Abstract
We aimed to assess age-related differences in compensatory hypoxic vasodilation during moderate-to-high dynamic exercise at absolute workloads. We hypothesized healthy older adults (n = 12, 61 ± 1 years) would exhibit impaired hypoxic vasodilation at a moderate absolute workload, and this effect would be exaggerated at a higher workload when compared to young adults (n = 17, 27 ± 2 years). Forearm blood flow (FBF) was measured with Doppler ultrasound. Dynamic forearm exercise (20 contractions/min) was completed at two absolute workloads (8 and 12 kg) under normoxic (0.21 FiO2, ~98% SpO2) and isocapnic hypoxic (~0.10 FiO2, 80% SpO2) conditions performed in random order. FBF was normalized as forearm vascular conductance (FBF / mean arterial blood pressure = FVC) to control for differences in blood pressure and to assess vasodilation. FVC increased with exercise and hypoxia (main effects, p < 0.05); vascular responses were not different between young and older adults (interaction effect exercise × group p = 0.37 and hypoxia × group p = 0.96). Results were confirmed when analyzed as either an absolute or relative change in FVC (ΔFVC and %ΔFVC, respectively). Although group responses to hypoxia were not different, individual results were highly variable (i.e., some adults constricted and others dilated to hypoxia). These data suggest (1) compensatory hypoxic vasodilation in older adults is not impaired during forearm exercise at both moderate and higher absolute exercise intensities, and (2) vascular responses to hypoxia are heterogeneous in both young and older adults. Results suggest unique individual differences exist in factors regulating vascular responses to hypoxia.
Keywords: Aging, Functional hyperemia, Blood flow
Introduction
Oxygen demands of skeletal muscle during exercise are met with a rise in blood flow to the working tissue. Impaired exercise blood flow is thought to be partially responsible for reduced exercise tolerance and functional capacity in older adults and clinical populations (Conley et al. 2000; Fleg et al. 2005; Poole et al. 2003). Normoxic exercise hyperemia is impaired in the leg of older adults (Koch et al. 2005; Proctor et al. 1998) but evidence in the forearm is conflicting; some studies show preserved forearm exercise hyperemia in older adults (Dinenno et al. 2005; Donato et al. 2006; Jasperse et al. 1994; Wray et al. 2010) while others show an impairment (Kirby et al. 2009).
In response to the stress of hypoxia during exercise, a compensatory vasodilation maintains oxygen delivery despite lowered hemoglobin saturation (DeLorey et al. 2004; Rowell et al. 1986; Wilkins et al. 2006, 2008). The mechanisms behind this increase in blood flow appear to be intensity-specific in young adults (Casey et al. 2010; Wilkins et al. 2008), however, much less is known about responses in older adults. Recent evidence from Casey et al. 2011 suggests older adults exhibit preserved compensatory hypoxic vasodilation during low intensity forearm exercise (10% maximal voluntary contraction, MVC), but responses are impaired at a moderate intensity (20% MVC).
The present investigation sought to strengthen and expand the understanding of vascular responses to hypoxia in aging. Such research has relevance to older populations with sleep apnea or those active in high altitude environments. Additionally, poor hypoxic vasodilation could impact ischemic events such as stroke or heart attack. We hypothesized blood flow responses to hypoxic exercise would be reduced in older adults at a moderate absolute exercise intensity and this impaired vasodilatory response would be exaggerated at a higher workload. These studies are warranted given conflicting blood flow results observed in older adults during forearm exercise under normoxic conditions and a solitary study indicating an intensity-specific impact of aging on hypoxic vasodilation.
Materials and methods
Subjects
Both younger (19–34 years) and older (56–67 years) men and women participated in the current study. Subjects were recreationally active, healthy, and free from known cardiovascular disease as judged from medical history, resting blood pressure measurements and fasting lipid and glucose levels. According to self-report, all subjects were non-smokers and were not taking any cardiovascular medications (i.e., ACE inhibitors, β-blockers, statins, etc.). Female subjects were not pregnant and young women were studied during the early follicular/placebo phase of the menstrual cycle (days 1–5; oral contraception was allowed, n = 4) to minimize any potential influences of female hormones (Minson et al. 2000). Older female subjects were post-menopausal and one was taking hormone replacement therapy (results were maintained when she was excluded, therefore her data were included in the final analysis).
Written informed consent was obtained from all subjects. All procedures were approved by the Institutional Review Board at the University of Wisconsin, Madison and conformed to the standards set by the Declaration of Helsinki. Subjects were instructed to refrain from exercise, NSAIDS, alcohol, and caffeine for 24 h prior to the study day.
Data acquisition and study protocol
Weight and height were measured and body mass index (BMI, kg m−2) was calculated. Forearm volume (FAV) was determined using water displacement. MVC of the non-dominant arm was determined as the average of the two highest measurements from five trials using a hand dynamometer (LaFayette Instruments; LaFayette, IN, USA). Heart rate (HR) and mean arterial blood pressure (BP) were measured by electrocardiography (Datex-Ohmeda; Helsinki, Finland) and automatic sphygmomanometer on the dominant arm (Datex-Ohmeda; Helsinki, Finland), respectively.
Subjects were supine with the non-dominant arm extended approximately 90° at heart level (Limberg et al. 2010, 2011; Schrage et al. 2004). Non-dominant forearm exercise was completed at two absolute workloads (8 and 12 kg) and required subjects to squeeze and release two handles together 4–5 cm to raise and lower a weight over a pulley at a rate of 20 times per minute (1 s contraction:2 s relaxation). An absolute increase in work should result in an absolute increase in oxygen consumption, allowing researchers to assess whether the increase in blood flow is appropriate to meet the metabolic challenge. Forearm blood flow (FBF; artery diameter, blood velocity) was measured with Doppler ultrasound (Vivid 7, General Electric; Milwaukee, WI, USA) (Limberg et al. 2010, 2011; Schrage et al. 2007). A 12 MHz probe was placed midway between the antecubital and axillary regions of the non-dominant forearm. The ultrasound probe operator continuously adjusted the probe position to maintain a fixed insonation angle < 60°.
A 5 cm, 20-gauge venous catheter was inserted into the antecubital vein in the exercising arm and was used to take blood samples throughout the study. Samples were drawn anaerobically in heparinized syringes over 10–20 s, were placed on ice and analyzed within 2 h of collection for venous blood gasses [oxygen (PvO2), carbon dioxide (PvCO2), pH] (ABL500, Radiometer, Copenhagen, Denmark). Samples were collected and analyzed in duplicate and all results were temperature-corrected. Pilot work showed measurements were not significantly different when analyzed 0.5 or 2.5 h after collection (p > 0.05, data not shown).
Subjects were instrumented with a nose clip, mouthpiece, and breathed through a low-resistance two-way non-rebreathing valve (model 2400, Hans Rudolph) for both normoxic and hypoxic trials. Inspiratory and expiratory flow rates, as well as inspired and expired gases were sampled at the mouth (MedGraphics, Ultima PFX; St. Paul, MN, USA). During normoxic trials, subjects breathed room air (0.21 FiO2). During hypoxic trials, the level of inspired oxygen was titrated to achieve an arterial oxygen saturation (SpO2) of 80% (~0.10 FiO2). SpO2 was assessed by pulse oximetry on the dominant hand (Datex-Ohmeda; Helsinki, Finland) and forehead (Nellcor, N-595; Pleaston, CA, USA). Gasses were blended (air-oxygen mixer, Puritan-Bennett; Los Angeles, CA, USA) in an attempt to maintain normoxic CO2 levels (isocapnia) while achieving desired SpO2.
Each subject completed a total of four trials (8 and 12 kg forearm exercise during normoxia and hypoxia) in random order with a 10-min normoxic rest period after each trial. With each trial, steady-state ventilation at the desired SpO2 was maintained for an average of 4 min prior to the collection of resting measures. Hypoxic and normoxic resting data are reported as the average of data collected prior to the respective 8 and 12 kg workloads. After quiet rest, subjects completed 3.5 min of dynamic exercise. All steady-state exercise data were collected during the last 30 s of dynamic exercise. Similar methods have been used previously by our laboratory and others (Casey et al. 2010; Limberg et al. 2010; Wilkins et al. 2006).
Data and statistical analysis
FBF was determined as published previously by our laboratory (Limberg et al. 2010, 2011). Briefly, FBF was calculated as the product of mean blood velocity (cm s−1) and vessel cross-sectional area (π radius2 with radius measured in cm) and was reported in ml min−1. Arterial blood velocity was continuously assessed throughout rest and each exercise workload. Beat-to-beat pulse-wave velocity was analyzed as an average of the last 30 s of rest and steady-state exercise. Artery diameters were obtained from B-mode images taken at rest and during the last 10 s of exercise. Diameter was determined in a longitudinal section of the artery running perpendicular to the ultrasound beam. Measurements were made off-line by a trained operator and final values are reported as the median of five measurements in late diastole during each resting and exercise period (between muscle contractions) to determine vessel cross-sectional area.
A commercial interface unit (Multigon Industries, Yonkers, NY, USA) processed the angle-corrected, intensity-weighted Doppler audio information from the GE Vivid ultrasound system into a flow velocity signal that was sampled in real time with signal-processing software (PowerLab, ADinstruments; Colorado Springs, CO, USA). All hemodynamic data were digitized, stored in a computer at 400 Hz, and analyzed off-line using PowerLab. Post-processing using PowerLab’s Chart application package yielded mean blood velocities. All ventilatory signals were sampled at 75 Hz and displayed on a separate chart recorder (MedGraphics Breeze Suite; St. Paul, MN, USA).
Due to group differences in BP, FBF measurements were normalized for simultaneous BP measures and are reported as vascular conductance (FVC; mL min−1 100 mmHg−1). Changes in FVC are indicative of changes in arteriole diameter and vascular responses to exercise and/or hypoxia. A change in FVC (ΔFVC) was calculated as FVCexercise – FVCnormoxia rest. The main dependent variable was a change in FVC due to hypoxia (compensatory vasodilation) and was assessed as a relative change in ΔFVC with hypoxia: %ΔFVC = [(ΔFVChypoxia – ΔFVCnormoxia) (ΔFVCnormoxia)−1 100%]. To better understand individual vascular responsiveness to a drop in SpO2 (a measure of hypoxia sensitivity), we compared the two-point slope of the relationship between SpO2 and FVC; a negative slope is indicative of hypoxic vasodilation and vice versa (Reichmuth et al. 2009).
Subject characteristics were compared via unpaired Student’s t test. Hemodynamic variables were analyzed using a proc mixed approach to determine the significance of the fixed effect of group (young, older), gas (normoxia, hypoxia), and/or intensity (Rest, 8, 12 kg) on parameters of interest. Bonferroni post hoc comparisons were performed when significant effects were observed. Analysis of covariance was conducted post hoc to estimate the significant effect of group (young, older) on FVC above effects of potential covariates (sex, MVC, FAV, glucose, triglycerides, total cholesterol, HDL, LDL, weight, waist circumference, BMI, HR, BP, minute ventilation, breathing frequency, tidal volume, end-tidal CO2). Previous research from our lab suggests adults with metabolic syndrome (obesity, dyslipidemia, hyperglycemia, hypertension) exhibit altered hypoxic-mediated vasodilation at rest; thus, we assessed potential effects of cardiovascular disease risk factors on blood flow responses (Limberg et al. 2011). All data are presented as mean ± standard error, and significance was determined a priori at p < 0.05. Statistical analysis was done using SAS 9.2 (Cary, NC, USA).
Results
Subject characteristics
Seventeen young (27 ± 2 years, males n = 12) and 12 older (61 ± 1 years, males n = 5) adults completed the current study. Data from a subset of younger participants (n = 13) were presented previously (Limberg et al. 2011). Subject characteristics are summarized in Table 1. There were no significant differences between groups in regard to weight nor fasting glucose, triglyceride, and cholesterol (total, LDL, HDL) levels (p > 0.05). Older adults exhibited smaller forearm size (FAV, p = 0.02) and lower handgrip strength (MVC, p < 0.01) when compared to the young control group. While within healthy ranges, older adults exhibited greater waist circumference (p < 0.01) and BMI (p = 0.04).
Table 1.
Subject demographics
| Young | Old | |
|---|---|---|
| Sex (M/F) | 12/5 | 5/7 |
| Age (years) | 27 ± 2 | 61 ± 1* |
| Height (cm) | 177 ± 2 | 168 ± 2* |
| Weight (kg) | 73 ± 3 | 69 ± 2 |
| BMI (kg/m2) | 23 ± 1 | 24 ± 1* |
| Waist (cm) | 78 ± 3 | 85 ± 3* |
| FAV (mL) | 1,022 ± 47 | 830 ± 43* |
| MVC (kg) | 41 ± 2 | 31 ± 3* |
| Glucose (mg/dL) | 76 ± 3 | 72 ± 4 |
| Triglyceride (mg/dL) | 108 ± 17 | 99 ± 20 |
| Total cholesterol (mg/dL) | 194 ± 15 | 160 ± 14 |
| HDL (mg/dL) | 57 ± 5 | 56 ± 7 |
| LDL (mg/dL) | 110 ± 10 | 86 ± 10 |
Data are presented as mean ± SE. Young n = 17, old n = 12 unless otherwise noted
Waist (young n = 16), LDL (young n = 14, old n = 11)
BMI body mass index, FAV forearm volume, MVC maximal voluntary contraction
p < 0.05 versus young
Hyperemic responses to exercise and hypoxia
Brachial artery diameter was significantly smaller (~0.02 cm) in older adults (Table 2; main effect, p < 0.05). FBF was greater in older subjects across workloads and gas conditions (main effect, p < 0.05); this difference was abolished when differences in BP were accounted for (FVC, p > 0.05 between groups). FBF and FVC increased with exercise and hypoxia in both groups (Table 2; main effects, p < 0.05) and this response was not different between groups (interaction effect exercise × group p = 0.37 and hypoxia × group p = 0.96, respectively). Whereas conclusions regarding FVC were maintained after adjusting for potential covariates (data not shown), glucose levels (p = 0.02) and minute ventilation (p = 0.02) had significant effects on FVC.
Table 2.
Hyperemic responses to exercise and hypoxia
| Rest
|
8 kg
|
12 kg
|
||||
|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | |
| Diameter (cm)A | ||||||
| Young | 0.42 ± 0.02 | 0.43 ± 0.02 | 0.44 ± 0.01 | 0.45 ± 0.01 | 0.45 ± 0.01a | 0.46 ± 0.01a |
| Old | 0.40 ± 0.01 | 0.40 ± 0.02 | 0.42 ± 0.02 | 0.43 ± 0.01 | 0.43 ± 0.01a | 0.43 ± 0.02a |
| Blood flow (FBF, mL/min)A,B | ||||||
| Young | 77 ± 10 | 79 ± 11 | 285 ± 22a | 348 ± 25a | 401 ± 33a,b | 425 ± 30a,b |
| Old | 75 ± 6 | 83 ± 11 | 343 ± 28a | 384 ± 22a | 428 ± 30a,b | 461 ± 32a,b |
| Conductance (FVC, mL/min × 100 mmHg)B | ||||||
| Young | 86 ± 12 | 87 ± 13 | 305 ± 23a | 374 ± 28a | 413 ± 30a,b | 438 ± 30a,b |
| Old | 76 ± 7 | 81 ± 10 | 317 ± 31a | 350 ± 24a | 367 ± 31a,b | 419 ± 31a,b |
Data are presented as mean ± SE. Young n = 17, old n = 12
Main effect of exercise:
p < 0.05 versus rest,
p < 0.05 versus 8 kg
Main effect of group (young vs. old),
main effect of gas condition (normoxia vs. hypoxia)
Given the potential for alternative conclusions based on data expression alone, we took a comprehensive approach to both data expression and analysis. Conclusions regarding responses to hypoxia between groups were maintained when vascular responses were analyzed as absolute (FVC, Table 2), absolute change (ΔFVC, Fig. 1), relative change (%ΔFVC, Fig. 2), hypoxia sensitivity (two-point slope, Fig. 3), and relative to forearm volume (ΔrFVC at average relative workloads, data not shown).
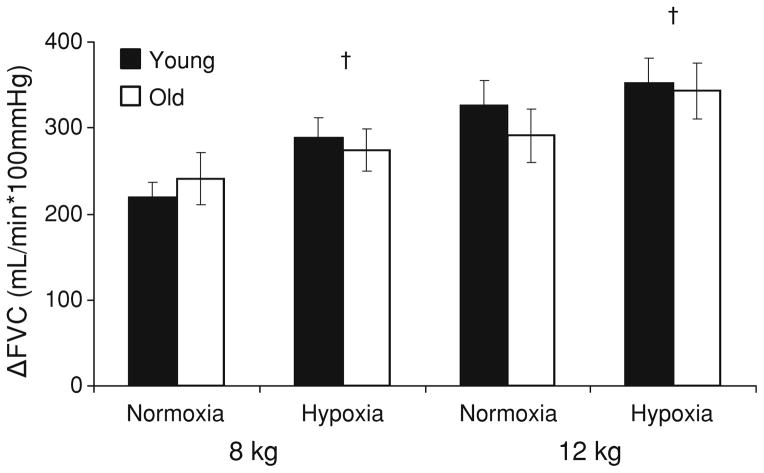
Fig. 1.
Increases in normoxic and hypoxic forearm vascular conductance with exercise. Data are presented as mean ± SE. A change in vascular conductance (ΔFVC) was calculated as FVCexercise – FVCnormoxia rest. Vascular responses to exercise (ΔFVC) increased with increasing exercise intensity (main effect of exercise: p < 0.05 12 vs. 8 kg) and were not different between groups (no effect of group). Both groups exhibited hypoxic-mediated vasodilation that was not different in magnitude between groups (†main effect of gas condition)
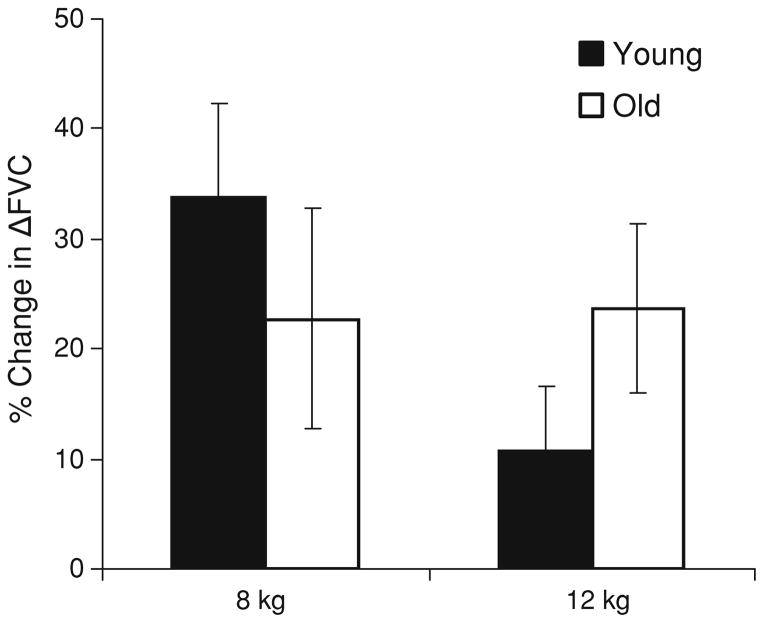
Fig. 2.
Compensatory responses to hypoxia during exercise. Data are presented as mean ± SE. Values represent a change in vascular conductance due to hypoxia (compensatory vasodilation) and are expressed as a relative change in ΔFVC with hypoxia . Vascular responses were not different between groups (No effect of group)
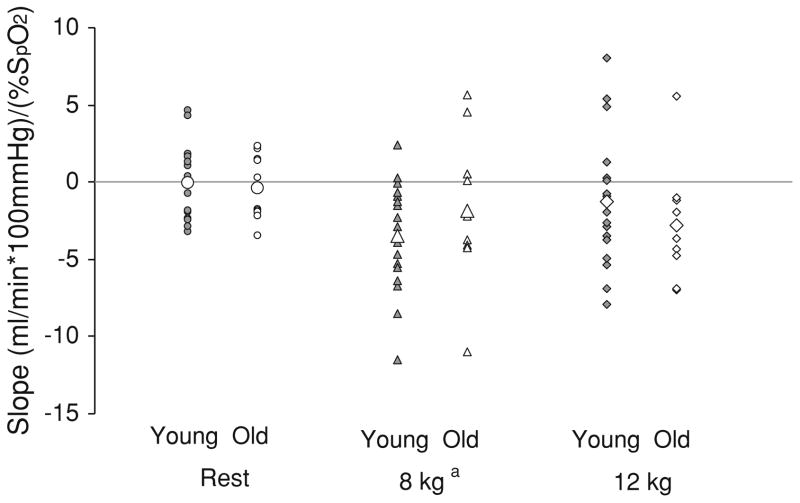
Fig. 3.
Individual stimulus–response relationships to hypoxia during rest and exercise. Data are presented as individual data points; large open symbols signify group means. Values were calculated as the two-point slope of the relationship between ΔFVC and arterial oxygen saturation (SpO2) to assess individual vascular responses to hypoxia. Negative values represent net hypoxia-mediated vasodilation; positive values represent vasoconstriction. Vascular responses to exercise increased at 8 kg exercise intensity (main effect of exercise: ap < 0.05 vs. rest) and were not different between groups (no effect of group)
Whereas group responses to hypoxic exercise were not different, individual responses were heterogeneous (Fig. 3). In response to hypoxia, some individuals exhibited vasoconstriction (positive values) and others exhibited vasodilation (negative values). Specifically, %ΔFVC during 8 kg exercise ranged from hypoxic-mediated vasodilation (up to 118%) to vasoconstriction (as low as −29%). Similar ranges were observed during 12 kg exercise (−35 to 78%). This heterogeneity was observed in both groups.
Systemic and venous blood gas responses
Older adults exhibited consistently higher BP and lower HR when compared to young controls (Table 3; main effect, p < 0.05); BP and HR increases from rest to exercise were not different between groups. In response to hypoxia, older adults had a smaller increase in HR when compared to young controls (group × gas interaction, p < 0.05). While statistically different (p < 0.05), SpO2 was physiologically similar (~1%) between groups, and decreased significantly in both groups with hypoxia (p < 0.05).
Table 3.
Systemic responses to exercise and hypoxia
| Rest
|
8 kg
|
12 kg
|
||||
|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | |
| Heart rate (beat/min)A,B,C | ||||||
| Young | 64 ± 2 | 81 ± 3 | 69 ± 2a | 84 ± 4a | 71 ± 2a | 85 ± 2a |
| Old | 63 ± 2 | 72 ± 3 | 68 ± 2a | 78 ± 3a | 71 ± 3a | 82 ± 3a |
| Mean arterial blood pressure (mmHg)A | ||||||
| Young | 90 ± 1 | 92 ± 2 | 93 ± 1a | 93 ± 2a | 96 ± 1a | 97 ± 1a |
| Old | 100 ± 4 | 101 ± 4 | 110 ± 4a | 112 ± 5a | 120 ± 6a | 112 ± 6a |
| SpO2 (%)A,B | ||||||
| Young | 98 ± 0 | 79 ± 0 | 98 ± 0 | 77 ± 1 | 99 ± 0 | 79 ± 1 |
| Old | 98 ± 0 | 79 ± 1 | 98 ± 0 | 78 ± 1 | 98 ± 0 | 78 ± 1 |
| Ventilation (BTPS) (L/min)B,C | ||||||
| Young (n = 11) | 10 ± 0 | 16 ± 3 | 10 ± 1 | 18 ± 3 | 11 ± 1 | 17 ± 3 |
| Old (n = 10) | 10 ± 1 | 12 ± 1 | 12 ± 1 | 14 ± 1 | 13 ± 1 | 15 ± 1 |
| Tidal volume (L)A,B | ||||||
| Young (n = 11) | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.2 |
| Old (n = 10) | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.0 | 0.7 ± 0.1 | 0.9 ± 0.1 |
| Breathing frequency (br/min)C | ||||||
| Young (n = 11) | 15 ± 1 | 17 ± 2 | 14 ± 2 | 19 ± 2 | 14 ± 1 | 17 ± 2 |
| Old (n = 10) | 15 ± 1 | 14 ± 2 | 18 ± 1 | 18 ± 1 | 18 ± 1 | 18 ± 2 |
| End-tidal CO2 (%)A,B | ||||||
| Young (n = 11) | 5.7 ± 0.2 | 5.2 ± 0.2 | 5.7 ± 0.2 | 5.2 ± 0.3 | 5.8 ± 0.2 | 5.3 ± 0.2 |
| Old (n = 10) | 5.3 ± 0.3 | 5.1 ± 0.3 | 5.3 ± 0.3 | 5.0 ± 0.3 | 5.3 ± 0.3 | 5.1 ± 0.3 |
Data are presented as mean ± SE. Young n = 17, Old n = 12 unless otherwise noted
Main effect of exercise:
p < 0.05 versus rest
Main effect of group (young vs. old),
main effect of gas condition (normoxia vs. hypoxia),
interaction between group and gas condition
Ventilatory data are presented from a subset of participants (young n = 11, older n = 10) due to a data storage error. Older adults exhibited lower tidal volume (Table 3; main effect, p < 0.05) but breathing frequency and resultant minute ventilation were not different when compared to young controls (p > 0.05). In response to hypoxia, both groups increased tidal volume and minute ventilation (Main effect, p < 0.05); however, younger adults appeared to have greater increases in breathing frequency and minute ventilation in response to the hypoxic stimulus (group × gas, p < 0.05). End-tidal CO2 (ETCO2) was consistently lower in older adults (Table 3; main effect, p < 0.05). With hypoxia, ETCO2 fell modestly (0.2–0.5%; main effect, p < 0.05) and similarly between groups (interaction effect group × gas, p = 0.45). These small differences in ETCO2 are not physiologically significant to forearm blood flow, given levels were within a normoxic, normocapnic range.
Venous blood gasses were not significantly different between groups (Table 4; p > 0.05). In response to hypoxia, both groups exhibited a decrease in PvO2 and an increase in PvCO2. The similar drop in PvO2 suggests increased muscle extraction in both groups—although extraction was not directly assessed with the current study design. Older adults exhibited consistently higher pH (Main effect, p < 0.05); the decrease in pH with exercise was not different between groups.
Table 4.
Venous blood gas responses to exercise and hypoxia
| Rest
|
8 kg
|
12 kg
|
||||
|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | |
| PvO2 (Torr)B | ||||||
| Young | 37 ± 2 | 28 ± 1 | 27 ± 1a | 23 ± 2a | 28 ± 1a | 22 ± 1a |
| Old | 34 ± 2 | 28 ± 1 | 28 ± 2a | 22 ± 1a | 28 ± 1a | 24 ± 1a |
| PvCO2 (Torr)B | ||||||
| Young | 41 ± 2 | 40 ± 2 | 51 ± 2a | 44 ± 3a | 49 ± 3a | 46 ± 3a |
| Old | 39 ± 2 | 36 ± 3 | 49 ± 4a | 44 ± 3a | 51 ± 3a | 47 ± 3a |
| Venous pH (units)A,B | ||||||
| Young | 7.37 ± 0.01 | 7.40 ± 0.01 | 7.32 ± 0.01a | 7.35 ± 0.01a | 7.30 ± 0.01a,b | 7.32 ± 0.01a,b |
| Old | 7.39 ± 0.01 | 7.40 ± 0.01 | 7.35 ± 0.02a | 7.35 ± 0.01a | 7.31 ± 0.01a,b | 7.34 ± 0.02a,b |
Data are presented as mean ± SE. Young n = 17, old n = 12
Main effect of exercise:
p < 0.05 versus rest,
p < 0.05 versus 8 kg
Main effect of group (young vs. old),
main effect of gas condition (normoxia vs. hypoxia)
Discussion
The current study examined potential age-related differences in compensatory hypoxic vasodilation. Novel findings demonstrate: (1) on average, compensatory hypoxic vasodilation in older adults is not impaired during forearm exercise at both moderate and higher absolute exercise intensities, and (2) vascular responses to hypoxia are variable in both young and older adults. The latter suggests heterogeneity in physiologic factors mediating hypoxic vasodilation.
Vascular responses to exercise with age
Evidence regarding an age-related impairment in forearm exercise hyperemia under normoxic conditions is conflicting. Some research suggests older adults have reduced blood flow during forearm exercise (Kirby et al. 2009), whereas others show responses to be preserved with age (Dinenno et al. 2005; Donato et al. 2006; Jasperse et al. 1994; Wray et al. 2010). It is possible differences in blood flow responses with aging are intensity-dependent (Proctor et al. 1998, 2003a, b). In the face of such controversy, results from the current study strengthen and expand current understanding of vascular control in aging; forearm exercise hyperemia is maintained with healthy aging at moderate and higher absolute exercise intensities (Table 2; Fig. 1).
Vascular responses to hypoxia with age
Investigation into the effect of aging on vascular responses to hypoxia and exercise has relevance to older populations with sleep apnea or those active in high altitude environments. Additionally, poor hypoxic vasodilation could impact ischemic events such as stroke or heart attack. A handful of studies have measured hypoxic vasodilation in the forearm of young adults at rest (Crecelius et al. 2011; Markwald et al. 2011; Weisbrod et al. 2001) and during exercise (Casey et al. 2010; Crecelius et al. 2011; Wilkins et al. 2006, 2008), however, much less is known about responses to hypoxia in older adults. Hypoxic vasodilation shares many pathways with the exercise response [Beta-adrenergic (Wilkins et al. 2008), nitric oxide (Casey et al. 2010; Crecelius et al. 2011; Markwald et al. 2011), prostaglandins (Crecelius et al. 2011; Markwald et al. 2011), adenosine (Leuenberger et al. 1999)] that may be impaired with aging (Andrawis et al. 2000; Nicholson et al. 2009; Schrage et al. 2007; Sindler et al. 2009; Taddei et al. 1995, 2000). Therefore, we hypothesized forearm hypoxic vasodilation would be impaired in older adults at a moderate absolute workload (8 kg) and this effect would be exaggerated at a higher workload (12 kg).
Contrary to our hypothesis, results from the current study show compensatory vasodilation to be maintained during both moderate and higher forearm exercise in older adults. These findings suggest older adults are capable of compensating for reduced oxygen delivery seen with hypoxia. Our results are in contrast to previously published findings during forearm exercise at a moderate relative intensity (20% MVC) (Casey et al. 2011). This was unexpected considering the forearm exercise model and level of hypoxemia appear nearly identical between studies. However, this is the first study to use absolute workloads at both moderate (8 kg) and high (12 kg) exercise intensities to assess age-related differences in blood flow responses. A specific, absolute increase in work should result in an absolute increase in oxygen consumption, allowing researchers to test whether the increase in blood flow (oxygen delivery) is appropriate to meet the metabolic challenge. Further, absolute exercise intensities have been shown to result in comparable increases in oxygen consumption between young and older adults (Donato et al. 2006; duManoir et al. 2010a, b) and are applicable to everyday activities. This becomes especially important when studying populations that differ, whether statistically or physiologically, in muscle mass and strength (Table 1) (Donato et al. 2010; Proctor et al. 1998, 2003a, b). Similar decreases in PvO2 between groups in response to hypoxia suggest increased extraction may contribute to preserved oxygen delivery similarly between groups (Table 4) and given group differences in strength, it is important to highlight expressing data in absolute (Fig. 1) or relative (data not shown) terms did not alter conclusions regarding hypoxic vasodilation.
Heterogeneity in blood flow responses to hypoxia
Vascular responses to hypoxia were heterogeneous despite tightly controlled hypoxic conditions (Fig. 3). This suggests compensatory vascular control mechanisms differ between individuals, irrespective of aging. This is in line with a growing body of evidence indicating heterogeneity in cardiovascular control (Charkoudian 2010; Parker et al. 2011). Given vascular control during hypoxia is a balance of sympathetically mediated constriction and local vasodilatory mechanisms, it may not be surprising some adults exhibit hypoxia-mediated vasodilation (Fig. 3, negative values) and others exhibit constriction (Fig. 3, positive values). Whereas the current study is descriptive in nature, the data make two important contributions to the literature: (1) the impact of aging on hypoxic vasodilation is not clearly established, and (2) a high degree of individual variability in hypoxic responses exists in both young and older healthy adults. Taken together, future studies are necessary to elucidate the physiological diversity in specific control mechanisms both within and between groups.
We explored predictors of compensatory vasodilation post hoc to determine whether the other factors could explain heterogeneous responses. Whereas group differences in FVC were not observed after adjusting for potential covariates (p = 0.25), glucose levels appeared to have a significant effect on FVC (p = 0.02). Therefore, factors secondary to aging (hyperglycemia) may play a more important role in blood flow responses than age per se. This is interesting considering all participants were relatively healthy and free from overt cardiovascular disease. These findings may have implications for comorbidities often associated with increasing age (i.e., diabetes).
Experimental considerations and limitations
Overlapping or redundant vascular control mechanisms may mask impairments in vascular control at low exercise intensities (Boushel et al. 2002; Mortensen et al. 2007; Schrage et al. 2004). To stress compensatory mechanisms beyond the point of redundancy, higher exercise intensities may be necessary. A novel aspect of the current study was the use of high intensity exercise. Interestingly, even at this higher intensity (12 kg), vascular responses to forearm exercise (FVC) were maintained in older adults. Previous research suggests vascular control mechanisms are altered or impaired with aging—such as blunted nitric oxide-mediated dilation (Schrage et al. 2007) and enhanced adrenergic-mediated vasoconstriction (Dinenno et al. 2005). However, it appears that alternative mechanisms may compensate for any such impairment in our research cohort. Additionally, research in animals supports the concept of altered flow distribution with aging (Musch et al. 2004) that could not be assessed with the current study design. Taken together, the impact of aging on hypoxic vasodilation is not clear and novel findings from the current study provide a basis for follow-up hypothesis-driven mechanistic research, including alpha-adrenergic constriction, beta-adrenergic dilation, and local metabolic factors.
Interestingly, we observed group-specific differences in heart rate responses to the hypoxic stimulus. Specifically, older adults had a smaller increase in HR in response to hypoxia when compared to young controls (Table 3). This observation is consistent with literature suggesting reduced beta-adrenergic sensitivity of the heart in older adults (Lakatta 1986). Beta-adrenergic vasodilation is also an important mechanism of hypoxia-mediated blood flow (Wilkins et al. 2006). However, altered beta-adrenergic responsiveness is unlikely to limit conclusions from the current study given beta-mediated hypoxic vasodilation is important only at low (10%, ~4 kg) forearm exercise intensities (Wilkins et al. 2008).
The potential for sex differences in hypoxic dilation deserves discussion. Including both sexes is an important strength, given the predominance of male-only physiologic studies in the literature. Recent evidence suggests sex plays an important role in vascular responses in the leg in older adults (Proctor et al. 1998, 2003a, b; Wray et al. 2010). On the other hand, previous research does not support sex differences in vascular control in the exercising forearm under normoxic conditions (Koch et al. 2005; Parker et al. 2007; Proctor et al. 1998, 2005; Ridout et al. 2005), and sex was not a significant covariate in the current study. Therefore, the inclusion of both men and women does not limit conclusions.
Conclusion
The current study assessed the impact of aging on the integrated vascular response to hypoxia. These findings indicate compensatory hypoxic vasodilation in older adults is not impaired during moderate and higher intensity forearm exercise. Heterogeneous vascular responses to hypoxia suggest unique individual differences exist in factors regulating blood flow under hypoxic conditions. The importance of physiological diversity in specific control mechanisms—in both health and aging—warrants further investigation.
Acknowledgments
We are grateful to the subjects for their participation. In addition, we extend many thanks to Jessica Danielson, John Harrell, Kathleen Grabowski, Adam Kiefer, Patrick Meyer, Caitlin Zillner, and Lee Linstroth for technical assistance. Thank you to Dr. Zhanhai Li, PhD and grant 1ULRR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research, National Institutes of Health for assistance with statistical analysis. This study was supported by grants from the American Federation on Aging Research #A08235 (WGS), American Heart Association pre-doctoral awards #0815622G (JKL) and #10PRE3870000 (JKL).
Footnotes
Communicated by Dag Linnarsson.
Conflict of interest There are no potential conflicts of interest.
Contributor Information
Jacqueline K. Limberg, Department of Kinesiology, School of Education, University of Wisconsin, 2000 Observatory Avenue, Rm 1149A, Madison, WI 53703, USA
Trent D. Evans, Department of Kinesiology, School of Education, University of Wisconsin, 2000 Observatory Avenue, Rm 1149A, Madison, WI 53703, USA
David F. Pegelow, Department of Kinesiology, School of Education, University of Wisconsin, 2000 Observatory Avenue, Rm 1149A, Madison, WI 53703, USA. The John Rankin Laboratory of Pulmonary Medicine, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA
Marlowe W. Eldridge, Department of Kinesiology, School of Education, University of Wisconsin, 2000 Observatory Avenue, Rm 1149A, Madison, WI 53703, USA. The John Rankin Laboratory of Pulmonary Medicine, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA. Department of Pediatrics, University of Wisconsin Hospital and Clinics, Madison, WI, USA
Joshua J. Sebranek, Department of Anesthesiology, University of Wisconsin Hospital and Clinics, Madison, WI, USA
Lester T. Proctor, Department of Anesthesiology, University of Wisconsin Hospital and Clinics, Madison, WI, USA
William G. Schrage, Email: wschrage@education.wisc.edu, Department of Kinesiology, School of Education, University of Wisconsin, 2000 Observatory Avenue, Rm 1149A, Madison, WI 53703, USA
References
- Andrawis N, Jones DS, Abernethy DR. Aging is associated with endothelial dysfunction in the human forearm vasculature. J Am Geriatr Soc. 2000;48:193–198. [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol. 2010;588:373–385. doi: 10.1113/jphysiol.2009.180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol. 2011;589(6):1477–1488. doi: 10.1113/jphysiol.2010.203539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N. Heterogeneity in human cardiovascular function contributes to a deeper understanding of integrative mechanisms. J Appl Physiol. 2010;108:473–474. doi: 10.1152/japplphysiol.01385.2009. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol. 2011;589(14):3671–3683. doi: 10.1113/jphysiol.2011.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey DS, Shaw CN, Shoemaker JK, Kowalchuk JM, Paterson DH. The effect of hypoxia on pulmonary O2 uptake, leg blood flow and muscle deoxygenation during single-leg knee-extension exercise. Exp Physiol. 2004;89:293–302. doi: 10.1113/expphysiol.2003.026864. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol. 2005;567:311–321. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. 2010;298:H671–H678. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- duManoir GR, DeLorey DS, Kowalchuk JM, Paterson DH. Differences in exercise limb blood flow and muscle deoxygenation with age: contributions to O2 uptake kinetics. Eur J Appl Physiol. 2010a;110:739–751. doi: 10.1007/s00421-010-1546-z. [DOI] [PubMed] [Google Scholar]
- duManoir GR, DeLorey DS, Kowalchuk JM, Paterson DH. Kinetics of VO2 limb blood flow and regional muscle deoxygenation in young adults during moderate intensity, knee-extension exercise. Eur J Appl Physiol. 2010b;108:607–617. doi: 10.1007/s00421-009-1263-7. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. J Physiol. 1994;474:353–360. doi: 10.1113/jphysiol.1994.sp020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587(9):1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch DW, Newcomer SC, Proctor DN. Blood flow to exercising limbs varies with age, gender, and training status. Can J Appl Physiol. 2005;30:554–575. doi: 10.1139/h05-141. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Diminished beta-adrenergic modulation of cardiovascular function in advanced age. Cardiol Clin. 1986;4:185–200. [PubMed] [Google Scholar]
- Leuenberger UA, Gray K, Herr MD. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J Appl Physiol. 1999;87:2218–2224. doi: 10.1152/jappl.1999.87.6.2218. [DOI] [PubMed] [Google Scholar]
- Limberg JK, De Vita MD, Blain GM, Schrage WG. Muscle blood flow responses to dynamic exercise in young obese humans. J Appl Physiol. 2010;108:349–355. doi: 10.1152/japplphysiol.00551.2009. [DOI] [PubMed] [Google Scholar]
- Limberg JK, Evans TD, Blain GM, Pegelow DF, Danielson JR, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Effect of obesity and metabolic syndrome on hypoxic vasodilation. Eur J Appl Physiol. 2011 Jun 9;2011 doi: 10.1007/s00421-011-2025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwald RR, Kirby BS, Crecelius AR, Carlson RE, Voyles WF, Dinenno FA. Combined inhibition of nitric oxide and vasodilating prostaglandins abolishes forearm vasodilatation to systemic hypoxia in healthy humans. J Physiol. 2011;589:1979–1990. doi: 10.1113/jphysiol.2011.205013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol. 2004;96(1):81–88. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension. 2009;53:973–978. doi: 10.1161/HYPERTENSIONAHA.108.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Jarvis SS, Ridout SJ, Pawelczyk JA, Proctor DN. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol. 2007;292:H1148–H1156. doi: 10.1152/ajpheart.00729.2006. [DOI] [PubMed] [Google Scholar]
- Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol. 2011;301(3):H1118–H1126. doi: 10.1152/ajpheart.00400.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284:H1251–H1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003a;95:1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA, Leuenberger UA. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol. 2003b;94(5):1859–1869. doi: 10.1152/japplphysiol.00898.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Le KU, Ridout SJ. Age and regional specificity of peak limb vascular conductance in men. J Appl Physiol. 2005;98:193–202. doi: 10.1152/japplphysiol.00704.2004. [DOI] [PubMed] [Google Scholar]
- Reichmuth KJ, Dopp JM, Barczi SR, Skatrud JB, Wojdyla P, Hayes D, Jr, Morgan BJ. Impaired vascular regulation in patients with obstructive sleep apnea: effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med. 2009;180:1143–1150. doi: 10.1164/rccm.200903-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout SJ, Parker BA, Proctor DN. Age and regional specificity of peak limb vascular conductance in women. J Appl Physiol. 2005;99:2067–2074. doi: 10.1152/japplphysiol.00825.2005. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduce forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol. 2001;537:613–621. doi: 10.1111/j.1469-7793.2001.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol. 2006;101:1343–1350. doi: 10.1152/japplphysiol.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol. 2008;586:1195–1205. doi: 10.1113/jphysiol.2007.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Donato AJ, Richardson RS. Human vascular aging: limb-specific lessons. Exerc Sport Sci Rev. 2010;38:177–185. doi: 10.1097/JES.0b013e3181f45413. [DOI] [PubMed] [Google Scholar]