Abstract
The human ear is capable of processing sound with a remarkable resolution over a wide range of intensity and frequency. This ability depends largely on the extraordinary feats of the hearing organ, the organ of Corti and its sensory hair cells. The organ of Corti consists of precisely patterned rows of sensory hair cells and supporting cells along the length of the snail-shaped cochlear duct. On the apical surface of each hair cell, several rows of actin-containing protrusions, known as stereocilia, form a “V”-shaped staircase. The vertices of all the “V”-shaped stereocilia point away from the center of the cochlea. The uniform orientation of stereocilia in the organ of Corti manifests a distinctive form of polarity known as planar cell polarity (PCP). Functionally, the direction of stereociliary bundle deflection controls the mechanical channels located in the stereocilia for auditory transduction. In addition, hair cells are tonotopically organized along the length of the cochlea. Thus, the uniform orientation of stereociliary bundles along the length of the cochlea is critical for effective mechanotransduction and for frequency selection. Here we summarize the morphological and molecular events that bestow the structural characteristics of the mammalian hearing organ, the growth of the snail-shaped cochlear duct and the establishment of PCP in the organ of Corti. The PCP of the sensory organs in the vestibule of the inner ear will also be described briefly.
Keywords: convergent extension, organ of Corti, hair cell, primary cilia, stereocilia
Introduction
The mammalian inner ear contains the cochlea and the vestibule for hearing and balance (Fig. 1), respectively. The functional aspects of the cochlea and the vestibule lend themselves greatly to the mechanical properties of the tissues and the precise arrangement and polarity of sensory hair cells in their sensory organs. The cochlea has one sensory organ, known as the organ of Corti and the vestibule has five sensory organs (Fig. 1A–C). Each hair cell has several rows of apical protrusions known as stereocilia in a staircase-like arrangement with the tallest stereociliary bundles located toward one side of the hair cell. The stereocilia of hair cells within each sensory organ is coordinately oriented (Fig. 1B,D). The coordinated orientation of stereocilia in inner ear sensory organs manifests perhaps the most distinctive form of a type of tissue polarity, known as planar cell polarity (PCP), in vertebrates (Figs. 1, 2).
Fig. 1. Planar cell polarity (PCP) of the inner ear sensory organs.

(A) The structure of a right inner ear is illustrated. The shaded areas represent one sensory organ in the cochlea, the organ of Corti and five sensory organs in the vestibule, maculae of the saccule and utricle (Sa, Ut) and cristae of lateral (LC), anterior (AC) and posterior (PC) semicircular canals. (B) The five vestibular sensory organs show distinctive forms of PCP. Hair cells (circles) on opposite sides of the striola (dashed lines) in the saccule and utricle have reversed planar polarity, as shown by the location of the kinocilium (red dots) which is placed near the tallest stereociliary bundles. The vestibular sensory organs in (B) were rotated counterclockwise 90° horizontally as they appear in (A) and the lateral crista was further rotated clockwise ~60° horizontally to show their whole mount views. (C–D) The cochlea is a fluid-filled labyrinth. The organ of Corti is suspended along the length of the cochlea and situated on the basilar membrane (C). In a whole mount surface view, the organ of Corti shows a mosaic arrangement of sensory hair cells (D, dark grey) and supporting cells (D, light grey). The stereociliary bundles (green) are arranged in a “V”-shaped staircase on the apical surface of each hair cell and the vertices of all the “V”-shaped stereocilia are uniformly oriented away from the center of the spiraling cochlea (D), displaying a PCP along the mediolateral axis of the cochlea. Abbreviations: IHC, inner hair cells; OHC1-OHC3, the first –third rows of outer hair cells; IPC, inner pillar cell; OPC, outer pillar cell; DC, Deiters’ cell.
Fig. 2. Stereotyped cellular patterning, polarity and neural connection in the organ of Corti stretch the entire length of the snail-shaped mammalian cochlea.

(A) A dissected mouse cochlea with YFP expressed in the spiral ganglion neurons to visualize the general morphology of the cochlea and innervation of its sensory organ along the length of the cochlea from the base to apex. The highest YFP signal is under the row of inner hair cells (IHC). (B) Schematic diagram showing the arrangement of auditory hair cells (blue) and their stereocilia bundles (red), with kinoclium (magenta) at the vertex, along the PCP axis. Supporting cells are not shown, but also exhibit distinct polarity. An example is the Dieter’s cells, each of which cup the base of an outer hair cells and form a phalangeal process that forms the separation between the more lateral row of outer hair cells at a distance along the longitudinal axis of the cochlea. (C) Hair cell polarity occurs in both the apical-basolateral axis and in a perpendicular axis within the plane of the epithelium, the PCP axis. Cell nuclei of myosin VI-labelled auditory hair cells (blue) are towards the basal regions of hair cells, whereas the phalloidin-labelled stereocilia bundles (red) are apical towards the lumen. On the apical surface, the stereocilia bundles are oriented uniformly along the mediolateral PCP axis. (D) Coordinately polarized orientation of the stereocilia bundles (red) on the apical surface of the auditory hair cells (blue) with kinocilium (magenta) seen at the vertices of the bundles. All four rows of the hair cells, with one row of inner hair cells and three rows of outer hair cells can be seen here. (E) YFP-expressing spiral ganglion neurons (green) show precise connections to auditory hair cells (blue). The spiral ganglion neurons and the sensory cells are derived from the same region within the otocyst and the development of spiral ganglion neurons influences the shape of the cochlea and its sensory organ. m, medial; l, lateral.
The entire inner ear is derived from a patch of ectodermal cells near the hindbrain known as the otic placode (Kikuchi et al., 1988, Morsli et al., 1998). How does such an incredibly complex structure develop from what is presumably an equivalent group of ectodermally-derived cells? The various sensory regions must be organized, orientated and innervated in an extremely well-controlled manner to allow for proper function. The overall structure and cellular organization require that tight temporal and spatial regulation be coordinated during development. The precise cellular patterning, morphological differentiation and innervation of the organ of Corti, therefore, depend on early developmental events and are determined by molecular pathways regulating terminal differentiation and morphogenesis. In this review, we briefly summarize the early development of the inner ear (for detailed discussions, see other reviews in this issue by Schimmang, Ohyama et al., Wu, Schneider-Maunoury and Pujades) and focus on the morphological and molecular events that create the uniform orientation of stereocilia in the organ of Corti and the lengthening of the cochlea during terminal differentiation. We integrate current data into a working model and discuss PCP regulation in the morphogenesis of the organ of Corti.
The organ of Corti
The organ of Corti is a continuous array of cells tonotopically organized on the basilar membrane along the length of the snail-shaped cochlea (Sher, 1971, Lim and Anniko, 1985). Graded variations of mechanic properties of the basilar membrane (von Bekesy, 1970), as well as electrical properties of hair cells (Hudspeth, 1989, Manley, 2000, Kruse and Julicher, 2005), enable tuning of sensory hair cells to a progression of frequencies corresponding to their location along the length of the cochlea. The tonotopical response is organized in ascending order from the apex to the base (von Bekesy, 1970). Along the length of the cochlea, there are four rows of hair cells interdigitated with several types of non-sensory cells to make up the organ of Corti. The innermost row toward the center (hereinafter referred to as “medial”) region of the cochlea and the three rows toward the peripheral (hereinafter referred to as “lateral”) region of the cochlea are known as the inner (IHCs) and outer hair cells (OHCs), respectively (Figs. 1C,D and 2). Several rows of “finger-like” extensions or hair bundles, known as the stereocilia (Figs. 1D, 2B–D), project from the apical surface of each hair cell and form a “V” shape, which is shallower in IHCs than in OHCs. Invariably, the vertices of all the “V”-shaped stereocilia point in the medial-to-lateral (hereinafter referred to as “mediolateral”) direction (Figs. 1C,D and 2B–D). A single primary cilium, known as kinocilium, is placed at the vertices of “V”-shaped stereocilia during development. Therefore, each hair cell is intrinsically asymmetrical in terms of the arrangement of stereociliary bundles and kinocilia and all the hair cells are uniformly polarized along the mediolateral axis of the cochlear duct (Figs. 1 and 2). The uniform orientation of stereocilia of hair cells along the mediolateral axis of the cochlea manifests a distinctive form of planar cell polarity (PCP) (Lewis et al., 1985, Lewis and Davies, 2002) (Figs. 1 and 2).
The nonsensory cells of the organ of Corti, commonly referred to as supporting cells, also have distinctive morphologies and organizations. Hair cells are separated from each other by supporting cells in a precise and invariable pattern. The supporting cells of the organ of Corti include the border cells, inner phalangeal cells (IPhC), the inner and outer pillar cells (IPC and OPC) and the Deiters’ cells (DC) (Figs. 1D, 2B) (Slepecky, 1996, Jones and Chen, 2007) The nuclei of these supporting cells are localized basally; from their soma, the supporting cells project phalangeal (“finger-like”) cellular processes toward the lumen of the cochlear duct (Fig. 2B). Their flattened ends of phalangeal processes separate hair cells from each other and form tight cellular contacts with the hair cells (Figs. 1D, 2B). Notably, the phalangeal processes of the supporting cells are highly polarized along the mediolateral axis of the cochlea. Morphologically, the pillar cells and DCs extend their cytoplasmic stalks and phalangeal processes along the mediolateral axis of the cochlea toward the periphery of the cochlea and contact the apical surface of the hair cells in the next row (Fig. 2B). Molecularly, several proteins display polarized subcellular localization along the mediolateral axis of the cochlea. The phalangeal processes of OPCs and DCs are also polarized along the longitudinal axis of the cochlea and contact hair cells at a distance (toward the apex of the cochlear duct) from the base of these supporting cells. Bundles of microtubules, intermediate filaments and microfilaments span the phalangeal processes of supporting cells to support for structural integrity of the organ of Corti (Slepecky and Ulfendahl, 1992, Henderson et al., 1995, Slepecky et al., 1995). The tight juxtaposition of hair cells and supporting cells at their apical surface allows the separation of the endolymphatic fluid that baths the apical domain from the perilymph in the basolateral domain for proper mechanotransduction (Slepecky, 1996). The unique morphology of supporting cells and their cellular organization with sensory hair cells allows a morphologically defined yet mechanically flexible structure to permit movement of the sensory epithelium in response to mechanical stimuli (Hudspeth, 2000).
In addition to sensory hair cells and supporting cells, the spiral ganglion neurons that innervate hair cells are important for the structural integrity and function of the organ of Corti (Fig. 2) (Rubel and Fritzsch, 2002). The tonotopic organization of the auditory sensory organ is maintained in the auditory neural pathway, beginning immediately postsynaptic to hair cells (Hudspeth, 2000). The innervation of hair cells by spiral ganglion neurons is required for the structural integrity of the organ of Corti (Ma et al., 2000, Rubel and Fritzsch, 2002, Matei et al., 2005). During development, the formation of the sensory lineage and the neuronal lineage within the developing inner ear is tightly coupled (Ma et al., 2000, Radde-Gallwitz et al., 2004, Matei et al., 2005, Satoh and Fekete, 2005). It is no surprise that not only the pathways directly involved in sensory differentiation are essential for the morphogenesis of the organ of Corti, but also the development of the neuronal lineage influences the shaping of the organ of Corti (Ma et al., 2000, Matei et al., 2005).
The specification and differentiation of the organ of Corti
The otic placode is recognizable around embryonic day 8.5 (E8.5) in mice (Kikuchi et al., 1988, Morsli et al., 1998, Riley and Phillips, 2003). By E10.5, the otic placode has invaginated and formed a hollow epithelium called the otocyst and at this time the regions of sensory and non-sensory epithelium begin to be determined. At these early stages of induction three-dimensional spatial cues, including Sonic hedgehog (Shh), Wnts, BMPs and FGFs (McKay et al., 1996, Morsli et al., 1998, Chang et al., 1999, Cole et al., 2000, Gerlach et al., 2000, Chang et al., 2002, Maroon et al., 2002, Riccomagno et al., 2002, Liu et al., 2003, Wright and Mansour, 2003, Chang et al., 2004, Ozaki et al., 2004, Solomon et al., 2004, Riccomagno et al., 2005, Fritzsch et al., 2006, Ohyama et al., 2006), play important roles in setting up sub-domains of the otocyst and controlling subsequent morphogenesis of various structures and specific cell types. Very soon after the initial specification, the newly formed otocyst begins specification and forms regions that will become the cochlea and vestibule, while the cochleovestibular neurons delaminate from the same regions in the otocyst that are designated to become the sensory epithelia. The regional specification of the otocyst is illustrated by expression of molecular markers in individual regions.
Soon after the cochlear duct has formed at the ventral region of the otocyst, sharp boundaries are formed to molecularly mark each half of the cochlear epithelium at E12.5. Otoconin90 (Zhao et al., 2007) is specifically expressed in the roof while the expression of several other genes including Isl1 (Radde-Gallwitz et al., 2004) and Sox2 (Kiernan et al., 2005b) (personal communication, A. Kiernan) is restricted to the floor of the duct. The pathways that set up the sharp boundaries of the cochlear duct at this stage are not known. Isl1 knockout mice die by E11.5 (Pfaff et al., 1996) and its role in inner ear development has yet to be determined. The analysis of molecular markers has not been reported in Sox2 mutants at E12.5 (Kiernan et al., 2005b) and the role of Sox2 for early cochlea development is unknown.
Regional specifications within the floor of the cochlear epithelium continue and result in the formation of a morphologically and molecularly distinctive sensory primordium. By E13.5–E14.5, the organ of Corti is recognized as a thickened ridge in the cochlear epithelium and marked by the expression of multiple genes. In particular, the precursor cells of the organ of Corti withdraw from the cell cycle around E14.5 (Ruben, 1967) and form a zone of non-proliferating cells (ZNPC) that is marked by two cyclin-dependent kinase inhibitors, p27/Kip1 and p19/Ink4d, and Isl1 and Sox2 (Chen and Segil, 1999, Chen et al., 2002, Chen et al., 2003, Radde-Gallwitz et al., 2004, Kiernan et al., 2005b, Matei et al., 2005, Lee et al., 2006). p27/Kip1 and Sox2 are required for the precursor cells of the organ of Corti to withdraw timely from the cell cycle (Chen and Segil, 1999) and for the specification of the sensory primordium (Kiernan et al., 2005b) in the cochlea, respectively. In addition, several genes in the Notch pathway show distinctive expression patterns in the vicinity of the sensory primordium, consistent with a role in specifying and/or restricting the sensory lineage in the cochlea (Haddon et al., 1998, Lanford et al., 1999, Eddison et al., 2000, Kiernan et al., 2005a, Brooker et al., 2006, Kiernan et al., 2006). Members of the BMP pathway are also expressed in the cochlear epithelium abutting the sensory primordium (Morsli et al., 1998). Their exact role in the specification and differentiation of the organ of Corti in mice, however, remains controversial (Li et al., 2005, Pujades et al., 2006).
Following specification of the sensory primordium in the cochlea, terminal differentiation of the organ of Corti initiates near the base of the cochlea. The gradient of hair cell differentiation begins with the onset of Math1 in the near-basal region and moves in both directions, finishing in the apical portions of the cochlea around E17.5 (Sher, 1971, Chen and Segil, 1999). Simultaneously with the longitudinal gradient of differentiation, a mediolateral, or inner-to-outer hair cells, gradient of differentiation is also observed. By E18.5, nearly all the cells of the organ of Corti along the length have differentiated into the highly patterned structure of one row of IHCs and three rows of OHCs (Fig. 2). The expression of p27/Kip1 is down-regulated in differentiating hair cells and remains in supporting cells (Chen and Segil, 1999), which differentiate and form the full complement of specialized supporting cells at the same time as the hair cells achieve their terminal morphology (Fig. 3A). The Notch pathway clearly plays an essential role in determining the fate of hair cells vs. supporting cells (Haddon et al., 1998, Kiernan et al., 2005a, Brooker et al., 2006).
Fig. 3. Convergent extension of the organ of Corti.
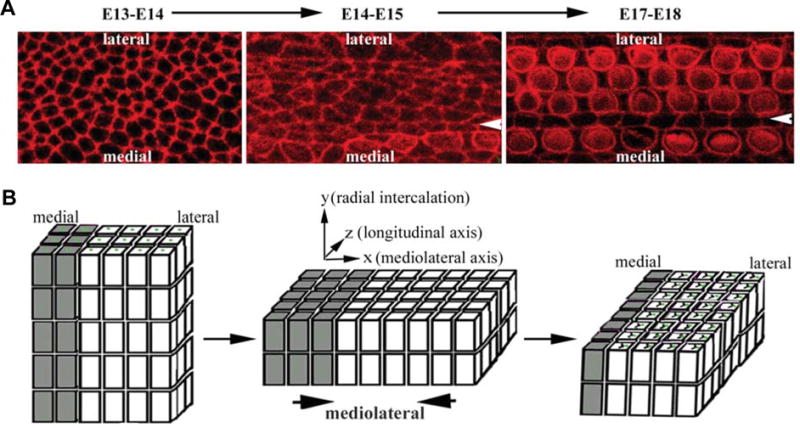
Whole mount surface views of the developing organ of Corti illustrate drastic shape change and remodeling of cell-cell contact from E13–E14 to E17–E18 in mice (A), which may underlie cellular rearrangement characteristic of convergent extension during the same period (B). The Arrowheads indicate the pillar cell region that separates IHCs from OHCs. The drawing in (B) is simplified, omitting the supporting cell population. Nevertheless, the supporting cells may play essential roles in PCP and proposed convergent extension cellular intercalation. The phenotypic defects in PCP mutants indicate both radial and mediolateral intercalations are involved and PCP signaling is only required for mediolateral intercalation. However, the sequential order of radial and mediolateral intercalations of the developing organ of Corti is only hypothesized and not established experimentally.
Morphogenesis of the organ of Corti: convergent extension and establishment of planar cell polarity (PCP)
Convergent extension in the developing organ of Corti
Convergent extension (CE) (Keller, 2002) is one of the most important cellular movements during gastrulation, contributing to the formation of three germ layers and the establishment of an elongated body plan with a distinctive anterior-posterior body axis from a blastula. It is a process of tissue narrowing along one axis (mediolateral axis) and concomitant extension along a perpendicular axis (anterior-to-posterior or A-P axis). During gastrulation, mesoderm cells undergo CE to establish the anterior-posterior body axis and to elongate along the anterior-posterior axis (Keller, 2002). CE also plays important roles in neurulation. At the neurula stage, cells in the neural ectoderm undergo CE to both elongate along the anterior-posterior axis and to close the neural tube (Keller, 2002). During gastrulation in Xenopus, it was shown, using Keller’s explants, that the mesoderm cells first undergo radial intercalation to form a thinner tissue and subsequently intercalate mediolaterally (Keller et al., 1985). The mediolateral intercalation of cells leads to the convergence (narrowing) of the tissue along the same (mediolateral) axis and elongation along the perpendicular anterior-posterior axis. While the cellular behavior and the driving force for radial intercalation is unclear, it is known that during mediolateral intercalation, cells are polarized or elongated along mediolateral axis and project mediolaterally oriented lamellapodia (Wilson and Keller, 1991, Keller, 2002). Computational models suggest that force generated by the protrusive activity of the cells along the mediolateral axis is sufficient to drive the intercalation of cells along the same axis (Zajac et al., 2003, Brodland, 2006, Brodland and Veldhuis, 2006). This hypothesis is consistent with the observation that actin-myosin cytoskeletal components are required for cellular intercalation (Zallen and Wieschaus, 2004). In mammals, morphogenetic changes indicate that similar CE cellular movement occurs during gastrulation and neurulation. However, the process of CE in mammals has yet to be documented directly.
During terminal differentiation from E14.5–E18.5, the postmitotic organ of Corti thins from an epithelium of 4–5 cells thick to a final two cell-layered structure and extends along the longitudinal axis significantly (Chen et al., 2002, McKenzie et al., 2004). The extension and thinning are independent of cell proliferation and death within the developing organ of Corti, respectively, suggesting that cellular rearrangement within a shorter and thicker sensory primordium leads to the formation of a longer and thinner mature organ (Chen et al., 2002). This type of cellular movement is remarkably similar to the process of CE which occurs during gastrulation and neurulation in Xenopus. Morphological and functional studies further suggest that radial and mediolateral intercalation of cells characteristic of CE is likely involved in extension of the cochlea and patterning of the hair cells (Fig. 3) (Montcouquiol et al., 2003, McKenzie et al., 2004, Wang et al., 2005, Jones and Chen, 2007).
Establishment of PCP in the organ of Corti
The terminal differentiation and extension of the organ of Corti occur in a ciliated epithelium between E14.5–E18.5 in mice. Both electron microscope and immunostaining show that cells in the developing cochlea are mono-ciliated, containing a microtubule-based primary cilium (Davis et al., 2006) known as the kinocilium (Figs. 1 and 2). The kinocilium is first seen centrally placed on the apex of nascent hair cells and surrounded by microvilli made up of actin filaments of uniform size (Sobkowicz et al., 1995). Subsequently, these microvilli begin to enlarge and become stereocilia. The kinocilium becomes displaced to the lateral side of the cell apex and stereocilia grow in a defined “V”-shaped pattern with the vertex of the “V” closely placed near the kinocilium (Frolenkov et al., 2004). The polarity of kinocilia appears to lead the polarization of stereocilia and the development of stereocilia and their polarity follow the differentiation gradient from the base to the apex along the longitudinal axis and from the inner to outer hair cells along the mediolateral axis of the cochlea. By E18.5 in mice, the polarity of stereocilia and kinocilia is established along the entire length of the cochlear duct and across the width of the organ of Corti. Once established, stereocilia continue to grow, mature and renew. In mammals, kinocilia in the cochlea regress postnatally. The transient presence and the polarity of kinocilia in the cochlea implicate a developmental role. However, the function of kinocilia in the development of the cochlea has not yet been reported.
Planar cell polarity pathway in shaping the cochlea and its sensory organ
PCP pathway
Intriguingly, as CE (Fig. 3B) was being proposed for the terminal morphogenesis of the organ of Corti (Chen et al., 2002), it was revealed that CE in vertebrates is regulated by a conserved genetic pathway, the planar cell polarity (PCP) pathway (Wallingford et al., 2000, Keller, 2002, Mlodzik, 2002, Wallingford et al., 2002).
The PCP pathway was first identified and characterized for its role in regulating various forms of PCP in Drosophila tissues (Gubb and Garcia-Bellido, 1982, Klein and Mlodzik, 2005, Strutt and Strutt, 2005). In the tissues that exhibit PCP, there is a well-defined planar polarity both in the intrinsically polarized structure of each individual cell and in the arrangement of different cells relative to each other within the group (Fig. 1). In the organ of Corti, the stereociliary bundles of each hair cell are arranged in an asymmetrical “V” shape. The asymmetrical nature of the “V”-shaped stereocilia represent the intrinsically polarized structure within each hair cell. Furthermore, all the stereocilia are uniformly oriented along the mediolateral axis of the cochlea, manifesting a precise coordination in the arrangement of the cells relative to each other within the group. The planar polarization of cells both in the intrinsic structure of each individual cell and in the arrangement of different cells relative to each other within the entire group requires a three-tiered regulation (Tree et al., 2002) through two alterative models (Fig. 4). Both models require: a global guidance cue for directional information (upstream PCP genes) and cellular factors to interpret the directional signal by adopting polarized asymmetric localization along the axis for polarity (core PCP genes) (Fig. 4). Upon the formation of polarized core PCP complexes, the third and final step of PCP signaling of planar polarization across the tissue can be achieved by cell-specific effectors downstream of core PCP genes (downstream PCP effector genes) that direct the formation of the asymmetrical structure within each cell and coordinate the polarization of all the cells across the entire tissue. Alternatively, the machinery that builds the asymmetrical structure within each cell is independent of PCP signaling. To achieve the third step in PCP signaling of planar polarization across the tissue in this second model, cellular mediators (downstream PCP mediator genes) link polarized core PCP complexes to the cellular machinery that creates the asymmetrical structure within individual cells to coordinate planar polarization in all the cells across the entire tissue (Fig. 4). In the first model, impaired PCP signaling will lead to defects in both the formation of the asymmetrical structure within individual cells as well as the coordination of all the cells across the tissue. In the second model, however, defective PCP signaling will only affect the coordination of planar polarization across the tissue but not the production of the asymmetrical structure within individual cells.
Fig. 4. A model of planar cell polarity (PCP) regulation in the cochlea.

The establishment of PCP across the organ of Corti requires a three-tiered regulation: (1) upstream factors for directional information along the mediolateral axis of the cochlea; (2) core PCP proteins form polarized complexes along the mediolateral axis of the cochlea; (3) unknown cellular mediators link polarized core PCP complexes to the machinery that builds the “V”-shaped stereocilia in individual hair cells and orient the vertices of the “V”-shaped stereocilia toward the periphery of the spiraling cochlea, establishing uniformly oriented stereocilia across the organ of Corti. The identity and the role of the upstream factors were hypothesized; and the role of Pk and Ankrd6/Diversin in inner ear was hypothesized based on their role in PCP signaling in Drosophila and in CE in zebrafish. Several core PCP proteins are observed at the boundaries between supporting cells and between hair cells and supporting cells in a polarized manner along the mediolateral axis of the cochlea. Their localization to one or both cells at the boundaries has not been determined unequivocally.
Genetic studies in Drosophila identified a set of core PCP genes that affect all known structures with PCP features (Tree et al., 2002, Klein and Mlodzik, 2005, Strutt et al., 2006). During establishment of PCP, core PCP proteins are sorted asymmetrically along the polarization axis and this polarized association of core PCP proteins is required for planar polarization across the tissue (Axelrod et al., 1998, Axelrod, 2001, Tree et al., 2002, Klein and Mlodzik, 2005, Strutt and Strutt, 2005). Studies in Xenopus and zebrafish revealed a conserved vertebrate PCP pathway that consists of a similar cassette of genes, including Frizzled (Fz) (Djiane et al., 2000), Dishevelled (Dvl) (Sokol, 1996, Wallingford et al., 2000), Ltap/Vangl2 (Goto and Keller, 2002, Jessen et al., 2002, Park and Moon, 2002) and homologs of Diego (Schwarz-Romond et al., 2002, Moeller et al., 2006) and Prickle (Veeman et al., 2003), that are required for CE during gastrulation and neurulation (Fig. 4). Core PCP proteins display polarized subcellular localization along the anterior-posterior axis (Jiang et al., 2005, Ciruna et al., 2006). Together with mediolaterally polarized sorting of Par complexes (Hyodo-Miura et al., 2006, Mlodzik, 2006), the core PCP proteins likely provide instructive roles to direct mediolateral intercalation of cells during CE (Mlodzik, 2006).
The core PCP component Fz can serve as a receptor for Wnt signaling molecules (Moon, 2005). The binding of Wnt to Fz can activate a canonical Wnt pathway in which β-catenin is stabilized for transcriptional activation of downstream target genes. PCP signaling does not involve β-catenin mediated transcriptional activation and, instead, involves cytoskeletal targets (therefore, known as the noncanonical Wnt pathway). Since the polarization of cells in the entire field requires directional information and Wnts as morphogens can fulfill such a directional role, Wnts have been extensively tested for their potential involvement in PCP. However, Wnts appear to be dispensable for PCP in several Drosophila tissues (Ma et al., 2003, Klein and Moldzik, 2005, Strutt and Strutt, 2005). A mathematic model (Amonlirdviman et al., 2005), whose predictions have been tested experimentally, further argues for a morphogen-independent feedback loop regulation for polarization over a long range. In contrast, two recent studies indicate an instructive role for Wnt and Hh in orienting the denticles (actin protrusions) on the epidermis of Drosophila embryos (Colosimo and Tolwinski, 2006, Price et al., 2006). Therefore, it is possible that epithelial planar polarization across the tissues may utilize a morphogen-independent mechanism for polarization over a long range, an instructive cue-dependent mechanism for polarization over a short range, or a combination of both. In contrast to their variable role in establishing epithelial PCP in Drosophila, upstream morphogens are involved in PCP signaling for vertebrate CE (Heisenberg et al., 2000, Smith et al., 2000, Tada and Smith, 2000, Kilian et al., 2003, Ohkawara et al., 2003). In Xenopus and zebrafish, Wnt5 and Wnt11 are required for CE (Heisenberg et al., 2000, Smith et al., 2000, Tada and Smith, 2000, Kilian et al., 2003, Ohkawara et al., 2003) and their role in CE appears to be permissive rather than instructive. In addition, graded anterior-posterior cues, such as activin and BMPs, are required for CE in Xenopus (Myers et al., 2002, Ninomiya et al., 2004). The exact mechanism underlying the involvement of graded anterior-posterior cues for CE is not clear.
Upon the formation of core PCP proteins on opposite sides of the cells along the axis for planar polarity, planar polarization across the tissue is achieved by coordinated morphological polarization. In Drosophila, three cytoskeleton-binding proteins, Inturned, Fuzzy and Fritz, function downstream of core PCP genes (Park et al., 1996, Turner and Adler, 1998, Yun et al., 1999, Adler et al., 2004, Collier et al., 2005). Mutations in Inturned, Fuzzy and Fritz cause the formation of multiple hairs at abnormal locations in wing cells (Adler et al., 2004, Collier et al., 2005), suggesting that these genes may function as downstream PCP mediators and link polarized core PCP complexes to the machinery that creates hairs in wing cells for coordinated planar polarization in all the cells across the wing. The generation of multiple hairs in these mutants also suggests that these downstream mediators directly regulate the formation of hairs by limiting the activity of the machinery that builds hairs in wing cells.
Two members of the Rho family of GTPase that are capable of modifying cytoskeletal components, RhoA and Rac, are implicated in the vertebrate PCP signaling downstream of a core PCP gene Dvl during CE in Xenopus (Habas et al., 2001, Marlow et al., 2002, Habas et al., 2003, Phillips et al., 2005). In addition, Inturned, Fuzzy and another cytoskeleton-binding protein Dub are required for CE in Xenopus and zebrafish (Oishi et al., 2006, Park et al., 2006). Notably, PCP signaling and Par complexes appear to be required only for polarizing lamellapodia protrusions mediolaterally (Wallingford et al., 2000, Hyodo-Miura et al., 2006). Disruptions in PCP signaling or Par complexes does not prevent lamellapodia protrusive activity, but rather results in random and unstable lamellapodia protrusions (Wallingford et al., 2000, Hyodo-Miura et al., 2006). Therefore, PCP signaling may guide only the coordinated polarization of cells across the tissue, but not the formation of the asymmetrical structure within individual cells during epithelial planar polarization nor the formation of lamellapodia during CE.
PCP pathway and morphogenesis of the organ of Corti
The establishment of PCP in the organ of Corti occurs concurrently with the extension of the cochlea involving cellular rearrangement characteristic of CE, raising the possibility that the mammalian PCP pathway may regulate both processes during terminal differentiation of the organ of Corti (Chen et al., 2002).
Indeed, mice defective in core PCP genes, including Ltap/Vangl2, Dvl1/2, Fz3/6 and Celsr1, show various degrees of misorientation of stereocilia (Fig. 5) (Curtin et al., 2003, Montcouquiol et al., 2003, Wang et al., 2005, Montcouquiol et al., 2006, Wang et al., 2006a, Wang et al., 2006b). A widened and shortened organ of Corti is also reported in several of these PCP mutants (Fig. 5) (Montcouquiol et al., 2003, Wang et al., 2005, Wang et al., 2006a). It was further demonstrated that the misorientation of stereocilia and the apparent CE defect are intrinsic to defective PCP signaling within the cochlea and not indirectly resulted from neural tube defects in these mutant animals (Wang et al., 2005). These data support that the establishment of uniform orientation of stereocilia and CE of the cochlea are regulated and linked by the mammalian PCP pathway.
Fig. 5. Typical PCP phenotypes in the cochlea.

Defective PCP signaling leads to formation of a shorter (A) and wider (B) cochlea in mutant (mut) mice, in comparison to wild-type (wt) animals. The widening of the cochlea and its sensory organ is most prominent toward the apical region (compare C and D) by staining for a hair cell marker (C, D). In addition, wild-type cochleae show a distinctive PCP, manifested with uniformly oriented stereocilia (E) while PCP mutant cochleae display misorientation of stereocilia (F). A single primary cilium, the kinocilium (C–F), is seen on the apical surface of each hair cell. lp: the loop tail alleles, a loss-of-function allele for core PCP gene Vangl2 (Ltap). Brackets and arrowheads (E, F) indicate the outer and inner hair cells, respectively. This image is modified from Wang et al. (2005), Nature Genetics. Authors’ Copyright.
However, the process of CE and cellular behavior during terminal differentiation of the organ of Corti has not been demonstrated directly. Neither is it known whether the rearrangement of cells is an active process within the developing organ of Corti, or a passive process due to active cellular intercalation in surrounding epithelial and/or mesenchymal cells and whether the actin/myosin cytoskeletal component is required. It is worth noting that in Math1−/− animals where no hair cell differentiation and some degree of supporting cell differentiation are observed (Bermingham et al., 1999, Chen et al., 2002, Woods et al., 2004, Matei et al., 2005), the cochlea appears to have a normal length (unpublished observation). This data suggests that the differentiation of the full complement hair cells and supporting cells is not essential for the extension of the cochlea and its sensory organ. The surrounding epithelial and mesenchymal cells may be sufficient to provide the driving force for CE of the cochlea.
An intriguing question is how the mammalian PCP pathway concurrently regulates the establishment of PCP, manifested with uniform oriented stereocilia of hair cells and CE in the cochlea during terminal differentiation. The two processes occur concurrently and defects in both processes are associated in PCP mutants. However, the establishment of PCP in the organ of Corti and CE are not mutually dependent. In Math1−/− animals, no apparent PCP manifest, morphologically polarized hair cells and supporting cells, is observed in the cochlear epithelium, while the extension of the cochlea appears to be normal. The two processes may utilize overlapping signaling pathways but contain differential molecular and cellular components. Consistent with this view, several core PCP genes are expressed in the entire cochlear epithelium with higher levels at the region medial to the developing organ of Corti. Within the developing organ of Corti, Ltap/Vangl2, Dvl2 and Fz3/6 display polarized subcellular localization along the mediolateral axis of the cochlea (Wang et al., 2005, Montcouquiol et al., 2006, Wang et al., 2006a, Wang et al., 2006b). In the region medial to the organ of Corti, the subcellular localization of these core PCP proteins are also polarized, but apparently along the base-to-apex axis of the cochlea that is perpendicular to the mediolateral axis observed for PCP (data not published). In addition, the contribution of underlying mesenchymal cells of the cochlea to uniform orientation of stereocilia and cellular rearrangement within the developing organ of Corti has not been examined.
To orient stereocilia uniformly along the mediolateral axis of the cochlea, information to differentiate the medial vs. lateral direction must be presented during development. Similar to Drosophila PCP studies, Wnts have been investigated for their potential role in PCP signaling in the cochlea. Wnt7a is expressed in pillar cells and addition of Wnt antagonists and Wnt7a in the organ of Corti culture leads to misorientation of stereocilia in vitro (Dabdoub et al., 2003). However, Wnt7a−/− animals do not have any apparent defects in PCP signaling (Dabdoub et al., 2003), suggesting that pathways compensatory or independent of Wnts provide directional information for the organ of Corti during development. Indeed, the expression of another Wnt molecule, Wnt5a, is restricted to the region medial to the developing organ of Corti (Qian et al., 2007). Genetic inactivation of Wnt5a leads to ~35% penetrance of a characteristic CE defect in the cochlea (Qian et al., 2007). Furthermore, Wnt5a genetically interacts with known PCP gene Ltap/Vangl2 in regulating the orientation of stereocilia, cochlear extension and neurulation (Qian et al., 2007). Together these data indicate a role of Wnt5a in PCP signaling in mice. Intriguingly, a Wnt antagonist, secreted Frizzled-related protein 3 (Sfrp3 or Frzb), is expressed in a reciprocal pattern with Wnt5a in the developing organ of Corti (Qian et al., 2007). However, it is not clear whether the reciprocal expression of Wnt5a and a Wnt antagonist is involved in generating a graded Wnt signal for an instructive role in PCP signaling.
Cell adhesion and PCP signaling in the organ of Corti
Ultimately, the affinity among constituent cells and the affinity between the cells and their extracellular matrix determine the cellular arrangement and consequently the morphology of a particular tissue or organ (Townes and Holtfreter, 1955, Nose et al., 1988, Steinberg and Takeichi, 1994, McNeill, 2000, Zajac et al., 2003). In vitro, differential cellular affinity can drive cellular aggregation and cellular rearrangement (Townes and Holtfreter, 1955, Nose et al., 1988, Steinberg and Takeichi, 1994, McNeill, 2000, Zajac et al., 2003, Strutt et al., 2004, Saburi and McNeill, 2005). In Drosophila, atypical cadherins, Fat and Dachsous and protocadherin Flamingo play essential roles in initiating and propagating PCP signaling (Fanto et al., 2003, Matakatsu and Blair, 2004, Simon, 2004, Strutt et al., 2004). A mammalian homolog of Flamingo, Celsr1, is required for PCP signaling in the cochlea (Curtin et al., 2003). However, the expression and function of atypical cadherins in PCP signaling in the cochlea has not been investigated. Furthermore, cellular packing geometry changes drastically during terminal differentiation of the organ of Corti (Fig. 3A). Cellular junctions must be remodeled during morphogenesis of the organ of Corti at the terminal differentiation stage. It is very likely that molecules mediating general cell-cell adhesion play important roles in PCP signaling and morpohogenesis of the organ of Corti. Investigation of cell adhesive activities and extracellular matrix properties in the cochlea will provide invaluable information to delineate the cellular and molecular components for PCP signaling in the cochlea.
PCP signaling and ciliogenesis in morphogenesis of the organ of Corti
Cilia consist of a microtubule-based axoneme tethered to the cell at the basal body and typically protrude from the apical surface of cells (Davis et al., 2006). The basal body consists of a pair of centrioles and is the organization center for both cytoskeletal microtubules and ciliary microtubules. The axoneme in the ciliary portion consists of microtubule doublets either with (9+2) or without (9+0) a centrally located pair (Davis et al., 2006). Protein synthesis does not occur in cilia. Cilia are assembled and maintained by intraflagellar transport (IFT), in which multimeric protein complexes called IFT particles are moved bidirectionally along the axomeme by the coordination of IFT motors, the anterograde (toward the plus ends of microtubules or the tip the cilium) molecular motor kinesin and the retrograde (toward the minus ends of microtubules) motor protein dynein along microtubules. The loss of IFT genes leads to ciliary defects.
The polarization of stereocilia in the hair cell is led by the polarity of the kinocilium, a primary cilium (Figs. 1 and 2). Kinocilia regress postnatally in the mammalian cochlea (Sobkowicz et al., 1995, Leibovici et al., 2005), implicating a developmental role. Recent studies revealed tantalizing links between cilia and PCP signaling (Ross et al., 2005, Park et al., 2006, Jones and Chen, 2007) and raised the possibility that PCP signaling is required for ciliogenesis and the possibility that cilia are involved in PCP signaling. These findings provided the first experimental indication that kinocilia may indeed have a developmental role in the cochlea.
Upon close examination, however, there are outstanding questions regarding the links between PCP signaling and ciliogenesis. Gene knockdown of PCP downstream effectors, Inturned and Fuzzy, in zebrafish causes a ciliogenesis defect (Park et al., 2006), suggesting that PCP signaling may be required for ciliogenesis. However, the PCP genes that are required for ciliogenesis are limited to these downstream cytoskeletal effectors. Kinocilia and other primary cilia appear to be normal in core PCP mutants in studies reported by several laboratories, including ours (Jones and Chen, 2007). These data argue against a requirement for PCP signaling in ciliogenesis, but rather a dual role of cytoskeletal effectors, such as Inturned and Fuzzy, for both ciliogenesis and PCP.
Receptors and mediators for hedgehog (Hh) and platelet-derived growth factor receptor (PDGFR) signaling pathways have been localized to cilia for transducing both pathways (Corbit et al., 2005, Haycraft et al., 2005, Liu et al., 2005, Schneider et al., 2005). This newly defined role of cilia as a specialized subcellular compartment for localizing and concentrating membrane bound signal receptors and complexes to relay signals from the cilium to the cell interior led to speculations that cilia may have a similar function and transduce Wnt signals (Ross et al., 2005, Simons et al., 2005). However, the first study implicating a role of cilia in PCP signaling has raised some issues(Ross et al., 2005). A PCP component, Ltap/Vangl2, was localized to the basal body and cilia in ciliated human respiratory epithelial cells from nasal brushing (Ross et al., 2005). Several Bardet-Biedl Syndrome (BBS) genes encode basal body proteins and mutations in these BBS genes cause ciliary defects and minor defects in stereociliary patterning (Ross et al., 2005). However, the stereociliary defect in BBS mutants is manifested with a low percentage of misshaped stereocilia, in contrast to misoriented stereocilia observed in PCP mutants. Ltap/Vangl2 or any other PCP proteins, such as Fz (Wnt receptors), have yet to be localized to the basal body or cilia in a tissue that exhibits PCP like the organ of Corti. Although the exact role of cilia or ciliary genes in PCP signaling and the morphogenesis of the organ of Corti remains obscure, the possibility for the involvement of cilia in PCP signaling is exciting and will be pursued vigorously by the general biological field.
Coalescing multiple signaling pathways in shaping the organ of Corti during terminal differentiation
Shortened and widened cochlear ducts and misorientation of stereociliary bundles are not limited to mouse mutants defective in PCP genes. In particular, genes for the Notch signaling are expressed in the developing cochlea during terminal differentiation and mutations in some of these genes result in certain characteristic PCP defects in the cochlea and its sensory organ (Kiernan et al., 2005a, Brooker et al., 2006). The role of the Notch pathway in sensory competency and in determining the fate of hair cells vs. supporting cells has been established (Daudet and Lewis, 2005, Kiernan et al., 2005a, Brooker et al., 2006, Kiernan et al., 2006). In the mice carrying mutations in two of the Notch ligands Jag2 and Dll1, however, misorientation of stereocilia is also apparent (Kiernan et al., 2005a). Shortened cochlear ducts with increased rows of hair cells were observed toward the apical region of the cochlea in mice mutated for Dll1 (Kiernan et al., 2005a, Brooker et al., 2006). It has been hypothesized that misorientation of stereociliary bundles in Jag2 and Dll1 mutants may be an indirect result of defects in patterning hair cell and supporting cell mosaic (Kiernan et al., 2005a). It has also been hypothesized that shortened and widened cochlear ducts in Dll1 mutants may be related to premature differentiation that shortens the period of growth and prevents the sensory primordium from elongating and narrowing normally (Brooker et al., 2006). While the molecular mechanisms underlying PCP-like defects in Notch pathway mutants are not determined, these data indicated a tight coupling of cellular differentiation and patterning with PCP signaling. It is possible that the specification and differentiation of hair cells and/or supporting cells produces specific surface or membrane components that are necessary for cell-cell communication during PCP signaling for establishing uniform orientation of stereocilia across the organ of Corti, and/or for mediolaterally polarized cellular protrusive activity to drive CE of the cochlea.
The development of spiral ganglion neurons also appears to influence the morphogenesis of the organ of Corti. In studies looking at the role of neurogenin1 in making afferent connections to inner hair cells, it has been found that not only do afferent connections have strong influence on the proper connections and autonomic innervations, but it also seems to have direct role in the development of the sensory epithelium (Ma et al., 2000). Although hair cells seem to differentiate normally in the absence of afferent innervation in Ngn1 null animals, other aspect of the phenotype, in striking resemblance of PCP defects (Ma et al., 2000), strongly implicates a role for neuronal influence on organ of Corti morphogenesis. The organ of Corti is shortened and expanded along the mediolateral axis in the apical region (Ma et al., 2000). Misorientation of stereocilia is also observed in the apical region of the cochlea (Ma et al., 2000).
In addition to the Notch pathway and neural influence, Hh, transforming growth factor-β (TGF-β) and FGF signaling may also contribute to PCP processes in the cochlea. In Drosophila embryos, Hh and Wnt molecules function together to orient the denticles, actin-based cell projections, on segmentally repeated subsets of ventral epidermal cells (Colosimo and Tolwinski, 2006, Price et al., 2006). The role of Hh in terminal differentiation of the organ of Corti is being actively investigated by several laboratories (Riccomagno et al., 2002, Riccomagno et al., 2005) and will help illustrate the complex upstream signaling molecules in PCP signaling. During gastrulation in Xenopus, anterior-posterior cues (along the extension axis), such as activin, a member of the TGF-β family, are required for convergent extension (Ninomiya et al., 2004). Interestingly, BMP4, one member of the TGF-β family, is expressed in an asymmetric manner in the cochlear epithelium along the longitudinal (extension axis) and mediolateral axes of the cochlea (Morsli et al., 1998). Multiple FGF ligands and receptors/mediators are also expressed in the cochlea during terminal differentiation (Pirvola et al., 2000, Mueller et al., 2002, Shim et al., 2005, Fritzsch et al., 2006). The role of Hh, BMP and FGF signaling in early patterning and differentiation has been well characterized. However, whether they also directly regulate PCP processes, the uniform orientation of stereocilia and the presumptive CE, in the cochlea remains uncharted.
A working model of PCP regulation in the cochlea and perspectives
The organ of Corti manifests perhaps the most distinctive form of planar cell polarity in vertebrates. It has emerged as a model system to illustrate the underlying mechanisms of the PCP pathway that is essential for development and function of multicellular organisms.
Based on current understanding of PCP signaling and mutations that affect uniform orientation of stereocilia and extension of the cochlea, we propose a working model that coalesces multiple signaling pathways for PCP regulation in the cochlea (Fig. 4). Upon upstream directional cues which may consist of both morphogens and proteins that mediate cell-cell adhesions, the core PCP complexes are sorted asymmetrically along the mediolateral axis of the cochlea. Cell-cell communications reinforce the sorting of core PCP proteins and the alignment of neighboring cells. Notably, all the known mice defective in core PCP genes maintain “V”-shaped stereocilia. Although it is possible that there is residual PCP signaling in all these known PCP mutants sufficient for the formation of “V”-shaped stereocilia, these observations point to a mechanism in which the formation of “V”-shaped stereocilia is independent of PCP signaling. Therefore, we propose that cellular mediators link polarized core PCP complexes to the machinery that directs the formation of “V”-shaped stereocilia in individual hair cells and align uniformly the vertices of the “V”-shaped stereocilia of all the hair cells along the mediolateral axis of the cochlea, displaying PCP across the entire organ of Corti.
Many questions remain in PCP signaling in the cochlea. The nature of upstream directional cues remains elusive. Wnt, Hh, BMPs, FGFs, Fat-Dachsous interaction, as well as neural influence may set up the initial polarity of the core PCP complexes. Similar to PCP regulation in Drosophila, core PCP complexes are also polarized along the axis for PCP in the mammalian cochlea. However, the detailed molecular interaction among core PCP proteins may deviate from that of the well-characterized Drosophila core PCP proteins. The identity of the putative cellular mediators that link polarized core PCP complexes to the machinery for the formation of “V”-shaped stereocilia and how these putative cellular mediators communicate with core PCP proteins and components of the stereocilia are not yet unknown. The apparent involvement of cilia and/or basal body in PCP signaling opened new directions for seeking the mechanisms underlying cochlea morphogenesis. It is tempting to hypothesize that cilia may function as a specialized apparatus for directional cues for PCP processes in the cochlea and that basal body as a microtubule organization center may function in sorting of core PCP complexes, and/or linking polarized core PCP complexes to stereocilia to coordinate their uniform orientation across the organ of Corti. The adhesive properties of the cells in the cochlea will also be critical for the understanding of the cellular and molecular components driving cellular rearrangements that shape the extended cochlear and its sensory organ. Research toward these directions is exciting and will not only advance our understanding of the underlying mechanism for the morphogenesis of the cochlea but also address fundamental issues in biology.
Acknowledgments
We thank Sharayne Mark and Kristen Radde-Gallwitz for their contribution to inner ear paint-fill, immunostaining and in situ hybridization images; Dalian Ding for helpful comments on inner ear structure. This work is supported by grants from the US National Institute of Health (to P.C.) and a predoctoral training grant from US National Institute of Health (to M.K.).
Abbreviations used in this paper
- BBS
Bardet-Biedl syndrome
- CE
convergent extension
- DC
Deiters’ cell
- Dvl
disheveled
- Fz
frizzled
- Hh
hedgehog
- IPhC
inner phalangeal cell
- IFT
intraflagellar transport
- IPC
inner pillar cell
- OPC
outer pillar cell
- PCP
planar cell polarity
- PDGFR
platelet-derived growth factor receptor
- Shh
sonic hedgehog
- TGF-β
transforming growth factor-β
- ZNPC
zone of non-proliferating cells
References
- Adler PN, Zhu C, Stone D. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr Biol. 2004;14:2046–51. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–6. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of dishevelled mediates frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–7. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of dishevelled provides signaling specificity in the planar cell polarity and wingless signaling pathways. Genes Dev. 1998;12:2610–22. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132:2115–24. doi: 10.1242/dev.01796. [DOI] [PubMed] [Google Scholar]
- Brodland GW. Do lamellipodia have the mechanical capacity to drive convergent extension? Int J Dev Biol. 2006;50:151–5. doi: 10.1387/ijdb.052040gb. [DOI] [PubMed] [Google Scholar]
- Brodland GW, Veldhuis JH. Lamellipodium-driven tissue reshaping: A parametric study. Comput Methods Biomech Biomed Engin. 2006;9:17–23. doi: 10.1080/10255840600554703. [DOI] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and delta1 in the mouse inner ear. Development. 2006;133:1277–86. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: Role of fgfs in sensory cristae. Development. 2004;131:4201–11. doi: 10.1242/dev.01292. [DOI] [PubMed] [Google Scholar]
- Chang W, Nunes FD, De Jesus-Escobar JM, Harland R, Wu DK. Ectopic noggin blocks sensory and nonsensory organ morphogenesis in the chicken inner ear. Dev Biol. 1999;216:369–81. doi: 10.1006/dbio.1999.9457. [DOI] [PubMed] [Google Scholar]
- Chang W, Ten Dijke P, Wu DK. Bmp pathways are involved in otic capsule formation and epithelial-mesenchymal signaling in the developing chicken inner ear. Dev Biol. 2002;251:380–94. doi: 10.1006/dbio.2002.0822. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. P27(kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–90. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor ink4d. Nat Cell Biol. 2003;5:422–6. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–4. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LK, Le Roux I, Nunes F, Laufer E, Lewis J, Wu DK. Sensory organ generation in the chicken inner ear: Contributions of bone morphogenetic protein 4, serrate1 and lunatic fringe. J Comp Neurol. 2000;424:509–20. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Collier S, Lee H, Burgess R, Adler P. The wd40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics. 2005;169:2035–45. doi: 10.1534/genetics.104.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosimo PF, Tolwinski NS. Wnt, hedgehog and junctional armadillo/beta-catenin establish planar polarity in the Drosophila embryo. PLoS ONE. 2006;1:e9. doi: 10.1371/journal.pone.0000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. Mutation of celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–84. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for notch activity in chick inner ear development: Specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–51. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Davis EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: New functional roles for an ancient organelle. Dev Cell. 2006;11:9–19. doi: 10.1016/j.devcel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Djiane A, Riou J, Umbhauer M, Boucaut J, Shi D. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000;127:3091–100. doi: 10.1242/dev.127.14.3091. [DOI] [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: Lessons from Drosophila. Proc Natl Acad Sci USA. 2000;97:11692–9. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, Kerridge S, Mcneill H. The tumor-suppressor and cell adhesion molecule fat controls planar polarity via physical interactions with atrophin, a transcriptional co-repressor. Development. 2003;130:763–74. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Beisel KW. Cells, molecules and morphogenesis: The making of the vertebrate ear. Brain Res. 2006;1091:151–71. doi: 10.1016/j.brainres.2006.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ. Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet. 2004;5:489–98. doi: 10.1038/nrg1377. [DOI] [PubMed] [Google Scholar]
- Gerlach LM, Hutson MR, Germiller JA, Nguyen-Luu D, Victor JC, Barald KF. Addition of the bmp4 antagonist, noggin, disrupts avian inner ear development. Development. 2000;127:45–54. doi: 10.1242/dev.127.1.45. [DOI] [PubMed] [Google Scholar]
- Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–81. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of rac and rho by wnt/frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/frizzled activation of rho regulates vertebrate gastrulation and requires a novel formin homology protein daam1. Cell. 2001;107:843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: Evidence from the mind bomb mutant. Development. 1998;125:4637–44. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Henderson CG, Tucker JB, Mogensen MM, Mackie JB, Chaplin MA, Slepecky NB, Leckie LM. Three microtubule-organizing centres collaborate in a mouse cochlear epithelial cell during supracellularly coordinated control of microtubule positioning. J Cell Sci. 1995;108(Pt 1):37–50. doi: 10.1242/jcs.108.1.37. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. Hearing. McGraw-Hill; New York: 2000. [Google Scholar]
- Hyodo-Miura J, Yamamoto TS, Hyodo AC, Iemura S, Kusakabe M, Nishida E, Natsume T, Ueno N. Xgap, an arfgap, is required for polarized localization of par proteins and cell polarity in Xenopus gastrulation. Dev Cell. 2006;11:69–79. doi: 10.1016/j.devcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–5. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Jones C, Chen P. Planar cell polarity signaling in vertebrates. Bioessays. 2007;29:120–32. doi: 10.1002/bies.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Keller RE, Danilchik M, Gimlich R, Shih J. The function and mechanism of convergent extension during gastrulation of Xenopus laevis. J Embryol Exp Morphol. 1985;89(Suppl):185–209. [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The notch ligands dll1 and jag2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005a;132:4353–62. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005b;434:1031–5. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The notch ligand jag1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Tonosaki A, Takasaka T. Development of apical-surface structures of mouse otic placode. Acta Otolaryngol. 1988;106:200–7. doi: 10.3109/00016488809106426. [DOI] [PubMed] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of ppt/wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–76. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: An emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–76. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Kruse K, Julicher F. Oscillations in cell biology. Curr Opin Cell Biol. 2005;17:20–6. doi: 10.1016/j.ceb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–92. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133:2817–26. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Leibovici M, Verpy E, Goodyear RJ, Zwaenepoel I, Blanchard S, Laine S, Richardson GP, Petit C. Initial characterization of kinocilin, a protein of the hair cell kinocilium. Hear Res. 2005;203:144–53. doi: 10.1016/j.heares.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The vertebrate inner ear. Boca Raton: CRC Press; 1985. [Google Scholar]
- Lewis J, Davies A. Planar cell polarity in the inner ear: How do hair cells acquire their oriented structure? J Neurobiol. 2002;53:190–201. doi: 10.1002/neu.10124. [DOI] [PubMed] [Google Scholar]
- Li H, Corrales CE, Wang Z, Zhao Y, Wang Y, Liu H, Heller S. Bmp4 signaling is involved in the generation of inner ear sensory epithelia. BMC Dev Biol. 2005;5:16. doi: 10.1186/1471-213X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DJ, Anniko M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol Suppl. 1985;422:1–69. [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of gli transcription factors. Development. 2005;132:3103–11. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan YL, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–24. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. Ptk7/cck-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Ma D, Yang CH, Mcneill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–7. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–43. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. Cochlear mechanisms from a phylogenetic viewpoint. Proc Natl Acad Sci USA. 2000;97:11736–43. doi: 10.1073/pnas.97.22.11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish rho kinase 2 acts downstream of wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–84. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, Mason I. Fgf3 and fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Interactions between fat and dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–94. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–50. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckay IJ, Lewis J, Lumsden A. The role of fgf-3 in early inner ear development: An analysis in normal and kreisler mutant mice. Dev Biol. 1996;174:370–8. doi: 10.1006/dbio.1996.0081. [DOI] [PubMed] [Google Scholar]
- Mckenzie E, Krupin A, Kelley MW. Cellular growth and rearrangement during the development of the mammalian organ of Corti. Dev Dyn. 2004;229:802–12. doi: 10.1002/dvdy.10500. [DOI] [PubMed] [Google Scholar]
- Mcneill H. Sticking together and sorting things out: Adhesion as a force in development. Nat Rev Genet. 2000;1:100–8. doi: 10.1038/35038540. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Planar cell polarization: Do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–71. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. A gap in convergent extension scores par. Dev Cell. 2006;11:2–4. doi: 10.1016/j.devcel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Moeller H, Jenny A, Schaeffer HJ, Schwarz-Romond T, Mlodzik M, Hammerschmidt M, Birchmeier W. Diversin regulates heart formation and gastrulation movements in development. Proc Natl Acad Sci USA. 2006 doi: 10.1073/pnas.0603808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of vangl2 and scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–7. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, et al. Asymmetric localization of vangl2 and fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–75. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT. Wnt/beta-catenin pathway. Sci STKE. 2005;2005:cm1. doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–35. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Jacques BE, Kelley MW. Fibroblast growth factor signaling regulates pillar cell development in the organ of Corti. J Neurosci. 2002;22:9368–77. doi: 10.1523/JNEUROSCI.22-21-09368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–7. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–38. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–75. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Oishi I, Kawakami Y, Raya A, Callol-Massot C, Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical wnt signaling mediator, duboraya. Nat Genet. 2006;38:1316–22. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, et al. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131:551–62. doi: 10.1242/dev.00943. [DOI] [PubMed] [Google Scholar]
- Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–5. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and hedgehog signaling. Nat Genet. 2006;38:303–11. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Park WJ, Liu J, Sharp EJ, Adler PN. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development. 1996;122:961–9. doi: 10.1242/dev.122.3.961. [DOI] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for lim homeobox gene isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–20. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via rhoa signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–9. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. Fgf/fgfr-2(iiib) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20:6125–34. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MH, Roberts DM, Mccartney BM, Jezuit E, Peifer M. Cytoskeletal dynamics and cell signaling during planar polarity establishment in the Drosophila embryonic denticle. J Cell Sci. 2006;119:403–15. doi: 10.1242/jcs.02761. [DOI] [PubMed] [Google Scholar]
- Pujades C, Kamaid A, Alsina B, Giraldez F. Bmp-signaling regulates the generation of hair-cells. Dev Biol. 2006;292:55–67. doi: 10.1016/j.ydbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska AK, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Developmental Biology. 2007 doi: 10.1016/j.ydbio.2007/03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, Chen P. Expression of islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol. 2004;477:412–21. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on sonic hedgehog. Genes Dev. 2002;16:2365–78. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of shh. Genes Dev. 2005;19:1612–23. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of bardet-biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–40. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: Primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220(Suppl):1–44. [PubMed] [Google Scholar]
- Saburi S, Mcneill H. Organising cells into tissues: New roles for cell adhesion molecules in planar cell polarity. Curr Opin Cell Biol. 2005;17:482–8. doi: 10.1016/j.ceb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–97. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. Pdgfralphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–6. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein diversin recruits casein kinase iepsilon to the beta-catenin degradation complex and acts in both canonical wnt and wnt/jnk signaling. Genes Dev. 2002;16:2073–84. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher AE. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol Suppl. 1971;285:1–77. [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing fgf signaling. Dev Cell. 2005;8:553–64. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Simon MA. Planar cell polarity in the Drosophila eye is directed by graded four-jointed and dachsous expression. Development. 2004;131:6175–84. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type ii, functions as a molecular switch between wnt signaling pathways. Nat Genet. 2005;37:537–43. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky NB. The cochlea. Springer; New York: 1996. [Google Scholar]
- Slepecky NB, Henderson CG, Saha S. Post-translational modifications of tubulin suggest that dynamic microtubules are present in sensory cells and stable microtubules are present in supporting cells of the mammalian cochlea. Hear Res. 1995;91:136–47. doi: 10.1016/0378-5955(95)00184-0. [DOI] [PubMed] [Google Scholar]
- Slepecky NB, Ulfendahl M. Actin-binding and microtubule-associated proteins in the organ of Corti. Hear Res. 1992;57:201–15. doi: 10.1016/0378-5955(92)90152-d. [DOI] [PubMed] [Google Scholar]
- Smith JC, Conlon FL, Saka Y, Tada M. Xwnt11 and the regulation of gastrulation in Xenopus. Philos Trans R Soc Lond B Biol Sci. 2000;355:923–30. doi: 10.1098/rstb.2000.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM, August BK. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol. 1995;24:633–53. doi: 10.1007/BF01179815. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–67. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kwak SJ, Fritz A. Genetic interactions underlying otic placode induction and formation. Dev Dyn. 2004;230:419–33. doi: 10.1002/dvdy.20067. [DOI] [PubMed] [Google Scholar]
- Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci USA. 1994;91:206–9. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Mundy J, Hofstra K, Strutt D. Cleavage and secretion is not required for four-jointed function in Drosophila patterning. Development. 2004;131:881–90. doi: 10.1242/dev.00996. [DOI] [PubMed] [Google Scholar]
- Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase iepsilon in Drosophila. Curr Biol. 2006;16:1329–36. doi: 10.1016/j.cub.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. Bioessays. 2005;27:1218–27. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus brachyury: Regulation of gastrulation movements via dishevelled, but not through the canonical wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Townes PL, Holtfreter J. Direct movements and selective adhesion of embryonic amphibian cells. J Exp Zool. 1955;128:53–120. doi: 10.1002/jez.a.114. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002;13:217–24. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Turner CM, Adler PN. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech Dev. 1998;70:181–92. doi: 10.1016/s0925-4773(97)00194-9. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical wnt/fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–5. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Von Bekesy G. Travelling waves as frequency analysers in the cochlea. Nature. 1970;225:1207–9. doi: 10.1038/2251207a0. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: The molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–5. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian pcp pathway to regulate convergent extension during neurulation. Development. 2006a;133:1767–78. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate pcp pathway. Nat Genet. 2005;37:980–5. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of frizzled3 and frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006b;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P, Keller R. Cell rearrangement during gastrulation of Xenopus: Direct observation of cultured explants. Development. 1991;112:289–300. doi: 10.1242/dev.112.1.289. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–8. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–90. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Yun UJ, Kim SY, Liu J, Adler PN, Bae E, Kim J, Park WJ. The inturned protein of Drosophila melanogaster is a cytoplasmic protein located at the cell periphery in wing cells. Dev Genet. 1999;25:297–305. doi: 10.1002/(SICI)1520-6408(1999)25:4<297::AID-DVG3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Zajac M, Jones GL, Glazier JA. Simulating convergent extension by way of anisotropic differential adhesion. J Theor Biol. 2003;222:247–59. doi: 10.1016/s0022-5193(03)00033-x. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–55. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yang H, Yamoah EN, Lundberg YW. Gene targeting reveals the role of oc90 as the essential organizer of the otoconial organic matrix. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


