Abstract
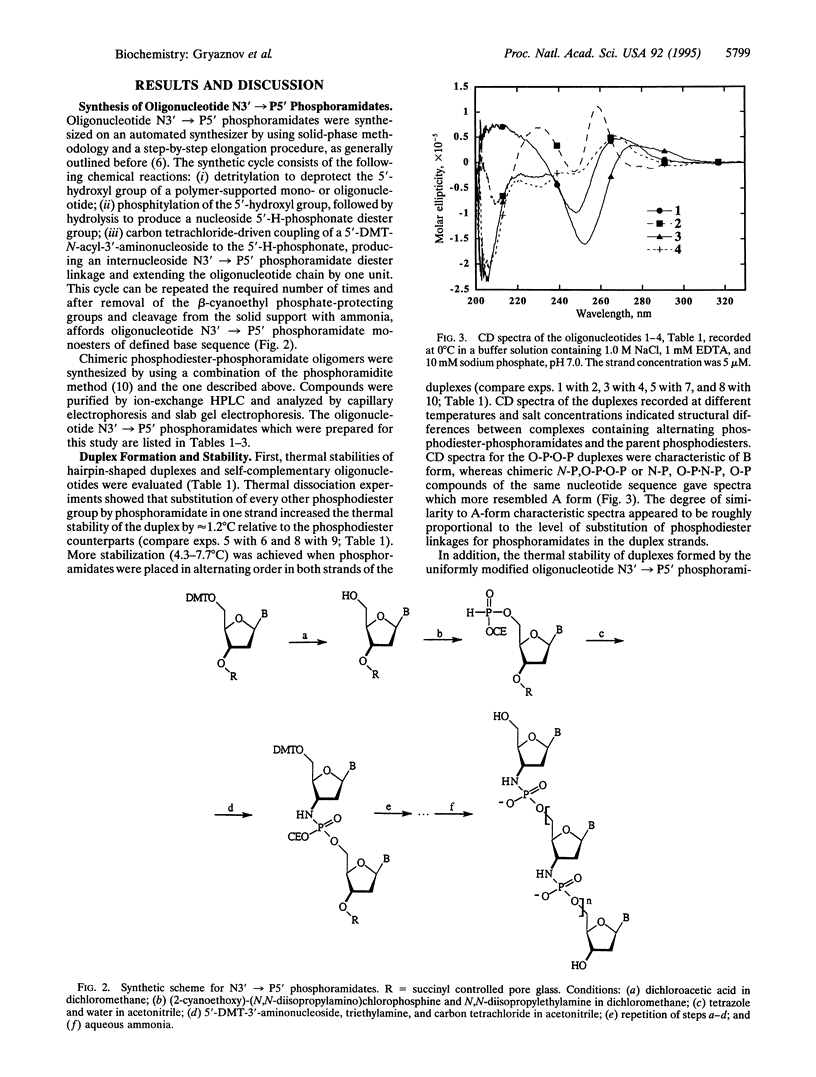
Synthetic oligonucleotides and their analogs have attracted considerable interest recently. These compounds may lead to highly specific therapeutic agents, as well as to powerful diagnostic tools. Here, we present the synthesis of uniformly modified oligodeoxyribonucleotide N3'-->P5' phosphoramidates containing 3'-NHP(O)(O-)O-5' internucleoside linkages and the study of their hybridization properties. Thermal dissociation experiments show that these compounds form very stable duplexes with single-stranded DNA, RNA, and with themselves following Watson-Crick base pairing. The duplex thermal stability was enhanced by 2.2-2.6 degrees C per modified linkage compared with phosphodiesters. The structure of complexes formed by phosphoramidates closely resembles that of RNA oligomers and corresponds to an A form, as judged by CD spectroscopy. N3'-->P5' phosphoramidates also form stable triplexes with double-stranded DNA under near-physiological conditions when natural phosphodiesters fail to do so. Physicochemical characteristics of the amidates are similar to those of RNA oligomers, even though they are composed of 2'-deoxyfuranose-based nucleosides.
Full text
PDF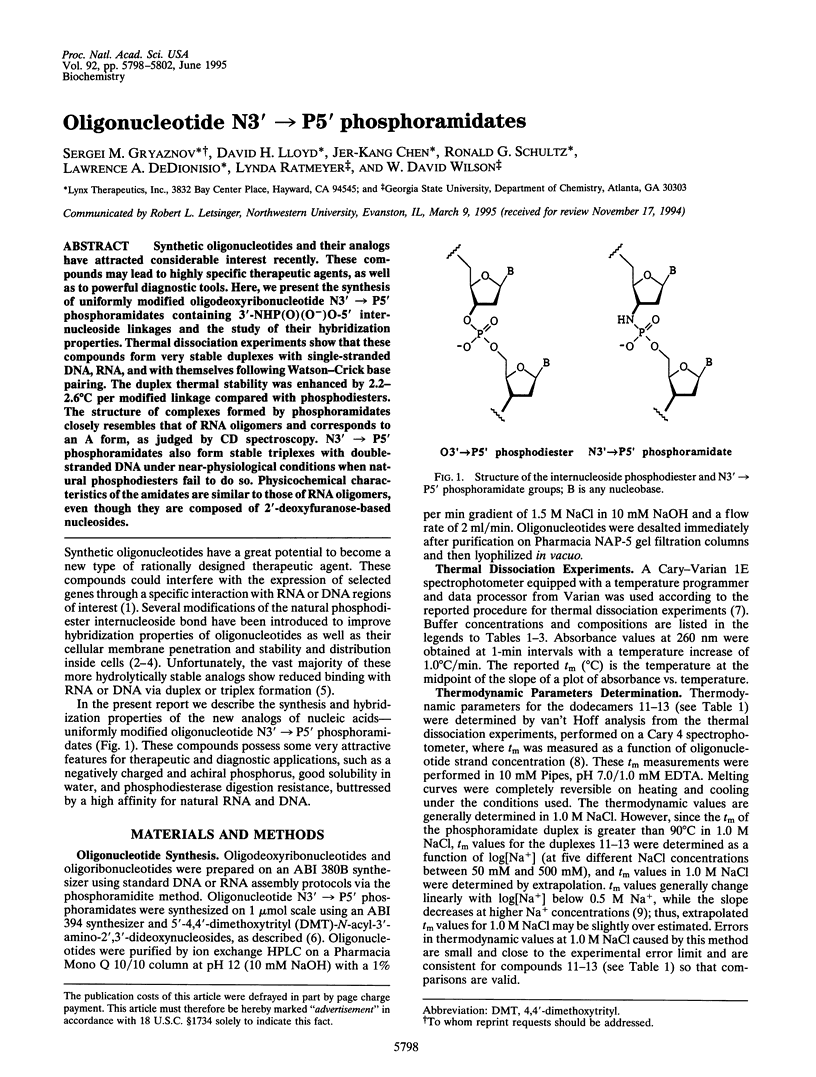


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caruthers M. H. Gene synthesis machines: DNA chemistry and its uses. Science. 1985 Oct 18;230(4723):281–285. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- Gryaznov S. M., Letsinger R. L. Synthesis and properties of oligonucleotides containing aminodeoxythymidine units. Nucleic Acids Res. 1992 Jul 11;20(13):3403–3409. doi: 10.1093/nar/20.13.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Dervan P. B. Sequence-specific recognition of double helical RNA and RNA.DNA by triple helix formation. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3806–3810. doi: 10.1073/pnas.90.9.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler-Herzog L., Zon G., Uznanski B., Whittier G., Wilson W. D. Duplex stabilities of phosphorothioate, methylphosphonate, and RNA analogs of two DNA 14-mers. Nucleic Acids Res. 1991 Jun 11;19(11):2979–2986. doi: 10.1093/nar/19.11.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zon G., Wilson W. D. Thermodynamics of DNA duplexes with adjacent G.A mismatches. Biochemistry. 1991 Jul 30;30(30):7566–7572. doi: 10.1021/bi00244a028. [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Caruthers M. H. Phosphorodithioate DNA as a potential therapeutic drug. Science. 1993 Mar 12;259(5101):1564–1570. doi: 10.1126/science.7681216. [DOI] [PubMed] [Google Scholar]
- Miller P. S. Oligonucleoside methylphosphonates as antisense reagents. Biotechnology (N Y) 1991 Apr;9(4):358–362. doi: 10.1038/nbt0491-358. [DOI] [PubMed] [Google Scholar]
- Olmsted M. C., Anderson C. F., Record M. T., Jr Importance of oligoelectrolyte end effects for the thermodynamics of conformational transitions of nucleic acid oligomers: a grand canonical Monte Carlo analysis. Biopolymers. 1991 Nov;31(13):1593–1604. doi: 10.1002/bip.360311314. [DOI] [PubMed] [Google Scholar]
- Roberts R. W., Crothers D. M. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science. 1992 Nov 27;258(5087):1463–1466. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]
- Zielinski W. S., Orgel L. E. Oligoaminonucleoside phosphoramidates. Oligomerization of dimers of 3'-amino-3'-deoxy-nucleotides (GC and CG) in aqueous solution. Nucleic Acids Res. 1987 Feb 25;15(4):1699–1715. doi: 10.1093/nar/15.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]




