Abstract
The pattern of neurodegeneration in Huntington’s disease (HD) is very characteristic of regional locations as well as that of neuronal types in striatum. The different striatal neuronal populations demonstrate different degree of degeneration in response to various pathological events in HD. In the striatum, medium spiny GABA neurons (MSN) are preferentially degenerate while others are relatively spared. Vulnerability of specific neuronal populations within the striatum to pathological events constitutes an important hallmark of degeneration in HD. In an attempt to explain a likely mechanism of degeneration of striatal neuronal populations in HD, possible causes underlying differential vulnerability of neuronal subtypes to excitoxic insults and neurotrophic factors are discussed in this paper.
Keywords: NMDA, Huntington's Disease, MSN
Introduction
Huntington’s disease (HD) is a genetically dominant neurodegenerative condition characterized by progressive loss of motor and cognitive function that is caused by degeneration of selected neuronal populations within the basal ganglia and the cerebral cortex. HD is mainly driven by a genetic defect on chromosome 4 that results in an increase of repetition CAG (>39 CAG repeat to manifest disease) at the encoding site of huntingtin protein.1 Profound effect in the degeneration of striatal projection neurons driven cognitive and motor impairments is the neuropathological signature of HD.2,3 Based on this observation, term “selective neuronal vulnerability” is proposed by numbers of investigators (Table 1).4 Overactivation of ionotropic glutamate receptors in response to endogenous or exogenous excitatory neurotransmitters via a pathological process that results in neuronal damage is well accepted as excitotoxicity phenomenon. Recent evidence suggests that excitotoxicity is one of the pathological pathways that is partly responsible for the degeneration of stratial neurons in HD.3
Table I: Differential vulnerability of specific cell populations in HD and its relationship to morphological and biochemical characteristics.
| Anatomical location | Cell type | Relative vulner-ability | Morphology | Afferents | Target | NT receptors | NT | Peptides | Other molecular markers |
|---|---|---|---|---|---|---|---|---|---|
| J Neurochem. 2010 June; 113(5): 1073–1091. | |||||||||
| Striatum | MSN (direct pathway) | +++ | projection neuron, long axon | Cortex (Glu), SNc (DA), Thalamus (Glu) | GPi, SNr | D1, NMDA, AMPA | GABA | Substance P/ Dynorphin | DARPP-32 GAD |
| MSN (direct pathway) | +++++ | projection neuron, long axon | Cortex (Glu), SNc (DA), Thalamus (Glu) | GPe | D2, NMDA, AMPA | GABA | Enkephalin | DARPP-32 GAD | |
| Interneurons | + | extensive dendritic net-work, amon projects locally | MSNs, other interneurons | MSNs, other interneu-rons | D2, NMDA, AMPA | Ach. | neuropetide Y, parvalbumin | iNOS somatostatin | |
| Cerebral Cortex | Pyramidal neurons (layers V/VI) | +++ | projection neuron, long axon | Thalamus, brainstem nuclei | Striatum, brainstem, thalamus | Glu, ACh, DA, NE, 5HT | Glu | - | MAP2 CaMK |
| Interneurons | + | extensive dendritic network, axon projects locally | Thalamus | Pyramidal neurons | Glu, GABA | GABA | Somatostain, neuropeptide Y | GAD | |
Striatal histology
95% of the striatal neurons are projection neurons and only 5% are interneurons. Striatal projection neurons (also known as Golgi type I cells) are all GABAergic. They have long axon, medium-sized cell body and spiny dendrite. Interneurons are cholinergic and morphologically distinguished by a large soma and wide dendritic arborisation.
Differential expression of glutamate receptor subtypes and excitotoxic neurodegeneration
Glutamate is a well-known excitatory amino acid transmitter in the CNS. It activates both N-methyl-D-aspartate (NMDA) and non- NMDA ionotropic glutamate receptors. The critical role of glutamate receptors in mediating excitotoxic neuronal death in various neurodegenerative diseases is widely accepted.5–8 Extensive studies show that abnormally sustained activation of NMDA receptors by glutamate can lead to prolonged increase in intracellular calcium via NMDA associated calcium channel.9 Subsequently calcium dependent enzymes are activated and nitric oxide (NO) is synthesized. Neuronal nitric oxide synthase (nNOS) by itself triggers a cascade that stimulates neuronal damage. Although both medium sized spiny neurons (MSNs) and interneurons have NMDA receptors, there is an obvious difference between MSNs and interneurons in terms of expression of glutamate receptor subunits. Intrastriatal injection of agonists for NMDA (quinolinic acid) and non-NMDA (kainic acid) to animal model has shown higher vulnerability of MSNs to glutamate-induced excitotoxicity, compared to interneurons.3,5 Lack of NMDA receptor subtype NR2B/NR2A in interneurons may make these cells less susceptible to excitotoxic insults. The second group of striatal interneurons are nicotinamide adenine dinucleotide phosphate (NADP) diaphorase positive that express very few NMDA receptors and are resistant to glutamate-induced excitotoxicity. Based on these observations, differential expression of glutamate receptor subtypes in striatal neuronal populations may participate in vulnerability of these cells to excitotoxic insults.
Selective protection of striatal neuronal subtypes by neurotrophic factors against excitotoxic insults.
Several protective mechanisms against different types of injuries exist in central nervous system. One of the mechanisms relies on neurotrophic factors that are involved in neuroprotection of neuronal cells. Among various neurotrophic factors in the striatum, members of neurotrophin and glial cell line derived neurotrophic factor (GDNF) are well known neurotrophic factors in striatum. Neurotrophic factors selectively protect specific neuronal populations against excitotoxic insults. Through useful experimental studies, engineered cells that released neurotrophins such as brain-derived neurotrophic factor (BDNF), NT-3, GDNF and neurturin were grafted in striatum before the intrastriatal injection of excitotoxic factors such as quinolinic acid or kainic acid. This study showed that BDNF and NT-3 equally protected both GABA/enkephalin and GABA/tackykinin positive neurons in striatum while GDNF and neurturin factors selectively protect striatal projection neurons of direct and indirect pathway, respectively. Cholinergic interneurons are only protected by GDNF.10,11 Based on the result of this study, selective protection of neurotrophic factors could be due to differential vulnerability of striatal neuronal populations. Another useful experimental study showed that intrastriatal injection of quinolinic acid increased expression of nerve growth factor (NGF) mRNA level while intrastriatal injection of Kainin acid induced expression of BDNF mRNA. Quinolinic acid and Kainin acid injection did not have any effect on expression of NT-3 (Fig. 1). Interestingly, down regulation of mRNA NT-3 was observed after intrastriatal injection of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA).12 This study supports the hypothesis, which suggests that activation of glutamate receptors in striatum by different excitotoxicity amino acids preferentially regulate mRNA expression of neurotrophic factors. This specific expression may explain differential vulnerability of striatal neuronal subtypes to these factors.
Fig. 1:
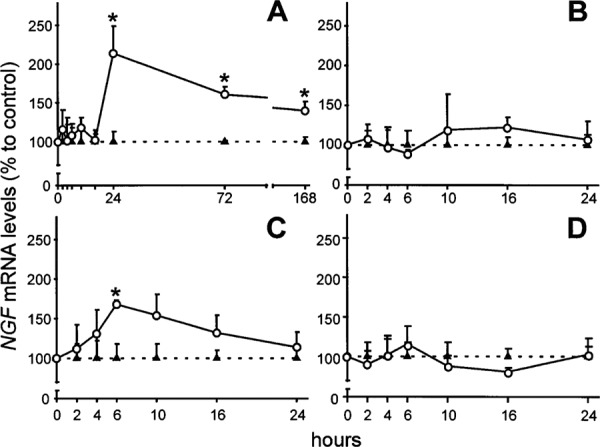
Regulation of NGF mRNA levels by glutamate receptor agonists. Intrastriatal QUIN injection induced a continuous increase of NGF mRNA from 24 h until the last time examined (A). Increased levels of NGF mRNA were also observed 6 h after AMPA intrastriatal injury (C). No changes in NGF mRNA levels were obtained in KA- (B) or ACPD- (D) injected striatal. Triangles represent shaminjected striatal, whereas circle represent results from EAA injection. Values are represented as mean 6 SEM (*P, 0.05). Neurobiology of Disease 5, 357–364 (1998).
Conclusion
Thus selective “vulnerability” of striatal neuronal populations to excitatory neurotransmitters and neurotrophic factors may constitute the mechanism underlying unique pattern of striatal degeneration in HD. The differential distribution of glutamate receptors and subunits in striatal neurons may alter vulnerability of striatal neuronal populations to excitotoxins. Recent studies also indicate that differential vulnerability of striatal neuronal subtypes to neurotrophic factors depends on level of expression of these factors and their receptors that are transiently up or down regulated through the activation of glutamate receptors. Since neurotrophic factors have a special characteristic to selectively protect striatal neuronal subtypes against excitoxic insults, they may be considered as a preventative and therapeutic approach for HD.
Footnotes
Article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Competing Interests: None,
Source of Funding: None
References
- 1.Cicchettia F, Saportac S, Hausere RA et al. Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proc. of Natl. Acad. Sci USA. 2009;106:12483–12488. doi: 10.1073/pnas.0904239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Precious Sophie V, Rosser Anne E. Producing striatal phenotypes for transplantation in Huntington’s disease. Experimental Biology and Medicine. 2012;237:343–351. doi: 10.1258/ebm.2011.011359. [DOI] [PubMed] [Google Scholar]
- 3.Cicchetti F, Soulet D, Freeman TB. Neuronal degeneration in striatal transplants and Hundtington’s disease: potential mechanisms and clinical implications. Brain. 2011;134:641–652. doi: 10.1093/brain/awq328. [DOI] [PubMed] [Google Scholar]
- 4.Ina H, YiMei Y, Jeffrey H K et al. Differential vulnerability of specific cell populations in HD and its relationship to morphological and biochemical characteristics. J Neurochem. 2010;113(5):1073–1091. [Google Scholar]
- 5.Coyle JT, Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington’s chorea. Nature. 1976;263:244–246. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- 6.Cicchetti F, Saporta S, Hauser RA et al. Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proc Natl Acad Sci USA. 2009;106:12483–12488. doi: 10.1073/pnas.0904239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funk CB, Prasad AN, Frosk P et al. Neuropathological, biochemical and molecular findings in a glutaric acidemia type 1 cohort. Brain. 2005;128:711–722. doi: 10.1093/brain/awh401. [DOI] [PubMed] [Google Scholar]
- 8.Flott-Rahmel B, Falter C, Schluff P et al. Nerve cell lesions caused by 3-hydroxyglutaric acid: a possible mechanism for neurodegeneration in glutaric acidaemia I. J Inherit Metab Dis. 1997;20:387–390. doi: 10.1023/a:1005342331229. [DOI] [PubMed] [Google Scholar]
- 9.Liu F, Patterson TA, Sadovova N et al. Ketamine-induced neuronal damage and altered N-methyl-D-aspartate receptor function in rat primary forebrain culture. Toxicol. Sci. 2013;131:548–557. doi: 10.1093/toxsci/kfs296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez N, Alberch J, Neveu I et al. Brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4/5 differentially regulate the phenotype andprevent degenerative change in striatal projection neurons after excitotoxicity in vivo. Neuroscience. 1999;91:1257–1264. doi: 10.1016/s0306-4522(98)00723-4. [DOI] [PubMed] [Google Scholar]
- 11.Perez- N, Arenas E, Marco S et al. Intrastriatal grafting of a GDNF-producing cell line protects striatonigral neurons from quinolinic acid excitotoxicity in vivo. Eur. J. Neuroscience. 1999;11:241–249. doi: 10.1046/j.1460-9568.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- 12.Josep MC, So nia M, Nu ria C et al. Differential Regulation of The expression of Nerve Growth Factor, Brain- Derived Neurotrophic Factor, and Neurotrophic-3 after Excitotoxicity in a Rat Model of Huntington’s Disease. Neurobiology of Disease. 1998;5:357–364. doi: 10.1006/nbdi.1998.0211. [DOI] [PubMed] [Google Scholar]


