Summary
Planar cell polarity (PCP) refers to the polarization of a field of cells within the plane of a cell sheet. This form of polarization is required for diverse cellular processes in vertebrates, including convergent extension (CE), the establishment of PCP in epithelial tissues and ciliogenesis. Perhaps the most distinct example of vertebrate PCP is the uniform orientation of stereociliary bundles at the apices of sensory hair cells in the mammalian auditory sensory organ. The establishment of PCP in the mammalian cochlea occurs concurrently with CE in this ciliated epithelium, therefore linking three cellular processes regulated by the vertebrate PCP pathway in the same tissue and emerging as a model system for dissecting PCP signaling. This review summarizes the morphogenesis of this model system to assist the interpretation of the emerging data and proposes molecular mechanisms underlying PCP signaling in vertebrates.
Introduction
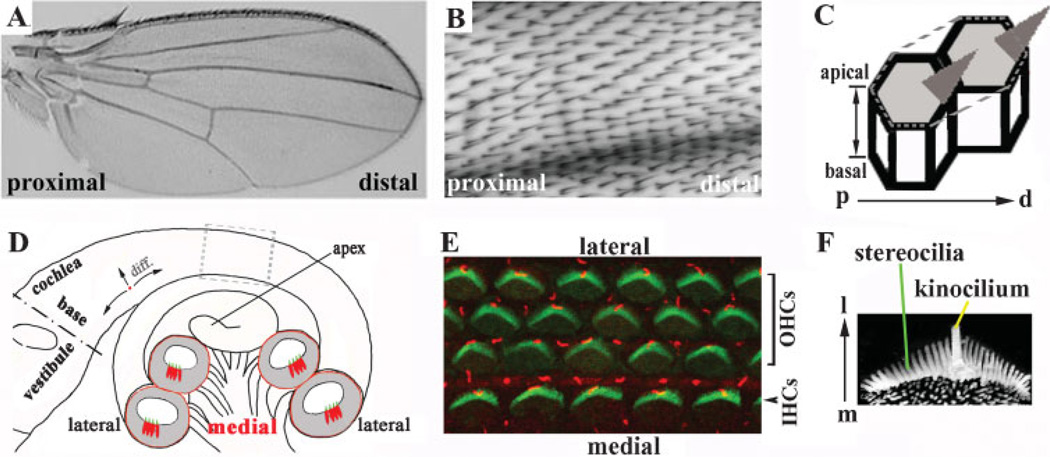
In addition to apical–basal polarization, cells within an epithelium often display a polarity across the plane of the epithelium known as epithelial ‘‘planar cell polarity’’ (PCP). Examples of PCP in invertebrates and vertebrates, respectively, are the uniform alignment in a proximal-to-distal orientation of the bristles at the apical surfaces of wing cells in Drosophila (Fig. 1A–C) and the uniform medial-to-lateral (mediolateral) orientation of precisely patterned stereociliary bundles at the apical surfaces of sensory hair cells in the organ of Corti, the mammalian auditory sensory organ (Fig. 1D–F).
Figure 1. Planar cell polarity.
A–C: PCP in the Drosophila wing. The bristles point from the proximal to distal direction and are uniformly oriented (A,B). The PCP displayed by the Drosophila wing is illustrated diagrammatically (C). Note that the uniform orientation of bristles manifests a polarity along the proximal-to-distal (p–d) axis of the plane formed by the epithelial sheet that is perpendicular to the basal–apical axis of the epithelium. D: A diagram of the cochlea. The cochlear duct forms a spiral and the tip of the cochlea, or apex, lies distally from the base of the cochlea, which is juxtaposed to the vestibule. (Note that the terms ‘‘apex’’ and ‘‘base’’ used for the cochlea are different from the terms used to describe the apical and basolateral domains of individual epithelial cells.) The longitudinal axis is along the length of the cochlear duct, while the mediolateral axis is from the center to the periphery of the cochlea. Shown in grey are cross-sections of the cochlear duct, illustrating the positions of the sensory hair cells (red) and the stereocilia bundles (green) protruding from their apical surfaces into the lumen of the duct. The gray dotted box indicates the region highlighted in panel E. E: The organ of Corti viewed from its surface in a confocal image. The stereociliary bundles (green) on the apical surface of each hair cell are patterned into a ‘‘V’’-shaped structure and all the ‘‘V’’s are uniformly oriented, showing a distinctive polarity that is parallel to the sensory epithelial sheet along the mediolateral axis. The kinocilia (red) are positioned at the vertices of the ‘‘V’’-shaped stereocilia. Note that the entire organ of Corti is ciliated. Some of the cilia (red) are from supporting cells (not shown). The single row of inner hair cells (IHCs) is located toward the center (medial) of the cochlear duct ,while the outer hair cells (OHCs) are located lateral to the inner hair cells toward the periphery of the cochlear duct. F: Stereocilia and a kinocilium of one hair cell, showing the stereociliary bundles arranged in a ‘‘V’’ shape with a kinocilium-positioned at the vertex of the V. m: medial; l: lateral.
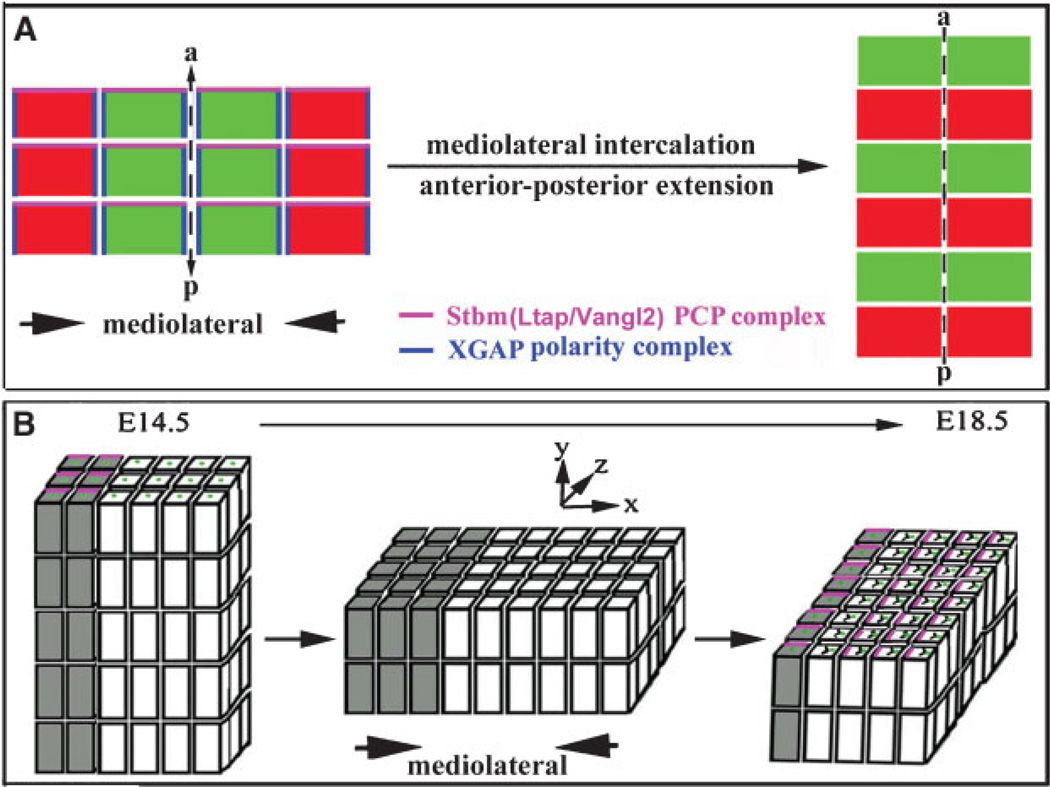
Genetic studies in Drosophila identified a set of genes involved in regulating PCP in all adult tissues of the fly.(1–3) In vertebrates, a similar group of genes homologous to the Drosophila PCP genes was found to control convergent extension (CE) (Fig. 2A),(4–13) a polarized cellular rearrangement that leads to the narrowing and concomitant lengthening of a tissue along two perpendicular axes.(14) Subsequently, the same set of genes was demonstrated to be required for establishing the precisely aligned orientations of sensory hair cell stereociliary bundles in the organ of Corti.(15–17) Most recently, several genes that regulate vertebrate PCP(18–20) were found to be required for ciliogenesis(21) and, conversely, genes previously known to be required for ciliogenesis were found to be involved in PCP.(22) The developing organ of Corti is a ciliated epithelium that undergoes polarized cellular rearrangement characteristic of CE during establishment of the PCP (Fig. 2B).(17,23,24) The discovery that these cellular processes are regulated by the vertebrate PCP pathway shines the spotlight on the organ of Corti for PCP study. The distinct morphology of the organ of Corti further commands an extensive application of this organ for dissecting the mechanism underlying PCP signaling in vertebrates. In this review, we first describe the morphological development of the mammalian organ of Corti for a basic understanding of the cellular processes involved. We will further summarize the emerging data and propose possible molecular mechanisms that may underlie PCP signaling in vertebrates.
Figure 2. Convergent extension.
A: A schematic illustration of CE during gastrulation and neurulation in Xenopus and zebrafish. Cells project mediolaterally oriented lamellipodia and exert traction along the mediolateral axis to drive convergence along the same axis and extension along the perpendicular axis (anterior–posterior). The PCP complex of Stbm and PK (magenta) are thought to be at the anterior ends of the cells during CE, and the XGAP polarity complex (blue) are localized to the mediolateral ends of the cells, orienting the cells at both axes for polarized cellular rearrangement. a, anterior; p, posterior. B: The proposed CE in the cochlea during terminal differentiation of the organ of Corti. The mature organ of Corti is derived from the shorter and thicker ‘‘primordial domain’’ (white boxes). Cells of the primordial domain are evidenced by a zone of non-proliferating cells around E13.5–E14.5 in mice. In PCP mutants, the organ of Corti is widened (X-axis) and shortened (Z-axis), suggesting that the developing organ of Corti undergoes mediolateral intercalation. While the organ of Corti is postmitotic after E13.5–E14.5, the cells adjacent to the developing organ of Corti (shaded boxes) continue to divide and show a distinct polarized localization of Ltap/Vangl2/Stbm (pink) at E14.5 and E18.5 along the extension axis. In the developing organ of Corti (white boxes), Ltap/Vangl2 (pink) apparently is localized to the medial end of hair cells, perpendicular to the polarized localization of Ltap/Vangl2 in the adjacent region (shaded boxes). The significance of the polarized subcellular localization of PCP proteins in the region medial to the organ of Corti has not been investigated. During the extension of the cochlea, the kinocilia (green) are displaced toward the lateral sides of the hair cells and the stereocilia develop and acquire the ‘‘V’’-shaped structure. The morphology of the developing cochlea and the localization of PCP proteins during the extension from E14.5–E18.5 are dynamic and have not been carefully characterized.
The planar cell polarity of the organ of Corti
The organ of Corti consists of four rows of sensory hair cells. The innermost row toward the center (hereinafter referred to as ‘‘medial’’) region of the cochlea and the three rows toward the peripheral (hereinafter referred to as ‘‘lateral’’) region of the cochlea are known as the inner (IHCs) and outer hair cells (OHCs), respectively (Fig. 1D,E). PCP within the organ of Corti is displayed by the uniform orientation of the surface protrusions of the sensory hair cells. Several rows of ‘‘finger-like’’ extensions or hair bundles, known as the stereocilia (Fig. 1E, green), project from the apical surface of each hair cell and form a ‘‘V’’ shape. Invariably, the vertices of the stereocilia ‘‘V’’s point in the medial-to-lateral (mediolateral) direction (Fig. 1D–F).
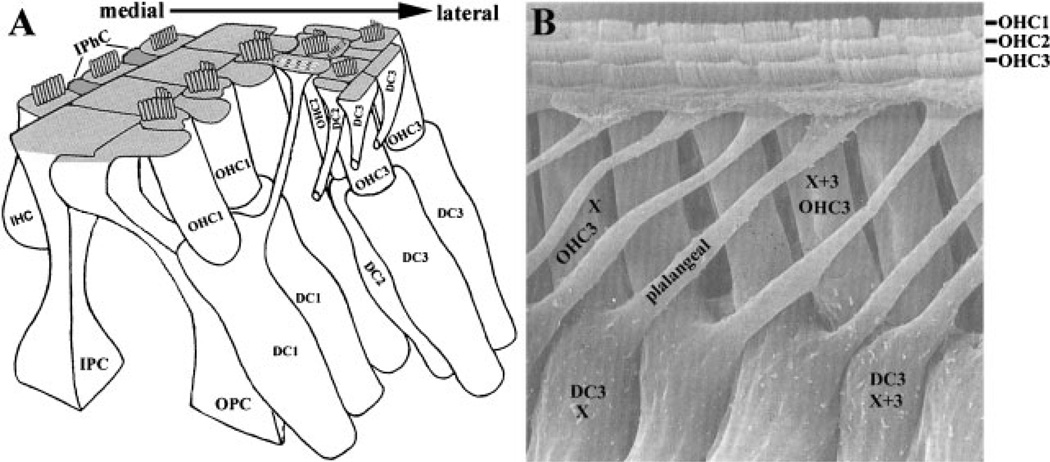
The hair cells are interdigitated with supporting cells in the organ of Corti (Fig. 3A,B). The supporting cells include the inner phalangeal cells (IPhC), the inner and outer pillar cells (IPC and OPC), and the Deiters cells (DC) (Fig. 3A). The nuclei of these supporting cells are localized basally and, from their soma, the supporting cells project phalangeal (‘‘finger-like’’) cellular processes toward the lumen of the cochlear duct (Fig. 3A,B). Their flattened ends of phalangeal processes separate hair cells from each other and extend along the lateral side of the apical surfaces of the hair cells where they form tight cellular contacts with the hair cells (through tight junctions and adherins junctions). The intricate cell–cell contacts in the organ of Corti are diagramed (Fig. 3A).
Figure 3. The polarity of supporting cells of the organ of Corti.
A: A diagram of the cellular architecture of the organ of Corti showing the intricate cell-cell contacts between hair cells and supporting cells and among the different types of supporting cells. The polarity of the phalangeal processes of supporting cells is also illustrated. The IHCs are separated from each other by the IPhCs at the apical surface. The IHCs are separated from the OHCs by the IPCs and OPCs. The OHCs within each row are separated by the phalangeal processes of the OPCs and the first and second rows of Deiters cells (DC1 and DC2). The phalangeal processes of the third row of DCs (DC3) form the lateral border for the sensory hair cells at the apical surface. IPhC, inner phalangeal cell; IPC, inner pillar cell; OPC, outer pillar cell; DC, Deiters cell; IHC, inner hair cell; OHC, outer hair cell. B: A scanning electron microscope image viewed from the lateral side of the organ of Corti to illustrate the polarity of the phalangeal processes of supporting cells around the third row of OHCs (OHC3). The hair cells (marked with OHC1, OHC2 and OHC3) at the surface are also visible. The phalangeal processes of the supporting cells project in precise orientations toward the lumen of the cochlear epithelium to separate hair cells from each other. For instance, a Deiters cell (DC3) at position X along the longitudinal axis projects its phalangeal processes toward the lateral direction to border the third row of outer hair cells at position X + 3 (toward the apex of the cochlear duct). (Panel A is modified from an illustration by Slepecky, 1996, with permission from Springer. Scanning electron micrograph in B is provided by Dr. Y. Raphael, University of Michigan, Ann Arbor, MI. From Raphael et al. 1991, reprinted by permission of John Wiley and Sons, Inc.).
Interestingly, in addition to the PCP exhibited by the sensory hair cell stereocilia bundles, the supporting cells also display a distinct polarity (Fig. 3). Notably, the phalangeal processes of the supporting cells are highly polarized along the same mediolateral axis of the cochlea (toward the periphery) observed for stereocilia. In addition, OPCs and DCs display a polarity along the longitudinal axis of the cochlea. The DCs extend their cytoplasmic stalks and phalangeal processes along the longitudinal axis of the cochlea and contact the apical surface of the outer hair cells in the second and third rows (OHC2, OHC3) at a distance (toward the apex of the cochlear duct) from the base of these supporting cells (Fig. 3). Rigid bundles of microtubules, intermediate filaments and microfilaments span the phalangeal processes of supporting cells.(25,26)
In summary, the precise patterning of hair cell stereociliary bundles in the organ of Corti displays a distinctive PCP, and the polarization of the phalangeal processes of supporting cells parallel to the sensory epithelium may also represent a previously unrecognized form of PCP in the organ of Corti. Many studies examining the mechanisms underlying PCP in the organ of Corti focus on the sensory hair cells and often overlook the intricate cellular polarity of supporting cells and their contacts with polarized hair cells. Thus, the interpretation of future studies must consider the indispensable role of supporting cells in PCP of the organ of Corti.
A morphogenetic trilogy in the organ of Corti: CE, establishment of PCP and ciliogenesis
The development of the organ of Corti is marked by distinctive morphogenetic events. The cochlear duct is formed at the ventral region of the developing inner ear by embryonic day 12.5 (E12.5) in mice and continues to grow in length through the end of embryogenesis. In the mouse, cells in the primordial organ of Corti withdraw from the cell cycle between E12 and E14 to form a postmitotic precursor domain that is four to five cells thick (Fig. 2B).(27,28) Subsequently, postmitotic cells of the primordium differentiate into hair cells and supporting cells in concurrent longitudinal and mediolateral directional gradients.(28–30) In other words, this gradient of differentiation occurs as the hair cells in the base differentiate prior to those in the apex (longitudinal gradient) and as the inner hair cells differentiate prior to the outer hair cells (mediolateral gradient). During terminal differentiation, the organ of Corti extends and becomes a two-cell-layered sensory epithelium with the layer of hair cells lying above the nuclei of the supporting cells. This extension has been shown to be unidirectional (from the base of the cochlea to the apex) in organ culture.(17) By E18.5, the cochlear duct has reached its mature length, and the organ of Corti along the entire cochlear duct has been patterned into one row of inner and three rows of outer hair cells. The extension and thinning is independent of cell proliferation and death within the developing organ of Corti,(17,23,28) suggesting the involvement of cellular rearrangements within the developing organ of Corti that lead to concomitant extension of the organ of Corti along the longitudinal axis and narrowing along a perpendicular axis. Such cellular rearrangements are characteristic of convergent extension (Fig. 2).
The establishment of PCP occurs concurrently with the terminal differentiation of hair cells and the extension of the organ of Corti between E14.5 and E18.5 in mice. A microtubule-based cilium, the kinocilium, is centrally placed on the apex of the nascent hair cell and surrounded by microvilli made up of actin filaments of uniform size.(29,31,32) Subsequently, microvilli begin to enlarge to become stereocilia. The kinocilium becomes displaced to the lateral side of the cell apex (Fig. 1E, red) and the stereocilia (Fig. 1E, green) grow in a defined pattern. The polarity of the kinocilia, therefore, parallels the PCP of the organ of Corti during development and appears to temporally lead the polarization of stereocilia at the apical surface of the hair cell. The development of the stereocilia and their polarity follow the differentiation gradient from the base to the apex of the cochlea.(32) By E18.5 in mice, the polarity of the stereocilia and kinocilia is established along the entire length of the cochlear duct (Fig. 1E). Once established, the stereocilia continue to grow, mature and renew. In contrast, the kinocilia regress postnatally in rodents. Like the hair cells, supporting cells are ciliated. As the hair cells undergo terminal differentiation and acquire PCP, supporting cells differentiate from columnar cells in the precursor domain and, in the process, undergo drastic morphological changes to form their polarized phalangeal processes. However, very little is known about the development of the supporting cell morphology during PCP.
The regulation of PCP in vertebrates
In the tissues that exhibit planar cell polarity, there is a well-defined planar polarity both in the arrangement of the different cells relative to each other within the group, and in the intrinsic polarized structure of each individual cell. This organization requires: (1) a global guidance cue for directional information and coordination of planar polarity across the entire tissue (upstream PCP genes), (2) cellular factors to interpret the directional signal by adopting polarized asymmetric localization within each cell along the axis for polarity (core PCP genes) and (3) tissue-specific effectors downstream of core PCP genes necessary to carry out specific morphogenetic events to achieve the polarity specific for individual tissues (downstream PCP effector genes).(2,3,33–35)
Core PCP genes
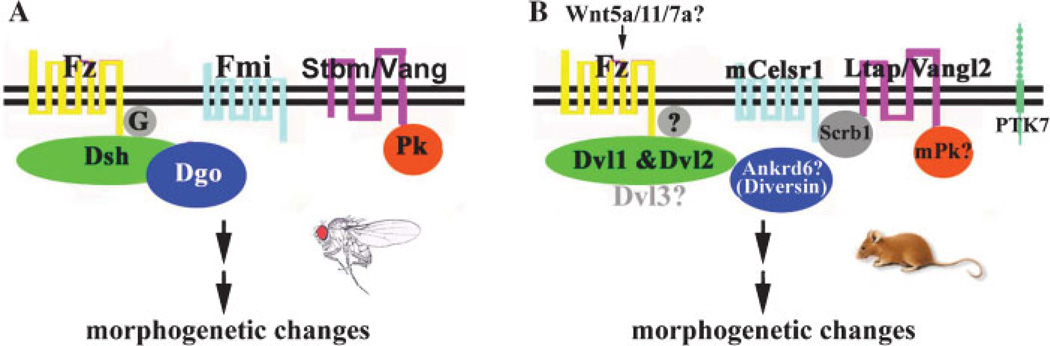
Genetic analyses of Drosophila mutants identified a set of core PCP genes(1–3) that affect all known structures in Drosophila with PCP features. The core PCP genes in Drosophila encode Frizzled (Fz),(36,37) Flamingo (Fmi),(38,39) Strabismus (Stbm)/Vang-gogh (Vang),(40,41) Prickle (Pk),(42) Dishevelled (Dsh),(43,44) Diego (Dgo),(45) and trimeric G protein Gαo (Table 1, Fig. 4A).(46) The PCP signaling pathway includes several components (Fz, Dsh and Gαo) of the ‘‘canonical’’ Wnt signaling pathway.(43,44,46–50) In contrast to the canonical Wnt pathway, the PCP signaling pathway is independent of β-catenin, comprises the core PCP proteins, targets cytoskeleton components, and is therefore known as a ‘‘noncanonical’’ Wnt pathway (for a review of the components of the canonical Wnt and the PCP pathways, see references(2,3,50)).In mice, mutations in core PCP genes (Fig. 4B, Table 1), including Fz,(51,52) Vangl2/Ltap,(16) Celsr1 (Flamingo homolog)(15) and Dvl/Dsh,(17,53) affect stereociliary orientation (Table 1). Mutations in PTK7(54) and Scrb1(16,55) also cause misorientation of the stereocilia. Hair pattern defects were observed in the mouse Fz6 mutant, demonstrating genetically another vertebrate tissue with PCP features.(51) In addition to the inner ear PCP phenotype, loss of function of these core PCP genes causes craniorachischisis (completely open neural tube).(15–17,52–54)
Table 1.
Vertebrate PCP genes and their molecular roles
| Subcellular Localization |
||||
|---|---|---|---|---|
| Gene | Structure | Mutant Phenotypes | Vertebrate | Drosophila homolog in wing cells |
| Ltap/Vangl2 core PCP gene | Putative 4-TM protein; | mCE, misorientation of stereocilia; CE* | medial (proximal) localization in OC | Stbm/Vang, proximal |
| Frizzled (Fz) core PCP gene | 7-TM receptor; known ligands are Wnts | mCE and stereocilia defects in Fz3 and Fz6 DKO, hair-patterning defect in Fz6 KO, CNS axonal defects in Fz3 KO mice | appears medial in OC; Fz3 possibly lateral in the SCs | Fz, distal; recruits Dsh and Dgo |
| Dishevelled (Dvl) core PCP gene | Cytoplasmic protein containing PDZ, DIX, and DEP domains | Mild misorientation of stereocilia, mCE defects in Dvl1 and Dvl2 DKO mice; cell division orientation in CE* | lateral (distal) in OC; the base of cilia in epidermal cells* | Dsh, distal; recruited to membrane by Fz |
| Celsr1 core PCP gene | Atypical cadherin, 7-TM domains | mCE, misorientation of stereocilia, CE* | Polarized in chick OC | Fmi/stan, proximal and distal |
| PTK7/CCK-4 core PCP gene | Putative 1-TM, Ig, tyrosine kinase domains | Craniorachischisis and misorientation of OHC3 stereocilia; implicated in CE* | Transient apical localization in OC | Otk, no known PCP phenotype |
| Scribbled (Scrb1) core PCP gene | PDZ domains mediate interactions with Vangl2 | Craniorachischisis and misorientation of OHC3 stereocilia | Uniform membrane localization in OC | Scrib, ts; apical-basal polarization |
| Prickle core PCP gene | Predicted PET and LIM-domain protein; inhibits Dsh to the same location | CE* | appear to be at the anterior ends of cells during CE* | pk, proximal; recruited by Stbm/Vang |
| Ankrd6 (Diversin) core PCP gene | Predicted ankyrin-repeat domains | CE*; Wnt pathway modulator; rescues the loss of Inversin* | RNA expressed in the cochlea | dgo,; distal localization in wing |
| Gαo | Trimeric G-protein | Unknown | Unknown | Go, recruited by Fz |
| Inturned (in) effector PCP gene | Putative TM protein | Ciliogenesis* and CE* | apical in epidermal cells* | in, proximal localization |
| Fuzzy (fy) effector PCP gene | Putative 7-TM protein | Ciliogenesis* and CE* | Unknown | fy, localization unknown |
| ADF/Cofilin effector PCP gene | Small actin-binding protein | CE*, NT closure defects in n-cofilin KO mice | Bind filamentous actin | tsr, localization unknown |
| Fritz effector PCP gene | Predicted WD40-repeat | Unknown | Unknown | frtz, localization unknown |
| Fat (ft) upstream factors | Putative protocadherin with EGF domains | Unknown | Unknown | ft, membrane |
| Dachsous (ds) upstream factors | Putative protocadherin | Unknown | Unknown | ds, membrane |
| Four-jointed (fj) upstream factors | Putative type II TM or secreted protein | Unknown | Unknown | fj, possible Golgi localization |
| Wnt upstream factors | Known ligands for Fz; downstream specificity depends on receptor and modulator context | Wnt7a-conditioned medium disrupts stereocilia orientation in vitro; Wnt5a and Wnt11 required for CE* | Wnt7a expressed in the cochlea during PCP | wingless (wg), segmentation gene; no known PCP phenotype |
| Bardet-Biedl Syndrome (BBS) BBS1-5, 7 BBS6/MKKS, BBS8/TTC8 | BBS3/ARL6, G-protein; BBS6/MKKS, putative chaperonin; BBS8, TPR-domain | BBS1-2, 4-5, 7-8 in ciliogenesis; subtle stereociliary defects in BBS4, MKKS null mice, BBS1 and Ltap double mutants; BBS4 in CE* | Basal body, pericentriolar and/or cilia | unknown |
The table summarizes the genes for which there are reported effects on PCP, CE and/or ciliogenesis in vertebrates, with special emphasis on the organ of Corti. In addition, vertebrate homologs of known Drosophila PCP genes that have not been functionally studied are also included. The mutant phenotypes and subcellular localizations are indicated for those tissues with characterized PCP or CE features.
CE: convergent extension; OC: organ of Corti; HC: hair cell; SC: supporting cell; OHC: outer hair cell; TM: transmembrane; KO: knockout; DKO: double knockout;
indicate functional assays or localization performed in Xenopus and/or zebrafish; mCE: mouse CE processes including craniorachischisis and widened and shortened cochlear duct; ts: tumor suppressor.
Figure 4. The PCP pathway.
A: Drosophila PCP pathway, showing the core PCP complexes of Fz–Dsh–Dgo and Stbm–Pk. Fmi is likely to be associated with both complexes. B: Vertebrate PCP pathway, showing additional components (PTK7 and Scribble) in PCP regulation in mice. The proposed interactions among the vertebrate PCP components are based on available data (see text). The association of Scrb1 with mCelsr1 is based on the ubiquitous localization of Scrb1 to apical cell membranes in the organ of Corti. The role of mouse Ankrd6, Pk and Dvl3 in PCP has not been demonstrated, and the role of Wnts in establishing epithelial PCP has yet to be tested.
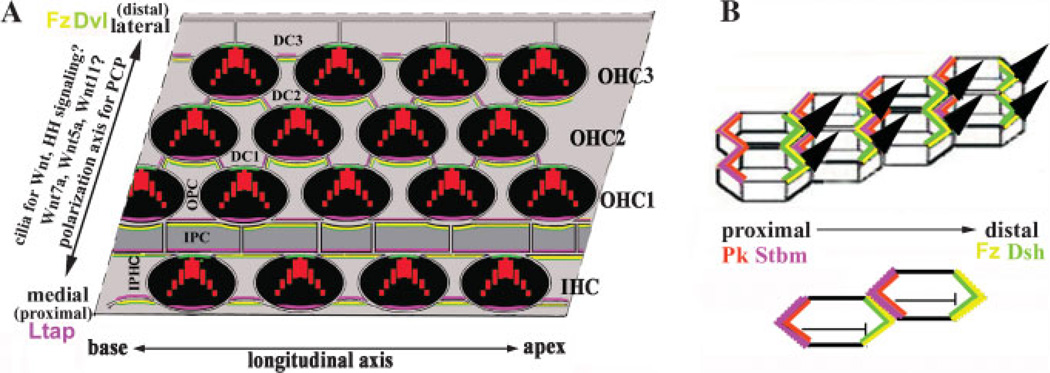
The hallmark of the PCP process in Drosophila is the asymmetric and polarized membrane association of PCP proteins.(2,3) Dsh and Dgo are cytoplasmic proteins and are recruited to the membrane by their association with each other and the association between the membrane PCP protein Fz and cytosolic Dsh.(45,56–58) Fmi is a seven-transmembrane protocadherin.(38) Stbm/Vang is a four-transmembrane protein and can recruit cytoplasmic protein Pk.(44,57–62) It is well established that Pk can interact with Dsh and antagonize its recruitment by Fz.(57–60,62) Through a feedback regulatory loop of the extracellular domains of Fz and Stbm at the interface of the membranes between the two neighboring cells, the complex of Stbm and Pk and the complex of Fz and Dsh and Dgo are localized to opposite sides of the cells along the polarization axis.(2,3,62) Fmi is thought to localize to both sides and plays a role in homophilic adhesion.(2,3,34,63–65) The asymmetric membrane distribution of the core PCP components is essential to direct PC. Failure to normally localize these proteins leads to a disruption in PCP.(56)
Studies of the membrane association of the mammalian core PCP proteins revealed polarized localizations of Dvl2,(17,53) Vangl2/Ltap,(55) Fz3 and Fz6(52) in the organ of Corti (Fig. 5). These localization studies show that the vertebrate PCP complexes may differ from those of Drosophila. It was reported that Dvl2 is localized to the lateral(17,53) (Fig. 5B), Ltap/Vangl2 to the medial(55) (Fig. 5A), and Fz3 and Fz6 to the medial sides (Fig. 5A) of the hair cells.(52) The localization of Dvl2 and Ltap/Vangl2 on the opposite side of the hair cells is consistent with the molecular interactions revealed in the fly PCP pathway (Fig. 6). It is unexpected that Dvl2 and Fz (Fz3 and Fz6) are not co-localized and that Fz is localized to the same side as Ltap/Vangl2. It is possible that the vertebrate PCP pathway utilizes a divergent polarization mechanism for the regulation of stereocilia orientation, and Dvl2 is recruited to the membrane by an unknown mechanism or by additional Fz protein(s) (other than Fz3 and Fz6).
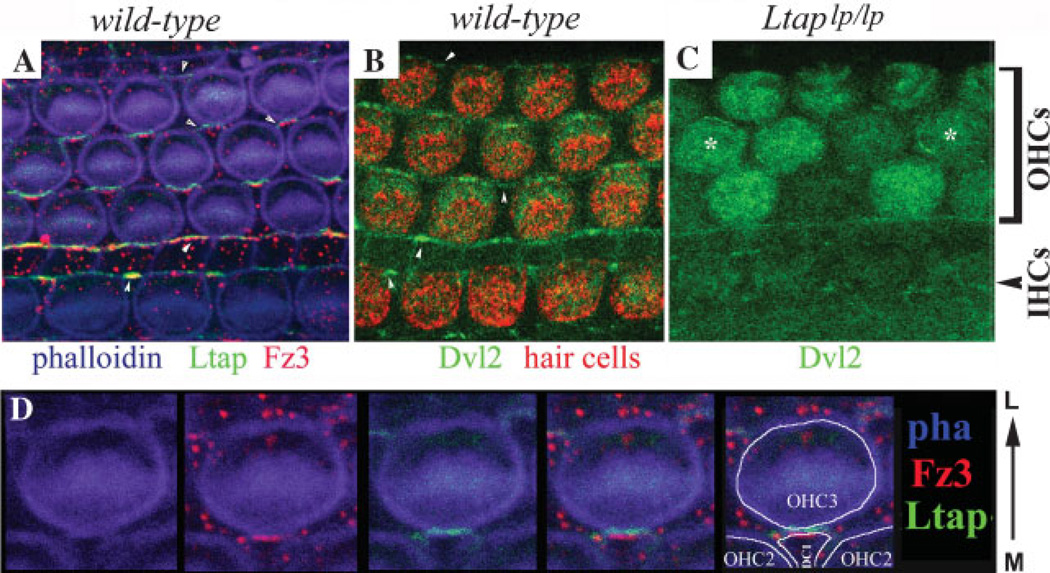
Figure 5. Polarized localization of Ltap/Vangl2, Dvl2 and Fz3 in the organ of Corti.
A: A whole-mount E17.5 organ of Corti that expresses Ltap–GFP(green) (D. Qian and P. Chen, unpublished data)was stained with an antibody against Fz3 (red). The tissue was also stained with phalloidin (blue) to outline the hair cells. Both Ltap–GFP and Fz3 appear to be on the medial side of the hair cells. However, non-overlapping localization of Ltap–GFP and Fz3 is observed. The Ltap–GFP signal is slightly lateral to that of Fz3. The arrowheads indicate the localization of Ltap–GFP and Fz3 to boundaries formed between supporting cells. B: Dvl2–GFP localization in the organ of Corti, showing an apparent localization (green) to the lateral end of the hair cells (red) at the apical surface of the organ of Corti. C: The polarized localization of Dvl2 is altered in PCP mutant LtapLp/Lp mice. The asterisks indicate two hair cells with altered membrane association of Dvl2–GFP. In the other hair cells, no enrichment of Dvl2 in the cell membrane was observed. The apical cytoplasmic GFP signal seems to intensify in some of the hair cells. Interestingly, the membrane association of Dvl2–GFP in the supporting cells that separate inner from outer hair cells appears to remain, albeit less intense. D: A large view of Fz3 (red) and Ltap–GFP (green) localization at the boundaries formed between a hair cell (OHC3) and a supporting cell (DC1) as in (A). Phalloidin (blue) outlines the cortex of cells and the V-shaped stereocilia on OHC3. Note that Fz3 is slightly lateral to Ltap–GFP at the boundary and may be contributed by DC1. M: medial; L: lateral.
Figure 6. PCP signaling in vertebrates.
A: A schematic drawing of the PCP regulation in the organ of Corti. The cellular interfaces of the organ of Corti are outlined. In this model, we tentatively placed Fz3 (yellow) on the lateral side of supporting cells (grey) and proposed that Fz3 recruits Dvl2 (green) to the same location. In the hair cells (black), Dvl2 is also localized to the lateral side. The mechanism for recruiting Dvl2 to the lateral side of the hair cells is not known. Ltap/Vangl2 (pink) is localized to the medial side of hair cells and supporting cells. The interaction between the extracellular domains of Ltap and Fz3 from the two adjacent cells enforces the polarization of PCP complexes in the organ of Corti. The detailed assignment of the PCP proteins to the individual side of the cells is based on experimental data in vertebrates and demonstrated mechanisms for fly PCP (B) regulation, but not definitive. Vertebrates may use completely different mechanisms for polarization of PCP complexes. The identity and the role of mouse Pk in PCP regulation in the organ of Corti is not known. A critical test of the proposed model is to determine whether mPk proteins play a similar antagonistic role for Dvl localization in the cochlea. The upstream factors involve Wnts, possibly including Wnt7a, Wnt5a, and Wnt11.The exact role of cilia in PCP regulation is not clear. They may be involved in direct signal transduction or in downstream effector gene functions. Since cilia are implicated in PCP signaling, it is possible that the signaling pathways mediated by cilia, such as Hedgehog pathway, may also be involved in PCP signaling. B: PCP regulation in Drosophila. Pk (orange) is recruited to the membrane by Stbm (pink) and inhibits the localization of Dsh (green) to the same site. The antagonist role of Pk to Dsh restricts Dsh/Fz complex to the opposite side of the cells. The interactions between the extracellular domains of Stbm and Fz (yellow) at the interface of the two adjacent cells stabilize the polarized localization of Stbm/Pk and Fz/Dsh to the opposite sides of the cells.
However, it is important to realize that the current assignments for the localization of Ltap/Vangl2, Dvl2, Fz3 and Fz6 may not be correct. The cellular boundaries in the organ of Corti are formed between hair cells and supporting cells and among different types of supporting cells (Figs. 3 and 6). It is apparent that some of the polarized localizations of PCP proteins in the organ of Corti are on the boundaries formed between supporting cells (Fig. 5A,B, indicated by arrow-heads). At the boundaries between hair cells and supporting cells, it is difficult to assign the localization of a protein to either the hair cell or the supporting cell. We sometimes observe non-overlapping localization of Ltap/Vangl2 and Fz3 at the boundaries formed between hair cells and supporting cells (Fig. 5A,D), suggesting that Ltap/Vangl2 and Fz3 are localized to the medial side of the contributing hair cell and the lateral side of the supporting cell, respectively (Fig. 6). An elegant study examined the localization of Fz6 in the organ of Corti from Fz6+/+ and Fz6−/− chimera mice.(52) The authors demonstrated a nice correlation between the loss of Fz6 localization and Fz6−/− hair cells, suggesting that the Fz6 is at the medial side of hair cells. However, it is possible that the contacting supporting cells were also Fz6−/− and that the disappearance of Fz6 at the boundaries is due to the loss of Fz in supporting cells.
It has not been directly demonstrated that polarized localizations of core PCP proteins are required for planar polarization in vertebrates. Our understanding of the composition of the vertebrate PCP pathway and the morphogenesis of the organ of Corti is far from complete. The dynamics of the subcellular localization of core PCP proteins and general morphology or packing of cells in the organ of Corti during PCP has not been investigated. Studies toward these areas are needed to decipher the molecular mechanism underlying planar polarization processes in vertebrates.
Upstream PCP genes
Because Fz can serve as a receptor for Wnt signaling molecules, and Wnt molecules are diffusible morphogens, capable of generating a gradient across the tissue, it has been hypothesized that members of the Wnt family may be directional cues for PCP signaling. To date, however, no Wnt has emerged from any study in Drosophila as a candidate for filling this role.(2,3,33) Instead, a mathematic model supported by experimental evidence has pointed to a feed-back loop regulation of a small initial difference in Fz activity along the axis of polarization and the involvement of protocadherins for the reinforcement and propagation of polarization over a long range.(34,62,66–69) The role of vertebrate homologs in PCP signaling has not been demonstrated (Table 1).
While Wnts appear to be dispensable for PCP signaling in Drosophila, Wnt molecules have been implicated in PCP regulation in the vertebrates. Several Wnts are expressed in the cochlear duct during terminal differentiation(70,71) (our unpublished data). In particular, Wnt7a is expressed in the pillar cells during terminal differentiation of the organ of Corti, and addition of Wnt7a or Wnt antagonists in the organ culture affects the orientation of stereocilia.(70) However, no PCP phenotype was observed in Wnt7a null mice.(70) It is possible that there are compensatory pathways for Wnt7a in PCP regulation in the organ of Corti, or the addition of Wnt7a or Wnt antagonists has a dominant negative effect on the other signaling pathways required for PCP. The nature of the signaling molecules for the PCP pathway remains to be determined, and a direct role of Wnt molecules in PCP regulation in the organ of Corti has yet to be demonstrated.(72)
Downstream effector PCP genes
In Drosophila, in,(18,72) fy(72) and frtz(73) function downstream of core PCP genes. Their role in the mammalian inner ear is unknown. The downstream cytoskeleton readout module of the asymmetrically assembled core PCP complex is also not known. The role of several cytoskeleton regulators involved in CE are known (described below), including DAAM1,(74) Rac,(75–77) Rho(74,76–81) and Rho kinase,(79) but their roles in stereocilia orientation have yet to be examined.
The regulation of CE by the vertebrate PCP pathway
In vertebrates, the first cellular process discovered to require the PCP pathway was CE during gastrulation and neurulation in Xenopus and zebrafish. Body axis is shortened when the PCP pathway is disrupted during gastrulation.(4,5,8,9,13,82–85) The neuroepithelium fails to undergo proper mediolateral intercalation when the PCP pathway is defective, resulting in a shortened and widened neural tube, which fails to meet at the midline and close.(53,86,87) Subsequently, mutations in mammalian PCP genes were found to show a completely open neural tube(15–17,52–54) (craniorachischisis, Table 1), presumably due to defective mediolateral intercalation of cells in these mutants.(53) These mutations also lead to widened and shortened cochlear ducts.(16,17) In organ culture, the mutant cochleae are defective in extension, consistent with a cochlear CE defect in the PCP mutants.(17) It is important to note that, in mammals, the role for CE in neural tube closure and cochlear extension is inferred by analogy to the analyses in Xenopus and zebrafish. The CE process in mammals has not been directly demonstrated.
Interestingly, germband extension at the onset of gastrulation in Drosophila also involves cell intercalation characteristic of CE.(88) However, Drosophila germline clones lacking Fz or Dsh function exhibit normal germband extension.(88) To date, it is unclear whether the PCP pathway plays any role in this process.(88) It appears that the regulation of CE by the PCP pathway has evolved in vertebrates.
Core PCP genes in CE
CE occurs in explants, which indicates a tissue-autonomous driving force for CE. During CE, cells extend stable lamellipodial protrusions at their medial and lateral ends to attach to mediolaterally located neighboring cells. The loss of polarity or stability of these mediolaterally oriented protrusions precedes the failure of CE, suggesting that the polarized and stable lamellipodial protrusions exert traction and pull the cells toward one another mediolaterally, thus are the autonomous driving force for CE.(10) Investigations into the mechanism underlying CE show that the ascidian PCP gene Prickle (Pk) is membrane associated in a polarized manner (anterior–posterior polarization) during CE, perpendicular to the mediolateral polarization in notochord cells(89) (Fig. 3B). During zebrafish neurulation, the Pk–Stbm complex appears to localize to anterior cellular borders within each cell.(86) These initial data suggest that polarized association of PCP proteins similar to the Drosophila PCP complexes (Pk–Stbm, Fz–Dsh–Dgo) may contribute to the formation of mediolaterally oriented lamellipodia that drive polarized cellular motility for CE. Interestingly, the localization of Vangl2/Ltap, as evidenced by an Ltap–GFP fusion protein, in the region medial to the organ of Corti shows a polarity along the longitudinal axis of the cochlea at E17.5–E18.5 in mice (data not shown), perpendicular to its mediolaterally polarized localization in the organ of Corti (Fig. 3). The polarity of Ltap–GFP in the region medial to the organ of Corti along the extension axis is similar to Pk–Stbm polarization (anterior–posterior along the extension axis) during zebrafish neurulation. It is possible that the entire cochlear duct undergoes CE and that other nonsensory regions of the cochlea play important roles in the process. In addition, the supporting cells of the organ of Corti differentiate and form the longitudinally polarized phalangeal processes of the supporting cells (Fig. 3) from densely packed undifferentiated precursors in the primordial organ of Corti. It is tempting to speculate that the polarization of phalangeal processes of the supporting cells along the mediolateral axis of the cochlea may contribute to the protrusive activities that promote mediolateral intercalation of the organ of Corti.
It is intriguing, however, how the polarization of PCP complexes along the extension axis could generate mediolaterally oriented cellular protrusions needed for the mediolateral cellular intercalation. A new study elegantly illustrated the role of an ArfGAP (XGAP) in CE in Xenopus(90) and provided a more complete picture for cellular polarization during CE. Gene knockdown of XGAP inhibits cellular intercalation during gastrulation, and XGAP is required to confine the cellular protrusive activity to the mediolateral ends of cells.(90) Furthermore, it was shown that XGAP is localized to the mediolateral ends of the cells and directs the PAR polarity proteins to the same location during gastrulation.(90) The polarization of XGAP/PAR proteins to the mediolateral ends and the localization of PCP complexes to the anterior–posterior ends of the cells have led to a hypothesized antagonist relationship between these regulators for stable cellular polarization.(91) The restricted localization of different polarity complexes to the mediolateral and anterior–posterior ends of the cells could provide the cell with distinctive directional information needed for polarized cellular intercalation during CE. The identification of additional regulators for CE may ultimately lead to the understanding of the mechanistic involvement of PCP signaling in the CE process.
Upstream PCP genes in CE
In contrast to the uncertain role of Wnt in the orientation of stereocilia, secreted Wnt molecules (Wnt11 and Wnt5) are known to be required for CE during Xenopus and zebrafish gastrulation.(82,84,85,92–94) Their role in this process appears to be permissive rather than instructive.(92) Additional factors, such as activin and other graded nodal signals, have also been shown to orient CE with respect to embryonic axes.(95) It will be interesting to test whether graded signals along both the convergence (mediolateral) and the extension (anterior–posterior) axes coordinate to polarize core PCP protein complexes and XGAP/PAR complexes along both axes for CE.(90) In mammals, however, the role(s) of Wnts or other morphogens in the regulation of CE within the organ of Corti or other mammalian tissues is unknown.
Downstream effector genes in CE
The downstream effector module of the PCP pathway in CE consists of cytoskeleton regulators in Xenopus and zebrafish. Distinctive domains of the Dvl protein(43,47) mediate its role in different downstream pathways. In particular, its DEP domain is PCP-specific. Wnts can activate the cytoskeletal regulators JNK, Rho kinase and Rac to regulate CE.(74,76,79,96) The activation of these cytoskeleton regulators by Wnts depends on the PCP domain of Dvl, indicating their involvement in the PCP regulation of CE.(92) A Formin-homology protein, DAAM1, mediates the formation of Dvl–Rho complex to regulate CE during gastrulation,(74) linking the core PCP apparatus with cytoskeleton regulation. The vertebrate homologs of Drosophila PCP effector genes Inturned and Fuzzy (18,72) are implicated in CE in Xenopus.(21) The mammalian cytoskeleton components of the PCP pathway in CE regulation are not known.
Ciliogenesis and the vertebrate PCP pathway
The kinocilia of the hair cells are primary cilia that protrude from the apical surfaces of the hair cells and whose assembly is regulated by basal-body-associated and intraflagellar transport (IFT) proteins.(97) Kinocilia are polarized toward the lateral side of the hair cell surfaces during terminal differentiation of the organ of Corti, and their polarization appears to precede that of stereocilia. The polarity of the kinocilia and their transient presence in the developing organ of Corti has led to a hypothesized role in stereocilia development. Only recently has experimental evidence linking ciliogenesis to PCP and CE started to emerge.
The ankyrin repeat-containing protein Inversin is a component of the node monocilia and a left–right determinant of the embryonic body plan during embryogenesis. Overexpression as well as knockdown of endogenous Inversin by morpholino antisense oligonucleotides impaired CE in Xenopus embryos 98. Loss of function of Inversin causes severe polycystic disease, a phenotype observed with mutations of several other ciliary proteins.(22) Significantly, a vertebrate candidate PCP gene, Diversin (also known as Ankrd6, the closest vertebrate homolog to Drosophila Dgo, Fig. 4, Table 1) shares homology with Inversin in the ankyrin repeat domain and can rescue the renal defect in Inversin mutant zebrafish,(98) suggesting that a candidate PCP component could function for a ciliary protein. A study with Bardet–Biedl Syndrome (BBS) ciliary proteins show that mutations in BBS ciliary genes, including Bbs1, Bbs4 and Mkks/Bbs6, lead to a mild phenotype in the orientation and shape of outer hair cell stereociliary bundles.(99) The kinocilia in E18.5 Mkks−/− mutants are displaced from the vertices of the V-shaped stereocilia.(99) Furthermore, Bbs1 genetically interacts with PCP gene Ltap/Vangl2 in stereocilary development, and Ltap/Vangl2 is localized to the basal body and along the ciliary axoneme in ciliated IMCD3 cells and human respiratory epithelial cells.(99) Parallel to the findings for the implication of cilia in PCP regulation, two PCP effector genes, Inturned and Fuzzy, have been shown to govern apical actin assembly and regulate the orientation of ciliary microtubules during ciliogenesis.(21)
These data together suggest a link between ciliogenesis and PCP signaling. However, in the mutants that are defective for core vertebrate PCP genes, Ltap/Vangl2, Celsr1, Dvl1/2, PTK7 and Fz3/6, ciliogenesis appears to be normal. Therefore, the role of the PCP apparatus in ciliogenesis appears to be limited to the downstream effectors that regulate the cytoskeleton, such as Inturned and Fuzzy. It is possible that the cytoskeletal role of Inturned and Fuzzy is required for both ciliogenesis and PCP signaling. However, their roles in ciliogenesis are independent of PCP signaling. On the contrary, BBS genes appear to affect PCP signaling and interact with a PCP gene Ltap/Vangl2, suggesting a direct role of cilia in PCP signaling.(99) The molecular role of cilia in PCP signaling, however, is not clear. Based on the findings that cilia mediate both Hedgehog and PDGF signaling(100) and that Inversin (and Diversin) can modulate noncanonical Wnt signaling in vitro.(98) it is tantalizing to hypothesize that cilia may also function in the signaling machinery for noncanonical Wnt/PCP signaling. Nevertheless, no direct functional data are available to support this view. An alternative view is that the role of the ciliary proteins in PCP-regulated processes may be cilia-independent. It is possible that the ciliary proteins linked to PCP have additional roles in protein sorting, transport(97) or cytoskeletal regulation required for planar polarization. These hypotheses can be effectively tested in the organ of Corti using a combination of mouse mutants and protein localization studies.
Summary and future perspectives
The relative ease of Xenopus and zebrafish embryo manipulations has prompted the identification of the vertebrate PCP pathway and the focus of vertebrate PCP study on CE. Studies on the mechanism of CE in these model systems will continue to be the major driving force for the understanding of the vertebrate PCP pathway and the underlying cellular processes. Meanwhile, the organ of Corti has emerged as an excellent model system to illustrate the multiple cellular processes controlled by vertebrate PCP signaling.
The combination of these model systems has provided an insightful view for a working model of PCP signaling in vertebrates (Fig. 6) that exploits similar polarization of PCP complexes observed in Drosophila for execution of cellular polarization. Despite the identification of some of the players and their molecular roles in PCP signaling, our understanding of the signaling cascade of PCP regulation is limited. Many questions remain in vertebrate PCP signaling (Fig. 6). For example, the nature of the global cues and their exact role (essential versus dispensable, instructive versus permissive) in PCP remain obscure. The molecular interactions among the vertebrate PCP proteins have yet to be delineated. The vertebrate core PCP genes may utilize different mechanisms for the polarization of PCP complexes. Furthermore, the mechanisms by which the core PCP proteins direct the downstream cytoskeletal changes remain elusive. The recently reported links between ciliogenesis and PCP signaling and the identification of additional polarization machinery (e.g. XGAP) in PCP-regulated cellular processes are exciting and provide additional routes to explore some of the outstanding issues in PCP signaling in vertebrates. It is possible that PCP signaling in vertebrates may employ additional apparatuses (cilia or other polarization machinery?) to sense signaling morphogens (Wnt and Hedgehog?), to direct the polarization of core PCP proteins, or to link the core PCP genes to the downstream effectors. A better characterization of the cellular morphology of the organ of Corti during PCP and the generation of additional mouse mutant and transgenic strains may address some of the outstanding questions in PCP regulation in vertebrates.
Acknowledgments
We would like to thank Drs. A. Wynshaw-Boris, J. Wang for providing us with Dvl2-GFP samples; Drs. Y. Wang and J. Nathans for the Fz3 antibody and plasmid for affinity purification of the antibody; Drs. Wynshaw-Boris, Wang and Nathans for discussions on the localization of PCP proteins in the cochlea, and Dr. Doug Falls for critical comments on the manuscript. We also like to thank Drs. D. Ding, D. Lim, and Y. Raphael for clarification and illustration of the cellular structure of the organ of Corti.
Abbreviations
- PCP
planar cell polarity
- CE
convergent extension
- IHC
inner hair cell
- OHC
outer hair cell
- DC
Deiters’ cell
- IPC
inner pillar cell
- OPC
outer pillar cell
- IPhC
inner phalangeal cell
References
- 1.Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- 2.Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. Bioessays. 2005;27:1218–1227. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- 3.Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 4.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 5.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, et al. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 6.Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- 7.Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 8.Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol. 2002;13:251–260. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 9.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 10.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- 12.Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 13.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 15.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 16.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Mark S, Zhang X, Qian D, Yoo SJ, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler PN, Charlton J, Park WJ. The Drosophila tissue polarity gene inturned functions prior to wing hair morphogenesis in the regulation of hair polarity and number. Genetics. 1994;137:829–836. doi: 10.1093/genetics/137.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yun UJ, Kim SY, Liu J, Adler PN, Bae E, et al. The inturned protein of Drosophila melanogaster is a cytoplasmic protein located at the cell periphery in wing cells. Dev Genet. 1999;25:297–305. doi: 10.1002/(SICI)1520-6408(1999)25:4<297::AID-DVG3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Adler PN. The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics. 2002;160:1535–1547. doi: 10.1093/genetics/160.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 22.Beales PL. Lifting the lid on Pandora’s box: the Bardet-Biedl syndrome. Curr Opin Genet Dev. 2005;15:315–323. doi: 10.1016/j.gde.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie E, Krupin A, Kelley MW. Cellular growth and rearrangement during the development of the mammalian organ of Corti. Dev Dyn. 2004;229:802–812. doi: 10.1002/dvdy.10500. [DOI] [PubMed] [Google Scholar]
- 25.Slepecky NB, Ulfendahl M. Actin-binding and microtubule-associated proteins in the organ of Corti. Hear Res. 1992;57:201–215. doi: 10.1016/0378-5955(92)90152-d. [DOI] [PubMed] [Google Scholar]
- 26.Henderson CG, Tucker JB, Mogensen MM, Mackie JB, Chaplin MA, et al. Three microtubule-organizing centres collaborate in a mouse cochlear epithelial cell during supracellularly coordinated control of microtubule positioning. J Cell Sci. 1995;108:37–50. doi: 10.1242/jcs.108.1.37. [DOI] [PubMed] [Google Scholar]
- 27.Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol Suppl. 1967;220:1–44. [PubMed] [Google Scholar]
- 28.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 29.Lim DJ, Anniko M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol Suppl. 1985;422:1–69. [PubMed] [Google Scholar]
- 30.Sher AE. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol Suppl. 1971;285:1–77. [PubMed] [Google Scholar]
- 31.Sobkowicz HM, Slapnick SM, August BK. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol. 1995;24:633–653. doi: 10.1007/BF01179815. [DOI] [PubMed] [Google Scholar]
- 32.Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ. Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet. 2004;5:489–498. doi: 10.1038/nrg1377. [DOI] [PubMed] [Google Scholar]
- 33.Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 34.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 35.Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 36.Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- 37.Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- 38.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 39.Lu B, Usui T, Uemura T, Jan L, Jan YN. Flamingo controls the planar polarity of sensory bristles and asymmetric division of sensory organ precursors in Drosophila. Curr Biol. 1999;9:1247–1250. doi: 10.1016/s0960-9822(99)80505-3. [DOI] [PubMed] [Google Scholar]
- 40.Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- 41.Heisenberg CP. Wnt signalling: refocusing on Strabismus. Curr Biol. 2002;12:R657–R659. doi: 10.1016/s0960-9822(02)01160-0. [DOI] [PubMed] [Google Scholar]
- 42.Gubb D, Green C, Huen D, Coulson D, Johnson G, et al. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krasnow RE, Wong LL, Adler PN. Dishevelled is a component of the frizzled signaling pathway in Drosophila. Development. 1995;121:4095–4102. doi: 10.1242/dev.121.12.4095. [DOI] [PubMed] [Google Scholar]
- 45.Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 46.Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 48.Boutros M, Mihaly J, Bouwmeester T, Mlodzik M. Signaling specificity by Frizzled receptors in Drosophila. Science. 2000;288:1825–1828. doi: 10.1126/science.288.5472.1825. [DOI] [PubMed] [Google Scholar]
- 49.Krasnow RE, Adler PN. A single frizzled protein has a dual function in tissue polarity. Development. 1994;120:1883–1893. doi: 10.1242/dev.120.7.1883. [DOI] [PubMed] [Google Scholar]
- 50.Moon RT. Wnt/beta-catenin pathway. Sci STKE. 2005;2005(271):cm1. doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- 51.Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proc Natl Acad Sci USA. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 55.Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- 58.Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- 59.Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- 60.Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. Embo J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mlodzik M. Spiny legs and prickled bodies: new insights and complexities in planar polarity establishment. Bioessays. 2000;22:311–315. doi: 10.1002/(SICI)1521-1878(200004)22:4<311::AID-BIES1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 62.Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, et al. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- 63.Das G, Reynolds-Kenneally J, Mlodzik M. The atypical cadherin Flamingo links Frizzled and Notch signaling in planar polarity establishment in the Drosophila eye. Dev Cell. 2002;2:655–666. doi: 10.1016/s1534-5807(02)00147-8. [DOI] [PubMed] [Google Scholar]
- 64.Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 65.Uemura T, Shimada Y. Breaking cellular symmetry along planar axes in Drosophila and vertebrates. J Biochem (Tokyo) 2003;134:625–630. doi: 10.1093/jb/mvg186. [DOI] [PubMed] [Google Scholar]
- 66.Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, et al. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- 67.Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 68.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 69.Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- 70.Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, et al. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- 71.Dabdoub A, Kelley MW. Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J Neurobiol. 2005;64:446–457. doi: 10.1002/neu.20171. [DOI] [PubMed] [Google Scholar]
- 72.Adler PN, Zhu C, Stone D. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr Biol. 2004;14:2046–2051. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Collier S, Lee H, Burgess R, Adler P. The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics. 2005;169:2035–2045. doi: 10.1534/genetics.104.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 75.Yao R, Natsume Y, Noda T. MAGI-3 is involved in the regulation of the JNK signaling pathway as a scaffold protein for frizzled and Ltap. Oncogene. 2004;23:6023–6030. doi: 10.1038/sj.onc.1207817. [DOI] [PubMed] [Google Scholar]
- 76.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol. 2000;10:979–988. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- 78.Habas R, He X. Activation of Rho and Rac by Wnt/frizzled signaling. Methods Enzymol. 2006;406:500–511. doi: 10.1016/S0076-6879(06)06038-1. [DOI] [PubMed] [Google Scholar]
- 79.Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 80.Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 81.Winter CG, Wang B, Ballew A, Royou A, Karess R, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 82.Ulrich F, Concha ML, Heid PJ, Voss E, Witzel S, et al. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development. 2003;130:5375–5384. doi: 10.1242/dev.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith JC, Conlon FL, Saka Y, Tada M. Xwnt11 and the regulation of gastrulation in Xenopus. Philos Trans R Soc Lond B Biol Sci. 2000;355:923–930. doi: 10.1098/rstb.2000.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 85.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 86.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- 88.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 89.Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 90.Hyodo-Miura J, Yamamoto TS, Hyodo AC, Iemura S, Kusakabe M, et al. XGAP, an ArfGAP, is required for polarized localization of PAR proteins and cell polarity in Xenopus gastrulation. Dev Cell. 2006;11:69–79. doi: 10.1016/j.devcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 91.Mlodzik M. A GAP in convergent extension scores PAR. Dev Cell. 2006;11:2–4. doi: 10.1016/j.devcel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 93.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, et al. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 94.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 95.Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–367. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- 96.Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, et al. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davis EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: new functional roles for an ancient organelle. Dev Cell. 2006;11:9–19. doi: 10.1016/j.devcel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 98.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 100.Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. doi: 10.1016/j.cell.2006.04.013. [DOI] [PubMed] [Google Scholar]