 mm, 3 mm, 4 mm and so on) in three directions for the two patients in Figure 4 are shown in Figure 5. For any displacement value, in any direction, the patient in panel A had the larger percentage of LAD volume displaced relative to the patient in panel B. For example, the patient from panel A had 26%, 39%, and 6% of LAD volume displaced by more than 5 mm in the TPEF, LR, and AP directions respectively; but the patient from panel B had none of LAD volume displaced by more than 5 mm in any direction.
Figure 5.
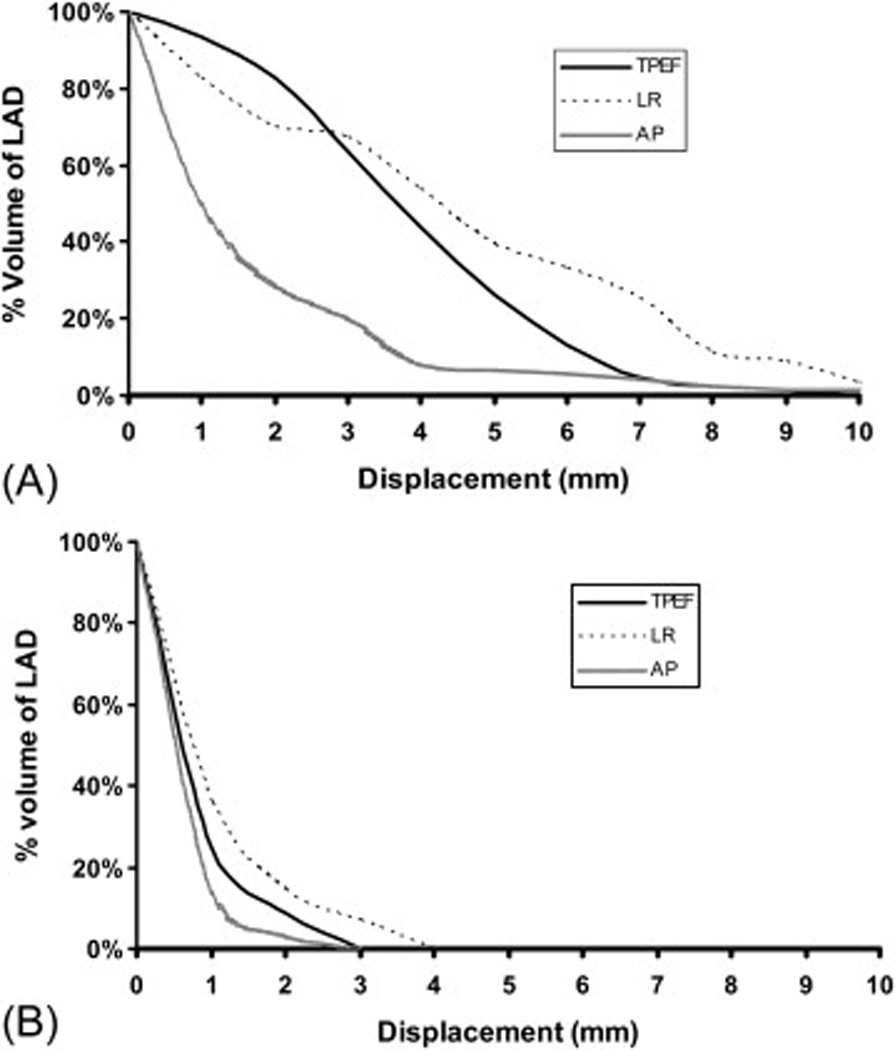
Percentages of LAD volumes (the y axis) exceeding certain set displacement values (e.g., 2 mm, 3 mm, 4 mm, etc; the×axis) in three directions for the two patients from Figure 4.
The extent of displacement of the LAD in the three directions for all 20 patients is shown in Table 2. The average mean displacement of the LAD for all 20 patients was 2.3 mm (range, 0.8–3.9 mm) in the TPEF direction, 2.6 mm (range, 1.0–6.8 mm) in the LR direction, and 2.3 mm (range, 0.6–6.5 mm) in the AP direction. The average maximum displacement of the LAD was 7.4 mm (range, 2.7–13.7 mm) in the TPEF direction, 7.1 mm (range, 3.6–15.5 mm) in the LR direction, and 8.2 mm (range, 2.9–14.8 mm) in the AP direction. No significant differences were found in the extent of LAD displacement between the TPEF, LR, and AP directions, except that the maximum displacement in the AP direction was significantly larger than the maximum displacement in the LR direction (p = 0.04).
Table 2.
Displacement of left anterior descending artery in three directions
| Pt. No. | Mean displacement (mm) | Maximal displacement (mm) | Minimal displacement (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TPEF | LR | AP | TPEF | LR | AP | TPEF | LR | AP | |
| 1 | 0.8 | 1.0 | 0.6 | 2.7 | 3.6 | 2.9 | 0.0 | 0.0 | 0.0 |
| 2 | 1.8 | 1.8 | 1.4 | 5.7 | 5.7 | 8.2 | 0.0 | 0.0 | 0.1 |
| 3 | 2.2 | 1.4 | 3.2 | 10.0 | 5.6 | 9.1 | 0.0 | 0.0 | 0.7 |
| 4 | 2.3 | 2.2 | 1.5 | 9.6 | 8.0 | 9.3 | 0.0 | 0.0 | 0.0 |
| 5 | 3.7 | 3.8 | 2.7 | 11.2 | 11.7 | 10.5 | 0.0 | 0.0 | 0.0 |
| 6 | 1.2 | 1.0 | 1.2 | 3.3 | 4.2 | 3.5 | 0.0 | 0.0 | 0.0 |
| 7 | 3.8 | 4.4 | 1.7 | 9.0 | 10.1 | 10.9 | 0.1 | 0.0 | 0.0 |
| 8 | 2.3 | 3.9 | 3.2 | 6.4 | 15.5 | 7.0 | 0.0 | 0.1 | 0.0 |
| 9 | 1.1 | 2.2 | 1.4 | 3.3 | 8.2 | 6.9 | 0.0 | 0.0 | 0.0 |
| 10 | 1.7 | 2.0 | 1.7 | 4.2 | 3.9 | 6.5 | 0.1 | 0.0 | 0.0 |
| 11 | 3.9 | 3.0 | 2.8 | 9.0 | 6.0 | 7.7 | 0.0 | 0.0 | 0.0 |
| 12 | 2.0 | 2.4 | 2.2 | 5.1 | 6.3 | 4.9 | 0.0 | 0.0 | 0.0 |
| 13 | 1.6 | 2.6 | 2.1 | 4.5 | 6.0 | 10.9 | 0.1 | 0.0 | 0.0 |
| 14 | 3.5 | 6.8 | 4.1 | 13.7 | 12.6 | 14.8 | 0.0 | 2.2 | 0.0 |
| 15 | 3.8 | 2.5 | 6.5 | 9.2 | 4.9 | 9.9 | 0.9 | 0.0 | 4.2 |
| 16 | 1.3 | 1.6 | 1.5 | 4.7 | 4.2 | 7.7 | 0.0 | 0.0 | 0.0 |
| 17 | 2.8 | 2.5 | 2.6 | 13.4 | 8.4 | 10.5 | 0.0 | 0.0 | 0.0 |
| 18 | 1.7 | 2.9 | 1.4 | 4.3 | 7.4 | 5.2 | 0.3 | 0.7 | 0.0 |
| 19 | 2.1 | 2.4 | 2.4 | 8.4 | 5.6 | 9.1 | 0.0 | 0.0 | 0.0 |
| 20 | 2.0 | 1.4 | 2.0 | 9.5 | 4.9 | 9.1 | 0.0 | 0.0 | 0.0 |
| Average | 2.3 | 2.6 | 2.3 | 7.4 | 7.1 | 8.2 | 0.1 | 0.2 | 0.3 |
| Minimum | 0.8 | 1.0 | 0.6 | 2.7 | 3.6 | 2.9 | 0.0 | 0.0 | 0.0 |
| Maximum | 3.9 | 6.8 | 6.5 | 13.7 | 15.5 | 14.8 | 0.9 | 2.2 | 4.2 |
| p | |||||||||
| TPEF vs. LR .189 | .701 | ||||||||
| TPEF vs. AP .964 | .07 | ||||||||
| TPEF vs. AP .076 | .037 | ||||||||
Abbreviations: Pt. No. = patient number; TPEF = toward posterior edge of treatment fields; LR = left–right; AP = anteroposterior.
Figure 6 shows the number of patients who had at least 10%, 20%, 30% and 50% of the LAD volume displaced by a certain specified value (e.g., 1 mm, 2 mm, 3 mm, and so on). Half (10) of the 20 patients had at least 10%, 20%, 30%, and 50% of the LAD volume displaced by more than 4 mm, 3 mm, 2 mm, and 2 mm respectively in the TPEF direction; 5 mm, 3 mm, 3 mm, and 2 mm respectively in the LR direction; and 4 mm, 3 mm, 2 mm, and 1 mm respectively in the AP direction. The numbers of patients who had at least 10%, 20%, 30% and 50% of the LAD volume displaced by more than 5 mm were 6, 5, 2, and 0 respectively in the TPEF direction; 10, 4, 3, and 1 respectively in the LR direction; and 8, 3, 2, and 1 respectively in the AP direction.
Figure 6.
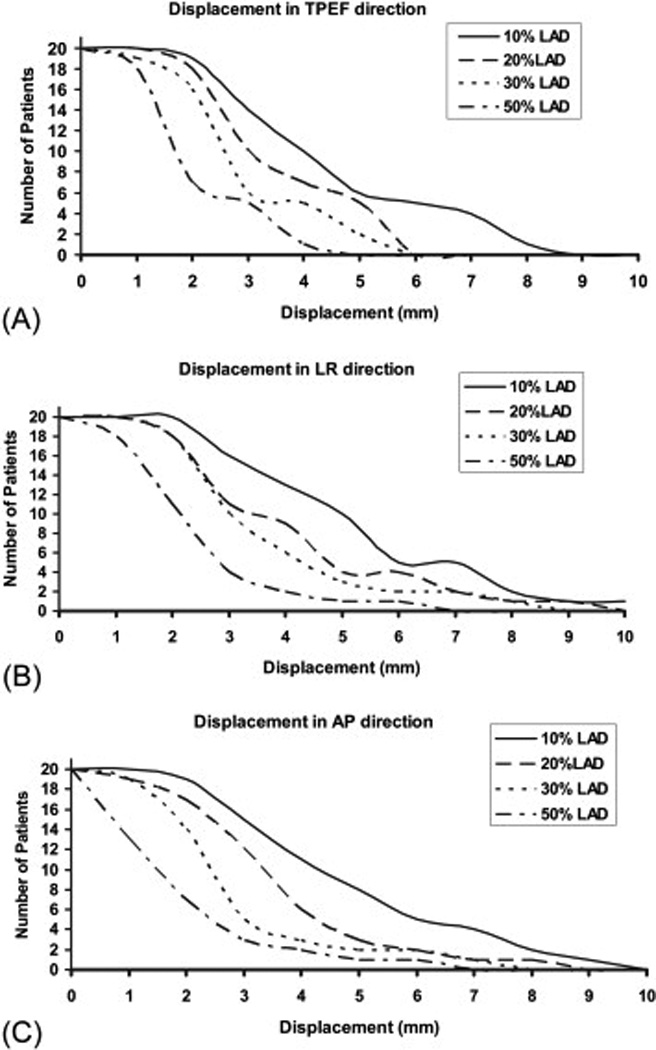
Numbers of patients (the y axis) who had at least 10%, 20%, 30% and 50% of the LAD volume displaced by a certain set value (e.g., 1 mm, 2 mm, 3 mm, etc; the × axis) in the (A) toward the posterior edge of the treatment field (TPEF), (B) left-right (LR), and (C) anteroposterior (AP) directions.
Discussion
DIBH is an effective method for limiting the motion of the heart caused by respiration and also for maximizing the distance between the LAD and the treatment fields for RT of the left breast. Several studies have demonstrated that DIBH can displace the heart posterior from the chest wall and reduce the heart dose without compromising the dose to the target (12–15). However, little information is available about the extent of heart movement, particularly of the LAD, resulting from cardiac activity without the influence of respiratory motion. Damage to the LAD, which supplies blood to critical regions of the myocardium, is a common cause of myocardial infarction and may be responsible for the increase in risk of heart disease after RT for breast cancer, particularly tumors in the left breast. In this study, we quantified heart motion in three dimensions in the absence of respiratory motion (i.e., while the subjects were in DIBH) by using MIP and mIP CT images. We also quantified the displacement of the LAD accurately visualized by CT angiography relative to typical treatment fields. This study provides useful information about intrinsic cardiac motion that could be applied clinically during RT for left breast cancer while subjects are in DIBH.
Although the extent of displacement of the entire heart owing to cardiac motion was notable, the largest displacements involved mainly the posterior portion of the heart, which was far away from the RT fields. Thus we conclude that the design of breast RT fields is unlikely to be affected by displacement of the heart periphery owing to cardiac motion. However, movement of the LAD during cardiac motion could affect the dose it receives during RT. Motion of the LAD in the SI direction is unlikely to affect the dose it receives from RT because of the quasi-linear shape of the LAD and its roughly longitudinal orientation along the orientation of treatment fields. Thus we chose to focus on displacement of the LAD in the TPEF, LR, and AP directions. We found substantial differences among patients in the extent of LAD displacement, ranging from 10 mm in one patient to 2 mm in another (Fig. 4). Displacement of different portions of the LAD during cardiac motion also varied within the same patient; for example, displacement of the superior and inferior portions of the LAD for the patient in Figure 4A was about 3 mm and displacement of the mid-portion was about 7 mm. Moreover, even though the average mean displacement of LAD in any direction was small (< 3 mm), the average maximum displacement of LAD in any direction was large (> 7 mm). These findings emphasize the substantial variance in displacement of the LAD both within and among patients.
Although displacement of the LAD varied substantially among patients, our results showed that about 10% of patients had at least 30% of LAD volume displaced by more than 5 mm in any direction during cardiac motion. This finding underscores the importance of protecting the LAD in designing breast RT fields, even when DIBH is to be used.
The displacement of LAD toward the edge of the treatment field (i.e., in the TPEF direction) is directly relevant to clinical practice because it provides a basis for estimating the margins necessary to avoid the LAD moving into the treatment fields for breast RT during a cardiac cycle. Displacement in the TPEF direction is related to the displacements in the LR and AP directions. When the treatment field is rotated to cover more lateral breast tissue, displacement of the LAD in the LR direction becomes increasingly important. Similarly, as the treatment fields are rotated to cover more medial portions of the breast, displacement of the LAD in the AP direction becomes more important. We found no significant differences in the extent of LAD displacement in any of these three directions.
The possible safe margin to protect LAD when designing radiation treatment field can be derived from the data shown in Figure 6, which showed that half of the patients had less than 10% of LAD volume move by more than 5 mm. We recommend a margin of at least 5 mm between the LAD and the posterior edge of the planned treatment field when the posterior edge of the treatment fields must be positioned close to the LAD. Because dose-volume response data for the LAD are generally lacking, this recommendation may need further assessment.
It could be argued that any movement of the LAD is sufficiently far from the treatment fields to have only minimal effects from the radiation. In this study, we placed the treatment fields so as to encompass the left breast tissue as much as possible and make the posterior edge of the fields less than 2 cm deep inside the lung as well. Even so, we found that in seven of the 20 patients studied, portions of the LAD were either inside the treatment fields in both cardiac phases or moved into the treatment field from one cardiac motion phase to the other. In general, the superior and inferior portions of the LAD were further from the beam edge than were the middle portion of the LAD. Moreover, the extent to which use of DIBH can spare the heart depends on the location of the target and the patient’s anatomy. For targets located in the lower pole of the left breast, where the heart is close to the chest wall, DIBH may not suffice to push the heart out of the treatment field. Some patients are also unable to take and hold a breath large enough to displace the heart from the chest wall. For those patients, use of DIBH techniques limits the motion of the heart resulting from respiration but does not increase the distance between the heart and the treatment fields. When the posterior edge of the treatment fields must be positioned close to the LAD, we recommend a margin of at least 5 mm between the LAD and the posterior edge of the planned treatment field (as discussed above), even when the DIBH technique is to be used with the goal of completely excluding the LAD from the field at all times.
As mentioned in previous sections, the patients in this study were randomly selected from those who underwent CT-based angiography, and they might not be diagnosed with breast cancer. Any potential differences in lung and cardiac function between the patients in this study and those with breast cancer patients may influence the result of this study. In addition, significant differences of radiation treatment position from this study may also influence the result of the study.
Conclusions
The most substantial displacement of the heart periphery during cardiac motion was at the posterior part of the heart, far from RT fields and unlikely to be affected by RT. Displacement of the LAD owing to cardiac motion during DIBH varied substantially both between and within patients, which could significantly affect the dose received by the LAD from breast RT. When the posterior edge of the treatment fields must be positioned close to the LAD, we recommend maintaining a distance of at least 5 mm between the LAD and the field edge when possible to do so with adequate clinical target volume coverage.
Footnotes
Conflicts of Interest Notification: The authors declare no conflicts of interest
References
- 1.Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. Early Breast Cancer Trialists' Collaborative Group. N Engl J Med. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 2.Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 3.Abe O, Abe R, Enomoto K, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease after radiotherapy for early breast cancer. Radiation Research. 2007;167:349–349. [Google Scholar]
- 5.Gyenes G, Rutqvist LE, Liedberg A, et al. Long-term cardiac morbidity and mortality in a randomized trial of pre- and postoperative radiation therapy versus surgery alone in primary breast cancer. Radiotherapy and Oncology. 1998;48:185–190. doi: 10.1016/s0167-8140(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 6.Harris EER, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. Journal of Clinical Oncology. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 7.Cho BCJ, Schwarz M, Mijnheer BJ, et al. Simplified intensity-modulated radiotherapy using pre-defined segments to reduce cardiac complications in left-sided breast cancer. Radiotherapy and Oncology. 2004;70:231–241. doi: 10.1016/j.radonc.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Fuller SA, Haybittle JL, Smith REA, et al. CARDIAC DOSES IN POSTOPERATIVE BREAST IRRADIATION. Radiotherapy and Oncology. 1992;25:19–24. doi: 10.1016/0167-8140(92)90190-6. [DOI] [PubMed] [Google Scholar]
- 9.Landau D, Adams EJ, Webb S, et al. Cardiac avoidance in breast radiotherapy: a comparison of simple shielding techniques with intensity-modulated radiotherapy. Radiotherapy and Oncology. 2001;60:247–255. doi: 10.1016/s0167-8140(01)00374-7. [DOI] [PubMed] [Google Scholar]
- 10.Taylor CW, McGale P, Povall JM, et al. Estimating cardiac exposure from breast cancer radiotherapy in clinical practice. International Journal of Radiation Oncology Biology Physics. 2009;73:1061–1068. doi: 10.1016/j.ijrobp.2008.05.066. [DOI] [PubMed] [Google Scholar]
- 11.Taylor CW, Povall JM, McGale P, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. International Journal of Radiation Oncology Biology Physics. 2008;72:501–507. doi: 10.1016/j.ijrobp.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 12.Korreman SS, Pedersen AN, Aarup LR, et al. Reduction of cardiac and pulmonary complication probabilities after breathing adapted radiotherapy for breast cancer. International Journal of Radiation Oncology Biology Physics. 2006;65:1375–1380. doi: 10.1016/j.ijrobp.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 13.Remouchamps VM, Vicini FA, Sharpe MB, et al. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. International Journal of Radiation Oncology Biology Physics. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 14.Sixel KE, Aznar MC, Ung YC. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. International Journal of Radiation Oncology Biology Physics. 2001;49:199–204. doi: 10.1016/s0360-3016(00)01455-3. [DOI] [PubMed] [Google Scholar]
- 15.Stranzl H, Zurl B. Postoperative irradiation of left-sided breast cancer patients and cardiac toxicity. Strahlentherapie Und Onkologie. 2008;184:354–358. doi: 10.1007/s00066-008-1852-0. [DOI] [PubMed] [Google Scholar]
- 16.Jagsi R, Moran JM, Kessler ML, et al. Respiratory motion of the heart and positional reproducibility under active breathing control. International Journal of Radiation Oncology Biology Physics. 2007;68:253–258. doi: 10.1016/j.ijrobp.2006.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan T, Sun XJ, Luo D. Improvement of the cine-CT based 4D-CT imaging. Medical Physics. 2007;34:4499–4503. doi: 10.1118/1.2794225. [DOI] [PubMed] [Google Scholar]


