Abstract
A correlation between expression of the glucose-regulated protein of 78 kDa (GRP78) in malignant melanoma tumors and poor patient survival is well established. In this study, in addition to demonstrating the expression of GRP78 in tumor tissue, we investigated the immune response against GRP78 in a group of patients with different progression stages of malignant melanoma. Furthermore, we analyzed the glycosylation status of GRP78 IgG autoantibodies at these stages and evaluated their capacities to affect the Akt signaling pathway and unfolded protein response signaling mechanisms, all known to promote malignant melanoma cell proliferation and survival. We found that progression of disease correlates not only with enhanced expression of GRP78 in the tumor but also with an increase in GRP78 autoantibody serum titers in these patients. We also found that the glycosylation status of anti-GRP78 IgG changes as the disease progresses. The anti-GRP78 IgG is abnormally glycosylated in the Fc region and asymmetrically glycosylated in the Fab region. We demonstrate that hyperglycosylated anti-GRP78 IgGs stimulate cell proliferation via Akt signaling pathways. They also mimic the effects of α2M* on the up-regulation of GRP78 and XBP-1, ATF6α, and IRE1α as ER stress biomarkers and show no effect on expression or activation of caspases 3, 9 or 12. In conclusion, the anti-GRP78 IgG autoantibodies down-regulate apoptosis and activate unfolded protein response mechanisms which are essential to promote melanoma cell growth and survival.
Keywords: melanoma, glycosylation, autoimmunity, GRP78, cancer progression
Introduction
The circulation of autoantibodies against antigens expressed by tumors is a common occurrence in cancer patients. One of these antigens, the glucose-regulated protein 78 (GRP78), induces synthesis of autoantibodies in prostate cancer patients whose anti-GRP78 IgG serum titers are positively correlated with disease progression and shorter overall survival [1]. In prostate cancer, the GRP78 antigenic region corresponds to an epitope defined by the tertiary structure peptide CNVSKDSC which mimics a complementary tertiary structure site in GRP78 (1). This site was found to be localized in the GRP78 primary amino acid sequence LIGRTWNDPSVQQDIKFL (Leu98-Leu115) which is also a binding site for its physiologic agonist, activated α2-macroglobulin (α2M*) [2]. Autoantibodies to GRP78 are also found in patients with ovarian cancer, and their presence has also been linked to poor prognosis and poor survival [3]. In a recent study in patients with malignant melanoma, the expression of GRP78 in tumor tissue was found to correlate with poor overall patient survival, but the presence of GRP78 autoantibodies, their epitope specificity, or their mechanisms of action were not assessed [4].
In prostate and ovarian cancer, the immune response against GRP78 is paradoxical in the sense that overexpression of this protein, rather than preventing tumor growth, induces growth and progression of the tumor [1,3]. Since binding of these autoantibodies to GRP78 promotes tumor growth, they behave as tumorigenic rather than as antitumor immune mediators. In a recent report [5], we show a causal link between a humoral response to GRP78 and the progression of cancer in a murine malignant melanoma model.
The reaction of GRP78 with its autoantibodies induces an autoimmune response that supports tumor-specific tolerance [6], a phenomenon extensively investigated and attributed to aberrant IgG glycosylation in women with ovarian and endometrial cancers [7]. Aberrantly glycosylated antibodies show an asymmetric oligosaccharide structure due to the presence of an oligosaccharide residue of the high-mannose type, inserted in the Fd fragment of the H chain of one of the Fab regions [8]. The addition of an N-linked carbohydrate chain to Asn 58 to only one of the IgG heavy chains [9] converts these IgG in univalent molecules because one of the paratopes is blocked by a carbohydrate chain. When specific to self-antigens, they are beneficial for the host because they block antigens and cannot trigger the biological reactions responsible for antigenic damage [10]. Taking this into consideration, it is possible to isolate these asymmetric IgG molecules from the total IgG present in normal or immune sera using concanavalin A-Sepharose chromatography [11]. Similar antibodies are also present in the serum from pregnant women and have been suggested as contributing to the suppression of maternal immune responses against the fetus [10].
GRP78 is essential as a sensor for endoplasmic reticulum (ER) stress. The pathogenic mechanism by which increased GRP78 expression results in an adverse prognosis in cancer is related to its capacity to limit apoptosis during ER stress or the unfolded protein response (UPR) [4]. In a previous work [2], we demonstrated that GRP78 autoantibodies from prostate cancer patients modulate proliferation of human prostate cancer cells in vitro. These antibodies mimic the proliferative effects induced by α2M* in prostate cancer cells [2]. Since we have already shown that anti-GRP78 autoantibodies from tumor-bearing or GRP78-immunized mice significantly accelerate tumor growth and induce Akt phosphorylation and proliferation of mouse B16F1 and human DM6 melanoma cells [5], we initiated a new study to assess the mechanisms by which anti-GRP78 autoantibodies stimulated human malignant melanoma growth. First, we evaluated their titers and epitope specificity, followed by a search of a possible aberrant glycosylation pattern in anti-GRP78 autoantibodies from a population of 32 patients with malignant melanoma. We analyzed the expression of GRP78 in different stages of primary and metastatic malignant melanoma progression in paraffin-fixed tumor sections and cell lines created from these tumors. Antibodies against this protein were identified in serum samples from this population. As the disease progressed, we observed a change in the ratio of oligosaccharides containing high mannose in both the Fab and Fc regions of the anti-GRP78 IgGs from these patients. These antibodies bind very efficiently to human melanoma cells and induce rapid in vitro cell proliferation. We also evaluated the capacity of the aberrantly glycosylated IgGs to induce Akt phosphorylation and unfolded protein response signaling mechanisms in human malignant melanoma cells. Our results suggest aberrant glycosylation as a possible mechanism by which anti-GRP78 autoantibodies promote malignant melanoma cell proliferation and survival.
Methods
Proteins and peptides
Recombinant GRP78, a kind gift from Dr. Sylvie Y. Blond from the University of Illinois at Chicago, was prepared as previously described [12]. Keyhole lymphet hemocyanin (KHL) was purchased from Sigma (St. Louis, MO, USA). The CNVSDKSC peptide was purchased from Genemed Synthesis, Inc. (San Francisco, CA, USA). The CNVSDKSC peptide of GRP78 was conjugated to KLH via its terminal cysteine residues by the heterobifunctional cross-linker sulfosuccinimidyl 4-(N-maleidomethyl)-cyclohexane-1-carboxylate (sulfo-SMMC) (Pierce, Rockford, Il, USA) and quantified as previously described [2, 13]. Protein A/G was purchased from Pierce Biotechnology (Rockford, Il, USA). Pure Canavalia ensiformis lectin (Concanavalin A) conjugated to alkaline phosphatase was purchased from EY Laboratories, Inc. (San Mateo, CA). A glycan differentiation kit containing a digoxigenin-labeled Galanthus nivalis lectin was purchased from Roche Diagnostics (Indianapolis, IN, USA).
Patients
Blood samples were taken from 32 patients at Duke University Medical Center who were suffering from invasive malignant melanoma. Control samples (n=15) were obtained from blood donor volunteers. The average age within the patient group was 39 years (range: 14 to 62 years) the majority of patients were males (1:10 F:M ratio). The anatomic locations of the malignant melanomas in order of frequency were as follows: back (12 cases), lower extremity (6 cases), upper extremity (4 cases), chest (4 cases), neck (2 cases), and unknown (2 cases). All patients had nodal involvement by malignant melanoma while 40% of the population had visceral metastasis. The Breslow thickness average was 5.57 mm (range: 1.78 to 13 mm). Patients were classified according to criteria implemented by The American Joint Committee on cancer staging for melanoma of the skin. Six of these tumors were stage IIIa, four were at stage IIIb, nine were at stage IIIc, and seventeen were at stage IV.
Antibodies
Human serum anti-GRP78 IgG was purified by affinity chromatography on Protein A/G-Sepharose, followed by immunoadsorption to the GRP78 peptide CNVSDKSC coupled to carboxyhexyl-Sepharose [2]. Non-immune human IgG was purchased from ICN Pharmaceuticals, Inc. (Aurora, Il, USA). Horseradish peroxidase-conjugated IgG (HRP-IgG) against human IgG, Fab' or Fc-specific, were purchased from Sigma (St. Louis, MO). Alexa Fluor® 488-conjugated to goat anti-human IgG was purchased from Invitrogen (Eugene, OR).
Analysis of serum anti-GRP78 autoantibody
Autoantibodies against GRP78 in the serum of melanoma cancer patients were assayed by an ELISA technique in 96-well culture plates coated with murine recombinant GRP78 or the GRP78 peptide CNVSDKSC conjugated to KLH (5 μg/ml) in 0.1 M Na2CO3, 0.01% NaN3, pH 9.3. Incubation of these plates with serum samples and data analysis were performed as previously described [2]. Quantification of the titers of anti-GRP78 autoantibodies was done using three dilutions of the patient sera (1:5000, 1:1000 and 1:500). Calibration curves were constructed with the two GRP78 antigens. We determined a linear range of response between 0-100 μg/ml using isolated anti-GRP78 IgG from patients. Patient serum (50 μl, 1:500 dilution) was used in all assays. At this dilution all titers fall within the linear range of the calibration curves. Briefly, plates were incubated with patient serum at 37°C for 2 h in 200 μl of 10 mM sodium phosphate-0.1 M NaCl (pH 7.4) containing 0.05% Tween 80 (PBS-Tween), and then rinsed with PBS-Tween. Plates were then incubated at 37°C for 2 h with an affinity purified anti-human IgG polyclonal alkaline phosphatase-conjugated rabitt IgG in PBS-Tween. After rinsing the plates with PBS-Tween, 200 μl of alkaline phosphatase substrate (1 mg/ml p-nitrophenylphosphate) in 0.1 M glycine, 1mM MgCl2, 1 mM ZnCl2 (pH 10.4) was added to the plate, and absorbance was monitored at a wavelength of 405 nm using an Anthos Labtec Kinetic Plate reader. Bound IgG expressed as ΔA405/min was converted to μg/ml using calibration curves constructed with purified patient anti-GRP78 IgGs.
Assay of α-D-mannose residues of anti-GRP78 autoantibodies
Specific detection of α-D-mannose residues of IgG autoantibodies against GRP78 was performed with a glycan differentiation kit (Roche Diagnostics, Indianapolis, IN, USA) on 96-well plates coated with the CNVSDKSC peptide conjugated to KLH, as described above. For these assays, serum aliquots (50 μl, 1:500 dilution) were added in triplicate in 200 μl final volume of PBS-Tween and incubated 2 h at 37°C, followed by rinsing in PBS-Tween and incubation with a digoxigenin-labeled Galanthus nivalis agglutinin which recognizes “high mannose” N-glycan chains [14] for 90 min at 37°C. After rinsing with PBS-Tween to remove unbound lectin, the plates were incubated for 1 h at 37°C with a polyclonal sheep anti-digoxigenin Fab fragment conjugated with alkaline phosphatase (7 units/well in 200 μl PBS-Tween) followed by rinsing with PBS-Tween. Detection of bound lectin was performed by incubating the plates with 200 μl alkaline phosphatase substrate (1 mg/ml p-nitrophenylphosphate) in 0.1 M glycine, 1 mM MgCl2, 1 mM ZnCl2, pH 10.4. Absorbance was monitored at 405 nm. The total D-mannose concentration of the specific anti-GRP78 IgG was calculated from calibration curves constructed with the glycoprotein carboxypeptidase Y [15] as suggested by the kit manufacturer.
Histologic and Immunohistochemistry
For immunohistochemical staining, 4-5 micrometer sections were preheated in 1× Dako Target Retrieval solution, pH 6.1, and heated for 20 min in a 100° C water bath. Following antigen retrieval, the tissue sections and retrieval solution were allowed to cool for 20 min. The slides were washed with several changes of deionized water and placed in Tris buffered saline (TBS). Tissue sections were blocked with 5% non-immune horse serum. Excess serum was drained from each slide and affinity purified GRP 78 goat polyclonal antibody (N-20) diluted 1:400 was applied and incubated for one hour. Detection of the bound primary antibody was accomplished by linking with biotinylated horse anti-goat IgG. Visualization of the bound immune complex was obtained using 3,3′-diaminobenzidine tetrahydrochloride (DAB) or permeant 3-amino-9-ethylcarbazole (AEC). Sections were counterstained with modified Harris hematoxylin, followed by traditional dehydration, clearing, and mounting.
Cell cultures
The melanoma tumor cell line DM6 was a kind gift from Dr. Hilliard F. Seigler, Department of Immunology, Duke University Medical Center. This cell line was established in vitro from melanoma involved lymph nodes [16]. These cells conserve the properties of the original tumor and express S-100, HMB-45 and MART-1 which are melanocyte-specific biological markers for metastatic melanoma [17].
Immunofluorescence microscopy
DM6 cells were plated at 5 × 105 cells/ml on Lab-Tek® II Chamber Slides (Nalge Nunc International, Naperville, IL) and allowed to adhere overnight. Cells were incubated for 1 h at 4°C in PBS containing 2% bovine serum albumin (BSA), 0.2 mg/ml goat IgG, and 0.01% NaN3 (staining buffer), followed by incubations with anti-GRP78 or nonimmune human IgGs (2 μg/ml) for 1 h at 4°C. Cells were washed in PBS and incubated for 1 h with an Alexa Fluor® 488-conjugated to goat anti-human IgG before washing and fixing in 4% paraformaldehyde. Immunofluorescence microscopy was performed using an Olympus BX-60 microscope (Olympus, Lake Success, NY).
Antibody binding studies by flow cytometry
DM6 cells were detached from the culture flasks (75 cm2) by incubation for 5 min at 37°C with Ca2+ and Mg2+-free phosphate-buffered saline (PBS) containing 4 mM EDTA and pelleted. The cells (1 × 106/ml) were washed with PBS before resuspension in ice-cold PBS containing 3% bovine serum albumin (staining buffer). The cell suspensions (500 μl) were incubated 30 min with human anti-GRP78 IgG (10 μg/ml). An equivalent quantity of non-immune human IgG was used as an isotype control. At this time the cells were washed, pelleted, and resuspended in 500 μl of ice-cold staining buffer. The cell suspensions were incubated for 30 min in the dark with AF488-conjugated goat anti-human IgG. The cells were washed twice with ice-cold staining buffer, resuspended in the same buffer and stored in the dark at 4°C for 10 min until analysis. Staining with propidium iodide (2 μg/ml) was performed immediately prior to flow cytometric analysis to exclude dead cells. Flow cytometry was conducted using the Guava EasyCyte Plus system (Guava Technologies, Hayward, CA). Data analysis and histogram preparation was performed using FlowJo® 8.6.3 software (Treestar Inc., Ashland, OR).
DM6 cell proliferation assays
DM6 cells suspended in RPMI 1640 containing 5% fetal bovine serum at a density of 1 × 105 cells/ml were plated in 96-well culture plates (0.1 ml/well) containing increasing concentrations of human melanoma patient anti-GRP78 IgG at a final volume of 0.2 ml/well. Cell proliferation was determined as previously described [2].
Stimulation of DM6 Melanoma Cells with Differentially Glycosylated IgG Fractions
DM6 cells plated in 96-well cell culture plates grew until roughly 90% confluent. After 5 h of serum starvation, we applied 100μl of RPMI with 100pM concanavalin A-Sepharose-separated IgG from individual melanoma patients. After 15 min stimulation at 37°C, the cells were rinsed twice with ice-cold PBS and then lysed in 100μl per well of PBS with 1mM EDTA, 1M urea, protease inhibitors, and phosphatase inhibitors (HALT Protease Inhibitor Cocktail and HALT Phosphatase Inhibitor Cocktail, Thermo Scientific, Rockford, IL) for 15 minutes on ice. Samples were centrifuged in v-bottom plates at 2000g for 5 mins, transferred to a clean plate, and diluted 1:1 with PBS with 1mM EDTA, 0.5% Triton X-100 and 5mM NaF. 100μl aliquots of each sample were used directly for ELISA quantitation of total and phosphorylated Akt (R&D Systems Inc., Minneapolis, MN) according to the manufacturer's protocol. All conditions were done in triplicate and performed twice. Measurements of phospho-Akt were normalized to total Akt levels.
SDS-PAGE and immunoblotting
Isolated IgGs were analyzed on 12 % polyacrylamide gels (1.2 mm thick, 14 × 10 cm) containing 0.1% SDS under reducing conditions. The IgG-derived Fc fragments were analyzed under reducing conditions on 15 % polyacrylamide gels. A discontinuous Laemli buffer system was used [18]. Transfer to nitrocellulose membranes by the Western blot method was carried out as described by Towbin et al. [19]. The molecular weights were assesed using a set of dye-conjugated Mr markers (Fermentas Life Sciences, Glen Burnie, MD).
Proteolytic digestion of IgG molecules and analysis of their fragments
Singly purified anti-GRP78 IgGs were digested with papain to identify the region in the molecule affected by aberrant glycosylation. The IgGs (200 μg) in 25 mM phosphate, pH 7.0, containing 10 mM EDTA and 10 mM cysteine were incubated with 50 μg of immobilized papain (Pierce Chemical Co., Rockford, IL) for 6 h at 37°C. The Fab and Fc fragments and undigested IgG were separated from the immobilized papain using Micro Bio-spin® chromatography columns (Bio-Rad, Hercules, CA). The digested material was applied to an immobilized protein A/G column equilibrated with PBS. The column was washed with PBS and the eluate was collected (5 ml). This unbound fraction contained the Fab fragments. The Fc fragments were eluted from protein A/G with 20 mM Glycine-HCl, pH 3.0, followed by dialysis against PBS, and concentrated using an Amicon centrifugal concentrator with a 10-kDa cutoff (Millipore Corporation, Bedford, MA). The Fab and Fc fragments were transferred to nitrocellulose membranes, reacted with specific rabbit anti-human Fab or Fc IgGs conjugated to HRP and then developed with the horseradish peroxidase substrate 4-chloro-1-naphtol. Densitometric analysis was performed using the program Image Quant®for Windows, Version 5.2, from Molecular Dynamics (Sunnyvale,CA).
Removal of N-linked oligosaccharides from GRP78 autoantibodies
Anti-GRP78 IgG (1 mg) in 20 mM sodium phosphate, pH 7.5, containing 150 mM NaCl and 0.02% sodium azide, was incubated with 100 milliunits of recombinant N-Glycanase (Peptide-N-Glycosidase F) from Glyko Inc. (Novato, CA) at 37°C for 16 h. The N-deglycosylated anti-GRP78 IgG was then separated from the N-Glycanase by chromatography on an immobilized protein A/G column equilibrated with PBS as described above.
Effect of GRP78 autoantibodies on expression of proteins of UPR signaling pathways
DM6 cells were plated on 6-well plates at a concentration of 3×105 cells per well. When cells reached 90% confluency, anti-GRP78 IgG, non-immune IgG control, or N-deglycosylated anti-GRP78 IgG (1ug/ml) in serum-free DMEM was added to the DM6 cell monolayers and incubated at 37°C for 24 h in triplicate. After aspirating culture medium and washing wells with PBS, cells were lysed with RIPA buffer (150mM NaCl, 0.5%D.O.C., 50mM Tris-HCl pH8.0, 1% NP-40, 0.1%SDS, 1× Halt™ Protease inhibitor) and sonicated. Protein concentration was determined using the bicinchonic acid procedure and loaded (35 μg) on each well of a 4-12% Bis-Tris gel (NuPAGE, Invitrogen). Proteins were then electroblotted to nitrocellulose membranes and singly reacted with antibodies against ATF-6α, IRE1α, XBP-1, N20 GRP78, procaspase-12, cleaved caspase-12, procaspase-9, cleaved caspase-9, cleaved caspase-3 and β-Actin purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The detection and quantification of these proteins in immunoblotts were performed by ECF and phosphorimaging using a STORM 860 ECF Imager System from General Electric (Piscataway, NJ).
Statistics
The levels of anti-GRP78 IgG were evaluated by a Student's t test using the program SYSTAT® for Windows: Statistics, Version 11 (Systat, Inc., Evanston, IL, USA).
Results
Anti-GRP78 IgG levels and analyses of their D-mannose content in serum from malignant melanoma patients
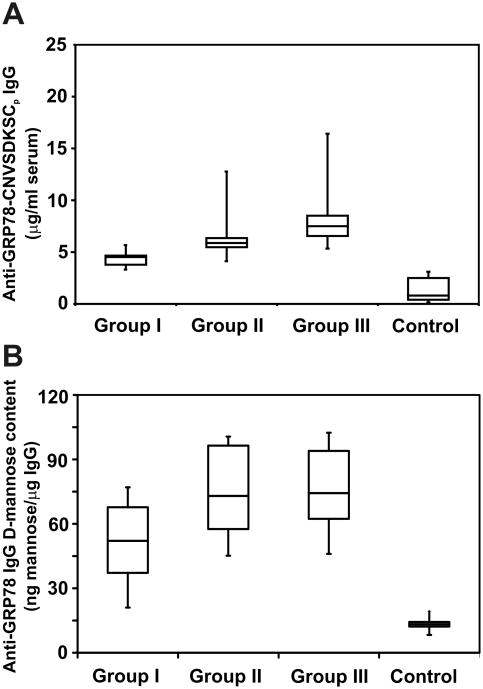
These analyses were performed in malignant melanoma patients (n=32) divided into three groups according to the growth phase of their tumors. Group I (n=6) contained patients with stage IIIa disease. Group II (n=9) represents patients with stage IIIb and IIIc disease. Group III (n=17) contained patients with stage IV disease. Their serum titers were compared with a group of normal controls (n=15). In a previous report [2], we found that circulating anti-GRP78 IgG autoantibodies in prostate cancer patients react mainly with the CNVSDKSC peptide, localized in the GRP78 N-terminal region. We performed similar assays with malignant melanoma patient serum using plates coated with total murine recombinant GRP78 and found titer values (data not shown) within the same order of magnitude of those determined using an ELISA assay with this peptide. The results in our subject groups (Fig. 1A) show titer increases that are statistically significant between the three groups of patients and controls (means, 4.4 ± 0.84, 6.38 ± 2.54, 8.39 ± 3.03 and 1.33 ± 1.05 μg/ml, respectively; P< 0.005). These experiments demonstrate that most of the reactivity (>90%) resides in the N-terminal region of GRP78, thereby confirming our previous observations in prostate cancer patients [2]. Then, we assessed the D-mannose content of anti-GRP78 autoantibodies using ELISA plates coated with this peptide and the Galanthus nivalis lectin as a detecting reagent. The results (Fig. 1B) show a significantly greater reactivity of this lectin with patient anti-GRP78 antibodies from the three groups compared to the control population (means, 56.2 ± 3.87, 73.58 ± 5.54, 78.33 ± 5.07 and 11.27 ± 1.23 ng D-mannose/μg anti-GRP78 IgG, respectively; P< 0.005).
Figure 1.
Serum levels of anti-GRP78 IgG and assay of their binding to concanavalin A in malignant melanoma patients (n=32) and normal controls (n=15). Group I (n=6) contains patients with stage IIIa disease. Group II (n=9) represents patients with stage IIIb and IIIc disease. Group III (n=17) contains patients with stage IV disease. Serum samples (50 μl, 1:500 dil) from patients and normal controls in 0.2 ml PBS-Tween were added to 96-well culture plates and assayed as described under Materials and Methods. A, comparison of anti-GRP78 serum titers of malignant melanoma patients and normal controls in plates coated with KLH-conjugated CNVSDKSC peptide. B, D-mannose content of the anti-GRP78 IgGs in the three groups of patients. Horizontal lines, mean titer values.
Specific identification of aberrant anti-GRP78 IgG in the serum of malignant melanoma patients
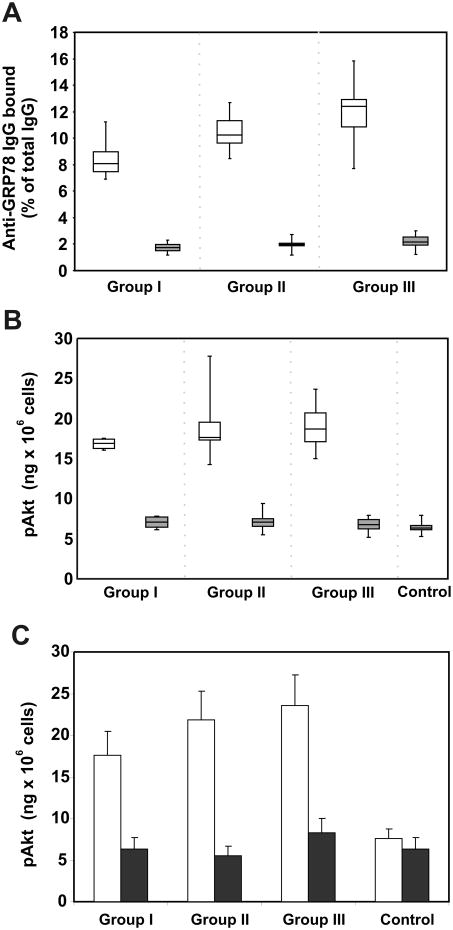
First, total IgG was isolated from each individual serum by chromatography on protein A/G-agarose, followed by chromatography on concanavalin A-Sepharose to separate the IgG populations with high and low D-mannose contents [11]. Then, similar concentrations (100 ng/well) of the three IgG populations were incubated in ELISA plates coated with the CNVSDKSC peptide as described above. Binding of anti-GRP78 was calculated and expressed as a percentage of total IgG. The results for the three groups of patients (Fig. 2A) show that the majority of the anti-GRP78 autoantibodies are found in the fractions with affinity for concanavalin A. We also examined the capacity of these IgGs (100 pM) to induce phosphorylation of Akt in DM6 malignant melanoma cells (Fig. 2B). These data show increased p-Akt in cells incubated with the IgG fraction with high affinity for concanavalin A, whereas the IgG fraction not retained by concanavalin A shows only a moderate effect. Next, we took IgG samples (100 pM) from each patient group and incubated these IgGs with this peptide (500 pM) before addition to DM6 cells. The results (Fig, 2C) from a representative high-mannose containing sample in the three groups of patients show that GRP78 in solution inhibits the effect of the IgG, thereby confirming that anti-GRP78 autoantibodies are responsible for Akt phosphorylation.
Figure 2.
Identification of aberrantly glycosylated anti-GRP78 IgGs in the serum of malignant melanoma patients, and their effect on Akt phosphorylation in melanoma DM6 cells. Assays are shown separately for each group of patients. Total IgG was purified from each single serum and then fractionated by chromatography on a concanavalin A-Sepharose resin. A, anti-GRP78 IgG in total IgG samples (100 ng) bound to concanavalin A (
 ), and not-bound to concanavalin A (
), and not-bound to concanavalin A (
 ). IgGs were added to 96-well culture plates, coated with the peptide CNVSDKSC conjugated to KLH. The anti-GRP78 IgG bound is expressed as a percentage of the total IgG used on each assay. B, Akt phosphorylation was calculated after incubation of DM6 cells with a single concentration (100 pM) of the IgG fractions bound (□) or not bound to concanavalin A (■). A group of normal control human IgGs (n=15) is also included. All experiments were done in triplicate. C, Akt phosphorylation in DM6 cells induced by a sample of IgGs (100 pM) from the three groups of patients in the absence (
). IgGs were added to 96-well culture plates, coated with the peptide CNVSDKSC conjugated to KLH. The anti-GRP78 IgG bound is expressed as a percentage of the total IgG used on each assay. B, Akt phosphorylation was calculated after incubation of DM6 cells with a single concentration (100 pM) of the IgG fractions bound (□) or not bound to concanavalin A (■). A group of normal control human IgGs (n=15) is also included. All experiments were done in triplicate. C, Akt phosphorylation in DM6 cells induced by a sample of IgGs (100 pM) from the three groups of patients in the absence (
 ) and presence (■) of recombinant murine GRP78 (500 pM).
) and presence (■) of recombinant murine GRP78 (500 pM).
Electrophoretic analyses of aberrant anti-GRP78 IgGs in malignant melanoma patients during the clinical course of the disease
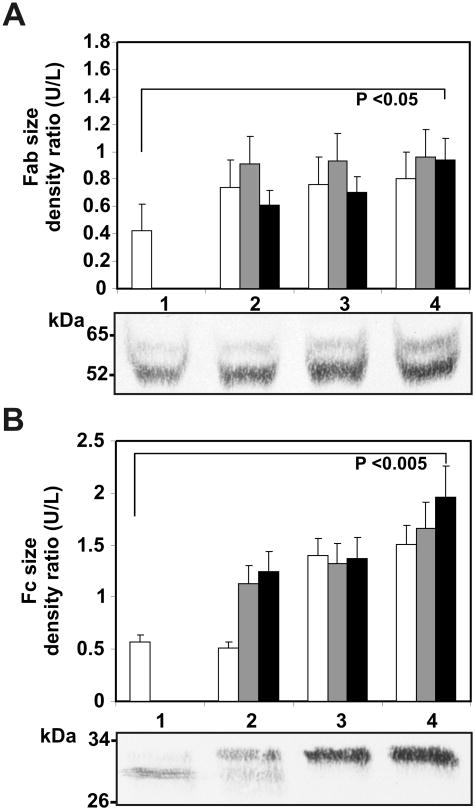
A large percentage of the aberrantly glycosylated IgGs appear to be directed against GRP78. We investigated any changes in the glycosylation of the GRP78 autoantibody in the serum of three patients during a 9 month period. Serum samples were obtained at the time of diagnosis, followed by samples at 7 and 9 months. The specific anti-GRP78 IgGs were purified by immunoaffinity chromatography on CNVSDKSC-carboxyhexyl-Sepharose. During this period, the antibody serum concentration increased significantly from 7.23 ± 2.54 μg/ml, 9.35 ± 3.21 μg/ml, and 8.76 ± 2.18 μg/ml at the beginning of the study to 12.47 ± 2.83 μg/ml, 17.42 ± 3.51 μg/ml, and 18.93 ± 3.62 μg/ml, respectively, for the three patients at 9 months (p<0.001).
Each single anti-GRP78 IgG was digested with papain and analyzed by SDS-PAGE. Representative IgG fragments obtained from patient 1 are shown. Fab or Fc protein fragments from IgG purified from three patients was quantified by densitometric analyses, and the change in size was expressed as the ratio between the upper (U) and lower (L) protein bands compared with normal IgG fragments. The Fab fragments from melanoma anti-GRP78 IgG exhibited a 55 kDa heavy chain and the progressive appearance of a 65 kDa band, when compared with normal IgG during this period (Figs. 3A). The Fc fragments show an even more dramatic shift in molecular weight from the control Fc (Figs. 3B), thereby suggesting that both the Fab and the Fc region of the anti-GRP78 IgG of melanoma patients is aberrantly glycosylated.
Figure 3.
Electrophoretic analysis of anti-GRP78 IgG glycosylation from three malignant melanoma patients. Specific GRP78 IgGs were purified by immunoaffinity chromatography and fragmented by papain digestion as described in Materials and Methods. Western blot images of patient 1 are shown. The analysis covers a period of nine months. Sizes of the IgG fragments during this period were compared with normal IgG fragments. A: comparison of the sizes of Fab fragments between normal IgG (□) and anti-GRP78 IgG at the beginning (2) for patient 1 (□), patient 2 (
 ) or patient 3 (■), followed by analyses at seven months (3) or nine months (4) after initiating the study. B: comparison of the sizes of Fc fragments between normal IgG and anti-GRP78 IgG at the beginning (2) for patient 1 (□), patient 2 (
) or patient 3 (■), followed by analyses at seven months (3) or nine months (4) after initiating the study. B: comparison of the sizes of Fc fragments between normal IgG and anti-GRP78 IgG at the beginning (2) for patient 1 (□), patient 2 (
 ) or patient 3 (■), followed by analyses at seven months (3) or nine months (4) after initiating the study.
) or patient 3 (■), followed by analyses at seven months (3) or nine months (4) after initiating the study.
Histologic and immunohistochemical studies
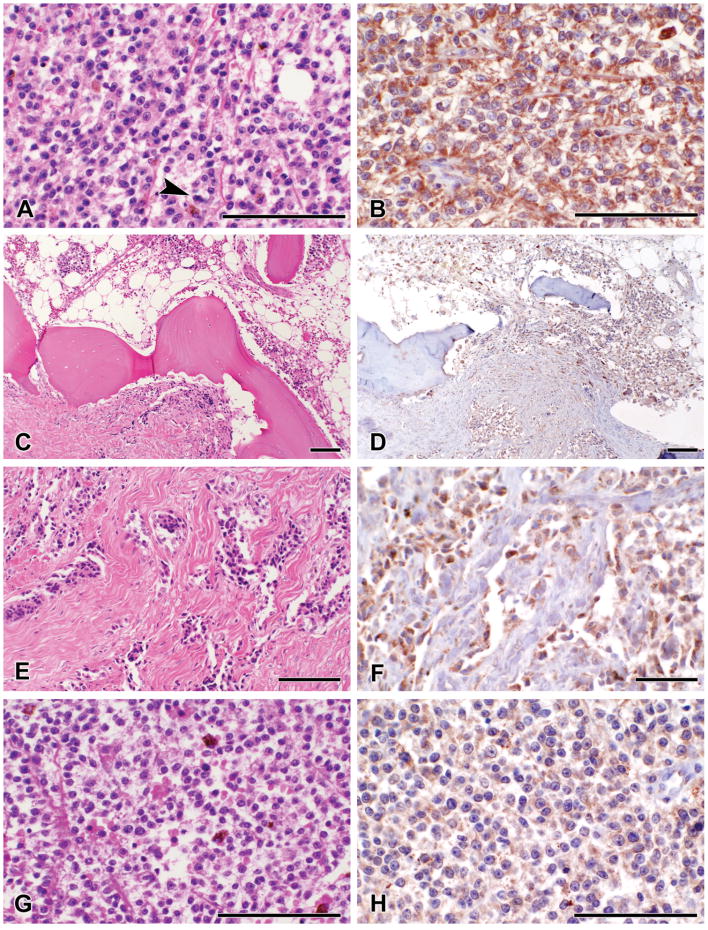
Expression of GRP78 in tissue from these patients was qualitatively evaluated during this period. Progression of the disease was examined by histologic and immunohistochemical analyses on three representative patients. Because similar changes were observed in all three patients tested, data is shown only for a representative patient (patient 3, 18.93 ± 3.62 μg/ml of anti-GRP78 IgG). The patient, a 20 year-old male, was diagnosed with invasive malignant melanoma of the left great toe. The latter was amputated at the metatarsal phalangeal joint. Gross examination of the specimen showed a subungueal ill-defined ulcerated mass measuring 4.5 cm in greatest dimension. Histologic examination of this area revealed a proliferation of enlarged melanocytes with high nuclear to cytoplasmic ratio and prominent nucleoli (Fig. 4A). These severely atypical melanocytes exhibited confluent growth with a significant pagetoid spread. The tumor invaded the dermis and subcutaneous tissue and was diagnosed as a malignant melanoma at Clark level V with a Breslow depth of 13 mm. High mitotic rate was seen (13 mitosis per mm2) (Fig. 4A arrow). Bone involvement was noted (Figs. 4C and 4E). Seven months after the amputation, a left groin node dissection revealed malignant melanoma replacing four of twenty-five lymph nodes. Sections from the involved lymph nodes showed melanocytes sharing the same histologic phenotype with those seen in the primary amputation (Fig. 4G).
Figure 4.
Immunohistochemical staining of GRP78 in tissue sections obtained from patient 3 during a period of 9 months. A, hematoxylin-eosin stained sections of the tumor at the beginning of the study, showing proliferation of severely atypical melanocytes with mitotic activity (arrow). B, GRP78 expression by the neoplastic cells at the beginning of the study. C, E, hematoxylin-eosin stained sections of the tumor showing invasion of the malignant melanoma to the bone at the beginning of the study. D, F, malignant melanocytes invading connective tissue and bone show expression of GRP78. G, metastatic malignant melanoma is detected in lymph nodes at 7 and 9 months of the study. H. the metastatic tumor maintains expression of GRP78 in both lymph node resections during this study.
Two months after the resection of the left groin lymph nodes, the patient showed progressive disease. Two of eleven left lymph nodes from the iliac region, and one of seven from the obturator region showed metastatic malignant melanoma. Histologic sections showed lymph node parenchyma replaced by severely atypical melanocytes. GRP78 was expressed in malignant melanoma cells in the epidermal as well as in the dermal compartments of the primary tumor (Fig. 4B). Tumor cells maintained expression of the protein at the level of the bone invasion (Figs. 4D and 4F). GRP78 was also expressed in the cytoplasm of malignant melanocytes in the lymph node metastases; however, the degree of expression is lower than in the primary tumor (Figure 4H).
Analyses of binding of melanoma anti-GRP78 IgG to human malignant melanoma cells by immunofluorescence microscopy and flow cytometry
We studied binding of the malignant melanoma anti-GRP78 IgG to DM6 melanoma cells grown on Chamber Slide Systems. Immunofluorescence staining of non-permeabilized DM6 cells incubated with anti-GRP78 IgG (Fig. 5A) or nonimmune human IgG (Fig. 5B) showed surface expression of GRP78 on these cells. These results were confirmed by flow cytometric analyses with anti-GRP78 IgG (Fig. 5C).
Figure 5.
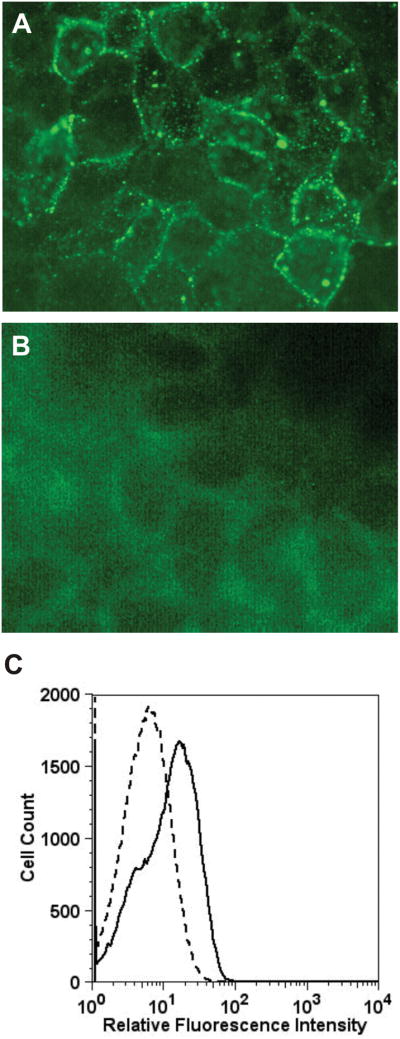
Binding of GRP78 to human melanoma DM6 cells (A) Immunofluorescence microscopy of non-permeabilized DM6 cells incubated with anti-GRP78 IgG. (B) Immunofluorescence microscopy of non-permeabilized DM6 cells incubated with nonimmune human IgG. (C) Flow cytometric analysis of anti-GRP78 IgG binding to DM6 cells. The solid line represents cells incubated with autoantibodies directed against GRP78. The dashed line represents binding of isotype control.
Effect of melanoma anti-GRP78 IgG on human melanoma cell proliferation and Akt phosphorylation
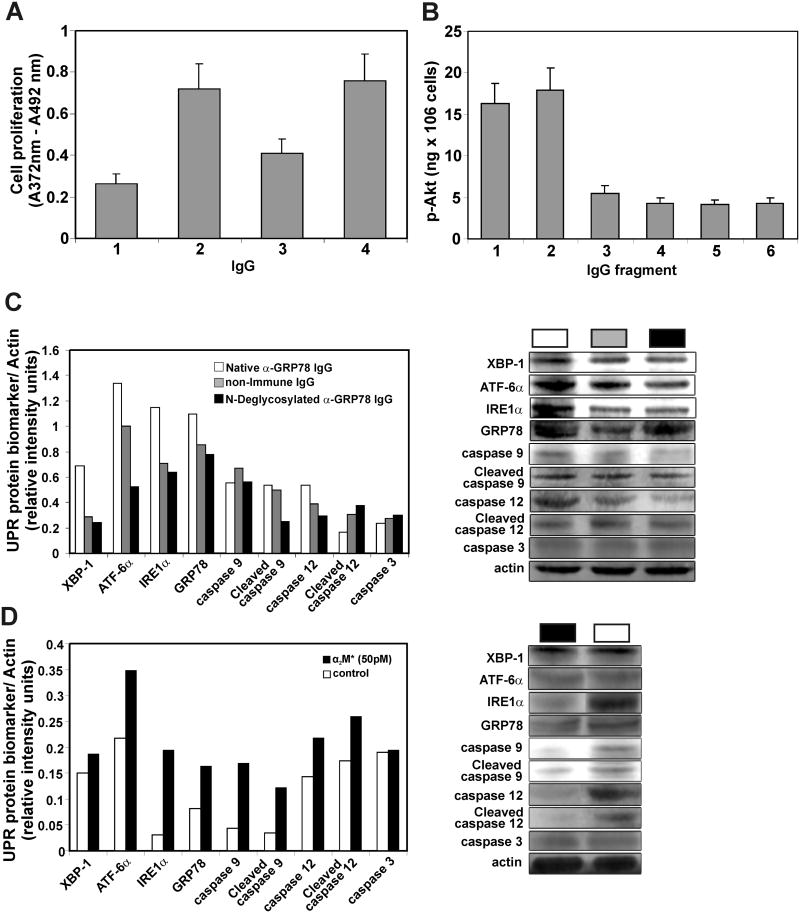
Cell proliferation was determined as described under Materials and Methods. Total anti-GRP78 IgG was purified by affinity chromatography from a serum pool (5 ml) mixing 0.5 ml of serum from each patient. DM6 cells were incubated for 72 h in RPMI 1640 culture medium containing 5% FBS with melanoma anti-GRP78 at 50 pM (Fig. 6A3) or 100 pM (Fig. 6A4). As a positive control, they were incubated with 100 pM prostate cancer anti-GRP78 IgG (Fig. 6A2). As a negative control, they were incubated with 100 pM nonimmune human IgG (Fig. 6A1). Under these conditions, DM6 cells show statistically significant increases in proliferation induced by both autoantibodies when compared with cells incubated with the non-immune human IgG (Fig. 6A2, p<0.0001; 6A3, P<0.004; 6A4, P<0.0001).
Figure 6.
Effect of patient anti-GRP78 on human melanoma cell proliferation and Akt phosphorylation. A, cell proliferation was determined using a BrdU labeling and colorimetric immunoassay detection method as described under Materials and Methods. DM6 cells were incubated for 72 h in RPMI 1640 culture medium containing 5% FBS with the IgG. A1, nonimmune human IgG (100 pM). A2, prostate cancer anti-GRP78 IgG (100 pM). A3, melanoma anti-GRP78 IgG (50 pM). A4, melanoma anti-GRP78 IgG (100 pM). B, for Akt phosphorylation assays, DM6 cells were incubated with a single concentration (100 pM) of the anti-GRP78 or normal non-immune human IgG and their fragments. B1, melanoma anti-GRP78 IgG. B2, Fab fragment from melanoma IgG. B3, Fc fragment from melanoma anti-GRP78 IgG. B4, non-immune IgG. B5, Fab fragment from non-immune IgG. B6, Fc fragment from non-immune IgG. All experiments were performed in triplicate. C, Up-regulation of UPR signaling components. Shown are XBP-1, ATF-6α, IREα, GRP78, procaspase-9, cleaved caspase-9, procaspase 12, caspase-12 and cleaved caspase 3 after incubation of DM6 cells with a single concentration (1 μg/ml) of native anti-GRP78 IgG (□), non-immune control IgG (
 ) or N-deglycosylated anti-GRP78 IgG (■) in serum-free DMEM as described under Materials and Methods. D, Up-regulation of UPR biological markers and caspases described above after incubation of DM6 cells with a single concentration of α2M* (50 pM). (□) Cells incubated in α2M*-free culture medium. (■) Cells incubated in α2M*-containing culture medium.
) or N-deglycosylated anti-GRP78 IgG (■) in serum-free DMEM as described under Materials and Methods. D, Up-regulation of UPR biological markers and caspases described above after incubation of DM6 cells with a single concentration of α2M* (50 pM). (□) Cells incubated in α2M*-free culture medium. (■) Cells incubated in α2M*-containing culture medium.
For Akt phosphorylation assays, DM6 cells were incubated with a single concentration (100 pM) of the anti-GRP78 or non-immune human IgG and their fragments (Fig. 6B). Both the intact anti-GRP78 IgG (Fig. 6B1) or its Fab fragment (Fig 6B2) induce Akt phosphorylation, whereas the melanoma anti-GRP78 Fc fragment (Fig 6B3) or the non-immune IgG or its Fab and Fc fragments (Figs. 6B4, 6B5 and 6B6, respectively) do not have any effect.
Effect of anti-GRP78 IgG on UPR signaling and caspase activation pathways
We have previously observed that activated α2-macroglobulin (α2M*) induces expression of GRP78 in a dose-dependent manner [20]. As observed above, the malignant melanoma anti-GRP78 IgG-stimulated DM6 cell proliferation mimics that induced by α2M*. Then, we measured expression of GRP78, XBP-1, ATF6α, and IRE1α as ER stress biomarkers at a single concentration (1 μg/ml) of anti-GRP78 IgG, non-immune IgG control or N-deglycosylated anti-GRP78 IgG in serum-free DMEM. The analysis (Fig. 6C) shows increases of GRP78, XBP-1, ATF6α, and IRE1α in cells incubated with native anti-GRP78 IgG, whereas the N-deglycosylated or non-immune control IgGs did not affect the levels of these ER stress biomarkers. During ER stress, caspases are activated through cleavage by upstream caspases, which lead to apoptotic cell death [21]. Activation of caspase-12 is specific to ER stress. Once activated, the catalytic subunit of caspase-12 is released into the cytosol, where it activates the caspase-9 cascade. For this reason, we also quantified levels of caspase-12, cleaved caspase-9 and cleaved caspase-3 (Fig. 6C). We observed no activation of caspase-12 or caspase-9 in DM6 cells incubated with either fully glycosylated or N-deglycosylated anti-GRP78 IgG. We also analyzed UPR signaling on cells stimulated with α2M* (Fig. 6D) and observed increases of GRP78, XBP-1, ATF6α, and IRE1α in DM6 cells, thus confirming previous observations in prostate cancer 1-LN cells [20]. Similarly, DM6 cells incubated with α2M* show no significant activation of caspases 3, 9 and 12 when compared with control cells incubated with culture medium alone. These results suggest that the anti-GRP78 IgG stimulates cell proliferation via up-regulation of ER stress biomarkers in a fashion analogous to that of α2M* which leads to prevention of apoptosis. Furthermore, they also suggest that hyper-glycosylation of the anti-GRP78 IgG is necessary for this mechanism.
Discussion
High levels of GRP78 expression in many types of cancers, such as breast [22], lung [23], gastric [24], colonic [25], prostatic [26] hepatocellular carcinoma [27] and malignant melanoma [4], have been correlated with an adverse prognosis. Conversely, augmented expression of GRP78 is also associated with a better prognosis in neuroblastoma [28] and is an indicator of favorable responsiveness to chemotherapy in breast cancer [29] and malignant gliomas [30]. These discrepancies demonstrate that expression of GRP78 needs to be carefully analyzed before considering it as a reliable prognostic biomarker in cancer. However, it is possible to associate GRP78 expression with the presence in the circulation of very high titers of an autoantibody against GRP78 in prostate and ovarian cancer [1, 3]. Expression of GRP78 in malignant melanoma was recently assessed [4], but these investigators did not correlate their findings with the autoimmune response to GRP78. We recently demonstrated a causal link between a humoral response to GRP78 and the progression of cancer in a murine malignant melanoma model [5].
In the present study, we assessed the titers of specific anti-GRP78 IgG autoantibodies in patients with malignant melanoma (n=32), divided in three groups according to tumor stage, and found that their levels are significantly higher than those found in the control population. The malignant melanoma anti-GRP78 autoantibodies recognize preferentially the primary amino acid sequence CNVKSDKSC, which contains a tertiary structural motif mimicking an epitope in GRP78 [2]. Their binding to the Galanthus nivalis lectin, which interacts with mannose-rich oligosaccharides [13], not normally present in circulating IgG, suggests that they are aberrantly glycosylated.
The highly mannosylated IgG fraction containing a greater percentage of GRP78 autoantibodies induced larger Akt phosphorylation levels in DM6 human melanoma cells when compared with the normally glycosylated IgG fraction. Akt phosphorylation was also stimulated by affinity purified malignant melanoma anti-GRP78 IgG, thereby suggesting their involvement in increased Akt phosphorylation levels. It is known that Akt deregulation promotes malignant melanoma cell proliferation and survival [31]
We correlated expression of GRP78 protein with autoantibody titers during the progression of malignant melanoma in three patients at disease stages III and IV. GRP78 was found in radial and vertical growth phases of primary malignant melanomas, thereby suggesting its involvement in primary tumor development. Furthermore, GRP78 continues to be expressed in malignant melanoma cells after they acquire metastatic potential involving lymph nodes and viscera. At the same time, there is an increase in titers of antibodies against this protein in serum, suggesting a correlation between these two events when the aggressiveness of malignant melanoma intensifies.
Analyses of purified anti-GRP78 IgGs from serum of these three patients, produced during this period, show that Fab and Fc regions increase in their glycosylation. The proportion of aberrantly glycosylated Fab fragment (55 to 65 kDa) is similar to that observed in ovarian cancer [7], whereas aberrant glycosylation, leading to a change in size of the Fc fragment (27 to 33 kDa), is almost complete in the malignant melanoma anti-GRP78 IgG. Changes in size of the Fc fragment indicate that the Asn 297 N-linked oligosaccharide is the target of this phenomenon, which probably results from lack of processing of D-mannosyl residues, thereby adding to the size of this region [9]. The presence of two Fab chains is an indication of asymmetric glycosylation. Therefore, the anti-GRP78 IgG is asymmetrically glycosylated at the Fab region and aberrantly glycosylated at the Fc. Functionally, the asymmetric Fab glycosylation matters, as reflected by its capacity to induce phosphorylation of Akt. A relationship between changes in the N-linked glycosylation of human serum IgG and progression of prostate, lung, and gastric cancer has been also reported [32-34].
The asymmetrical glycosylation of the anti-GRP78 IgG is essential for up-regulation of GRP78 and XBP-1, ATF6α, and IRE1α as ER stress biomarkers [20]. Since α2M* up-regulates these biological markers in DM6 cells in a fashion similar to that observed with prostate cancer 1-LN cells [20], we infer that the anti-GRP78 IgG also mimics the effects of α2M* in these cells. Similarly, α2M* did not promote activation of caspase-3, caspase-9 or caspase-12, thereby confirming the function of these autoantibodies to GRP78 as protective to tumor cells.
In summary, here we show that treatment of DM6 malignant melanoma cells with antibodies to GRP78 isolated from malignant melanoma patients promotes their proliferation by activating Akt. To overcome the stress induced by rapid growth in the presence of asymmetrically glycosylated autoantibodies to GRP78 which mimic the function of α2M*, these cells trigger UPR signaling which in turn may be regulated by ER- and plasma membrane-associated GRP78.
Abbreviations used
- GRP78
glucose-regulated protein of 78 kDa
- Akt
protein B-dependent protein kinase
- XBP1
X-box binding protein 1
- ATF6α
activating transcription factor 6
- IRE1α
serine/threonine-protein kinase/endoribonuclease precursor
Footnotes
Financial Support: NIH CA 131235.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mintz PJ, Kim J, Do KA, Wang X, Zinner RG, Cristofanilli M, et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nature Biotech. 2003;21:57–63. doi: 10.1038/nbt774. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Gronow M, Chucacovich M, Llanos C, Urzua C, Gawdi G, Pizzo SV. Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 2006;66:11424–11431. doi: 10.1158/0008-5472.CAN-06-1721. [DOI] [PubMed] [Google Scholar]
- 3.Chinni SR, Gerçel-Taylor C, Flachetto RA, Shabamovitz J, Hunt DF, Taylor DD. Cathepsin D and glucose-regulated protein 78 recognized by the humoral response of ovarian cancer patients. Clin Cancer Res. 1997;3:1557–1564. [PubMed] [Google Scholar]
- 4.Zhuang L, Scoyler RA, Lee CS, McCarty SW, Cooper WA, Zhang XD, et al. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462–470. doi: 10.1111/j.1365-2559.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 5.de Ridder GG, Gonzalez-Gronow M, Ray R, Pizzo SV. Autoantibodies against cell surface GRP78 promote tumor growth in a murine model of melanoma. Melanoma Res. 2011 doi: 10.1097/CMR.0b013e3283426805. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor DD, Gerçel-Taylor C. Tumor-reactive immunoglobulins in ovarian cancer: diagnostic and therapeutic significance. Oncol Rep. 1998;5:1519–1524. doi: 10.3892/or.5.6.1519. [DOI] [PubMed] [Google Scholar]
- 7.Gerçel-Taylor C, Bazzett LB, Taylor DD. Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol Oncol. 2001;81:71–76. doi: 10.1006/gyno.2000.6102. [DOI] [PubMed] [Google Scholar]
- 8.Margni LA, Binaghi RA. Nonprecipitating asymmetric antibodies. Annu Rev Immunol. 1988;6:535–554. doi: 10.1146/annurev.iy.06.040188.002535. [DOI] [PubMed] [Google Scholar]
- 9.Wright A, Morrison SL. Antibody variable region glycosylation: biochemical and clinical effects. Springer Semin Immunopathol. 1993;15:259–273. doi: 10.1007/BF00201106. [DOI] [PubMed] [Google Scholar]
- 10.Margni RA, Bortel IM. Paradoxical behaviour of asymmetric IgG antibodies. Immunol Rev. 1998;163:77–87. doi: 10.1111/j.1600-065x.1998.tb01189.x. [DOI] [PubMed] [Google Scholar]
- 11.Leoni J, Labeta M, Margni RA. The asymmetric IgG non-precipitating antibody. Localization of the oligosaccharide involved by Concanavalin A interaction. Mol Immunol. 1986;23:1397–1400. doi: 10.1016/0161-5890(86)90026-x. [DOI] [PubMed] [Google Scholar]
- 12.King L, Berg M, Chevalier M, Carey A, Elguindi EC, Blond SY. Isolation expression, and characterization of fully functional nontoxic Bip/GRP78 mutants. Prot Exp Pur. 2001;22:1481–158. doi: 10.1006/prep.2001.1424. [DOI] [PubMed] [Google Scholar]
- 13.Smith PK, Krohn RI, Hermanson GT. Measurement of protein using bicinchonic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 14.Shibuya N, Goldstein J, Van Damme EJM, Peumans WJ. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem. 1988;263:728–734. [PubMed] [Google Scholar]
- 15.Hashimoto C, Cohen RE, Zhang WJ, Ballou CC. Carbohydrate chains on yeast carboxypeptidase Y are phosphorylated. Proc Natl Acad Sci USA. 1981;78:2244–2248. doi: 10.1073/pnas.78.4.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darro WL, Slingluff CL, Seigler HF. The role of HLA class I antigens in recognition of melanoma cells by tumor-specific cytotoxic T lymphocytes. J Immunol. 1989;142:3329–3335. [PubMed] [Google Scholar]
- 17.Zubovits J, Buzney E, Yu L, Duncan LM. HMB-45, S-100, NK1/C3 and MART-1 in metastatic melanoma. Hum Pathol. 2004;35:217–223. doi: 10.1016/j.humpath.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NFκB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006;281:13694–13707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Hendershot LM. The role of the unfolded protein response in tumor development; fried or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez PM, Tabbara SO, Jacobs LK, Manning FC, Tsangaris TN, Schwartz AM, et al. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- 23.Uramoto H, Sugio K, Oyama T, Nakata S, Ono K, Yoshimatu T, et al. Expression of endoplasamic reticulum molecular chaperone GRP78 in human lung cancer and its clinical significance. Lung Cancer. 2005;49:55–62. doi: 10.1016/j.lungcan.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Song MS, Park YK, Lee JN, Park K. Induction of glucose-regulated protein 78 by chronic hypoxia in human gastric tumour cells through a protein kinase C-ε/ERK/AP-1 signalling cascade. Cancer Res. 2001;61:8322–8330. [PubMed] [Google Scholar]
- 25.Xing XM, Lai MD, Wang YH, Xu EP, Huang Q. Overexpression of glucose-regulated protein 78 in colon cancer. Clin Chim Acta. 2006;364:308–315. doi: 10.1016/j.cca.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, Cai J, et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, et al. Activation of the ATF6, XBP1 and GRP78 genes in hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–614. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 28.Hsu WM, Hsieh FJ, Jeng YM. GRP78 expression correlates with histologic differentiation and favorable prognosis in neuroblastic tumors. Int J Cancer. 2005;113:920–927. doi: 10.1002/ijc.20693. [DOI] [PubMed] [Google Scholar]
- 29.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 30.Pyrko P, Schönthal Ah, Hoffman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 31.Robertson GP. Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev. 2005;24:273–285. doi: 10.1007/s10555-005-1577-9. [DOI] [PubMed] [Google Scholar]
- 32.Kanoh Y, Mashiko T, Danbara M. Changes in serum IgG oligosaccharide chains with prostate cancer progression. Anticancer Res. 2004;24:3135–3139. [PubMed] [Google Scholar]
- 33.Kanoh Y, Ohara T, Mashiko T, Abe T, Masuda N, Akahoski T. Relationship between N-linked oligosaccharide chains of human serum immunoglobulin G and serum tumor markers with non-small cell lung cancer progression. Anticancer Res. 2006;26:4293–4298. [PubMed] [Google Scholar]
- 34.Kanoh Y, Ohara T, Tadano T, Kanoh M, Akahoski T. Changes to N-linked oligosaccharide chains of human serum immunoglobulin G and matrix metalloproteinase-2 with cancer progression. Anticancer Res. 2008;28:715–720. [PubMed] [Google Scholar]