Abstract
Background
Late radiation-associated dysphagia (late-RAD) is a rare delayed toxicity, commonly associated with lower cranial neuropathies, in oropharyngeal cancer (OPC) survivors. Prevention of late-RAD is paramount because the functional impairment can be profound, progressive, and refractory to standard therapies. The objective of this analysis was to identify candidate dosimetric predictors of late-RAD and associated lower cranial neuropathies after radiotherapy (RT) or chemo-RT (CRT) for OPC.
Methods
An unmatched retrospective case-control analysis was conducted. OPC patients treated with definitive RT or CRT (1999–2006) were included. Late-RAD was defined by new or progressive pharyngeal dysphagia per modified barium swallow (MBS) studies after recovery from acute toxicity with an interval of adequate function. As a secondary endpoint, predictors of late lower cranial neuropathies associated with late-RAD cases were examined. Controls were followed prospectively on an institutional trial a minimum of 4 years without symptoms of late-RAD. Dysphagia-aspiration related structures (DARS) and regions of interest containing cranial nerve paths (RCCNPs) were retrospectively contoured. Dose volume histograms were calculated. Non-parametric bivariate associations were analyzed with Bonferroni correction and multiple logistic regression models were fit.
Results
Thirty-eight patients were included (12 late-RAD cases, 26 controls). Median latency to late-RAD was 5.8 years (range: 4.5–11.3 years). Lower cranial neuropathies were present in 10 of 12 late-RAD cases (IX=4, X=6, XII=8), 7 of whom had multiple nerve palsies. IMRT was delivered in all controls and 25% of cases (p<0.001); the remainder received 3D-CRT. Smoking history, tumor subsite, fractionation schedule, neck dissection, and concurrent chemotherapy did not significantly differ between cases and controls (p>0.05). T-stage was significantly higher in late-RAD cases (p=0.002); N-stage was higher in controls (p=0.109). Mean superior pharyngeal constrictor (SPC) dose was higher in cases relative to controls (median: 70.5 vs 61.6 Gy). Adjusting for T-stage or total RT dose, mean SPC dose significantly predicted late-RAD (p=0.036) and related cranial neuropathies (p=0.019). RCCNPs did not significantly predict late-RAD or cranial neuropathies.
Conclusions
SPC dose may predict for late-RAD and related lower cranial neuropathies. These data, and those of previous studies that have associated SPC dose with classical dysphagia endpoints, suggest impetus to constrain dose to the SPCs when possible.
Keywords: Dysphagia, Oropharyngeal Cancer, Radiotherapy, Pharyngeal Constrictors, Quality-of-Life
Background
Dysphagia is a dose-limiting toxicity and potential long-term sequelae of radiation therapy (RT) or chemoradiotherapy (CRT) for head and neck cancer (HNC). Classical radiation-associated dysphagia (C-RAD) presents during radiation therapy and persists for weeks after treatment, but may become permanent [1]. That is, C-RAD constitutes an acute toxicity or a consequential late effect of early toxicities that persists into long-term survivorship. This has traditionally been thought to reflect muscle edema, fibrosis and atrophy. Recently, we have reported a unique group of patients with late-onset radiation-associated dysphagia (late-RAD) who have shown a different time course developing clinically significant dysphagia five years or more after RT [2]. Late-RAD manifests as delayed onset or progression of pharyngeal dysphagia preceded by a long interval of adequate functional recovery from the acute toxicities of radiotherapy. A unique feature of late-RAD in oropharyngeal cancer survivors is that it is commonly associated with delayed mono and polyneuropathies of the lower cranial nerves (IX, X, and XII). Distinct from dysphagia as a consequential late effect that is often attributable to pharyngoesophageal stricture, a lack of pharyngeal propulsion owing to limited mobility of dysphagia-aspiration related structures (DARS) drives impairment with late-RAD. Often coupled with lower cranial neuropathy, this leads to a highly inefficient swallow with substantial pharyngeal residue and a tendency for silent aspiration. Pneumonia eventually develops as a consequence of intractable aspiration in up to 85% of long-term survivors with late-RAD, and two-thirds of late-RAD cases in our prior series ultimately necessitated lifelong gastrostomy despite exhaustive rehabilitation efforts [2].
Dysphagia-specific organs-at-risk (DARS) have been examined as they correlate with C-RAD. Previously we and others have demonstrated a dosimetric correlation between superior pharyngeal constrictor (SPC) dose and C-RAD endpoints [3,4]. Mean SPC dose has been shown to predict a variety of dysphagia outcomes including gastrostomy use during RT [3], and oropharyngeal swallowing efficiency after sequential CRT [4]. Other proposed DARS include the larynx [3], esophageal inlet [3], floor of mouth [5], and anterior oral cavity [4]. The purpose of this case-control study was to identify candidate organs-at-risk (OARs) and dosimetric parameters that may predict for late-RAD and associated lower cranial neuropathies in long-term oropharyngeal cancer survivors.
Methods
Study Design and Sampling Method
A retrospective unmatched case-control analysis was conducted with institutional review board approval from the University of Texas, MD Anderson Cancer Center (MDACC). Thirty-eight (38) patients (12 late-RAD cases and 26 controls) receiving definitive RT or chemo-RT for oropharyngeal cancer from the years 1999 to 2006 were included. Late-RAD was defined as new or progressive pharyngeal dysphagia per modified barium swallow (MBS) studies preceded by an interval of adequate function after recovery from acute toxicity (minimum latency of 4 years after RT or CRT). Lower cranial nerve palsies associated with late-RAD (nerves IX, XI, and/or XII) were assessed by clinical cranial nerve examination at the time of MBS studies and at head and neck cancer surveillance visits. The sample was drawn from 23 consecutive cases who presented with late-RAD after definitive radiotherapy (±chemotherapy) at MDACC for oropharyngeal cancer and were referred for MBS studies from 04/2004 through 07/2012; 11 cases treated in 2000 or earlier were excluded because radiotherapy treatment plans could not be restored to analyze dose. Thus, 12 late-RAD cases (median latency: 69 months, 49–136) were included in the analysis.
Controls were derived from an institutional trial in which swallowing function was assessed at baseline and longitudinally through 2 years with serial MBS studies.6 Controls were followed a minimum of 4 years after definitive RT or CRT without symptoms of late RAD or CN palsy (median follow-up: 84 months, 73–92). Thirty-four eligible controls were identified, 8 were excluded because RT treatment plans could not be restored. Thus, 26 controls (without Late-RAD or lower cranial nerve palsy) were included in this analysis.
Data Collection
Clinical data were retrospectively obtained for all cases and controls. Data included age, gender, TNM classification, tumor subsite, smoking history, fractionation schedule, history of neck dissection, type of RT (3D conformal radiotherapy [3D-CRT] versus IMRT), and concurrent chemotherapy during RT.
Treatment plan and dosimetric data were restored from the Pinnacle3 (Philips) system. Candidate organs-at-risk for Late-RAD and associated lower cranial neuropathies were retrospectively contoured onto each patient’s RT treatment plan. DARS volumes were modeled after our prior study [4]. Additional structures, collectively termed regions of interest containing cranial nerve paths (RCCNPs), were included to trace lower cranial nerve tracts. Specifically the following DARS and RCCNPs (defined in Table 1) were retrospectively contoured: cerebellopontine angle (CP angle), medulla, bilateral jugular foramina, bilateral peripheral cranial nerve tracts, suprahyoid musculature, base of tongue, superior (SPC), middle (MPC) and inferior pharyngeal constrictors (IPC), parotid glands, superior and inferior larynx, hard and soft palates, retropharyngeal space, intrinsic tongue, submandibular glands and anterior and posterior oral cavity. For the jugular foramina, cranial nerve tracts, parotid glands and submandibular glands, separate contours were drawn for regions ipsilateral to the primary tumor and contralateral to the primary tumor. Example contours are illustrated in Figure 1. Dose-volume histograms were calculated for each structure.
Table 1.
Description of OAR Contours
| Structures | Definition |
|---|---|
| Cerebellopontine Angle | Brainstem visible between the superior most aspect of the facial canal to the superior most aspect of the jugular foramina |
| Medulla | Brainstem visible between the superior most aspect of the jugular foramina to the foramen magnum to include brain stem nuclei of CN IX, X and XII |
| Ipsilateral and Contralateral Jugular Foramina | Entire jugular foramina contoured in bone window to include CN tracts of IX and X |
| Ipsilateral and Contralateral Cranial Nerve Tracts | Carotid artery and jugular vein from the exit of the jugular foramen posterior to the styloid process to include the nerve tracts of CN IX, X, XII |
| Suprahyoid musculature | Combination of musculature (including mylohyoid, geniohyoid) superior and anterior to the hyoid up to the floor of mouth |
| Base of Tongue | Tongue posterior to the retromolar trigone if teeth were visible or the portion of the tongue up until 1 cm anterior to the angle of the mandible if patient was edentulous |
| Superior Pharyngeal Constrictor | Constrictor muscles extending superiorly to the skull base and inferiorly to the most superior surface of the hyoid including the insertions onto the mylohyoid line |
| Middle Pharyngeal Constrictor | Constrictor muscles extending through the levels of the hyoid (from superior to inferior edge of hyoid) |
| Inferior Pharyngeal Constrictor | Constrictor muscles extending superiorly to the most inferior surface of the hyoid and cricoid cartilage |
| Parotid Glands | Parotid glands contoured in the head and neck window |
| Superior Larynx (i.e., glottic and supraglottic) | Larynx from its most superior extent inferiorly to the level of and including the glottis |
| Inferior Larynx (i.e., subglottic) | Larynx inferior to the glottis |
| Hard Palate | Hard palate contoured in bone window |
| Soft Palate | Soft palate contoured in soft tissue window including the uvula |
| Retropharyngeal space | Potential nodal space posterolateral to the pharyngeal constrictors superiorly to the skull base and inferiorly to the level of the hyoid |
| Intrinsic Tongue | Musculature of the tongue excluding the suprahyoid musculature |
| Anterior and Posterior Oral Cavity | Mucosal surfaces of the oral cavity including the buccal mucosa, the lips, the floor of mouth and the tongue divided equally in the sagittal plane |
Figure 1.
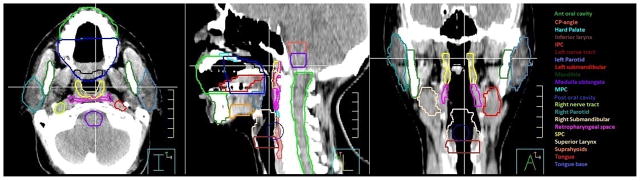
Example Contours for Candidate Organs at Risk
Statistical Methods
Statistical analyses were performed using JMP Statistical Software (SAS, Cary, NC, http://www.jmp.com/). The presence or absence of late-RAD as well as the presence or absence of cranial neuropathies were treated as binary outcomes. Bivariate analysis for clinical factors between groups (cases vs. controls) was performed using multiparametric Wilcoxon tests at α = 0.05. Bivariate analysis for dose data was performed using multiparametic Wilcoxon analysis at a Bonferroni correction of p < 0.002 (α = 0.05/23 structures) as a predictor for each of the binary outcomes. Multiple logistic regression analysis was then used to adjust for confounding by tumor burden and RT dose. Recursive partitioning analysis was used in exploratory post hoc analyses to estimate threshold doses. Post hoc receiver-operator characteristic (ROC) curves were fit in the study sample but require validation in an independent sample for the purposes of hypothesis-validation.
Results
Sample Characteristics
Patient, tumor and treatment characteristics of both cases and controls are summarized in Table 2. On bivariate analysis three clinical factors were significantly different between cases and controls: total RT dose (p = 0.01), RT type (p < 0.001) and T stage (p = 0.002). All controls received IMRT whereas 3 of 12 (25%) cases received IMRT with the remainder receiving conventional 3D-CRT. RT dose and T-stage were higher in cases; N-stage was higher in controls. Most cases had T2 or T3 disease, whereas most controls had T1 or T2 disease. Only 1 Late-RAD case had a positive retropharyngeal node. All controls received induction PCC (paclitaxel, carboplatin, cetuximab) on an institutional trial.6 Concurrent chemotherapy was more common in cases than controls, but this was not significantly different. N-stage, smoking history, tumor subsite, fractionation schedule, neck dissection, and concurrent chemotherapy did not significantly differ between cases and controls (p>0.05). HPV status was not standardly collected in the years of this study (cancers diagnosed 1999 through 2006). Roughly half of patients in each group (cases and controls) were never smokers which might provide a surrogate for the proportion of patients with HPV-associated disease.
Table 2.
Clinical Characteristics of Cases and Controls
| Cases (n=12) | Controls (n=26) | p-value | |
|---|---|---|---|
| Gender | 0.90 | ||
| Male | 9 (75%) | 19 (73%) | |
| Female | 3 (25%) | 7 (27%) | |
| Age at XRT | |||
| Mean | 52.3 (0%) | 57 (0%) | |
| Median | 53 (0%) | 55 (0%) | |
| Range | 32–53 | 33–55 | |
| T Stage | 0.001* | ||
| T1 | 0 (0%) | 13 (50%) | |
| T2 | 5 (42%) | 9 (35%) | |
| T3 | 6 (50%) | 4 (15%) | |
| T4 | 1 (8%) | 0 (0%) | |
| N Stage | 0.11 | ||
| N0 | 1 (8%) | 0 (0%) | |
| N1 | 1 (8%) | 0 (0%) | |
| N2A | 1 (8%) | 0 (0%) | |
| N2B | 6 (50%) | 17 (65%) | |
| N2C | 2 (17%) | 8 (31%) | |
| N3 | 1 (8%) | 1 (4%) | |
| Subsite | 0.16 | ||
| Base of Tongue | 5 (42%) | 18 (69%) | |
| Tonsil | 7 (58%) | 8 (31%) | |
| Cigarettes at Diagnosis | 0.91 | ||
| Never (<100 cig/lifetime) | 6 (50%) | 14 (54%) | |
| Former (quit > 1 year) | 4 (33%) | 9 (34%) | |
| Current | 2 (17%) | 3 (12%) | |
| Race | 0.39 | ||
| White | 10 (83%) | 24 (92%) | |
| Black | 1 (8%) | 0 (0%) | |
| Latin American | 1 (8%) | 1 (4%) | |
| Asian | 0 (0%) | 1 (4%) | |
| Accelerated Fractionation | 0.06 | ||
| Single | 4 (33%) | 17 (65%) | |
| Accelerated | 8 (67%) | 9 (35%) | |
| Concurrent | 0.07 | ||
| No | 5 (42%) | 19 (73%) | |
| Yes | 7 (58%) | 7 (27%) | |
Late-RAD and Lower Cranial Neuropathies
The median latency to late-RAD was 5.8 years (range: 4.5–11.3 years) after RT or CRT. Lower cranial neuropathies were present in 10 of 12 (83%) late-RAD cases (IX=4, X=6, XII=8), 7 of whom had multiple nerve palsies on physical examination. Only 1 late-RAD case had stricture co-existing with physiologic pharyngeal dysphagia on MBS.
Mean Superior Pharyngeal Constrictor Dose Predicted for Late-RAD and Lower Cranial Neuropathies
Summary dose statistics for cases and controls are summarized in Table 3. Upon bivariate analysis, mean SPC dose was significantly higher amongst late-RAD cases (median 70.5 Gy, SD 3.7) compared with controls (median 61.6 Gy, SD 5.5, p = 0.0003) after a Bonferroni correction (Figure 2). All but 2 late-RAD cases had mean SPC dose ≥65 Gy. Additionally, mean ipsilateral parotid dose was higher amongst cases (median 66.1 Gy, SD 1.5) compared with controls (median 30.0 Gy, SD 1.0, p < 0.0001). No other DARS or RCCNPs showed significantly elevated doses in cases compared with controls after Bonferroni correction. In adjusted logistic regression models controlling for T-stage or RT dose, the difference in mean SPC dose between cases and controls remained significant (p < 0.05). Mean SPC dose of 62.1 Gy was suggested as a dichotomization point for the late-RAD endpoint by recursive partition analysis (ROC AUC = 0.865).
Table 3.
Dosimetric Parameters of DARs and RCCNPs for Cases and Controls
| Mean Dose [Median(Mean±SD)] | ||
|---|---|---|
| Cases | Controls | |
| Anterior Oral Cavity | 17.9 (18.4±11.2) | 32.2 (32.8±8.1) |
| Base of Tongue | 72.3 (71.6±3.3) | 67.5 (64.7±14.0) |
| Cerebellopontine Angle | 10.7 (15.3±12.1) | 18.4 (19.6±9.3) |
| Contralateral Jugular Foramen | 34.3 (34.7±11.1) | 46.7 (44.1±13.3) |
| Contralateral Nerve Tract | 50.3 (51.9±7.4) | 56.9(56.7±8.3) |
| Contralateral Parotid | 72.5 (69.5±9.1) | 60.4 (62.7±7.6) |
| Contralateral Submandibular | 71.2 (62.4±20.8) | 61.0 (58.3±15.0) |
| Hard Palate | 27.2 (24.9±13.9) | 26.2 (29.0±10.7) |
| Inferior Larynx | 14.9 (24.4±25.3) | 20.4 (20.7±9.0) |
| Inferior Pharyngeal | ||
| Constrictor | 27.8 (31.5±19.4) | 27.4 (28.8±10.4) |
| Intrinsic Tongue | 40.2 (40.9±12.9) | 51.6 (52.0±9.8) |
| Ipsilateral Jugular Foramen | 43.3 (41.6±14.7) | 54.8 (52.7±9.5) |
| Ipsilateral Nerve Tract | 67.7 (64.6±9.3) | 64.1 (65.2±6.7) |
| Ipsilateral Parotid* | 66.1 (60.2±15.4) | 30.0 (32.2 ± 9.6) |
| Ipsilateral Submandibular | 72.7 (61.0±28.5) | 70.6 (67.5±14.4) |
| Medulla | 33.2 (32.8±8.0) | 35.9 (35.0±7.4) |
| Middle Pharyngeal | ||
| Constrictor | 68.4 (67.4±5.7) | 63.1 (60.3±9.3) |
| Posterior Oral Cavity | 61.3 (58.4±12.6) | 53.6 (54.0±8.3) |
| Retropharyngeal Space | 68.7 (62.8±2.0) | 61.7 (61.0±8.9) |
| Soft Palate | 71.0 (66.4±9.8) | 60.0 (57.5±10.2) |
| Superior Larynx | 52.0 (53.1±15.3) | 58.1 (53.1±15.0) |
| Superior Pharyngeal | ||
| Constrictor* | 70.5 (70.1±3.7) | 61.6 (62.9±5.5) |
| Suprahyoid musculature | 60.4 (58.0±9.7) | 56.7 (55.5±13.8) |
Dose higher in cases than controls, significant at an α = 0.05/23 structures
Figure 2.
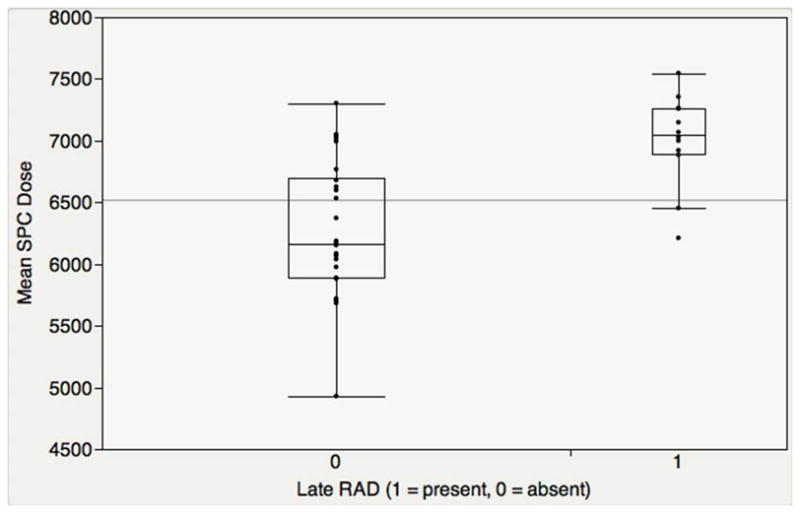
Mean SPC Dose predicted Late-RAD
Further, bivariate analysis showed that amongst all 23 structures using a Bonferroni correction, only mean SPC dose was higher amongst late-RAD cases with late lower cranial neuropathies (median 71.1 Gy, SD 3.6) compared with controls without cranial neuropathy (median 61.8 Gy, SD 5.5, p = 0.0002, Figure 3). All but 2 late-RAD cases with lower cranial neuropathies had mean SPC dose ≥70 Gy. In adjusted logistic regression models controlling for T stage or RT dose, this difference in mean SPC dose between late-RAD cases with lower cranial neuropathies and controls remained significant (p < 0.05). Mean SPC dose of 69.2 Gy was suggested as a numeric breakpoint for the lower cranial nerve palsy endpoint by recursive partition analysis (ROC AUC = 0.896).
Figure 3.
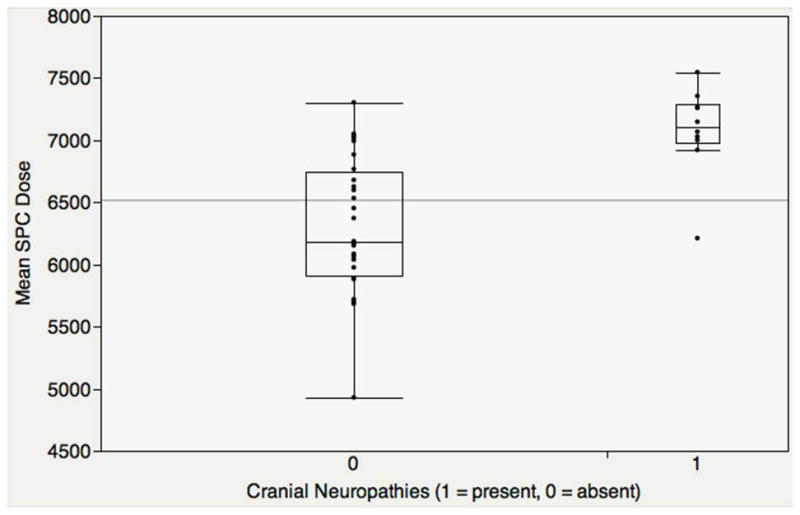
Mean SPC Dose predicted Late Lower Cranial Neuropathies
Discussion
We present, to our knowledge, the first dosimetric study of Late-RAD and lower cranial neuropathy after definitive radiotherapy for oropharyngeal cancer. Late-RAD is uncommon with modern HNC treatment, but when it occurs it is associated with severe dysfunction, largely refractory to standard therapies [2]. The association of late-RAD with lower cranial neuropathies in long-term OPC survivors is only recently recognized, and likely explains the progressive and incurable nature of the problem in many cases. In the era of HPV-associated oropharyngeal cancers, prevention of late-RAD is particularly important for patients with HPV-associated disease who tend to be younger, with less comorbidity and better expected longevity after treatment. Historically, tobacco-related HNC was diagnosed in older patients with a long history of smoking, often debilitating comorbidities and poor long-term survival. However, HPV-associated disease now accounts for the majority of incident oropharyngeal cancer cases in the US [7]. Young age at diagnosis and high rates of long-term survival amongst these patients suggest that the pool of patients at risk for developing late-RAD is ever increasing in contemporary practice. Risk-reduction of this devastating late effect requires understanding of treatment-related factors that predispose to late-RAD.
Results of this unmatched case-control analysis suggest that mean SPC dose is associated with the development of both Late-RAD and delayed lower cranial neuropathies that often drive this condition, after controlling for tumor burden and treatment intensity. Multiple studies have demonstrated the role of pharyngeal constrictor dose in the development of C-RAD, and particularly the SPC dose. Table 4 summarizes swallowing outcomes associated with SPC dose in published papers. Previously, Schwartz et al. [4] demonstrated that elevated dose (V55 > 80%, V65 > 30%) to the superior portion of the SPC delineated by the C1 vertebral body and dose to the anterior oral cavity (V30 > 65%, V35 > 35%) predicted for long-term C-RAD as measured by oropharyngeal swallowing efficiency longitudinally to 2-years in a study of 31 patients with Stage IV oropharyngeal cancer. We assessed this split of the SPC by the C1 vertebral body in the current analysis to determine if dose to the superior aspect of the SPC was uniquely predictive of late-RAD, but both dose to the superior and inferior aspect of the SPC remained predictive of late-RAD (p<0.002). Eisbruch et al. [8] also demonstrated the relationship between mean dose of each PC and long-term C-RAD as measured by summary videofluoroscopy scores and baseline-adjusted aspiration through 2 years in a prospective long-term study of 73 oropharyngeal cancer patients. Importantly, mean SPC dose showed the highest correlation with C-RAD amongst the PCs, and mean dose significantly correlated with volumetric dose. Our Late-RAD data further emphasize the role that SPC dose may play in dysphagia after radiotherapy for oropharyngeal cancer. Moreover, these data suggest that the predictive value of mean SPC dose on dysphagia may extend decades into survivorship after oropharyngeal irradiation.
Table 4.
Summary of Studies Correlating SPC Dose with C-RAD Outcomes
| Study | Reported SPC Dose Relationship | Swallowing Outcome Measured | Time Point |
|---|---|---|---|
| Levendag et al. (2008) [17] | Mean dose < 51 Gy* | Severe dysphagia as characterized by at least 1 of: RTOG Score Dysphagia ≥ 3, PSS≤50, MDADI≤50 and H&N35 q35–q38 | Longitudinal |
| Feng et al. (2007) [18] | V40 < 95, V50 < 90, V60 < 80* | Videofluoroscopy summary score & aspiration | 3 months post-RT |
| Teguh et al. (2008) [19] | Mean dose | Fiberoptic endoscopic evaluation of swallowing score | ≥ 1 year post RT |
| Caudell et al. (2010) [20] | V65 < 33* | Pharyngeal stricture requiring dilation | ≥ 1 year post RT |
| Schwartz et al. (2010) [4] | V55 < 80%* | Oropharyngeal swallowing efficiency (OPSE) | 6, 12 and 24 months post- RT |
Abbreviations: RTOG, Radiation Therapy Oncology Group, PSS, Performance Status Scale-Head Neck, MDADI, MD Anderson Dysphagia Inventory
Volumetric doses associated with favorable swallowing outcomes
Late-RAD is characteristically different from C-RAD. We have previously detailed phenotypic characteristics of these patients [2]. Late-RAD is driven by immobility of DARS leading to poor bolus propulsion, pharyngeal stasis, and delayed aspiration. Fibrosis and neuropathy are considered the primary source of late-RAD, and co-existing stricture is extremely rare. Lower cranial neuropathy, typically reported as a late effect of nasopharyngeal cancer irradiation, is increasingly recognized in clinical practice as a potential late effect of oropharyngeal irradiation. While Huang et al. have recently reported the presence of cranial neuropathies in three cases who received definitive radiation treatment, including IMRT, for oropharyngeal cancers [9], our analysis is unique as it highlights nerve palsies as the driver of late-RAD in many cases [2]. Lower cranial neuropathies are extremely common in patients with late-RAD, present in 10 of 12 cases in this series and 48% of cases in our earlier series. Clinically, this points to a potential peripheral nerve injury-based etiology of late-RAD. This association is supported by our analysis confirming a high prevalence of comorbid lower cranial neuropathies among Late-RAD cases and the independent association of these neuropathies with SPC dose. Anatomically, the SPC is a region-of-interest because it contains a significant number of small nerve tracts as well as constrictor muscles driving medial constriction and longitudinal pharyngeal muscles that shorten the pharynx during deglutition (Figure 4), injuries to which would produce the characteristic findings of Late-RAD related to pharyngeal propulsion [10]. That is, the SPC region that was associated with late-RAD and lower cranial neuropathy is not just the superior constrictor musculature but rather a region of complex neuroanatomical significance to swallowing function. This is further supported by case reports demonstrating cranial nerve palsies and other late effects in patients receiving radiotherapy for nasopharyngeal carcinoma where high dosage to the SPC region is unavoidable [11–13]. Surprisingly, despite the large number of polyneuropathies, cranial nerve palsies were not associated with higher doses to either the brainstem or peripheral nerve tracts. This is of particular interest, since Rosenthal et al. [14] previously demonstrated significant differences in beam path toxicities including higher brainstem doses in IMRT versus 3D-CRT planning. Matched comparisons in an IMRT cohort are needed to fully exclude this association as a driver of late-RAD and associated nerve palsies.
Figure 4.
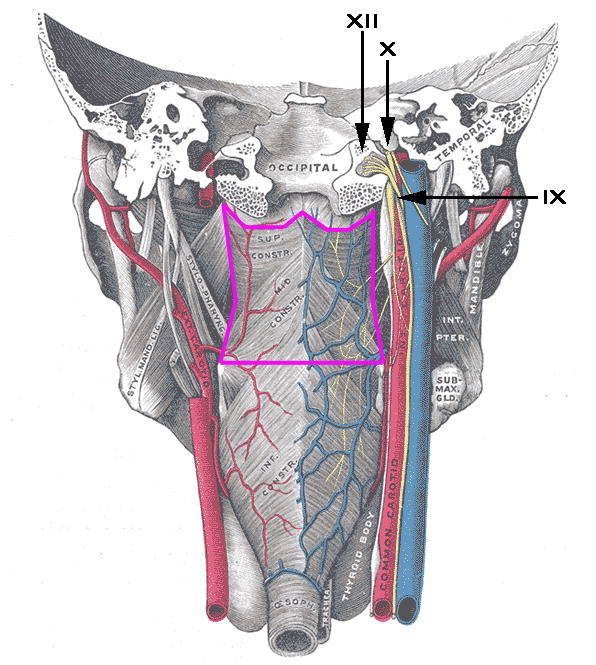
Anatomic rendering of SPC contour region
In designing our case-control study, two pivotal factors led to our selection of cases and controls: 1) the development of a uniform case grouping, and 2) the presence of consistent, long-term functional swallowing data in controls. For our case grouping, we limited selection of Late-RAD cases to oropharyngeal cancers for these primary reasons: the increased incidence of HPV-associated oropharyngeal cancer, the relevance of late effects among OPC survivors in the context of greater expected longevity with HPV-driven disease, and the uniformity of dose distribution. In selecting controls, we selected OPC from a previous prospective clinical trial due to their standardized, functional swallowing follow-up. This was critically important in distinguishing cases from controls as the presentation and onset of dysphagia in the controls was well-characterized and closely monitored on trial.
Both tumor subsite and radiation technique might contribute to SPC dose differences between our unmatched cases and controls. Relative to base of tongue cancers, tonsillar cancers are closer to the SPC region and thus presumably patients receiving radiation for tonsillar tumors would have higher SPC doses. However there was no significant difference in subsite between cases and controls. Thus, the principal differential in our unmatched cases and controls was the type of radiation technique used. IMRT was more common in controls than cases. Thus, differences in dose to DARS could be attributed in part to radiation treatment method. However, amongst all DARs and RCCNPs, the only significantly higher dose in cases versus controls was in the SPC region, and this maintained after both Bonferonni correction and multivariate adjustment for tumor burden and treatment intensity. Further, Cartmill et al. have previously reported on the dosimetric parameters for DARs in 3D-CRT for oropharyngeal cancer [15]; 12 patients were treated to 66 Gy using concomitant boost therapy with a 1.0–1.5 PTV expansion. The mean SPC dose in this cohort was 59.9 Gy, 6.1 Gy less than the treatment dose. Our cases were treated to higher doses, typically 70–72 Gy, and the mean SPC dose of 70.1 Gy in our cases was on parallel with our treatment dose. This disparity reflects that SPC dose was not a standard constraint in the 3D-CRT era rather than an intrinsic limitation of 3D-CRT as a treatment modality. Dose constraints to DARS were not appreciated until the pioneering work of Eisbruch et al. that predated treatment of many patients in this study [16]. Future controlled comparisons are needed to confirm these preliminary findings, and determine if SPC dose constraints are feasible and may lessen the risk for late-RAD and related lower cranial neuropathies in long-term OPC survivors.
Conclusion
Late-RAD and associated lower cranial nerve palsies are rare but significantly debilitating outcomes of oropharyngeal irradiation. We present a hypothesis-generating case-control study demonstrating an association between elevated mean SPC dose and the development of Late-RAD (median 5.75 years) and associated lower cranial neuropathies. Alongside multiple other studies demonstrating the association between SPC dose and classical radiation-associated dysphagia, we provide further evidence for consideration of the SPC in dosimetric constraints, when possible.
Acknowledgments
Abdallah Mohamed has been supported by a UICC American Cancer Society Beginning Investigators Fellowship funded by the American Cancer Society. Katherine A. Hutcheson acknowledges funding from the UT Health Innovation for Cancer Prevention Research Fellowship, The University of Texas School of Public Health – Cancer Prevention and Research Institute of Texas (CPRIT) grant #RP101503.
List of Abbreviations
- RAD
Radiation-Associated Dysphagia
- Late-RAD
Late Radiation-Associated Dysphagia
- C-RAD
Classical Radiation-Associated Dysphagia
- DARs
Dysphagia-Associated Organs-at-Risks
- CP Angle
Cerebellopontine Angle
- OARs
Organs-At-Risk
- HNC
Head and Neck Cancer
- RT
Radiotherapy
- IMRT
Intensity Modulated Radiotherapy
- 3D-CRT
3-Dimensional Conformal Radiotherapy
- PCs
Pharyngeal Constrictors
- SPC
Superior Pharyngeal Constrictors
- MPC
Middle Pharyngeal Constrictors
- IPC
Inferior Pharyngeal Constrictors
- PTV
Planning Treatment Volume
- HPV
Human Papilloma Virus
- ROC
Receiver-Operator Characteristic
Footnotes
Competing Interests
The authors declare that there are no competing interests.
Disclaimer: The content is soley the responsibility of the authors and does not necessarily represent the official views of the CPRIT.
Authors Contributions
MJA and ASRM contributed equally to this work.
CDF and KAH contributed equally to this work.
MJA segmented organs-at-risk, designed organs-at-risk, performed dose calculations and statistical analysis, contributed to authorship. ASRM segmented the organs-at-risk, contributed to authorship of manuscript. CAB segmented the organs-at-risk, contributed to dose calculations. GBG designed organs-at-risk, contributed to authorship of manuscript. DIR designed organs-at-risk, contributed to authorship of manuscript. CH obtained functional swallowing data, contributed to authorship of manuscript. DSS designed the organs-at-risk, obtained functional swallowing data for controls. JSL maintained and obtained clinical and functional swallowing data for cases and controls. CDF designed the organs-at-risk, participated in statistical analysis. KAH conceived project, contributed to authorship of manuscript, participated in statistical analysis, designed organs-at-risk, obtained clinical data for cases and controls, obtained swallowing functional data.
Contributor Information
Musaddiq J. Awan, Email: muawan@gmail.com.
Abdallah S. R. Mohamed, Email: abdallah.sherif@alexmed.edu.eg.
Jan S. Lewin, Email: jlewin@mdanderson.org.
Charles A. Baron, Email: cbaron@wesleyan.edu.
G. Brandon Gunn, Email: gbgunn@mdanderson.org.
David I. Rosenthal, Email: dirosenthal@mdanderson.org.
F. Christopher Holsinger, Email: holsinger@stanford.edu.
David S. Schwartz, Email: dschwartz516@gmail.com.
Clifton D. Fuller, Email: cdfuller@mdanderson.org.
Katherine A. Hutcheson, Email: karnold@mdanderson.org.
References
- 1.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 2.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118:5793–5799. doi: 10.1002/cncr.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanguineti G, Gunn GB, Parker BC, Endres EJ, Zeng J, Fiorino C. Weekly dose-volume parameters of mucosa and constrictor muscles predict the use of percutaneous endoscopic gastrostomy during exclusive intensity-modulated radiotherapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2011;79:52–59. doi: 10.1016/j.ijrobp.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1356–1365. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar R, Starmer H, Alcorn E, Murano YL, Quon H. Radiation dose to the floor of mouth muscles predicts swallowing complications after chemoradiation in oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;84:S206–S207. doi: 10.1016/j.oraloncology.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010;28:8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisbruch A, Kim HM, Feng FY, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011;81:e93–99. doi: 10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang AT, Song S, Dominguez LM, Nguyen J, Goldman RA, Reiter ER. Delayed lower cranial neuropathies following primary radiotherapy for oropharyngeal squamous cell carcinoma. Laryngoscope. 2013;123:1207–1209. doi: 10.1002/lary.23938. [DOI] [PubMed] [Google Scholar]
- 10.Pearson WG, Jr, Hindson DF, Langmore SE, Zumwalt AC. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2013;85:735–740. doi: 10.1016/j.ijrobp.2012.07.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chew NK, Sim BF, Tan CT, Goh KJ, Ramli N, Umapathi P. Delayed post-irradiation bulbar palsy in nasopharyngeal carcinoma. Neurology. 2001;57:529–531. doi: 10.1212/wnl.57.3.529. [DOI] [PubMed] [Google Scholar]
- 12.King AD, Leung SF, Teo P, Lam WW, Chan YL, Metreweli C. Hypoglossal nerve palsy in nasopharyngeal carcinoma. Head Neck. 1999;21:614–619. doi: 10.1002/(sici)1097-0347(199910)21:7<614::aid-hed5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Kong L, Lu JJ, Liss AL, et al. Radiation-induced cranial nerve palsy: a cross-sectional study of nasopharyngeal cancer patients after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1421–1427. doi: 10.1016/j.ijrobp.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal DI, Chambers MS, Fuller CD, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72:747–755. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cartmill B, Cornwell P, Ward E, et al. Emerging understanding of dosimetric factors impacting on dysphagia and nutrition following radiotherapy for oropharyngeal cancer. Head Neck. 2013;35:1211–1219. doi: 10.1002/hed.23040. [DOI] [PubMed] [Google Scholar]
- 16.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 19.Teguh DN, Levendag PC, Sewnaik A, et al. Results of fiberoptic endoscopic evaluation of swallowing vs. radiation dose in the swallowing muscles after radiotherapy of cancer in the oropharynx. Radiother Oncol. 2008;89:57–63. doi: 10.1016/j.radonc.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76:403–409. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]


