Abstract
Mutations in the gene calreticulin (CALR) occur in the majority of JAK2- and MPL-unmutated patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF); identifying CALR mutations contributes to the diagnostic pathway of ET and PMF. CALR mutations are heterogeneous spanning over the exon 9, but all result in a novel common protein C terminus. We developed a polyclonal antibody against a 17-amino-acid peptide derived from mutated calreticulin that was used for immunostaining of bone marrow biopsies. We show that this antibody specifically recognized patients harboring different types of CALR mutation with no staining in healthy controls and JAK2- or MPL-mutated ET and PMF. The labeling was mostly localized in megakaryocytes, whereas myeloid and erythroid cells showed faint staining, suggesting a preferential expression of calreticulin in megakaryocytes. Megakaryocytic-restricted expression of calreticulin was also demonstrated using an antibody against wild-type calreticulin and by measuring the levels of calreticulin RNA by gene expression analysis. Immunostaining using an antibody specific for mutated calreticulin may become a rapid, simple and cost-effective method for identifying CALR-mutated patients complementing molecular analysis; furthermore, the labeling pattern supports the preferential expansion of megakaryocytic cell lineage as a result of CALR mutation in an immature hematopoietic stem cell.
Introduction
The discovery of the JAK2V617F mutation has greatly facilitated the diagnosis of patients with chronic Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs). Polycythemia vera is almost invariably associated with JAK2 mutations (JAK2V617F and JAK2 exon 12 mutations), whereas only 50–60% of essential thrombocythemia (ET)1, 2, 3 and primary myelofibrosis (PMF)4, 5, 6 patients are JAK2V617F mutated. Mutations at codon 515 of the gene encoding the thrombopoietin receptor (MPL) can be detected in 3–5% of JAK2-unmutated ET and 5–8% of PMF patients.7, 8, 9 JAK2 and MPL mutations were elected to constitute major diagnostic criteria in the 2008 World Health Organization (WHO) classification of MPN.10, 11 Recently, in 50–80% of patients with JAK2 and MPL unmutated ET and PMF, mutations of CALR, the gene encoding the endoplasmic reticulum Ca2+-binding chaperone calreticulin, were discovered.12, 13 Therefore, the proportion of ET and PMF patients with a known molecular marker is currently set at up to 80–85%. Owing to their high frequency in, and the selective association with, ET and PMF, CALR mutations are expected to be endorsed by the WHO committee for being included among the major diagnostic criteria for ET and PMF together with JAK2 and MPL mutations.14 However, bone marrow (BM) biopsy remains mandatory for discriminating between ET and myelofibrosis, including prefibrotic myelofibrosis, as well as for the diagnosis of JAK2/MPL/CALR-unmutated (‘triple negative') patients.15
CALR mutations are highly heterogeneous, with at least 40 different types reported16 to date, represented by insertions or deletions, all located in exon 9. The two commonest mutations accounting for ≈80% of mutated cases are a 52-bp deletion (CALRdel52/Type I; c.1092_1143del; L367fs*46) and a 5-bp insertion (CALRins5/Type II; c.1154_1155insTTGTC; K385fs*47). However, all known mutations are predicted to cause a frameshift to a unique alternative reading frame that results in a novel protein C terminus of a minimum of 36 amino acids. This novel sequence contains several positively charged amino acids, at variance with the largely negatively charged wild-type protein; this novel sequence, which disrupts the endoplasmic reticulum-signaling peptide, could influence subcellular localization, stability and/or function of calreticulin. Evidence of effective translation of the mutated protein was obtained by transient expression in human embryonic kidney cells,13 whereas data about a possible subcellular mislocalization are contrasting.12, 13
Herein, we show that an antibody specifically raised against a peptide located in the novel C terminus portion of mutated calreticulin can be used to stain mutated cells in BM biopsies of ET and PMF patients carrying CALR mutations. Furthermore, the results of immunostaining being differentially associated with hematopoietic cell lineages provided some clues about potential mechanisms underlying the strict association of CALR mutations with disorders characterized by preferential involvement of the megakaryocytic cell lineage, as are ET and PMF.
Materials and methods
Patients
Patients with a diagnosis of polycythemia vera, ET and PMF fulfilling the 2008 WHO criteria who were followed at the Hematology Department, University of Florence, and for whom an archived paraffin-embedded BM biopsy was available, were randomly selected from our database. Patients had provided an informed written consent for the use of biological material and clinical information for investigational purposes. The JAK2V617F and MPLW515L/K mutations were assessed by real-time quantitative PCR17, 18 and/or high-resolution melting (HRM) analysis followed by bidirectional Sanger sequencing (for MPL mutations only).19 Mutations in exon 9 of CALR were assessed by bidirectional Sanger sequencing;12 all mutations were assessed in blood granulocytes.
Anti-CALR antibody preparation
A 17-mer peptide derived from the C-terminal of mutated calreticulin was synthesized on the Symphony automatic peptide synthesizer (Protein Technologies, Tucson, AZ, USA), conjugated with ovalbumin and then purified on a Sephadex column by standard techniques; the conjugated peptide was used to immunize rabbits with four injections on day 0, 21, 28 and 35. Following confirmation of the presence of peptide-specific antibodies in the rabbit plasma by standard enzyme-linked immunosorbent assay, a last boost was delivered on day 45 and animals were bled 10 days later. Peptide synthesis and animal immunization were performed at Primm srl (Milano, Italy). The immunoglobulin fraction was purified by protein G-Sepharose 4 Fast Flow (GE Health Care Life Science, Uppsala, Sweden) column and then passed over a CNBr-activated Sepharose 4B containing the immobilized peptide to recover peptide-specific antibodies.
Production of recombinant mutated calreticulin
A synthetic form of CALRdel52 was expressed in the BL21 DE3 RIPL and JM109 DE3 Escherichia coli strains by standard procedures; exponentially growing bacterial cultures were extracted and both the insoluble and the soluble fractions were collected and analyzed in a denaturing polyacrylamide gel electrophoresis.
Western blot analysis
Granulocytes were isolated from peripheral blood of patients and healthy donors, resuspended in RIPA lysis buffer and extracted by sonication; samples normalized by protein content were then submitted to polyacrylamide gel electrophoresis under denaturating conditions and blotted. Membranes were probed with primary antibodies against mutated calreticulin and GAPDH as loading control (Sigma-Aldrich, St Louis, MO, USA). Suitable peroxidase-conjugated IgG preparations (Sigma-Aldrich) were used as secondary antibodies; the enhanced chemiluminescence procedure was employed for blot development. Images were collected by Image Quant apparatus (GE Healthcare, Buckinghamshire, UK).
Immunostaining
For immunostaining, 3-μm-thick formalin-fixed, paraffin-embedded BM sections were deparaffinized in xylene and hydrated in graded alcohols. Antigen retrieval was performed in ethylenediaminetetraacetic acid buffer (1 mM, pH 8.0) for two 15-minute cycles at max power in a microwave oven, and slides were then incubated with the anti-mutated calreticulin antibody at a final dilution of 1:1000 in Tris-buffered saline for 1 h at room temperature. Following incubation with a horseradish peroxidase-labeled goat anti-rabbit antibody, detection steps were performed using 3, 3′-diaminobenzidine substrate according to standard procedures and the sections were finally counterstained with hematoxylin. A rabbit polyclonal antibody specifically directed against the N-terminal 50–150 amino-acid region of wild-type calreticulin was obtained from Abcam (ab39897; www.abcam.com).
Calreticulin gene expression analysis
CD34+ samples were obtained from the BM of normal volunteer donors20 by immunomagnetic separation (EasySep Human CD34 Positive Selection Kit, StemCell Technologies, Vancouver, BC, Canada). Erythroblasts were obtained by culturing CD34+ cells in Iscove's modified Dulbecco's media supplemented with 20% bovine serum albumin, insulin and transferrin (StemCell Technologies), 50 ng/ml stem cell factor and 4 U/ml erythropoietin (R&D Systems, Minneapolis, MN, USA) for 8–10 days.21 Erythroid differentiation was monitored by examination of May Grunwald and Giemsa-stained cytospins and glycophorin A expression by flow cytometric analysis.22 For obtaining megakaryocytes, CD34+ cells were cultured in Iscove's modified Dulbecco's media supplemented with 20% bovine serum albumin, insulin and transferrin, 50 ng/ml stem cell factor and 100 ng/ml thrombopoietin (Genzyme, Boston, MA, USA) for 14–16 days; megakaryocytes were purified by immunomagnetic sorting using monoclonal anti-CD41a antibody (Dako, Milan, Italy). Granulocytes were collected by Ficoll separation of peripheral blood samples; after removal of contaminating red cells by hypotonic lysis, CD16-positive neutrophils were purified using magnetic microbeads (Miltenyi, Auburn, CA, USA).23 The final purity of the different cell fractions was always higher than 90%, as assessed by flow cytometry.
Total cellular RNA was isolated from 0.5–1 × 106 cells from each sample by means of the RNeasy Mini Kit (Qiagen, Valencia, CA, USA); the concentration and quality of RNA samples were determined with disposable RNA chips (RNA 6000 Nano LabChip kit, Agilent, Santa Clara, CA, USA) using the Agilent 2100 bioanalyzer. RNA samples from five different normal donors were pooled in order to obtain at least 3 μg of total cellular RNA for target synthesis according to the Affymetrix protocol (Affymetrix, Santa Clara, CA, USA). Biotin-labeled cRNA was synthesized by means of the Affymetrix One Cycle Target Labeling and Control Reagents Kit and then hybridized to Affymetrix HG-U133A GeneChip arrays for 16 h. GeneChips were washed and stained by means of Affymetrix GeneChip Fluidics Station 450 and scanned using the Affymetrix GeneChip Scanner 3000. The probe level data were normalized and converted into expression values using the robust multiarray average procedure.24 Expression data were then per gene normalized to the median expression level across all samples. Expression heatmap as well as the bar graphs were generated using Microsoft Excel.
Results
Development and characterization of an anti-mutated calreticulin antibody
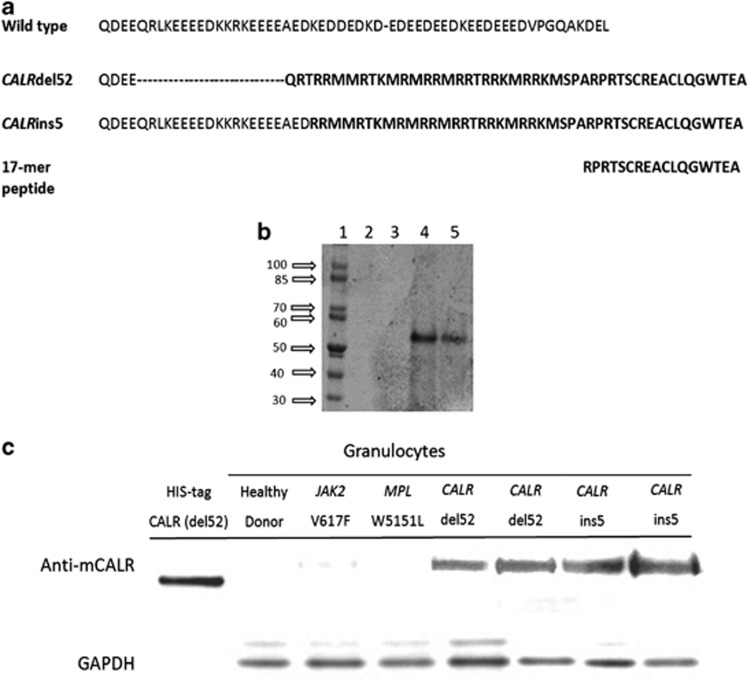
We selected the 17-mer C-terminal peptide RPRTSCREACLQGWTEA as the most suitable for immunization based on preliminary bioinformatic analysis (Figure 1a). An antibody was developed in rabbits against the peptide and purified. We also prepared a recombinant HIS-tagged protein corresponding to the amino-acid sequence corresponding to the CALRdel52 mutation (Figure 1b). We used this recombinant protein as a reference in western blots prepared with proteins extracted from purified granulocytes obtained from healthy donors, JAK2V617F- or MPLW515L-mutated ET and PMF, and patients with a CALRdel52, CARLins5 and CALRindel genotype. One representative experiment is shown in Figure 1c. Granulocytes from CALR-mutated patients reacted with the anti-mutated CALR antibody, unlike healthy donors and JAK2/MPL-mutated patients, indicating the selectivity of the antibody for the mutated calreticulin.
Figure 1.
Generation and characterization of the anti-mutated CALR antibody. In panel a, the sequence of the 17-mer peptide used for generation of the anti-mutated CALR antibody is shown in relation with the amino-acid sequence of wild-type CALR and the predicted sequence of mutated CALR originated from the del52 and ins5 abnormalities. In panel b, gel electrophoresis of the mutated calreticulin prepared in BL21DE3RIPL (lanes 2 and 3) and JM109DE3 (lanes 4 and 5) E. Coli strains; only in the latter strain successful production of calreticulin was obtained. Calreticulin has a molecular weight of 47 kDa, but migrates in these conditions at an apparent higher molecular weight likely owing to a specific conformational pattern. In panel c, western blot analysis shows anti-mutated CALR antibody selectivity for mutated protein vs the wild-type form. CALR recombinant protein expressed in E. Coli was used as a positive control. GAPDH was used as a loading control.
Specific immunostaining of BM cells in CALR-mutated patients
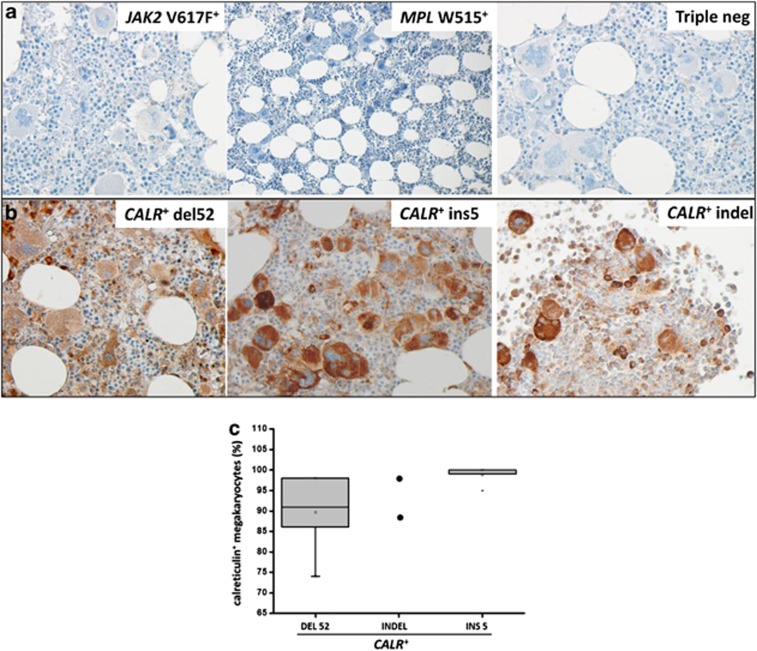
For immunostaining, we used BM biopsies from 10 to 20 individuals of the following categories: non-hematologic disorders (solid cancers, used as controls), JAK2V617F-mutated polycythemia vera, ET and PMF patients; MPL-mutated ET or PMF (n=8); JAK2/MPL/CALR triple-negative patients; CALR-mutated ET or PMF patients, including the CALRdel52, CALRins5 and CALRindel (ins5′-TCCTTCAG-delGCAGAGAAACAAATGAAGGACAAACAGGACG-3′) (n=2) mutations. Results are presented in Figure 2. Subjects with non-hematologic disorders (not shown), MPN patients harboring the JAK2V617F and MPLW515 mutation and triple-negative ET and PMF patients, all resulted negative with the antibody raised against anti-mutated calreticulin (panel 2a). Conversely, the three variants of CALR mutations showed strong immunostaining (panel 2b), thus establishing the specificity of the immunostaining depending on the genetic background of the patients. We observed that immunostaining was localized mainly in megakaryocytes, both large, mature forms as seen in ET (Figure 3a two positive and one negative megakaryocytes from an ET patient are shown) and atypical megakaryocytes as found in advanced fibrotic PMF (Figures 3b–d), including small megakaryocytes (panel b). The mean percentage of immunostained megakaryocytes was 91% (range, 74–98%) in CALRdel52-mutated patients, 92% (88–98%) in CALRins5 and 87%/98% in the two CALRindel patients (Figure 2c). Conversely, the staining was much fainter in myeloid and erythroid cells, compared with megakaryocytes (Figures 3e and f).
Figure 2.
Immunostaining of bone marrow biopsies with the anti-mutated CALR antibody. Representative sections from CALR-unmutated ET/PMF patients (JAK2V617F mutated, MPLW515L mutated and triple-negative mutation) and from CALR-mutated patients (CALRdel52, CALRins5 and CALRindel) are shown in panel a and panel b, respectively. Sections were stained according to the procedure described in the Materials and Methods section using a 1:1000 dilution of the anti-mutated CALR antibody. Pictures were taken with a LEICA DM LS2 microscope using a N-Plan × 40/0.65 objective. The number of immunostained megakaryocytes over total number of morphologically recognizable megakaryocytes was calculated by counting at least 100 megakaryocytes/slide from 10 patients with ET and 10 patients with PMF (n=2 for the CALR indel), and expressed as percent of total megakaryocytes (c); as there was no significant difference between the two groups, all individual data were pooled.
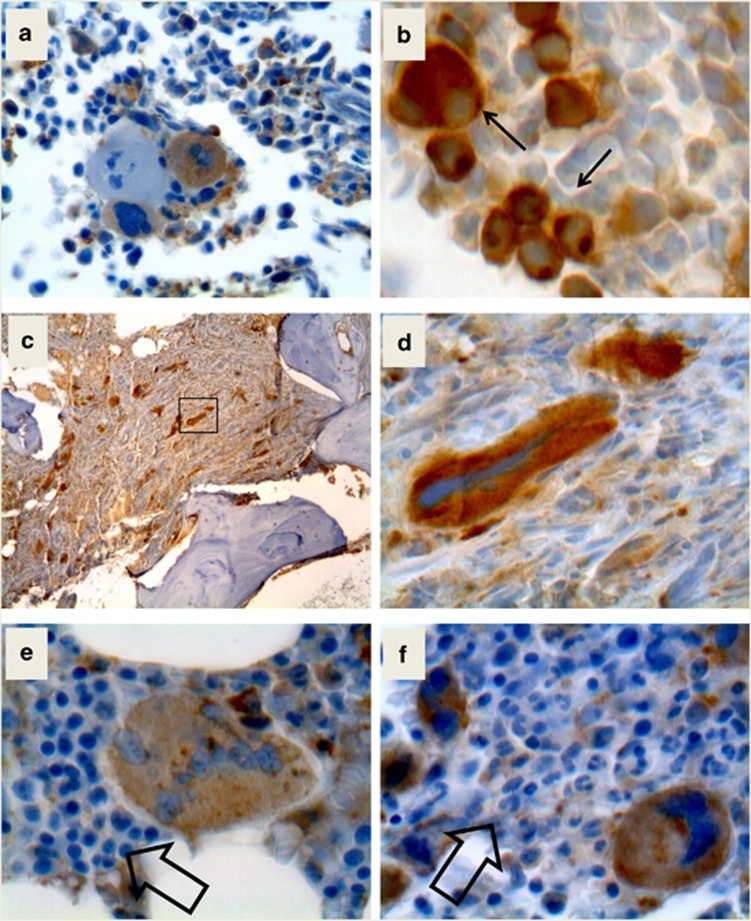
Figure 3.
Immunostaining of bone marrow biopsies with the anti-mutated CALR antibody. Panel a shows two megakaryocytes labeled by the anti-mutated calreticulin antibody together with a negative one. In panel b, abnormal, small megakaryocytes in the bone marrow of a CALRins5 PMF patient are shown. Panel c shows a low-resolution picture of an advanced fibrosis in a CALRdel52 patient and d is a high-power field from panel c (square) to show the abnormally shaped large megakaryocytes within buddles of fibers. In panels e and f, the faint label of erythroid and myeloid cells is presented at higher magnification.
Preferential expression of calreticulin in cells of megakaryocytic lineage
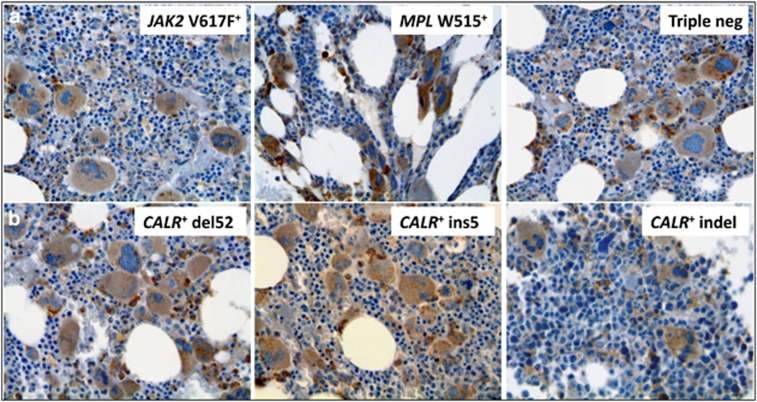
We then asked whether the much more pronounced staining observed in megakaryocytes compared with cells of the myeloid and erythroid cell lineages was a consequence of the acquisition of the CALR mutation itself or rather it reflected a physiologic megakaryocytic lineage-associated overexpression of calreticulin. To this end, we used a commercially available antibody directed against the N terminus of calreticulin, therefore expected to label the protein in both CALR-mutated and CALR-unmutated MPN patients as well as in healthy controls. As anticipated, cell-associated immunoreactivity could be observed in controls (not shown) and in patients with any molecular subtype of MPN (JAK2V617F-, MPLW515- and CALR-mutated and triple-negative patients) (Figure 4). Intriguingly, the immunostaining was associated predominantly with megakaryocytes, whereas myeloid and erythroid cells showed a uniformly faint labeling, the overall picture mimicking that observed in CALR-mutated patients probed with the anti-mutated calreticulin antibody. We interpreted these findings as suggestive of a physiologic preferential overexpression of calreticulin along the megakaryocyte lineage compared with myeloid and erythroid cell lineages.
Figure 4.
Immunostaining of bone marrow biopsies with the anti-wild-type calreticulin antibody. Representative sections from CALR-unmutated ET/PMF patients (JAK2V617F mutated, MPLW515L mutated and triple-negative mutation) and from CALR-mutated patients (CALRdel52, CALRins5 and CALRindel) are shown in panel a and panel b, respectively.
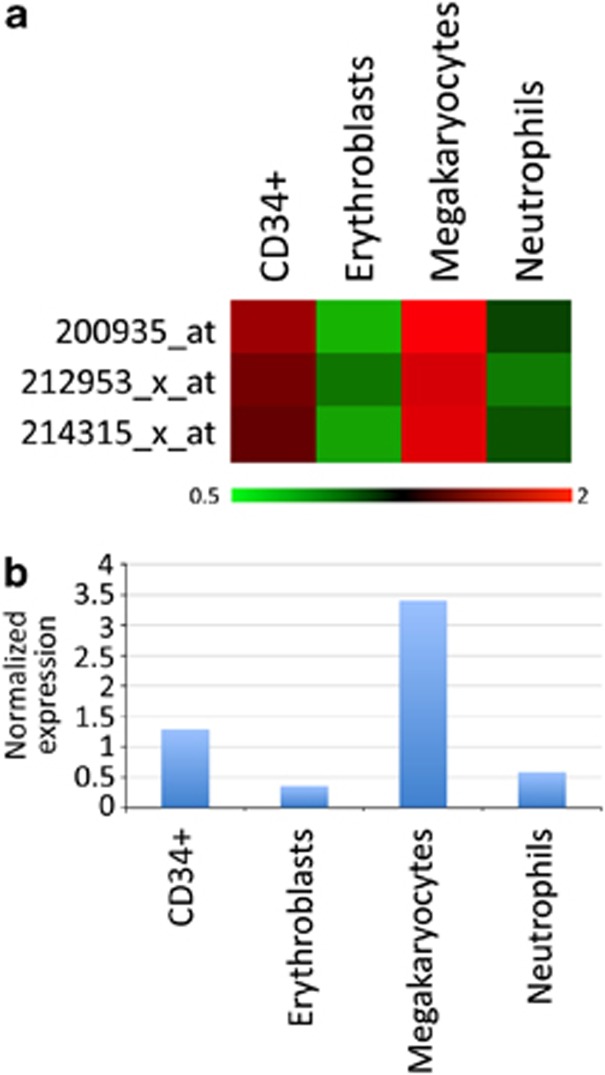
With the aim to support those findings, we measured the levels of calreticulin mRNA in normal hematopoietic cells by a gene expression array. To this end, we re-analyzed our own unpublished microarray data set of gene expression obtained from cells at specific stages of hematopoietic differentiation, either freshly immunoselected from peripheral blood (neutrophils) or BM (CD34+) of healthy controls or obtained from normal CD34+ progenitor cells induced to unilineage differentiation toward the erythroid and megakaryocytic differentiation in liquid cultures. Figure 5 shows the relative abundance of calreticulin mRNA in these cell populations and reveals that CALR expression decreases during normal erythroid and granulocyte maturation, whereas it is significantly upregulated in megakaryocyte differentiation.
Figure 5.
CALR expression in hematopoietic progenitors and differentiated cells. CALR expression profile were analyzed in purified CD34+ cells, erythroblasts, megakaryocytes and neutrophils. (a) Heatmap showing the mRNA expression detected by the three different CALR probesets present on the HG-U133A array. Gene expression coloring was based on normalized signals, as shown at the bottom. The expression values were normalized on the median of all samples. (b) Bar graph displaying the average normalized expression detected by the three CALR probesets in the populations examined.
Discussion
Mutations in the gene calreticulin (CALR) have been recently reported in about 15–25% of patients with ET, PMF and post-ET myelofibrosis who are negative for the more common JAK2V617F and MPL exon 10 mutations.12, 13 The occasional simultaneous positivity for JAK2V617F and CALR mutation, reported to date in a handful of patients,25, 26 may possibly reflect the presence of two different clones; clonal mixture is a well-described phenomenon in MPN27 and has been extensively characterized for the co-occurrence of JAK2 and MPL mutations.28, 29 CALR mutations have not been detected in acute myeloid leukemia, lymphoproliferative disorders, a variety of solid tumors and chronic myelogenous leukemia.12, 13 Only a few occasional patients with chronic myelomonocytic leukemia (3%), atypical chronic myeloid leukemia (3%), myelodysplastic syndromes (8.3%) including 3 of 24 patients with refractory anemia and ringed sideroblasts, and thrombocytosis (RARS-T)13 were found CALR mutated in the two seminal studies,12, 13 but subsequent larger series of MDS patients showed that CALR mutations are indeed very infrequent in this category of patients.30, 31 A novel single-nucleotide variant (CALRE398D c1194G>T) was reported in one of eight patients with CSF3R-mutated chronic neutrophilic leukemia.32
Known mutations of CALR are all located at exon 9 and are somatic deletions or insertions; the two commonest abnormalities (del52 and ins5) account for ≈80% of cases, whereas in the remaining 20% lesions are highly heterogeneous. Unlike for JAK2V617F and MPL exon 10 mutations, where the single-nucleotide nature of the mutations has facilitated the development of specific and highly sensitive methods, including commercially available kits that are amenable to interlaboratories standardization,33 the nature of CALR mutations and their variability currently require bidirectional Sanger for assessment, if one wants to include all possible variants. This approach is routinely used in tertiary hematology centers, but may not be easily available in smaller hematology units that, on the other hand, are commonly involved in the initial phases of the diagnostic pathway. Alternative screening methods might therefore be warranted. Having this in mind, we exploited the fact that all exon 9 mutations result in a novel C terminus of the protein with the acquisition of a minimum 36-amino-acid stretch in place of 27 amino acids that are lost from the normal sequence. Therefore, we developed a polyclonal antibody against a 17-peptide residue in the variant C terminus of mutated calreticulin that proved able to label selectively BM sections from CALR-mutated patients, distinguishing them from normal subjects and ET and PMF patients harboring the JAK2 or MPL mutations. Therefore, immunostaining could serve as a specific diagnostic tool for molecular categorization of the patients.
The inspection of immunostained BM sections from CALR-mutated patients also provided intriguing information that could have relevance for the understanding of pathogenetic mechanisms associated with CALR mutations. In fact, we observed that the immunostaing was almost entirely confined to cells of the megakaryocyte lineage, whereas myeloid and erythroid cells showed modest labeling. By immunostaining with an antibody recognizing wild-type calreticulin, we showed that preferential expression of calreticulin in megakaryocytes is a physiologic phenomenon, and may likely reflect cell-specific promoter-dependent regulation of calreticulin. To further support these suggestions, analysis of gene expression profile in purified hematopoietic cells showed that the levels of calreticulin in normal cells are significantly downmodulated from the CD34+ cells to erythroblasts and granulocytes (although the degree of expression still allows the identification of the protein by western blotting as presented in Figure 1c), whereas they are maintained at even higher levels in megakaryocytes. To the best of our knowledge, there is no information available concerning the comparative expression of CALR in various types of myeloid cells; however, our results are consistent with some previous observations suggesting a crucial role of calreticulin in platelet function. Calreticulin is expressed on the surface of human platelets, and antibodies against calreticulin cause platelet activation and induce FcgammaRIIa-independent platelet aggregation. Moreover, surface calreticulin is associated with collagen receptors on the platelet surface, where it may have a role in the modulation of the platelet–collagen interaction.34 Furthermore, the interaction of platelet surface calreticulin with its ligand C1q, which is part of the first component of complement C1, leads to cellular activation followed by release of biological mediators and expression of adhesion molecules, contributing directly or indirectly to the inflammatory process.35 On the other hand, the decrease of calreticulin expression during granulocyte differentiation was documented by Clark et al,36 who showed that the stimulation of granulocyte differentiation in HL60 cells induces a rapid decline in calreticulin mRNA and protein levels. Differentiation also greatly reduced the Ca2+ content of HL60 cells, reflecting an endoplasmic reticulum remodeling as a crucial feature of granulocytic differentiation.36 As CALR mutations occur in multipotent progenitors capable of both erythroid and myeloid differentiation,12 these findings may have relevance for further analysis aimed at dissecting the specific role of mutated calreticulin in disorders that preferentially manifest with involvement of the megakaryocytic lineage, as are ET and PMF.
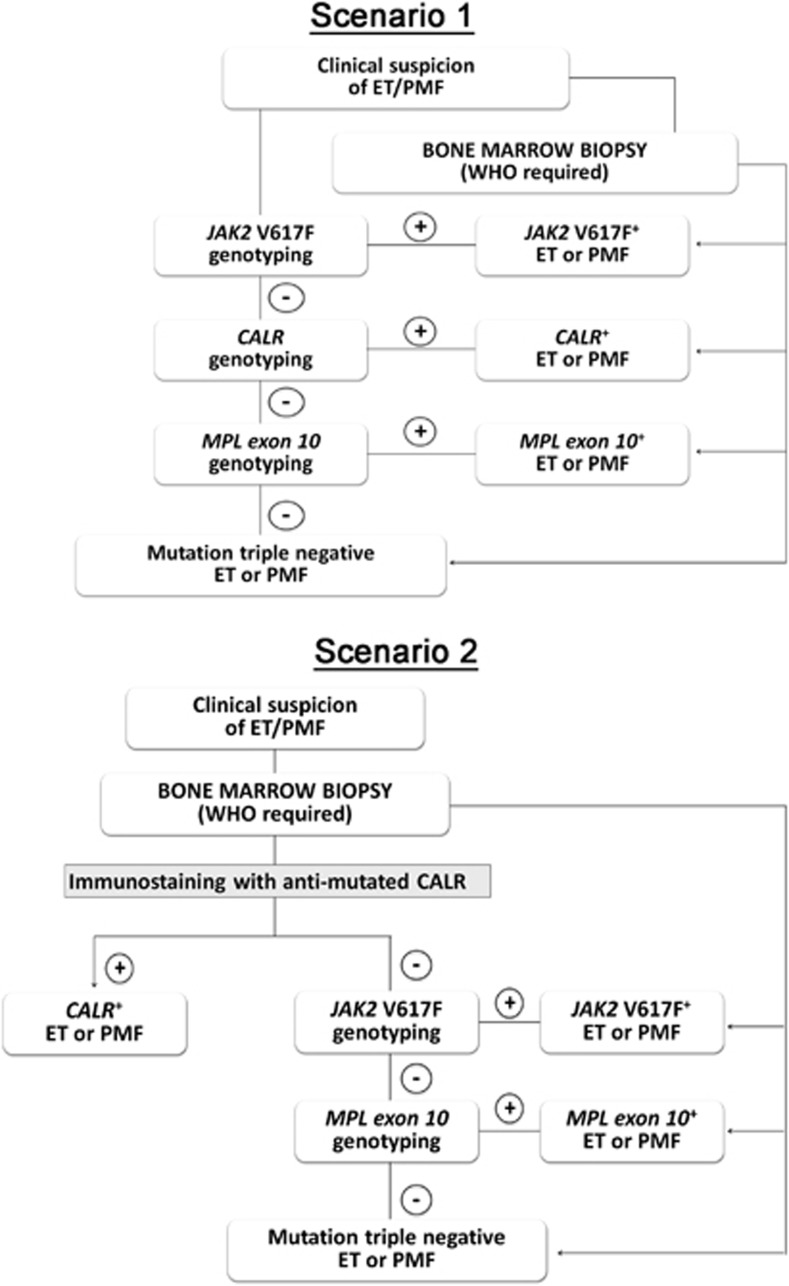
Identification of CALR mutations has been proposed14 as a novel major criteria for the upcoming revision of 2008 WHO diagnostic criteria for ET and PMF11 together with a restricted list of clinical and hematological variables and the BM biopsy. In this study, we provide evidence that immunostaining in BM biopsies can be conveniently used for identifying patients harboring CALR mutations. Considering the specificity of the immunostaining procedure, its feasibility in any routine histopathology laboratory, the lower cost compared with molecular tests and the fact that obtaining a BM biopsy is mandatory for the diagnosis of ET and PMF according to the WHO criteria, we propose that this approach could be applied during the initial steps of the diagnostic algorithm for patients suspected to have ET and PMF, as suggested in Figure 6 scenario 1. Accordingly, immunostaining for mutated CALR would come before the mutational genotyping for the more common JAK2V617F mutation, leaving MPL mutation as the third step. We want to make it clear that this approach is not intended as a substitute for the optimized algorithm based on molecular genotyping,14 which remains the gold standard to date, Figure 6 scenario 2. Rather, we argue that immunostaining with anti-mutated calreticulin antibody could help saving time and money for those hematology services where sequencing platforms may not be routinely available, reserving the use of genotyping for JAK2V617F and MPL mutations thereafter. Finally, the use of a mutation-specific antibody may be useful for studying different aspects of hematopoiesis and particularly megakaryocytopoiesis in patients harboring CARL mutations.
Figure 6.
Diagnostic algorithms for CALR-mutated myeloproliferative neoplasms. In scenario 1, a standard ‘genotype-exclusive' diagnostic algorithm is presented, whereas in the flow chart shown in scenario 2 immunostaining with anti-mutated calreticulin is used at the time of bone marrow biopsy to rule out a CALR-mutated MPN before genotyping for JAK2V617F and MPL mutations. See Discussion for details.
Acknowledgments
This study was supported by a special grant from Associazione Italiana per la Ricerca sul Cancro—‘AIRC 5 per Mille'— to AGIMM, ‘AIRC-Gruppo Italiano Malattie Mieloproliferative' (#1005); for a description of the AGIMM project and list of investigators, see www.progettoagimm.it).
Author contributions
AMV and PG designed the study, analyzed the data and wrote the manuscript; NB and LC developed and characterized the antibody; MB prepared the antibody; GRo, AP and CM performed genotyping; GRo, GRa and VC performed and analyzed immunohistochemistry; RM performed microarray analysis; LP, RF, AB, PG and AMV contributed samples and clinical information. All authors have read the final version of the manuscript and agreed on its content.
The authors declare no conflict of interest.
References
- Antonioli E, Guglielmelli P, Pancrazzi A, Bogani C, Verrucci M, Ponziani V, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia. 2005;19:1847–1849. doi: 10.1038/sj.leu.2403902. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366:1945–1953. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- Wolanskyj AP, Lasho TL, Schwager SM, McClure RF, Wadleigh M, Lee SJ, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol. 2005;131:208–213. doi: 10.1111/j.1365-2141.2005.05764.x. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Schwager SM, Steensma DP, Mesa RA, Li CY, et al. The JAK2 tyrosine kinase mutation in myelofibrosis with myeloid metaplasia: lineage specificity and clinical correlates. Br J Haematol. 2005;131:320–328. doi: 10.1111/j.1365-2141.2005.05776.x. [DOI] [PubMed] [Google Scholar]
- Barosi G, Bergamaschi G, Marchetti M, Vannucchi AM, Guglielmelli P, Antonioli E, et al. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110:4030–4036. doi: 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Huang J, Finke C, Mesa RA, Li CY, et al. Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia. 2008;22:756–761. doi: 10.1038/sj.leu.2405097. [DOI] [PubMed] [Google Scholar]
- Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- Guglielmelli P, Pancrazzi A, Bergamaschi G, Rosti V, Villani L, Antonioli E, et al. Anaemia characterises patients with myelofibrosis harbouring Mpl mutation. Br J Haematol. 2007;137:244–247. doi: 10.1111/j.1365-2141.2007.06565.x. [DOI] [PubMed] [Google Scholar]
- Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110:1092–1097. doi: 10.1182/blood-2007-04-083501. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. (eds)WHO classification of Tumors of Haematopoietic and Lymphoid Tissues International Agency for Research on Cancer: Lyon; 2008 [Google Scholar]
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Thiele J, Vannucchi AM, Barbui T.An overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms Leukemia 2014. e-pub ahead of print 20 January 2014doi: 10.1038/leu.2014.35 [DOI] [PubMed]
- Barbui T, Thiele J, Vannucchi AM, Tefferi A. Problems and pitfalls regarding WHO-defined diagnosis of early/prefibrotic primary myelofibrosis versus essential thrombocythemia. Leukemia. 2013;27:1953–1958. doi: 10.1038/leu.2013.74. [DOI] [PubMed] [Google Scholar]
- Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes Blood 2013; e-pub ahead of print 23 December 2013. [DOI] [PMC free article] [PubMed]
- Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–1959. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- Pancrazzi A, Guglielmelli P, Ponziani V, Bergamaschi G, Bosi A, Barosi G, et al. A sensitive detection method for MPLW515L or MPLW515K mutation in chronic myeloproliferative disorders with locked nucleic acid-modified probes and real-time polymerase chain reaction. J Mol Diagn. 2008;10:435–441. doi: 10.2353/jmoldx.2008.080015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi E, Pietra D, Guglielmelli P, Bordoni R, Casetti I, Milanesi C, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood. 2013;121:4388–4395. doi: 10.1182/blood-2013-02-486050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredini R, Zini R, Salati S, Siena M, Tenedini E, Tagliafico E, et al. The kinetic status of hematopoietic stem cell subpopulations underlies a differential expression of genes involved in self-renewal, commitment, and engraftment. Stem Cells. 2005;23:496–506. doi: 10.1634/stemcells.2004-0265. [DOI] [PubMed] [Google Scholar]
- Tenedini E, Fagioli ME, Vianelli N, Tazzari PL, Ricci F, Tagliafico E, et al. Gene expression profiling of normal and malignant CD34-derived megakaryocytic cells. Blood. 2004;104:3126–3135. doi: 10.1182/blood-2003-07-2597. [DOI] [PubMed] [Google Scholar]
- Grande A, Piovani B, Aiuti A, Ottolenghi S, Mavilio F, Ferrari G. Transcriptional targeting of retroviral vectors to the erythroblastic progeny of transduced hematopoietic stem cells. Blood. 1999;93:3276–3285. [PubMed] [Google Scholar]
- Gemelli C, Montanari M, Tenedini E, Zanocco Marani T, Vignudelli T, Siena M, et al. Virally mediated MafB transduction induces the monocyte commitment of human CD34+ hematopoietic stem/progenitor cells. Cell DeathDiffer. 2006;13:1686–1696. doi: 10.1038/sj.cdd.4401860. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons Leukemia 2014. e-pub ahead of print 9 January 2014;doi: 10.1038/leu.2014.3 [DOI] [PubMed]
- Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms Blood 2014; e-pub ahead of print 29 January 2014. [DOI] [PubMed]
- Kralovics R. Genetic complexity of myeloproliferative neoplasms. Leukemia. 2008;22:1841–1848. doi: 10.1038/leu.2008.233. [DOI] [PubMed] [Google Scholar]
- Li S, Kralovics R, De Libero G, Theocharides A, Gisslinger H, Skoda RC. Clonal heterogeneity in polycythemia vera patients with JAK2 exon12 and JAK2-V617F mutations. Blood. 2008;111:3863–3866. doi: 10.1182/blood-2007-09-111971. [DOI] [PubMed] [Google Scholar]
- Lasho TL, Pardanani A, McClure RF, Mesa RA, Levine RL, Gilliland DG, et al. Concurrent MPL515 and JAK2V617F mutations in myelofibrosis: chronology of clonal emergence and changes in mutant allele burden over time. Br J Haematol. 2006;135:683–687. doi: 10.1111/j.1365-2141.2006.06348.x. [DOI] [PubMed] [Google Scholar]
- Hou H-A, Kuo Y-Y, Chou W-C, Chen P-H, Tien H-F.Calreticulin mutation was rarely detected in patients with myelodysplastic syndrome Leukemia 2014. e-pub ahead of print 17 February 2014doi: 10.1038/leu.2014.71 [DOI] [PubMed]
- Broséus J, Lippert E, Harutyunyan AS, Jeromin S, Zipperer E, Florensa L, et al. Low rate of calreticulin mutations in refractory anaemia with ring sideroblasts and marked thrombocytosis Leukemia 2014. e-pub ahead of print 30 January 2014doi: 10.1038/leu.2014.49 [DOI] [PubMed]
- Lasho TL, Elliott MA, Pardanani A, Tefferi A.CALR mutation studies in chronic neutrophilic leukemia Am J Hematol 2014. e-pub ahead of print 13 January 2014doi: 10.1002/ajh.23665 [DOI] [PubMed]
- Jovanovic JV, Ivey A, Vannucchi AM, Lippert E, Oppliger Leibundgut E, Cassinat B, et al. Establishing optimal quantitative-polymerase chain reaction assays for routine diagnosis and tracking of minimal residual disease in JAK2-V617F-associated myeloproliferative neoplasms: a joint European LeukemiaNet/MPN&MPNr-EuroNet (COST action BM0902) study. Leukemia. 2013;27:2032–2039. doi: 10.1038/leu.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton CM, Smethurst PA, Eggleton P, Farndale RW. Physical and functional interaction between cell-surface calreticulin and the collagen receptors integrin alpha2beta1 and glycoprotein VI in human platelets. Thromb Haemost. 2002;88:648–654. [PubMed] [Google Scholar]
- Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004;41:173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Clark RA, Li SL, Pearson DW, Leidal KG, Clark JR, Denning GM, et al. Regulation of calreticulin expression during induction of differentiation in human myeloid cells. Evidence for remodeling of the endoplasmic reticulum. J Biol Chem. 2002;277:32369–32378. doi: 10.1074/jbc.M205269200. [DOI] [PubMed] [Google Scholar]