Abstract
Primary cilia are essential components of diverse cellular processes. Many of the requirements can be linked to the apparent signaling function of primary cilia. Recent studies have also uncovered a role for primary cilia in planar cell polarity (PCP) signaling. PCP refers to the coordinated orientation of cells along an axis parallel to the plane of the cell sheet. In vertebrates, the inner ear sensory organs display distinctive forms of PCP. One of the inner ear PCP characteristics is the coordinated positioning of a primary cilium eccentrically in every sensory hair cell within each organ. The inner ear, therefore, provides an opportunity to explore the cellular role of primary cilia in PCP signaling. In this chapter, we will introduce the PCP of the inner ear sensory organs, describe the conserved mechanism underlying the establishment of the planar polarity axis in invertebrates and vertebrates, and highlight a unique requirement for primary cilia in PCP regulation in vertebrates. Additionally, we will discuss a potentially ubiquitous role for cilia in cellular polarization in general.
1. Introduction
Cellular polarity underlies many complex developmental processes in higher organisms, such as the generation of different cell lineages from a single fertilized egg, the establishment of the asymmetric body axes from the blastula, the directional migration of cells, the specification of the axon and dendrites in a neuron, the compartmentalization of apical–basal domains of epithelial cells, and the precise orientation of cells within a tissue. The acquisition of each of these polarities often involves common molecular and cellular components that act in a cell context-dependent manner (Lawrence et al., 2007).
Primary cilia are microtubule-based organelles assembled from the centriole-derived basal body and extend from the surfaces of cells (Davis et al., 2006). The assembly of primary cilia and transport of proteins along the length of the ciliary axonemes depends upon intraflagellar transport (IFT) (Davis et al., 2006; Scholey and Anderson, 2006; Taulman et al., 2001). Consistent with their antennae-like morphology, cilia can apparently receive remote information and transduce the signal to the cell body largely through IFT proteins. The signaling function of cilia has been linked to many cellular processes, including mechanotransduction, cellular proliferation and differentiation, cell migration, and signaling transduction for Hedgehog (Hh) and platelet-derived growth factor receptor (PDGFR) (Christensen and Ott, 2007; Christensen et al., 2007; Corbit et al., 2005; Davenport et al., 2007; Davis et al., 2006; Haycraft et al., 2005, 2007; Liu et al., 2005; Michaud and Yoder, 2006; Ou et al., 2007; Schneider et al., 2005; Scholey and Anderson, 2006; Singla and Reiter, 2006; Yoder et al., 2002). Recent studies have also revealed links between cilia and planar cell polarity (PCP) signaling in the mouse inner ear and in the zebrafish (Jones et al., 2008; Park et al., 2006, 2008; Ross et al., 2005; Wallingford, 2006).
In the inner ear sensory organs, each sensory hair cell consists of a hair bundle that is made up of specialized F-actin-filled microvilli, known as stereocilia, and a single primary cilium, known as the kinocilium. On the apical surface of each hair cell, stereocilia are arranged with graded heights into a staircase that is distinctively shaped for each inner ear sensory organ. The kinocilium sits snugly near the tallest stereocilia (Fig. 8.1). The position of the kinocilium and the organization of the stereociliary bundle mark an intrinsic polarity and the orientation of each hair cell. All of the hair cells in each inner ear sensory organ are coordinately oriented, conferring a precise polarity within the plane of the sensory epithelium (Fig. 8.1) (Lewis and Davies, 2002). The regulation of PCP therefore requires directional information, the establishment of the planar polarity axis that is relayed to all of the cells across the entire tissue, and transformation of polarity signals to the formation of the polarized structure of hair bundles. Genetic studies identified a set of conserved genes encoding the so-called core PCP proteins that are asymmetrically partitioned to define the planar polarization axis and to coordinate the orientation of hair cells across the sensory epithelium (Fig. 8.3) (Curtin et al., 2003; Lu et al., 2004; Montcouquiol et al., 2003, 2006; Wang et al., 2005, 2006a,b). However, the molecular and cellular apparatus that receives directional information is obscure (Dabdoub et al., 2003; Qian et al., 2007), and the precise mechanism that determines the intrinsic polarity of hair cells has not been determined.
Figure 8.1.
Planar cell polarity of inner ear sensory epithelia. (A) Mouse inner ear isolated at E18.5. The white tracing outlines the fluid-filled labyrinth that connects the vestibule and the cochlea of the inner ear. A Math1-GFP transgene (green) is expressed in hair cells and highlights the sensory epithelia of the six sensory organs: the organ of Corti of the cochlea (CO); the maculae of the utricle (UT) and the saccule (SA); and the three perpendicular cristae: the posterior crista (PC), anterior crista (AC), and lateral crista (LC). (B)–(G) The planar cell polarity of vestibular (B)–(D) and cochlear (E)–(G) sensory organs isolated from a mouse embryo at E18.5 viewed by immunohistochemistry and confocal microscopy (B), (C), (E), (F), and the intrinsic polarity of vestibular (D) and cochlear (G) hair cells illustrated by schematic diagrams. The hair cells are polarized across the sensory epithelium along the medial-to-lateral axis (M, medial; L, lateral). (B) The utricle viewed at a level just beneath the cell cortex. Actin (green) is enriched at the cell membranes. Spectrin (red) accumulates in the cuticular plates of hair cells, is excluded from the pericentriolar region surrounding the basal body and serves as a convenient marker of hair cell polarity. The dotted line (purple) demarcates the line of polarity reversal, where the polarities of the hair cells are reversed in the utricle. (E) The surface of the organ of Corti. Actin (green) accentuates the microvilli that make up the stereociliary bundles. Tubulin (red) marks both the cilia associated with stereociliary bundles atop the sensory hair cells and the cilia of intervening nonsensory supporting cells. The black arrowhead marks the separation of the inner hair cells from the outer hair cells, which are uniformly oriented toward the abneural, or lateral, edge of the sensory epithelium. (C) and (F) Confocal projections of vestibular (C) and cochlear (F) hair cell bundles at high magnification. The kinocilia (C, red; F, yellow) are assembled from the eldest of two centrioles (C, not shown; F, red) that make up the basal bodies, and are closely apposed to the hair cell bundles (C, F, green). Kinocilia and stereocilia of the vestibule (C) are considerably longer than those of the cochlea (F). (D) and (G) Diagrams of a typical vestibular (D) and cochlear (G) hair cell bundle showing the array of stereocilia rows increasing in height and the eccentrically placed kinocilium which protrudes from behind the tallest row of stereocilia. Note the round morphology of the vestibular hair cell bundle in comparison to the “V”-shaped morphology of the cochlear hair cell bundle.
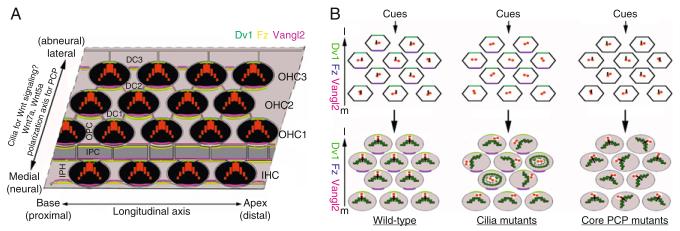
Figure 8.3.
Models of PCP regulation in the ear. (A) Schematic drawing of the organ of Corti viewed from its surface. Hair cells (black) are arranged into four parallel rows and are separated from each other by nonsensory supporting cells (gray). Hair cells are synchronously aligned across the sensory epithelium, displaying planar cell polarity. The apical and asymmetric distribution of conserved PCP proteins, Dvl (green), Fz (yellow), and Vangl2 (magenta) along the planar polarity axis is required to direct planar cell polarity of the hair cells in the cochlea. Wnt7a and Wnt5a have been implicated in regulating planar cell polarity of hair cells. The role of primary cilia in planar cell polarity has recently been confirmed, and cilia may act as receivers for Wnts or an unknown polarizing signal for PCP regulation (OHC, outer hair cell; IPC, inner pillar cell; IPHC, inner phalangeal cell; DC, Deiters cell). (B) Model of the role of cilia in the morphological polarization of hair cells in the organ of Corti. An unknown polarization signal directs the asymmetric membrane sorting of core PCP proteins Dvl (green), Fz (blue), and Vangl2 (magenta) in wild-type animals and ciliary mutants. Cell–cell interactions reinforce the distribution of PCP proteins along the planar polarity axis. In contrast, the PCP proteins fail to organize properly in core PCP mutants. Consequently, in wild-type cells, the kinocilia (black lines) and hair cell bundles (green) are polarized and all the hair cells are uniformly aligned along the medial-to-lateral, or planar polarization, axis. Like wild-type animals, PCP mutants exhibit normally polarized kinocilia and hair cell bundles, but the bundles are randomly oriented. Ciliary mutants lack kinocilia and exhibit both randomly polarized hair cell bundles and bundles that have lost their intrinsic polarity. Therefore, the distribution of core PCP proteins is necessary but not sufficient to direct polarization of the kinocilia and the stereociliary bundle. Basal bodies, through the positioning and alignment of the centrioles (red), may direct the organization of the cytoskeletal network to build the polarized structure of the stereociliary bundles. It is likely that the interactions between core PCP proteins and the basal bodies, together with their associated cytoskeletal components, cooperate to direct the coordination of stereociliary bundles across the sensory epithelium.
During development, the positioning of the primary cilium of the hair cell, the kinocilium, leads and predicts the polarity of each stereociliary bundle, supporting the hypothesis that kinocilia direct the polarization of hair cells (Cotanche and Corwin, 1991; Denman-Johnson and Forge, 1999; Tilney et al., 1992). This review summarizes recent results from inner ear studies that revealed a surprising but likely ubiquitous role for primary cilia in cellular polarity regulation, through the positioning of the basal body to direct the polarity of cells (Absalon et al., 2007; Benzing and Walz, 2006; Boisvieux-Ulrich and Sandoz, 1991; Boisvieux-Ulrich et al., 1985; Jones et al., 2008; Mitchell et al., 2007).
2. Planar Cell Polarity and Ciliogenesis of the Inner Ear Sensory Organs
2.1. Planar cell polarity of the mammalian inner ear sensory organs
The mammalian inner ear is comprised of two distinct regions: the cochlea, which regulates auditory function, and the vestibule, which perceives motion and balance (Fig. 8.1). There are six sensory organs within the inner ear that are largely responsible for regulating these specialized functions (Fig. 8.1). The auditory sensory organ, called the organ of Corti, runs along the length of the spiraling cochlea (Fig. 8.1). The two macular sensory organs, the saccule and the utricle, reside in the center of the inner ear and detect gravity and linear acceleration (Fig. 8.1). Each of three cristae lies at the base of three perpendicular semicircular canals of the vestibule to detect rotational motion and contribute to the maintenance of balance (Fig. 8.1).
The auditory and vestibular sensory organs contain polarized epithelial cells of two general types: the mechanosensory hair cells and the nonsensory supporting cells. The hair cells have elongated cell bodies whose lateral membranes are tightly connected to the surrounding supporting cells by adherens and tight junctions. Together, these cells of the sensory epithelia make up a cellular mosaic, such that hair cells are separated from adjacent hair cells by supporting cells. In the organ of Corti, the mechanosensory hair cells are arranged into four parallel rows, one row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs) (Fig. 8.1). The mechanosensory hair cells also alternate with supporting cells to form a characteristically shaped patch in each of the vestibular sensory epithelia (Fig. 8.1).
The mammalian cochlea and vestibule can process sound and positional signals, respectively, with remarkable resolution and sensitivity. This ability depends largely on an extraordinary mechanotransduction apparatus present atop the mechanosensory hair cells. At the apical surfaces of the mechanosensory hair cells are a bundle of modified actin-rich microvilli called stereocilia, often referred to as stereociliary bundles (Fig. 8.1). Each bundle is made up of tightly packed rows of stereocilia that are connected by extracellular linkages and increase in height to form a staircase-like structure (Fig. 8.1). Behind and central to the tallest row of stereocilia sits a single true cilium, or kinocilium, which is linked to the stereociliary bundle via extracellular kinocilial links (Fig. 8.1). The stereocilia are anchored beneath the cell cortex to the cuticular plate, a meshwork of crosslinked actin filaments and associated cytoskeletal proteins. The basal body at the base of the kinocilium is closely apposed to the cuticular plate and connects the filaments to apical microtubules that radiate along the periphery of the cell cortex and along the length of the lateral cell membranes.
The eccentric position of the kinocilium and the organization of the stereociliary bundle mark the intrinsic polarity and the orientation of the hair cell. The orientations of all of the stereociliary bundles are coordinately aligned across the plane of the epithelium of each sensory organ, displaying a distinct cellular synchrony called planar cell polarity (Jones and Chen, 2007; Kelly and Chen, 2007; Lewis and Davies, 2002). In the cochlea, the stereociliary bundle has a “V” shape, and the vertices of the “V”-shaped stereociliary bundles invariantly point toward the abneural, or lateral, edge of the epithelium (Fig. 8.1). The vestibular hair cell bundles of the cristae are uniformly oriented toward one side of the epithelia. In the macular sensory organs of the vestibule, the stereociliary bundles are also oriented along the mediolateral axis of the epithelia (Fig. 8.1). However, the cells on either side of a line of polarity reversal, which lies lateral to a physiologically and morphologically distinct striolar region, have opposite orientations (Fig. 8.1) (Deans et al., 2007; Denman-Johnson and Forge, 1999; Kelly and Chen, 2007).
PCP of the inner ear sensory organs is continuous across each sensory epithelium and contributed by both hair cells and supporting cells. The supporting cells of the inner ear sensory organs that interdigitate between hair cells are also polarized. In the cochlea, the supporting cells are characterized by long phalangeal processes, which are oriented coordinately across the sensory epithelium (reviewed by Jones and Chen, 2007). In the vestibule, asymmetric subcellular localization of proteins reveals the intrinsic polarity and coordinated orientation of supporting cells (Deans et al., 2007; Wang et al., 2006b).
2.2. Kinocilia and development of the stereociliary bundle
The hair cell bundles are intrinsically polarized, bilaterally symmetrical structures (Figs. 8.1 and 8.2). In the organ of Corti, the kinocilium sitting precisely at the vertex of each “V”-shaped stereociliary bundle. Moreover, the bundle is oriented so that the kinocilium and tallest stereocilia row face the cell periphery; while the shortest stereocilia row faces the cell interior. The vestibular hair cell bundles exhibit a similar staircase structure, and the placement of the kinocilium in relation to the bundle is preserved. However, the vestibular hair bundle morphology is circular in shape, and the lengths of stereocilia and kinocilia are considerably longer (Fig. 8.1).
Figure 8.2.
Kinocilium and basal body polarization. Confocal images of cochlear whole mounts (A)–(D) and a schematic diagram (E) focusing on a single cochlear hair cell bundle at successive stages in mouse ear development. Extension of the cochlear spiral unidirectionally from the base (b) to the distal tip, or apex (a), occurs concurrently with hair cell (HC) differentiation in the organ of Corti. In an immature hair cell, the kinocilium (red) projects centrally from the apical cell membrane. The kinocilium proceeds to polarize the growing microvilli (green) toward the lateral edge of the cell surface. By the end of embryogenesis, the polarized V-shaped morphology of the stereociliary bundle becomes apparent. The orientation of the hair cell bundle is refined postnatally until the kinocilium associated with the vertex of the stereociliary bundle points distally along the medial-to-lateral axis. The medial (neural) and the lateral (abneural) sides of the sensory epithelium face the interior and periphery of the cochlea, respectively. The kinocilium regenerates postnatally prior to the onset of hearing in mammalian cochleae. (F) Diagram illustrating the positioning of the ciliary basal body during cochlear hair cell bundle development. The pair of centrioles that make up the basal body undergoes similar polarization movements during hair cell maturation. The movements can be described as a four-step process: (1) the pair of centrioles is positioned centrally beneath the cell cortex in newly differentiating hair cells; (2) the pair of centrioles migrates to the lateral cell surface; (3) one of the centrioles leads the other toward the distal edge of the sensory epithelium; (4) the pair of centrioles go through a presumed period of refinement as they align themselves along the medial-to-lateral axis, such that the daughter centriole is ultimately positioned distally (laterally), while the elder maternal centriole, from which the kinocilium is assembled, is positioned proximally (medially) and is closely apposed to the stereociliary bundle (not shown) (M, medial; L, lateral).
The polarized structures of the stereociliary bundles have functional significance. Deflection of the bundle either toward or away from the tallest stereocilia row (parallel to the planar polarity axis) leads to opposite mechanosensory responses. Deflections of the bundle perpendicular to the planar polarity axis have no effect, supporting the directional sensitivity of the hair cell bundle (Hudspeth, 1989, 2000). Moreover, mutations in genes that disrupt the morphology of hair cell bundles cause impaired hearing and vestibular dysfunction (El-Amraoui and Petit, 2005). It is conceivable that the coordinated orientation of hair cell bundles across the epithelium of each inner ear sensory organ is essential for their physiological functions and contributes to the sensitivity of the inner ear.
Temporal observations of stereociliary bundles by scanning electron microscopy during mammalian ear development have revealed that the development of the stereociliary bundle is carefully orchestrated during hair cell maturation (Fig. 8.2) (Frolenkov et al., 2004). In the mouse, the microvilli that will give rise to the stereocilia are apparent on the apical surfaces of newly differentiated hair cells by embryonic day 13.5 (E13.5) in the vestibule and E15.5 in the cochlea. Initially, the microvilli are of a uniform height at the cell cortex. Near the center of the immature bundle sits a single kinocilium, easily distinguishable at this stage because it is longer than the microvilli surrounding it (Fig. 8.2) (Frolenkov et al., 2004; Kikuchi and Hilding, 1965; Kikuchi et al., 1988). By E15.5 in the vestibule and E16.5 in the cochlea, the kinocilium migrates to the periphery of the bundle toward the lateral side of the cell surface membrane. Over the next couple of days, the microvilli nearest the kinocilium begin to elongate, followed by microvilli progressively a distance away from the kinocilium. Stereocilia elongation ceases in a defined order leading to the formation of the staircase pattern. Some of the stereocilia filaments extend basally into the apical cytoplasm and become anchored to the developing cuticular plate. By E18.5, the kinocilia and tightly associated stereociliary bundles are relatively close to their final orientations. Furthermore, the characteristic “V”-shape morphology of the hair bundles is well defined in most of the cochlea. Postnatally, the orientation of the hair cell bundle goes through a period of refinement as the cuticular plate locks the bundle in place (Dabdoub and Kelley, 2005). Prior to the onset of hearing at postnatal day 10 (P10), the kinocilia in the cochlea are resorbed (Leibovici et al., 2005; Sobkowicz et al., 1995). In contrast, the kinocilia in the vestibular sensory organs persist throughout the life of the animal. Together, these observations provided three lines of evidence suggest that the kinocilium may play an initial role in stereociliary bundle development and orientation. The first evidence is the observation that the polarization of the kinocilium precedes the polarization of the stereociliary bundle during hair cell bundle development (Fig. 8.2) (Frolenkov et al., 2004). Secondly, the direction of kinocilium migration during bundle development is not random, but is biased toward the final orientation of the bundle (Dabdoub et al., 2003). Thirdly, the kinocilia in the mammalian cochlea degenerate prior to the onset of hearing (Leibovici et al., 2005; Sobkowicz et al., 1995).
Hair cell regeneration studies in which mechanically induced injury was applied to cochlear explants obtained from newborn mice also provided some insightful observations pertaining to a potential role of the kinocilium in stereociliary bundle formation during regeneration of hair cells (Sobkowicz et al., 1995). The authors observed that regenerating kinocilia reformed prior to hair cell recovery, even when the injury was induced at 10 days in vitro, at which time the kinocilia are normally absent in culture, as in the intact animal. Furthermore, the regenerating kinocilium often assumed a central position in the recovering hair cells and proceeded to polarize the newly formed hair cell bundle through the typical developmental sequence, supporting a morphogenetic role for the kinocilium in hair cell development.
Notably, not only are mechanosensory hair cells ciliated, but also the nonsensory supporting cells (Fig. 8.1). The supporting cells in the cochlea can be subdivided into three subclasses based upon their distinct cellular morphology: inner pillar cells, inner phalangeal cells, and Dieters cells (Fig. 8.3). Their cilia also appear to be developmentally transient. In the vestibule, the cilia of supporting cells persist alongside their hair cell counterparts, but are much shorter than the kinocilia of hair cells. However, ciliogenesis in the supporting cells has not been examined, nor is their relationship to the establishment of PCP known.
2.3. Physiological function of primary cilia in the inner ear sensory organs
A mechanosensory role has been proposed for the vestibular kinocilia, which remain tightly associated to the stereociliary bundle (Eatock et al., 1998). Kinocilia of vestibular hair cells may participate partially or wholly in mechanotransduction. It has been proposed that the kinocilia may relay fluid-induced movements of the overlying otoconial membrane to the deflection of stereociliary bundles in the macular sensory organs. Because of the extracellular kinocilial links that connect the kinocilium to the stereociliary bundle, kinocilial deflection affects deflection of the stereociliary bundle, making it difficult to experimentally distinguish a separate mechanosensory role for the kinocilia. Both the kinocilia and stereocilia of vestibular hair cells of mice are considerably longer than those in the cochlea and vary in length amongst the different vestibular sensory organs. The variations in the length of both vestibular kinocilia and stereocilia presumably contribute to the transduction sensitivity of these cells in their respective organs. A mechanosensory role for the kinocilium in the nonmammalian cochlea where it persists has not been ruled out.
3. Planar Cell Polarity Regulation for the Development of the Inner Ear
3.1. Planar cell polarity pathway in orienting the sensory cells of the inner ear
PCP is displayed in many forms. In Drosophila, the best known examples are the uniformly oriented fly wing bristles and the regular arrangement of ommatidia in the adult fly eye (Adler, 1992; Gubb and Garcia-Bellido, 1982). In these tissues that exhibit PCP, there is a defined polarity both in terms of the structure of each unit, such as the pointed bristle that sits at the vertex of each wing cell or the precise arrangement of photoreceptors within each ommatidium, and in terms of the arrangement of the different units relative to each other, such as the uniform orientation of all the wing bristles that point distally and the regular orientation of ommatidia in the fly compound eye. Therefore, the establishment of PCP requires a signaling network consisting of multiple regulatory components: (1) global guidance cues that provide directional information, (2) cellular factors to interpret the directional signals and establish the PCP axis, and (3) tissue-specific effectors necessary to build the intrinsic polarity of each individual cell (Klein and Mlodzik, 2005; Lawrence et al., 2007; Ma et al., 2003; Strutt and Strutt, 2005; Tree et al., 2002).
In Drosophila, it appears that a network of adhesive molecules may propagate and amplify an initial molecular gradient to provide the directional information (Lawrence et al., 2007; Strutt and Strutt, 2005); a set of transmembrane and membrane-associated proteins, known as core PCP proteins, form complexes at opposing sides of the cell to define the PCP axis (Axelrod, 2001; Klein and Mlodzik, 2005; Ma et al., 2003); and several cytoskeleton-binding proteins act downstream of core PCP genes to affect cellular morphological polarization (Adler et al., 1990, 1994; Collier et al., 2005; Park et al., 1994, 1996; Turner and Adler, 1998; Yun et al., 1999).
In vertebrates, PCP was first described for a process known as convergent extension (CE) (Wallingford et al., 2000). CE occurs during gastrulation and neurulation (Keller and Tibbetts, 1989; Keller et al., 1985) and involves the mediolateral polarization of cells that drives the convergence of cells toward the midline and the extension of the tissue along the perpendicular anterior–posterior axis (Brodland, 2006; Brodland and Veldhuis, 2006; Zajac et al., 2003; Zallen and Wieschaus, 2004). Studies in Xenopus and zebrafish identified a vertebrate PCP pathway that consists of a similar cassette of conserved core genes, including Celsr1,Frizzled (Fz), Disheveled (Dvl), and Ltap/Vangl2, required for CE during gastrulation and neurulation (Ciruna et al., 2006; Djiane et al., 2000; Goto and Keller, 2002; Jessen et al., 2002; Jiang et al., 2005; Mlodzik, 2006; Moeller et al., 2006; Park and Moon, 2002; Schwarz-Romond et al., 2002; Sokol, 1996; Veeman et al., 2003; Wallingford et al., 2000). Two members of the Rho family of GTPases that are capable of modifying cytoskeletal components, RhoA and Rac, are implicated in vertebrate PCP signaling downstream of Dvl during CE in Xenopus (Habas and He, 2006; Habas et al., 2001, 2003; Marlow et al., 2002; Phillips et al., 2005). The vertebrate homologs of fly PCP effector proteins, Inturned and Fuzzy, regulate PCP signaling downstream of core PCP genes and participate in CE regulation in Xenopus and zebrafish (Park et al., 2006).
Protein localization and loss-of-function studies established essential roles for conserved core PCP genes in coordinated cellular orientation in inner ear sensory organs. During the establishment of epithelial PCP in the cochlea, Fz3 and Fz6, Disheveled 2 (Dvl2), and Vangl2, display polarized subcellular localization and are distributed asymmetrically across the cochlear epithelium along the mediolateral axis of the cochlear epithelium (Fig. 8.3) (Montcouquiol et al., 2006; Wang et al., 2005, 2006b). Mutations in core PCP genes lead to the misorientation of stereociliary bundles (Curtin et al., 2003; Lu et al., 2004; Montcouquiol et al., 2006; Wang et al., 2005, 2006b). These data together suggest that the vertebrate PCP pathway may utilize a similar molecular mechanism in establishing the PCP axis in the inner ear sensory epithelia.
Notably, mutations in mouse core PCP genes do not affect the formation of the polarized stereociliary bundles and their association with the kinocilium but only the coordinated orientation of all the stereociliary bundles in the sensory epithelia (Fig. 8.3) (Curtin et al., 2003; Lu et al., 2004; Montcouquiol et al., 2003; Wang et al., 2005, 2006b), indicating that the cell–cell coordination but not the intrinsic polarity of hair cells is affected. This idea is consistent with the observed subcellular asymmetry of some of the core PCP proteins in reference to the polarity of hair cells. One of the mammalian homologs of Drosophila core PCP protein Prickle (Pk), Pk2, is preferentially localized to the medial side of cells on both sides of the line of polarity reversal in the utricle (Deans et al., 2007), regardless of the reverse polarity of hair cells across the line of polarity reversal (Fig. 8.1). Similar to Pk2, the subcellular localization of Fz6 on the lateral side of cells does not appear to differ in hair cells with opposite polarity on either sides of the line of polarity reversal (Deans et al., 2007). There are three implications from the observations of core PCP protein localization and the phenotypes of core PCP mutants: (1) the polarity of core PCP protein localization does not determine the direction of the cellular polarity, (2) a cellular mechanism independent of core PCP proteins is responsible for the formation of the intrinsic polarity of hair cells, and (3) the cellular mechanism for cell-intrinsic polarity must interact with the core PCP proteins for coordinated alignment of cells across the tissue in a cell context-dependent manner.
The molecules that translate the polarity of core PCP proteins to the orientation of hair cells in a cell context-dependent manner have not been identified. The role of any of the genes known to act downstream of core PCP genes in regulating vertebrate CE and Drosophila PCP has yet to be examined in the inner ear. As discussed in Section 4 below, recent studies of primary cilia have identified a crucial requirement for ciliary and basal body genes in PCP regulation in vertebrates and expose them as attractive candidates underlying cellular morphological polarization (Jones et al., 2008; Ross et al., 2005). In particular, the asymmetric localization of core PCP proteins in the auditory sensory epithelium is maintained in ciliary mutants, although the hair cells are misoriented or completely lose their intrinsic polarity in ciliary mutants (Fig. 8.3) (Jones et al., 2008). These observations suggest that cilia are essential components in regulating cell-intrinsic polarity and must interact with core PCP proteins for coordinated orientation of all the hair cells (Fig. 8.3). However, the direct molecular link between cilia or the basal body to core PCP proteins is missing.
In addition, the extracellular cues that provide directional information to core PCP proteins to establish the PCP axis remain unresolved. In contrast to an apparently dispensable role for Wnts in PCP regulation in Drosophila, ligands for Fz receptors, Wnt5 and Wnt11, were shown to play essential roles in convergent extension in Xenopus and zebrafish (Heisenberg et al., 2000; Kilian et al., 2003; Ohkawara et al., 2003; Smith et al., 2000; Tada and Smith, 2000). Several Wnts are expressed in the cochlea (Dabdoub et al., 2003; Qian et al., 2007). Inactivation of one of them, Wnt5a, results in characteristic PCP phenotypes in the cochlea (Qian et al., 2007). However, it is yet to be determined whether Wnts play an instructive role in generating directional information or merely a permissive role for vertebrate PCP signaling. Furthermore, recent identification of the primary cilium as a signaling apparatus that represses β-catenin-dependent canonical Wnt signaling supports the possibility that primary cilia may promote Wnts for the noncanonical PCP signaling pathway (Corbit et al., 2008; Gerdes et al., 2007; He, 2008; Kishimoto et al., 2008).
3.2. Planar cell polarity pathway in patterning and growth of the inner ear sensory organs
During ear development, the precursor cells that give rise to mechanosensory hair cells and nonsensory supporting cells of the mouse auditory sensory organ form a zone of nonproliferating cells in the cochlea (Chen and Segil, 1999; Chen et al., 2002; Ruben, 1967). Accompanying the acquisition of the highly polarized structure of hair cells and supporting cells during terminal cellular differentiation and the establishment of PCP in the cochlea, the length of the organ of Corti is more than doubled from the shorter and thicker nonproliferating sensory primordium. Such an elongation of the sensory organ in the absence of additional cell divisions suggests cellular rearrangements characteristic of convergent extension during terminal differentiation of the organ of Corti (Chen and Segil, 1999; Chen et al., 2002).
The morphological defects observed in PCP mutants support the concurrence of CE and establishment of PCP in the cochlea. Associated with misorientation of stereociliary bundles, the cochlear duct is shortened and widened in Wnt5a and several core PCP mutants (Montcouquiol et al., 2003; Qian et al., 2007; Wang et al., 2005, 2006b). Instead of the invariable four rows of hair cells, there are additional rows of hair cells, especially toward the distal end of the cochlear duct (Montcouquiol et al., 2003; Qian et al., 2007; Wang et al., 2005, 2006b). These growth defects of the cochlear duct and inappropriate patterning of its sensory organ are consistent with a defect in CE.
As discussed below, apparent cochlear CE defects were also observed in ciliary mutants (Jones et al., 2008). The association of CE and cellular orientation defects in the cochlea of both PCP mutants and ciliary mutants suggests shared molecular components by both processes. However, it is not clear what the molecular roles are for Wnt5a and core PCP genes in CE of the organ of Corti, nor is it known whether the same cellular polarization process simultaneously drives CE and causes the coordinated polarization of hair cells and supporting cells in the cochlea.
4. Cilia and PCP Regulation
4.1. Evidence linking cilia and PCP signaling in vertebrates
Much of the evidence that links cilia and PCP signaling has come from studies of polarized cellular movements during convergent extension of the vertebrate body axis during Xenopus and zebrafish gastrulation. Mutations in conserved PCP genes or ciliary genes independently lead to typical CE phenotypes, such as shorter and wider body axes. The relative ease of Xenopus and zebrafish embryo manipulations, morpholino-based knock-down approaches and the accessibility of ciliated tissues have made them leading vertebrate models in exploring the relationship between cilia and PCP signaling.
The coupling of cilia and PCP has predictably included the examination of whether ciliary protein functions involve PCP signaling, also known as noncanonical Wnt signaling activity. A recurring theme appears to be that a common function of ciliary genes is to constrain canonical β-caten-independent Wnt signaling activity while simultaneously promoting the noncanonical Wnt signaling activity (Corbit et al., 2008; Gerdes et al., 2007; He, 2008; Kishimoto et al., 2008). Analyses of various ciliary mutants have led to the proposal that ciliary proteins may participate as switches between canonical and noncanonical Wnt signaling pathways to interpret the Wnt signals into appropriate cellular responses during development.
For example, knockdown of the ciliary gene Inversin in gastrulating zebrafish and Xenopus embryos causes defects in convergent extension, which are enhanced in the trilobite mutants, mutants of core PCP gene, Vangl2 (Ross et al., 2005; Simons et al., 2005). Inversin morphants also manifest renal defects that can be rescued by Diversin, a putative vertebrate homolog of the fly PCP protein, Diego. Inversin may indirectly promote PCP processes by negatively regulating canonical Wnt signaling through the targeting of Dvl for proteasome-mediated degradation (Simons et al., 2005).
Many of the genes implicated in Bardet-Biedl syndrome (BBS) have been linked to cilia and/or basal body assembly and function. bbs1,bbs4, and bbs6 morphants exhibit gastrulation defects consistent with defects in convergent extension (Gerdes et al., 2007; Ross et al., 2005). Like inversin, convergent extension defects are enhanced when bbs4 morpholinos are injected into the trilobite mutant background (Ross et al., 2005). Moreover, bbs1,bbs4, and bbs6 interact with noncanonical Wnt11 and Wnt5b in convergent extension movements (Gerdes et al., 2007). Suppression of bbs gene expression leads to the stabilization of β-catenin, suggesting that they normally inhibit canonical Wnt signaling. The stabilization of β-catenin and Dvl is phenocopied by suppressing another ciliary gene, kif3a, the anterograde kinesin required for ciliogenesis (Corbit et al., 2008; Gerdes et al., 2007).
The zebrafish gene duboraya is required for ciliogenesis, left–right body patterning and convergent extension (Oishi et al., 2006). Morphants of the PCP gene, frizzled-2, could phenocopy the laterality and ciliogenesis defects of duboraya morphants; and frizzled-2 genetically interacts with duboraya to regulate left–right patterning. Duboraya function is regulated by phosphorylation, which is mediated by frizzled-2-dependent noncanonical Wnt signaling activity (Oishi et al., 2006). Whether Duboraya can inhibit canonical Wnt activity has not been reported.
Zebrafish gene seahorse is enriched in ciliated tissues, and seahorse morphants phenocopy ciliary mutants in the manifestations of kidney cysts and left–right asymmetry defects (Kishimoto et al., 2008). In addition, seahorse morphants show dorsalization phenotypes and ectopic Wnt target transcription, indicative of ectopic β-catenin-dependent signaling activity. The ectopic activity can be suppressed by seahorse mRNA injection, suggesting that seahorse normally inhibits canonical Wnt signaling. Unlike seahorse morphants alone, knockdown of seahorse simultaneously with either the ciliary gene inversin or the PCP gene prickle induces gastrulation phenotypes consistent with PCP defects (Kishimoto et al., 2008). In addition, Seahorse can be found in a complex with Dvl, which is shared by both Wnt pathways, consistent with the idea that Seahorse may play a role in modulating signaling down either pathway.
4.2. Cilia in regulating PCP of the vertebrate inner ear
Many parallels between PCP regulation in flies and mice have been reported. However, there is no evidence that the mechanosensory cilia in flies play any role in PCP establishment or maintenance. It is possible that the role of primary cilia in PCP regulation may have evolved in vertebrates. Yet, the exact nature of the relationship of primary cilia to PCP signaling has been obscure. The first genetic evidence that the relationship between cilia and PCP signaling was conserved in mammalian ear development was revealed through studies of bbs mouse mutants. Not only were there abnormal stereociliary bundle morphologies in the cochleae of bbs-deficient mice, but the kinocilia lost their close associations with the stereociliary bundles. Furthermore, mice that were simultaneously mutated for bbs genes and the PCP gene, Vangl2, showed an enhancement of the cochlear phenotype. However, since kinocilia remained in the bbs mutants, it was difficult to assess the specific role of kinocilia in planar polarity of the hair cells.
To test the role of cilia in PCP regulation in the ear, Cre-mediated recombination was used to inactivate a floxed allele of IFT protein 88 (IFT88) in the mouse ear at approximately E10.5, thereby disrupting kinocilia formation in the inner ear primordium several days prior to hair cell differentiation in the organ of Corti (Jones et al., 2008). The authors observed misoriented stereociliary bundles in the cochleae of IFT88 mutant mice and cochlear extension defects consistent with aberrant convergent extension. In addition, there were both a significant increase in the number of misrotated bundles in mice mutated for IFT88 and Vangl2 and an enhancement of the cochlear extension defect in the double mutants. Similar observations were made with mice lacking Kif3a, the anterograde kinesin required for ciliogenesis. Together, these findings provided compelling evidence for a direct role for cilia in planar polarization processes and a genetic relationship between the ciliogenic and PCP pathways.
A direct role for the kinocilium in the development of the intrinsic polarity and orientation of the stereociliary bundle was also revealed in the study of IFT88 mutant cochleae. A proportion of the hair cell bundles in IFT88 mutants exhibited abnormal morphologies, most of which were circular in shape. The circular bundles were perhaps reminiscent of immature bundles of the cochlea that had arrested in their development, indicating that the cilium is essential for the polarized structure of the hair cell bundle. The circular bundle morphology exhibited low penetrance in the mutant animals. One explanation could be delayed or variable Cre-mediated recombination at the floxed sites and therefore remnant IFT88 activity. Alternatively, the orientations of the stereociliary bundles were established prior to the time in which the Cre recombinase was active, and aberrant bundle morphologies were consequently indicative of the failure to maintain the intrinsic polarity of the hair bundle.
Normally, the centrioles, integral components of the basal body, are aligned along the planar polarity axis in the cochleae of wild-type mice (Jones et al., 2008). In addition, the position of the maternal centriole, the template for kinocilium assembly, is positioned more proximally to the daughter centriole and thus is closely associated to the stereociliary bundle (Fig. 8.1). Ablation of kinocilia in the IFT88 mutant cochleae left the basal bodies intact. Interestingly, there was a strong correlation between the positions of the basal bodies and the orientation of the stereociliary bundles; that is, the bundles were consistently misoriented in the same direction as the basal bodies in the ciliary mutants. This observation suggested that the basal body, through the placement and alignment of the centrioles, is likely the cell-intrinsic regulator of stereociliary bundle polarity and orientation. The basal body is an ideal candidate for the intrinsic regulator of bundle polarity because of its own intrinsic chirality, both in terms of the asymmetric structure of the centrioles and the molecular and structural distinction between the two centrioles. Consistent with this suggestion is the observation that some circularly shaped hair bundles in IFT88 mutants had centrally positioned basal bodies. The positioning of basal bodies underlying planar polarization processes in multiciliated cells has also been reported, and may be a general intracellular mechanism that is coordinated to polarize cells across a tissue (Marshall and Kintner, 2008; Mitchell et al., 2007). Interestingly, core PCP protein Dvl is required for the apical positioning and planar polarization of basal bodies in multiciliated mucociliary epithelia of the Xenopus epidermis (Park et al., 2008). Furthermore, the loss of either Dvl or PCP effector protein Inturned leads to a failure to apically localize basal bodies and consequently affects apical cytoskeletal organization (Park et al., 2008).
4.3. Crosstalk between cilia and conserved PCP proteins
How the ciliogenic and PCP signaling pathways interact to coordinate the alignment of the stereociliary bundles is not known. Surprisingly, the study of IFT88 mutants (Jones et al., 2008) revealed that core PCP proteins, Fz3 and Vangl2, were polarized normally along the planar polarity axis in IFT88 mutants, suggesting that IFT88, or the cilium, is not required for the PCP proteins to receive and interpret cues to affect their polarized distribution in these IFT88 mutant animals. However, the study did not exclude the possibility that kinocilia may be involved in transducing directional cues to core PCP proteins in the mouse prior to the time that IFT88 was inactivated, nor the possibility that a polarization signal was provided non-autonomously from cells in adjacent tissues where the Cre recombinase may not have been active. In addition, the structures of kinocilia and their tight associations with stereociliary bundles in all PCP mutants examined thus far appear normal. However, it is possible that while their structures appear normal, the sensory functions of kinocilia may be perturbed in PCP mutants and have not yet been determined.
The identities of the molecular players involved in the crosstalk between the ciliogenic and PCP pathways are not known. The basal body may serve as an organizing center for ciliogenic and PCP signaling proteins. The accumulation of PCP proteins Dvl and Vangl2 at the basal body in Xenopus in vivo (Park et al., 2006, 2008; Simons et al., 2005) and cultured cells in vitro was observed (Ross et al., 2005). However, neither the localization of PCP proteins to the basal bodies in cochlear or vestibular cells nor the direct association between these proteins in cochlear or vestibular extracts has been determined. The normal polarization of PCP proteins in IFT88 mutants suggests that the IFT88 and/or cilia may function downstream of the PCP signaling pathway. It is possible therefore that the PCP complexes recruit ciliary proteins transiently. For example, the PCP proteins may recruit PCP effector proteins to the basal body to cause the changes in the cytoskeleton necessary to orient the cells across the entire epithelium. Studies conducted in other vertebrate models support this idea. For example, knockdown of PCP effector genes, Inturned and Fuzzy, in Xenopus affects both convergent extension movements and cilia morphogenesis (Park et al., 2006). The ciliogenic defects in Inturned and Fuzzy morphants are attributed to a failure to orient ciliary microtubules during ciliogenesis (Park et al., 2006). Similar cytoskeletal disorganization was reported in duboraya morphants (Oishi et al., 2006). Furthermore, knockdown of Dvl leads to decreased apical actin accumulation, reduced ciliogenesis in multiciliated cells, and misorientation of cilia rootlets, supporting an upstream role for core PCP genes in cytoskeletal alterations for the formation and polarity of cilia (Park et al., 2008).
5. Cilia and Determination of the Intrinsic Cellular Polarity of Inner Ear Cells
5.1. The molecular machinery for building the intrinsically polarized hair bundles
The intrinsic polarity of hair cells is marked by the position of the kinocilium and the polarity of the stereociliary bundle. The molecular components of the machinery that builds the stereociliary bundle in hair cells have been partially identified from genetic studies (El-Amraoui and Petit, 2005). Usher syndrome (USH) is the most frequent cause of hereditary deafblindness in humans. USH can also be associated with vestibular dysfunction, reduced odor identification, and sperm motility (Kremer et al., 2006). There are three clinical subtypes, USH1–3, according to the severity and onset of the hearing impairment, vestibular dysfunction, and retinitis pigmentosa. Each USH subtype is genetically heterogeneous, and at least 12 chromosomal loci are assigned to USH. Five USH1 genes have been cloned, including those encoding an unconventional myosin VIIa for USH1B (el-Amraoui et al., 1996; Weil et al., 1996), a PDZ domain-containing protein harmonin for USH1C (Verpy et al., 2000), cadherin 23 for USH1D (Bolz et al., 2001; Bork et al., 2001; Di Palma et al., 2001), a cadherin-related protein protocadherin 15 for USH1F (Ahmed et al., 2001; Alagramam et al., 2001b), and a putative scaffold protein with three ankyrin repeats and a sterile alpha motif (SAM) domain and a C-terminal PDZ domain, Sans, for USH1G (Weil et al., 2003). Also, three USH2 and one USH3 genes were identified that encode transmembrane protein usherin (USH2A) (Adato et al., 2000; Dreyer et al., 2000; Rivolta et al., 2000; Weston et al., 2000), a G protein-coupled seven-transmembrane receptor VLGR1b (USH2C) (Weston et al., 2004), a PDZ domain- and proline-rich domain-containing scaffold protein whirlin (USH2D) (Adato et al., 2005a), and a four-transmembrane domain protein clarin-1 (USH3A) (Adato et al., 2002). Mouse models for USH1 and USH2D linked USH1 and USH2D genes to the development of hair cell stereociliary bundles.
Mice that carry mutated loci for USH1B gene myosin VIIa (shaker-1) (El-Amraoui et al., 1996; Weil et al., 1997), USH1C gene harmonin (deaf circler) (Verpy et al., 2000), USH1D gene cadherin 23 (waltzer) (Di Palma et al., 2001), USH1F gene protocadherin 15 (Ames waltzer) (Alagramam et al., 2001a), USH1G gene Sans (Jackson shaker) (Kikkawa et al., 2003), or USH2D gene whirlin (whirler) (Mburu et al., 2003) all display stereocilia defects as revealed by scanning electron microscope (SEM) analysis. Stereocilia contain up to 2000 parallel actin filaments that are anchored in an actin meshwork of the cuticular plate at the apical cortex of hair cells and are arranged in three to four rows of increasing height toward the kinocilium. The stereocilia are interconnected by ankle links, lateral and tip links, and to the nearby kinocilium by fibrous extracellular links. The USH proteins are often present in multiple isoforms in hair cells, and show dynamic and overlapping expression patterns to various regions of the stereociliary bundle or the kinocilium during development of hair bundles. Protein–protein interaction assays in vitro further suggest that USH proteins function in a protein network and affect actin dynamics. In particular, scaffold proteins harmonin and whirlin can integrate other USH proteins and alter their association with transmembrane adhesion molecules and actin cytoskeleton (Adato et al., 2005b; El-Amraoui and Petit, 2005). Consistent with their localization and protein–protein interactions, mutations in any of the USH1 and USH2D genes leads to common stereocilia defects, including disorganization of stereocilia and misshapening of the hair bundle, loss of the graded heights of stereocilia, shortened or enlarged and fused stereocilia, and displacement of the kinocilium (Di Palma et al., 2001; Holme and Steel, 2002; Johnson et al., 2003; Kazmierczak et al., 2007; Lagziel et al., 2005; Lefevre et al., 2008; Libby et al., 2003; Mogensen et al., 2007; Mustapha et al., 2007). Although it has not been determined how exactly the formation of the polarized structure of hair bundles is regulated by USH genes, the genetic, biochemical, and cell biological studies together identified USH protein complexes as components of a protein network that builds the precise structure of the hair bundles.
5.2. Cilia determine intrinsic cellular polarity
Despite the shared defect of the loss of the uniform orientation of stereociliary bundles in the cochlea of both core PCP and ciliary mutants, the mechanisms underlying their defect differ. In known core PCP mutants, the polarized structure of the hair bundle, including graded heights of stereocilia, the staircase arrangement of stereocilia, the shape of the stereociliary bundle, and the placement of the kinocilium and the basal body near the tallest stereocilia, are not affected (Curtin et al., 2003; Lu et al., 2004; Montcouquiol et al., 2003; Wang et al., 2005, 2006b). In contrast, circular stereociliary bundles are present (Jones et al., 2008), and remaining kinocilia are often shorter and displaced from their association with the tallest stereocilia in ciliary mutants (Jones et al., 2008; Ross et al., 2005). This difference between core PCP mutants and ciliary mutants indicates that known core PCP genes are not essential for the intrinsic polarity of hair cells while ciliary genes are required.
The examination of the cellular alignment in core PCP mutants and ciliary mutants revealed an additional fundamental difference in the two types of mutants. In looptail mutants that carry a loss-of-function allele for core PCP gene Vangl2, the polarized subcellular distributions of Dvl2, Fz, and Pk2 are affected (Deans et al., 2007; Wang et al., 2005, 2006b), suggesting a failure in normal cell–cell alignment that is communicated through core PCP proteins. In contrast, core PCP proteins, Vangl2 and Fz3, are normally partitioned along the mediolateral axis of the cochlea and show polarized subcellular localization across the cochlear sensory epithelium in ciliary mutants (Fig. 8.3) (Jones et al., 2008), indicating normal cell–cell communication by core PCP proteins but a failure in responding to the polarization signals from the asymmetrically partitioned core PCP proteins in ciliary mutants.
As morphological studies of the inner ear and studies of basal body and ciliary genes in inner ear development implicated a role for cilia in positioning of the basal body and as well as the basal body as a determinant of the intrinsic polarity of hair cells, three critical issues emerged. (1) What is the signal for cilia to position the basal body? (2) How does the basal body regulate the intrinsic polarity of the cell? (3) How do cilia and/or the basal body fulfill an essential role in transducing the polarity signals from core PCP proteins?
It is possible that cilia respond to an extracellular directional cue, either the same cue as that received by the core PCP proteins or an entirely independent ocue, for the positioning of cilia and the basal body. Another possibility is that ciliogenesis is first required to acquire or maintain a particular configuration of the pair of centrioles or the basal body to respond directly to a directional cue transduced from asymmetrically partitioned core PCP proteins. Alternatively, although the results obtained by conditionally knocking out ciliary genes revealed normal partitioning of core PCP proteins, there may be remnant cilia activity in these conditional ciliary mutants that is sufficient for an early role in transducing a directional signal for planar polarization, including the positioning of cilia and basal body, and the polarization of core PCP proteins. No experimental data is available to support or exclude any of the above possibilities for the positioning of the basal body.
The advancement of USH gene studies, on the other hand, has implicated a common genetic pathway for ciliary genes and USH genes in the determination of intrinsic cellular polarity. In particular, a recent study by Lefevre et al. (2008) systematically examined hair bundle polarity in mutant mice for five USH1 genetic forms. Together with several earlier studies, a core cochlear phenotype that shares many similarities with ciliary mutants was revealed. The stereocilia phenotype in USH1 mutant mice includes the replacement of the V-shaped stereociliary bundle by clusters of stereocilia, the presence of circularly shaped stereocilia, the loss of graded heights of stereocilia, and the reduction of lateral links between stereocilia. In particular, in mice mutated for cadherin 23, protocadherin 15, or Sans, some stereociliary bundles lack polarity and become clustered or circular, and the kinocilium is frequently dissociated from the stereociliary bundle or cluster. The localization of cadherin 23, protocadherin 15, and Sans further implicates a potential interaction between the basal body and USH protein complexes. During development, the full-length protocadherin 15 protein is enriched at the base of the tallest row of stereocilia that are adjacent to the base of the kinocilium (Haywood-Watson et al., 2006; Lefevre et al., 2008). Cadherin 23 has been localized along the kinocilia in the vestibule, and has also been detected in the kinocilia in the distal tip of the cochlear duct (Lagziel et al., 2005). Prior to the morphological polarization of hair cells and the formation of distinct stereocilia at E14 in mice, cadherin 23 is initially detected in the centrosome in cells of the developing organ of Corti. Sans is enriched in the immediate vicinity of the basal body of the auditory kinocilia in neonatal mice (El-Amraoui and Petit, 2005), and shows a pattern that is very similar to pericentriolar γ-tubulin staining (P. Chen, unpublished data).
As USH syndrome affects hearing and vision, studies of USH proteins in vertebrate photoreceptor cells provide new insight regarding the molecular role for USH proteins, and support a direct interaction between the basal body and USH protein complexes. USH proteins are highly expressed in photoreceptors, another type of cells with specialized cilia, and are collectively localized at the periciliary region (Maerker et al., 2008). In particular, USH1G Sans, which is concentrated at the proximity of the basal body of the outer hair cell kinocilium, is also localized to the basal body and the connecting cilium in mouse and Xenopus photoreceptor cells. In NIH3T3 cells, Sans is detected in the centrosome. In addition, Sans is associated with microtubules and its subcellular distribution can be altered by destabilizing microtubules in NIH3T3 and photoreceptor cells. Together, these observations implicated a direct interaction between USH proteins and the basal body and the microtubules.
It is conceivable that to act as a cellular polarity determinant, the basal body must interact with the machinery that builds the asymmetric structure of stereociliary bundles in hair cells. Sans, Cadherin 23, or protocadherin 15 directly interact with the basal body, which may serve as a compass to orient cytoplasmic microtubules and coordinate the activity of USH proteins. Sans, cadherin 23, and protocadherin 15 may then integrate the positional information provided by the basal body to the other USH proteins to direct the formation of the polarized structure of stereociliary bundles in hair cells.
6. Conclusions and Perspectives
The arrangement of the primary cilium of the inner ear sensory hair cell, the kinocilium, and the stereociliary bundle atop each inner ear sensory hair cell marks an intrinsic polarity of the hair cell, and the coordinated orientation of all of the hair cells in each of the six inner ear sensory organs confers a polarity within the plane of the epithelium of each inner ear sensory organ. The establishment of PCP in the inner ear sensory organs requires a set of conserved core PCP proteins that response to directional cues and undergo asymmetrical partitioning to define the PCP axis for downstream morphological polarization. The involvement of a ciliary and basal body genes in mediating Wnt signaling (Corbit et al., 2008; He, 2008; Kishimoto et al., 2008; Simons et al., 2005; Singla and Reiter, 2006), the functional complementation of the loss of ciliary function by PCP proteins (Simons et al., 2005), and the role of several Wnt transducers, including Fz and Dvl, in establishing the planar polarization axis have implicated a role for cilia in PCP regulation. Since cilia can function as specialized subcellular compartments for localizing and concentrating membrane bound signal receptors and complexes in order to relay signals from the extracellular environment to the cell interior, it was thought that the primary cilium of the inner ear sensory hair cell, the kinocilium, may function as a signaling apparatus to transduce polarization signals. Indeed, two recent studies on BBS genes and IFT genes in inner ear development have also established an essential role for ciliary and basal body genes in vertebrate PCP signaling.
Surprisingly, the study of ciliary mutant cochleae showed that the proper positioning of ciliary basal bodies and the formation of the polarized cellular structure of inner ear sensory hair cells, the stereociliary bundles, are disrupted in ciliary mutants, whereas core PCP proteins are partitioned normally along the polarization axis (Jones et al., 2008). These data uncover a distinct requirement for ciliary genes downstream of core PCP proteins in basal body positioning and a role for the basal body in the determination of the intrinsic polarity, possibly the intrinsic polarized organization of cytoskeleton, of hair cells. Although the putative polarization signal remains elusive, the positioning of basal bodies may be a general mechanism underlying other cellular polarization processes involving cilia (Absalon et al., 2007; Boisvieux-Ulrich et al., 1985; Feldman et al., 2007; Mitchell et al., 2007).
However, despite of the abundance of genes known to be involved in stereocilia development, the dearth of information on proteins that act downstream of core PCP proteins to affect cytoskeletal polarization leaves open the question how do cilia through the positioning of the basal body determine the intrinsic polarity of the hair cell? Two of the major challenges that remain are (1) to identify the molecular link that connects the basal body and the protein network that builds the polarized structure of the stereociliary bundle and (2) to illustrate how the basal body communicates with polarized core PCP proteins to coordinately orient all of the hair cells within each inner ear sensory epithelium. In addition, the existing data do not rule out the possibility that the kinocilium may also have a role in transducing a signal for core PCP proteins. While the conditional inactivation of IFT genes in inner ear sensory epithelia have effectively bypassed the requirement of these genes in early embryonic processes and targeted studies on cellular polarization processes in ear development, conditional knockout approaches may not sufficiently inactivate cilia function at all times required to affect polarization processes during ear development. More effective control of temporal IFT gene inactivation in the inner ear will help resolve this issue. Until then, it is tempting to speculate that the kinocilium acts as a signaling apparatus and transduces polarization signals to core PCP proteins, which in turn regulate the positioning and polarization of the basal body that collaborate with an intracellular protein network to build and orient the intrinsically polarized structure of stereociliary bundles in hair cells.
REFERENCES
- Absalon S, et al. Basal body positioning is controlled by flagellum formation in Trypanosoma brucei. PLoS ONE. 2007;2:e437. doi: 10.1371/journal.pone.0000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adato A, et al. Three novel mutations and twelve polymorphisms identified in the USH2A gene in Israeli USH2 families. Hum. Mutat. 2000;15:388. doi: 10.1002/(SICI)1098-1004(200004)15:4<388::AID-HUMU27>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Adato A, et al. USH3A transcripts encode clarin-1, a four-transmembrane-domain protein with a possible role in sensory synapses. Eur. J. Hum. Genet. 2002;10:339–350. doi: 10.1038/sj.ejhg.5200831. [DOI] [PubMed] [Google Scholar]
- Adato A, et al. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum. Mol. Genet. 2005a;14:3921–3932. doi: 10.1093/hmg/ddi416. [DOI] [PubMed] [Google Scholar]
- Adato A, et al. Interactions in the network of Usher syndrome type 1 proteins. Hum. Mol. Genet. 2005b;14:347–356. doi: 10.1093/hmg/ddi031. [DOI] [PubMed] [Google Scholar]
- Adler PN. The genetic control of tissue polarity in Drosophila. BioEssays. 1992;14:735–741. doi: 10.1002/bies.950141103. [DOI] [PubMed] [Google Scholar]
- Adler PN, et al. Molecular structure of frizzled, a Drosophila tissue polarity gene. Genetics. 1990;126:401–416. doi: 10.1093/genetics/126.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler PN, et al. The Drosophila tissue polarity gene inturned functions prior to wing hair morphogenesis in the regulation of hair polarity and number. Genetics. 1994;137:829–836. doi: 10.1093/genetics/137.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, et al. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am. J. Hum. Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam KN, et al. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat. Genet. 2001a;27:99–102. doi: 10.1038/83837. [DOI] [PubMed] [Google Scholar]
- Alagramam KN, et al. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum. Mol. Genet. 2001b;10:1709–1718. doi: 10.1093/hmg/10.16.1709. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing T, Walz G. Cilium-generated signaling: A cellular GPS? Curr. Opin. Nephrol. Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- Boisvieux-Ulrich E, Sandoz D. Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol. Cell. 1991;72:3–14. doi: 10.1016/0248-4900(91)90072-u. [DOI] [PubMed] [Google Scholar]
- Boisvieux-Ulrich E, et al. The orientation of ciliary basal bodies in quail oviduct is related to the ciliary beating cycle commencement. Biol. Cell. 1985;55:147–150. doi: 10.1111/j.1768-322x.1985.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Bolz H, et al. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat. Genet. 2001;27:108–112. doi: 10.1038/83667. [DOI] [PubMed] [Google Scholar]
- Bork JM, et al. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am. J. Hum. Genet. 2001;68:26–37. doi: 10.1086/316954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodland GW. Do lamellipodia have the mechanical capacity to drive convergent extension? Int. J. Dev. Biol. 2006;50:151–155. doi: 10.1387/ijdb.052040gb. [DOI] [PubMed] [Google Scholar]
- Brodland GW, Veldhuis JH. Lamellipodium-driven tissue reshaping: A parametric study. Comput. Methods Biomech. Biomed. Eng. 2006;9:17–23. doi: 10.1080/10255840600554703. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, et al. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Christensen ST, Ott CM. Cell signaling. A ciliary signaling switch. Science. 2007;317:330–331. doi: 10.1126/science.1146180. [DOI] [PubMed] [Google Scholar]
- Christensen ST, et al. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- Ciruna B, et al. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S, et al. The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics. 2005;169:2035–2045. doi: 10.1534/genetics.104.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, et al. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, et al. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Corwin JT. Stereociliary bundles reorient during hair cell development and regeneration in the chick cochlea. Hear. Res. 1991;52:379–402. doi: 10.1016/0378-5955(91)90027-7. [DOI] [PubMed] [Google Scholar]
- Curtin JA, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Kelley MW. Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J. Neurobiol. 2005;64:446–457. doi: 10.1002/neu.20171. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, et al. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- Davenport JR, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EE, et al. The emerging complexity of the vertebrate cilium: New functional roles for an ancient organelle. Dev. Cell. 2006;11:9–19. doi: 10.1016/j.devcel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Deans MR, et al. Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J. Neurosci. 2007;27:3139–3147. doi: 10.1523/JNEUROSCI.5151-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman-Johnson K, Forge A. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J. Neurocytol. 1999;28:821–835. doi: 10.1023/a:1007061819934. [DOI] [PubMed] [Google Scholar]
- Di Palma F, et al. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat. Genet. 2001;27:103–107. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- Djiane A, et al. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000;127:3091–3100. doi: 10.1242/dev.127.14.3091. [DOI] [PubMed] [Google Scholar]
- Dreyer B, et al. Identification of novel USH2A mutations: Implications for the structure of USH2A protein. Eur. J. Hum. Genet. 2000;8:500–506. doi: 10.1038/sj.ejhg.5200491. [DOI] [PubMed] [Google Scholar]
- El-Amraoui A, Petit C. Usher I syndrome: Unravelling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. J. Cell Sci. 2005;118:4593–4603. doi: 10.1242/jcs.02636. [DOI] [PubMed] [Google Scholar]
- el-Amraoui A, et al. Human Usher 1B/mouse shaker-1: The retinal phenotype discrepancy explained by the presence/absence of myosin VIIA in the photoreceptor cells. Hum. Mol. Genet. 1996;5:1171–1178. doi: 10.1093/hmg/5.8.1171. [DOI] [PubMed] [Google Scholar]
- Feldman JL, et al. The mother centriole plays an instructive role in defining cell geometry. PLoS Biol. 2007;5:e149. doi: 10.1371/journal.pbio.0050149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolenkov GI, et al. Genetic insights into the morphogenesis of inner ear hair cells. Nat. Rev. Genet. 2004;5:489–498. doi: 10.1038/nrg1377. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat. Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev. Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Habas R, He X. Activation of Rho and Rac by Wnt/frizzled signaling. Methods Enzymol. 2006;406:500–511. doi: 10.1016/S0076-6879(06)06038-1. [DOI] [PubMed] [Google Scholar]
- Habas R, et al. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Habas R, et al. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, et al. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Haywood-Watson RJ, II, et al. Ames Waltzer deaf mice have reduced electroretinogram amplitudes and complex alternative splicing of Pcdh15 transcripts. Invest. Ophthalmol. Vis. Sci. 2006;47:3074–3084. doi: 10.1167/iovs.06-0108. [DOI] [PubMed] [Google Scholar]
- He X. Cilia put a brake on Wnt signalling. Nat. Cell Biol. 2008;10:11–13. doi: 10.1038/ncb0108-11. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Holme RH, Steel KP. Stereocilia defects in waltzer (Cdh23), shaker1 (Myo7a) and double waltzer/shaker1 mutant mice. Hear. Res. 2002;169:13–23. doi: 10.1016/s0378-5955(02)00334-9. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. Hearing. McGraw-Hill, New York; New York: 2000. [Google Scholar]
- Jessen JR, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, et al. Ascidian prickle regulates both mediolateral and anterior–posterior cell polarity of notochord cells. Curr. Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Johnson KR, et al. Mouse models of USH1C and DFNB18: Phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum. Mol. Genet. 2003;12:3075–3086. doi: 10.1093/hmg/ddg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Chen P. Planar cell polarity signaling in vertebrates. BioEssays. 2007;29:120–132. doi: 10.1002/bies.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- Kazmierczak P, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Keller R, Tibbetts P. Mediolateral cell intercalation in the dorsal, axial mesoderm of Xenopus laevis. Dev. Biol. 1989;131:539–549. doi: 10.1016/s0012-1606(89)80024-7. [DOI] [PubMed] [Google Scholar]
- Keller RE, et al. The function and mechanism of convergent extension during gastrulation of Xenopus laevis. J. Embryol. Exp. Morphol. 1985;89(Suppl.):185–209. [PubMed] [Google Scholar]
- Kelly M, Chen P. Shaping the mammalian auditory sensory organ by the planar cell polarity pathway. Int. J. Dev. Biol. 2007;51:535–547. doi: 10.1387/ijdb.072344mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y, et al. Mutations in a new scaffold protein Sans cause deafness in Jackson shaker mice. Hum. Mol. Genet. 2003;12:453–461. doi: 10.1093/hmg/ddg042. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Hilding D. The development of the organ of Corti in the mouse. Acta Otolaryngol. 1965;60:207–222. doi: 10.3109/00016486509127003. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, et al. Development of apical-surface structures of mouse otic placode. Acta Otolaryngol. 1988;106:200–207. doi: 10.3109/00016488809106426. [DOI] [PubMed] [Google Scholar]
- Kilian B, et al. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech. Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Kishimoto N, et al. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev. Cell. 2008;14:954–961. doi: 10.1016/j.devcel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: An emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Kremer H, et al. Usher syndrome: Molecular links of pathogenesis, proteins and pathways. Hum. Mol. Genet. 2006;15(Spec No. 2):R262–R270. doi: 10.1093/hmg/ddl205. [DOI] [PubMed] [Google Scholar]
- Lagziel A, et al. Spatiotemporal pattern and isoforms of cadherin 23 in wild type and waltzer mice during inner ear hair cell development. Dev. Biol. 2005;280:295–306. doi: 10.1016/j.ydbio.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, et al. Planar cell polarity: One or two pathways? Nat. Rev. Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre G, et al. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135:1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- Leibovici M, et al. Initial characterization of kinocilin, a protein of the hair cell kinocilium. Hear. Res. 2005;203:144–153. doi: 10.1016/j.heares.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lewis J, Davies A. Planar cell polarity in the inner ear: How do hair cells acquire their oriented structure? J. Neurobiol. 2002;53:190–201. doi: 10.1002/neu.10124. [DOI] [PubMed] [Google Scholar]
- Libby RT, et al. Cdh23 mutations in the mouse are associated with retinal dysfunction but not retinal degeneration. Exp. Eye Res. 2003;77:731–739. doi: 10.1016/j.exer.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Liu A, et al. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Lu X, et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Ma D, et al. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- Maerker T, et al. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum. Mol. Genet. 2008;17:71–86. doi: 10.1093/hmg/ddm285. [DOI] [PubMed] [Google Scholar]
- Marlow F, et al. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr. Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Marshall WF, Kintner C. Cilia orientation and the fluid mechanics of development. Curr. Opin. Cell Biol. 2008;20:48–52. doi: 10.1016/j.ceb.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mburu P, et al. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat. Genet. 2003;34:421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Res. 2006;66:6463–6467. doi: 10.1158/0008-5472.CAN-06-0462. [DOI] [PubMed] [Google Scholar]
- Mitchell B, et al. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. A GAP in convergent extension scores PAR. Dev. Cell. 2006;11:2–4. doi: 10.1016/j.devcel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Moeller H, et al. Diversin regulates heart formation and gastrulation movements in development. Proc. Natl Acad. Sci. USA. 2006;103:15900–15905. doi: 10.1073/pnas.0603808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen MM, et al. The deaf mouse mutant whirler suggests a role for whirlin in actin filament dynamics and stereocilia development. Cell Motil. Cytoskeleton. 2007;64:496–508. doi: 10.1002/cm.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapha M, et al. Whirler mutant hair cells have less severe pathology than shaker 2 or double mutants. J. Assoc. Res. Otolaryngol. 2007;8:329–337. doi: 10.1007/s10162-007-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara B, et al. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- Oishi I, et al. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat. Genet. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- Ou G, et al. Sensory ciliogenesis in Caenorhabditis elegans: Assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol. Biol. Cell. 2007;18:1554–1569. doi: 10.1091/mbc.E06-09-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- Park WJ, et al. The frizzled gene of Drosophila encodes a membrane protein with an odd number of transmembrane domains. Mech. Dev. 1994;45:127–137. doi: 10.1016/0925-4773(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Park WJ, et al. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development. 1996;122:961–969. doi: 10.1242/dev.122.3.961. [DOI] [PubMed] [Google Scholar]
- Park TJ, et al. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Park TJ, et al. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HM, et al. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ. Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- Qian D, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta C, et al. Missense mutation in the USH2A gene: Association with recessive retinitis pigmentosa without hearing loss. Am. J. Hum. Genet. 2000;66:1975–1978. doi: 10.1086/302926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220(Suppl.):1–44. [PubMed] [Google Scholar]
- Schneider L, et al. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. doi: 10.1016/j.cell.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, et al. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Smith JC, et al. Xwnt11 and the regulation of gastrulation in Xenopus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000;355:923–930. doi: 10.1098/rstb.2000.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, et al. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J. Neurocytol. 1995;24:633–653. doi: 10.1007/BF01179815. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. BioEssays. 2005;27:1218–1227. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: Regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Taulman PD, et al. Polaris, a protein involved in left–right axis patterning, localizes to basal bodies and cilia. Mol. Biol. Cell. 2001;12:589–599. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, et al. Actin filaments, stereocilia and hair cells of the bird cochlea. VI. How the number and arrangement of stereocilia are determined. Development. 1992;116:213–226. doi: 10.1242/dev.116.1.213. [DOI] [PubMed] [Google Scholar]
- Tree DR, et al. A three-tiered mechanism for regulation of planar cell polarity. Semin. Cell Dev. Biol. 2002;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Turner CM, Adler PN. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech. Dev. 1998;70:181–192. doi: 10.1016/s0925-4773(97)00194-9. [DOI] [PubMed] [Google Scholar]
- Veeman MT, et al. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Verpy E, et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat. Genet. 2000;26:51–55. doi: 10.1038/79171. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Hum. Mol. Genet. 2006;15(Spec No. 2):R227–R234. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, et al. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006a;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 2006b;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, et al. Human myosin VIIA responsible for the Usher 1B syndrome: A predicted membrane-associated motor protein expressed in developing sensory epithelia. Proc. Natl Acad. Sci. USA. 1996;93:3232–3237. doi: 10.1073/pnas.93.8.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, et al. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat. Genet. 1997;16:191–193. doi: 10.1038/ng0697-191. [DOI] [PubMed] [Google Scholar]
- Weil D, et al. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum. Mol. Genet. 2003;12:463–471. doi: 10.1093/hmg/ddg051. [DOI] [PubMed] [Google Scholar]
- Weston MD, et al. Genomic structure and identification of novel mutations in usherin, the gene responsible for Usher syndrome type IIa. Am. J. Hum. Genet. 2000;66:1199–1210. doi: 10.1086/302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MD, et al. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am. J. Hum. Genet. 2004;74:357–366. doi: 10.1086/381685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK, et al. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Yun UJ, et al. Theor inturned protein of Drosophila melanogaster is a cytoplasmic protein located at the cell periphery in wing cells. Dev. Genet. 1999;25:297–305. doi: 10.1002/(SICI)1520-6408(1999)25:4<297::AID-DVG3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Zajac M, et al. Simulating convergent extension by way of anisotropic differential adhesion. J. Theor. Biol. 2003;222:247–259. doi: 10.1016/s0022-5193(03)00033-x. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]