Abstract
Several basic helix-loop-helix (bHLH) genes have been shown to be essential for the generation of the auditory sensory hair cells or the spiral ganglion (SG) neurons that innervate the hair cells in the cochlea, as well as a variety of cell types in the other nervous systems. However, it remains elusive what cellular context-dependent mechanisms confer the inner ear–specific neuronal or sensory competency/identities. We explored the possibility that one of the mechanisms responsible for generating cellular diversity in the nervous system through cooperative action of bHLH and LIM-homeodomain (LIM-HD) transcriptional factors might also contribute to the inner ear–specific sensory and/or neuronal competency. Here, we show that Islet1 (Isl1), a LIM-HD protein, is expressed early in the otocyst in the region that gives rise to both the auditory sensory organ, the organ of Corti, and SG neurons. Subsequently, the expression of Isl1 is maintained in SG neurons but is transitory in the sensory lineage. At embryonic day 12 (E12) in mice, the expression of Isl1 marks distinctively the ventral portion of the nascent cochlear epithelium encompassing the primordial organ of Corti. At E13, Isl1 is maintained at relatively high levels in the sensory primordium while down-regulated in the other regions of the cochlear duct. As the sensory epithelium starts to differentiate, it is down-regulated in the entire cochlear epithelium. The expression of Isl1 in the developing inner ear reveals an early and likely a common step in the development of both sensory and neuronal lineages of the inner ear, and suggests its potential role in the inner ear-specific sensory and neuronal cell development.
Indexing terms: organ of Corti, spiral ganglion neurons, sensory hair cells, LIM-HD, bHLH, Math1
The inner ear is derived entirely from the otic placode, recognized initially as a thickening near the hindbrain at embryonic day (E) 8 in mice (Fig. 1A). Once it is established, the otic placode invaginates to form an enclosed otocyst by E10.5. As the otic vesicle closes, cochleovestibular neurons start to delaminate from the otic epithelium around E9.5 (see reviews by Fritzsch et al., 2002, and Romand and Varela-Nieto, 2003). From E10.5 to E11.5, neurogenesis in the enclosed otocyst can be observed near the ventral region (the neurogenic region) of the otic epithelium (Ma et al., 1998, 2000; Kim et al., 2001; Liu et al., 2000). By E12, the cochleovestibular ganglion (CVG) neurons have delaminated from the otic epithelium, and the cochlear duct has formed as an outpocketing from the ventral region of the otocyst and continues to grow until E18 (Morsli et al., 1998; see review by Fritzsch et al., 1997). During the growth of the cochlear duct, the cells in the primordial organ of Corti exit the cell cycle between E13.5 and E14.5 to establish a distinct zone of nonprolif-erating cells delimited by the expression of the cyclin-dependent kinase inhibitors p27Kip1 and p19Ink4d in the cochlea (Ruben, 1967; Chen and Segil, 1999; Chen et al., 2003). Subsequently, hair cells and supporting cells differentiate within the sensory primordium to form a precise mosaic of hair cells and supporting cells (Sher, 1971; Lanford et al., 1999; Chen et al., 2002; see review by Kelley and Bianchi, 2001).
Fig. 1.
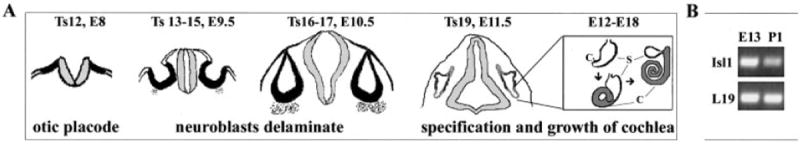
Detection of Islet1 (Isl1) transcripts in the developing inner ear. A: The distinct stages of the developing mouse inner ear are illustrated, including the initial recognition of the placode, neurogenesis, and cochlea specification and growth. B: Isl1 was detected at higher levels at embryonic day (E) 13.5 than at postnatal day (P)1, compared with comparable expression of a house-keeping gene L19 at both stages.
Morphological and expression studies show that the cochlear neurons or the spiral ganglion (SG) neurons and the auditory sensory organ they innervate are derived within the same region in the otocyst and that the same progenitors in the sensory primordium in the cochlea give rise to both hair cells and supporting cells (Lewis, 1996; see review by Kelley and Bianchi, 2001, and Fritzsch et al., 2002). Molecular mechanisms regulating the generation of the neuronal and sensory cells of the inner ear have also been reported (see reviews by Bryant et al., 2002, Gao, 2003; Riley, 2003). The neurogenic basic helix-loop-helix (bHLH) transcriptional factors, such as Neurogenin1, NeuroD, and Math1, are shown to play a vital role in the development of various nervous systems (Ma et al., 1996, 1999; Lee, 1997a,b; Morrow et al., 1999; Ben-Arie et al., 1997; Gowan et al., 2001; Bermingham et al., 2001; Lumpkin et al., 2003). In the inner ear, Neurogenin1 (Ma et al., 2000) and NeuroD (Liu et al., 2000, Kim et al., 2001) are required for the generation or delamination and survival of SG neurons, respectively. In the cochlear epithelium, Math1 was found to be necessary (Bermingham et al., 1999; Chen et al., 2002) and sufficient (Zheng and Gao, 2000; Kawamoto et al., 2003) for the generation of sensory hair cells, as well as for the survival of hair cell precursors (Chen et al., 2002). Nevertheless, it remains unknown whether the organ of Corti and the SG neurons are derived from the same common progenitors, and we have yet to identify the molecular mechanisms governing the generation of inner ear-specific cell types (Fekete and Wu, 2002).
To further dissect molecular pathways involved in the development of the SG neurons and the sensory cells in the cochlea, we profiled gene expression patterns of the cochlear epithelium at two different stages: E13.5 and postnatal day 1 (P1). We identified Islet1 (Isl1; Pfaff et al., 1996), a LIM-HD gene, as a candidate gene expressed early in the cochlear epithelium and differentially regulated during development. Members of LIM-HD transcriptional factors have been shown to regulate neuronal cell fates in both invertebrates and vertebrates (Bachy et al., 2001). It has been demonstrated that LIM-HD factors cooperate with neurogenic bHLH genes to synergistically regulate subtype-specific gene expression (Allan and Thor, 2003; Lee and Pfaff, 2003; Thaler et al., 2004). In the early developing chicken inner ear, it has also been shown that Isl1 and/or its close homolog Islet2 (Isl2) were expressed in the neurogenic patch within the otic epithelium that gives rise to delaminating cochleovestibular ganglion neurons (Adam et al., 1998). The detection of Isl1 at E13.5 in the cochlear epithelium after the SG neurons have delaminated from the epithelium in our array and reverse transcriptase-polymerase chain reaction (RT-PCR) assays suggested that it may also present in one or more lineages in the cochlear epithelium in addition to the neuronal lineage in the inner ear. The potential coupling of bHLH and LIM-HD factors in the development of the mammalian inner ear neuronal and sensory lineages prompts us to study its expression in the mouse inner ear at critical developmental stages. We found that Isl1 is expressed early in the otocyst in the region that gives rise to both the cochlea and SG neurons. Subsequently, the expression of Isl1 is maintained in the SG neurons while expression in the cochlea epithelium is transient. At E12 in mice, the presence of Isl1 protein is seen in the entire ventral portion of the nascent cochlear duct encompassing the primordial organ of Corti. At E13, as cells in the developing organ of Corti exit cell cycle, the levels of Isl1 protein are relatively higher in the postmitotic sensory primordium demarcated by the expression of p27Kip1. By E14, Isl1 is down-regulated in the organ of Corti as hair cells start to differentiate. Therefore, although the expression of Isl1 does not confirm the common origin for the sensory and neuronal lineages in the inner ear, it reveals an early and common step in the development of both lineages. Furthermore, its expression is consistent with a potential role in specifying inner ear neuronal and sensory competency and identity in coordination with neurogenic genes, such as bHLH genes.
MATERIALS AND METHODS
Mouse strains and animal care
The generation of the mouse strain expressing green fluorescent protein (GFP) under the control of Math1 enhancers (Helms et al., 2000) was as described (Chen et al., 2002, Lumpkin et al., 2003). The original transgenic mouse was generated in the B6D2F1 genetic background and was crossed with CD-1 mice for more than 10 generations for the current studies. The day the vaginal plug was found was identified as E0.5. In addition, for embryos younger than E9.5, the staging system of Theiler (1989) was used to more accurately determine the developmental stages. Animal care and use was in accordance with NIH guidelines and was approved by the Animal Care and Use Committee of House Ear Institute, University of Rochester, and Emory University.
Preparation of tissue sections for GFP and immunofluorescence
Embryos from timed pregnant females were harvested. The entire embryo (E8.5, E9.5, E10.5, and E11.5), partially dissected inner ears (E12.5), or dissected inner ears (E13.5 and older) were fixed in fresh 4% paraformaldehyde in phosphate buffered saline (PBS) for 1 hour at 4°C, soaked in 20% sucrose in PBS on ice for up to 6 hours, embedded in OCT, and cryosectioned at 12–16 μm. The sections were then stored at −20°C. GFP fluorescence can be preserved under this condition for more than 4 months. For embryo sections, both transverse (dorsoventral) and vertical (anteroposterior) sections were prepared. For dissected ears, only vertical sections were prepared.
For immunofluorescence, cryosections were air-dried before being rehydrated with PBS. The rehydrated sections were incubated with 10% normal sera in 0.1% Triton X-100 for half an hour, and then with primary antibodies in PBS containing 4% normal sera at 4°C overnight. After three washes with PBS, the sections were incubated with secondary antibodies in PBS for 1 hour at room temperature. The sections were washed in PBS twice, mounted for examination with either Zeiss Axio100 upright microscope or Olympus IX71 inverted microscope fitted with epifluorescence, or Zeiss LSM510 confocal microscope (Carl Zeiss). The primary antibodies used for this study: mouse monoclonal Tuj1 against neuronal-specific β-tubulin (BabCO, 1:100), mouse monoclonal antibody against p27Kip1 (Neomarkers, clone DCS-72.F6, 1:100), mouse monoclonal antibody against Isl1 (DSHB, partially purified Ig, 1:100 or 1:33 for detection of low levels of expression), rabbit antibody against myosin VIIa (1:1,000, courtesy of A. El-Amraoui and C. Petit, Pasteur Institut), rabbit antibody against GFP (Molecular Probe, A-6466, serum, 1:1,000), goat polyclonal antibody against NeuroD (Santa Cruz, N-19, 1:100), and rabbit polyclonal Pax2 (Covance, 1:100). Secondary antibodies were obtained from Jackson ImmunoLab. The secondary antibodies used are conjugated with Cy2, rhodamine red-X, and Cy5.
In situ hybridization
In situ hybridization was performed as described previously (Yang et al., 2003). Briefly, inner ear sections were fixed in 4% paraformaldehyde, treated with proteinase K, acetylated in 0.1 M triethanolamine-HCl containing 0.25% acetic anhydride, and post-fixed in 4% paraformaldehyde. The sections were then dehydrated in ethanol and air-dried. Hybridization solution was applied directly to dried sections for prehybridization, followed by application of probe-containing hybridization solution for over night incubation. The wash and visualization were performed according to standard procedures.
RT-PCR
Pure epithelial tissues of cochlea were prepared as described (Chen et al., 2003). Total RNA was prepared by using RNeasy Mini kit (Qiagen, Valencia, CA) and subjected to reverse transcription with random primers and oligodT using both OminiScriptase (Qiagen) and MMLV (Promega, Madison, WI) reverse transcriptases. Resulted cDNA populations were analyzed by PCR to detect expression of housekeeping gene L19 and Isl1. Primers used are as follows: L19, 5′-GGTCTGGTTGGATCCCAATG-3′ and 5′-CCCGGGAATGGACAGTCA-3′; Isl1, 5′-CAGCAAGAACGACTTCGTGA-3′ and 5′-GGACTGGCTACCATGCTGTT-3′.
RESULTS AND DISCUSSION
Identification of Isl1 expression in the cochlear epithelium
To identify genes involved in the development of the organ of Corti in the cochlea, we isolated pure cochlear epithelia from E13.5 and P1 mice (Chen et al., 2002) and profiled gene expression using Affymetrix Mouse 430A GeneChip. Among the genes that are differentially expressed as determined by gene array assays and verified by semiquantitative RT-PCR, Isl1 was detected at high levels at stages before hair cell differentiation (E13.5) and down-regulated following hair cells differentiation (P1; Fig. 1B, Isl1 bands), while a housekeeping gene L19 is expressed at the same levels at both stages (Fig. 1B, L19 bands). Isl1 expression in the chicken inner ear neuronal lineage (Adam et al., 1998) suggested that its mammalian homolog may be expressed in the neuronal lineage in mammalian inner ear. However, our cochlear epithelial sample at the early stage was prepared from E13.5 embryos after the SG neurons have delaminated from the cochlear epithelium (Fritzsch et al., 2002; Romand and Varela-Nieto, 2003). This result indicates that Isl1 is also expressed in a lineage(s) in the cochlear epithelium. Based on the importance of Isl1 and other Lim-HD transcriptional factors in neurogenesis, we decided to study Isl1 expression in the mouse inner ear at each critical developmental stage.
Expression of Isl1 marks the neuronal and sensory lineages in the otocyst
We compared the expression of Isl1 with molecular markers for vestibular–cochlear ganglion (VCG) neurons and for the sensory lineage in the epithelium, to determine whether it is expressed in the mammalian inner ear neuronal lineage as was observed in chicken (Adam et al., 1998) and to identify the lineage(s) that expresses Isl1 in the cochlear epithelium. For VCG neurons, we used an antibody (Tuj1) against the neuronal-specific β-tubulin (Lee et al., 1990) that recognizes delaminated VCG neurons and antibody against NeuroD (Santa Cruz) that is expressed transiently in VCG precursors and in delaminated VCG neuroblasts. For the sensory lineage in the cochlear epithelium, we used an antibody against p27Kip1 for the postmitotic primordial organ of Corti that will give rise to both hair cells and supporting cells before terminal differentiation (Chen and Segil, 1999; Lowenheim et al., 1999), and an antibody against myosin VIIa for differentiated hair cells (Hasson et al., 1995; Sahly et al., 1997). In addition, we used a mouse strain in which the expression of the GFP is under the control of Math1 enhancers (Math1/GFP; Helms et al., 2000) to mark hair cells from the precursor to early postnatal stages (Chen et al., 2002; Lumpkin et al., 2003). An antibody against Pax2, which marks the specific regions of the early inner ear anlage (Lawoko-Kerali et al., 2002), was also used as a reference marker.
For generating embryos at specific stages of the development, we did time-mating of mice, and the day of plug identification was determined to be E0.5. Because slight differences of the age of embryos yield considerable morphological and molecular changes among young embryos, we also used the staging system of Theiler (1989) to assess the exact stage of individual embryos up to E9.5. At E8.5 or Theiler’s stage 13–14 (Fig. 2A–D), the otic placode has started to invaginate, and Pax2 is expressed in the otic placode (Fig. 2A). A couple of cells near the otic placode express NeuroD (Fig. 2B, indicated by arrows). These NeuroD-positive cells also express Isl1 (Fig. 2C,D, indicated by arrows). The position of these Isl1+ cells and their expression of NeuroD are indicative of their identity as the likely delaminated neuroblasts from the otic placode.
Fig. 2.
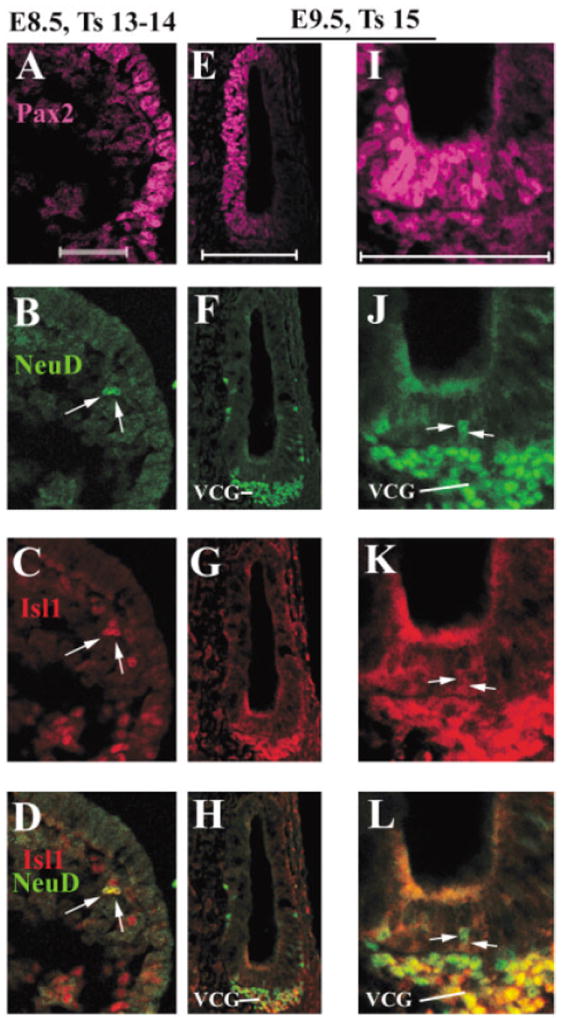
Expression of Islet1 (Isl1) in the inner ear neuronal lineage from embryonic day (E) 8.5 to E9.5. A–L: A vertical section from E8.5 (A–D) and a transverse ventral section from E9.5 (E–L) embryos were triply stained with antibodies against Pax2 (A,E,I, magenta), NeuroD (B,F,J, green), and Isl1 (C,G,K, red). D,H,L: Overlays of NeuroD and Isl1 staining. I,J,K,L: Higher magnified images of E,F,G,H, respectively. For E8.5 vertical section, dorsal is at the top, and for E9.5 transverse section, anterior is at the top. Medial is at the left for both the sections. VCG, vestibular–cochlear ganglion neurons. Scale bars = 50 μm in A (applies to A–D), E (applies to E–H), I (applies to I–L).
One day later at E9.5 or Theiler’s stage 15, the otic placode has formed an invaginated open vesicle (Fig. 2E–L). Pax2 is expressed in medial regions of the otic vesicle as shown in the transverse sections in mid-ventral region (Fig. 2E,I). The expression of NeuroD marks the delaminated vestibular–cochlear ganglion neurons (Fig. 2F,H,J,L, VCG) and a few neuroblast precursor cells within the otic epithelium (Fig. 2J, indicated by arrows). Isl1 is also expressed in the delaminated VCG neurons (Fig. 2G,H,K,L) and in the neuroblast precursor cells within the otic epithelium (Fig. 2K,L, arrows).
At E10.5 (Fig. 3A–H), the medial regions of the otocyst are still delimited by the expressed of Pax2 (Fig. 3E,G). Delaminated vestibular–cochlear ganglion neurons all express NeuroD and Isl1 (Fig. 3C,F,H, VCG, cells expressing both are yellow), and the neuronal β-tubulin has started to be expressed in the VCG neurons (Fig. 3D). In the otic epithelium, the number of cells expressing Isl1 has increased dramatically (Fig. 3A,C, between two arrows, and compare with Fig. 2K–L). By this stage, no Math1/GFP expression is detectable in the otocyst (data not shown).
Fig. 3.
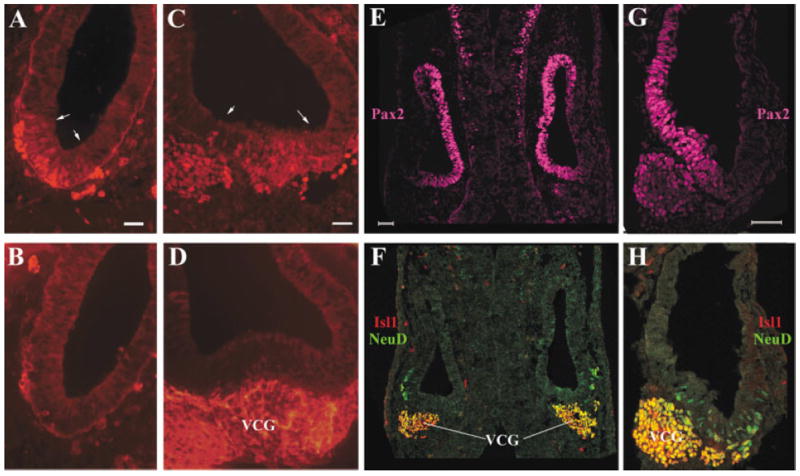
Expression of Islet1 (Isl1) in the inner ear neuronal lineage at embryonic day (E) 10.5. A–D: Adjacent vertical (A,B) or transverse (C,D) E10.5 sections were stained for Isl1 (A,C) or Tuj-1 (C,D). Arrows in A and C delimit the expression domain of Isl1 in the otic epithelium at this stage. E–H: Transverse (E,F) and vertical (G,H) sections were triply labeled for Pax2 (E,G, magenta), Isl1 (F,H, red), and NeuroD (F,H, green). F,H: The staining for Isl1 and NeuroD was overlaid. VCG, vestibular–cochlear ganglion neurons. Scale bars = 50 μm in A (applies to A,B), C (applies to C,D), E (applies to E,F), G (applies to G,H).
By E11.5, different regions of the otocyst have acquired distinct structural features (Fig. 4A,B; Fritzsch et al., 1997; Kelley and Bianchi, 2001). The endolymphatic sac is apparent (Fig. 4A,B, dorsal of the otic vesicle with a tubular structure), and the majority of VCG neurons have delaminated from the ventral medial region of the otocyst. Isl1 expression continues to exclusively mark the population of cells that are delaminated VCG neurons expressing the neuronal-specific β-tubulin (compare Fig. 4A with B, C with D). By this stage, NeuroD is expressed in the newly delaminated VCG neurons closer to the otic vesicle (Fig. 4E,F, yellow cells expressing both NeuroD and Isl1), while down-regulated in VCG neurons born earlier and moved farther from the otic vesicle (Fig. 4E,F, red cells only express Isl1 but not NeuroD).
Fig. 4.
Islet1 (Isl1) expression in the vestibular-cochlear ganglion neurons and in the otic epithelium at embryonic day (E) 11.5. A–D: Adjacent otocyst sections at E11.5 (A,B and C,D) were stained with an antibody against Isl1 (Isl1, A,C) or neuronal β-tubulin (Tuj1, B,D). C,D: Higher magnifications of the ventral region in A and B, respectively. The green signal in A–D is from green fluorescent protein (GFP) expressed under the control of Math1 enhancers (Chen et al., 2002). Arrows in A and C indicate the boundaries of the Isl1-expressing ventral–medial region of the otocyst. Arrowheads in C and D indicate cells expressing the nucleus-localized GFP under the control of Math1 enhancers within the otocyst epithelium. AE,F: A transverse section triply stained for Isl1 (E,F, red), NeuroD (E,F, green), and Pax2 (E,F, magenta). E: The staining of Isl1 and NeuroD was overlaid to indicate specific staining for each (red for Isl1 and green for NeuroD) or for double labeling (yellow). F: All three labels were overlaid to indicate the relative expression domains of each gene. Note that the magenta colored Pax2 staining covers over other staining. Only complementary staining to Pax2 staining of Isl1 and NeuroD can be showed in F. Arrows in E,F indicate the Isl1-expressing domain in the otic epithelium that is negative for NeuroD staining. VCG, vestibular–cochlear ganglion neurons. Scale bars = 50 μm A (applies to A,B), C (applies to C,D), E (applies to E,F).
Together, the comparison of NeuroD and Isl1 expression patterns at this (Fig. 4) and earlier stages (Figs. 2, 3) indicated that Isl1 is expressed in the inner ear neuronal lineage and that its expression persists after the transitory expression of NeuroD in the same lineage.
In Addition, Isl1 expression is also seen in the epithelial lineage(s) by E11.5 (Fig. 4E,F) in addition to the neuronal lineage of the inner ear. At E11.5, Isl1 is detected in cells that do not express NeuroD in the ventral region of the otic epithelium (Fig. 4E,F, red cells between two arrows). The earliest expression of Math1/GFP (Fig. 4A,C, arrowheads indicating Math1/GFP expressing cells), whose expression is limited to the sensory lineage in the inner ear, is also detected within the Isl1+ ventral region of the otocyst (Fig. 4A,C, the region bracketed by arrows), suggesting that at least some of the Isl1+ ventral epithelial cells represent the epithelial cells for the sensory lineage of the inner ear. It is worth noting that the Math1/GFP+ cells detected at E11.5 are likely the earliest born saccule hair cell precursors. The ventral region of the otocyst gives rise to both saccule and cochlea (Fritzsch et al., 1997). In the cochlea, Math1/GFP or endogenous Math1 is not detected until E13.5 (Chen et al., 2002; Lanford et al., 2000) while differentiation of the sensory epithelia in the vestibule occurs earlier (Ruben, 1967; Sher, 1971).
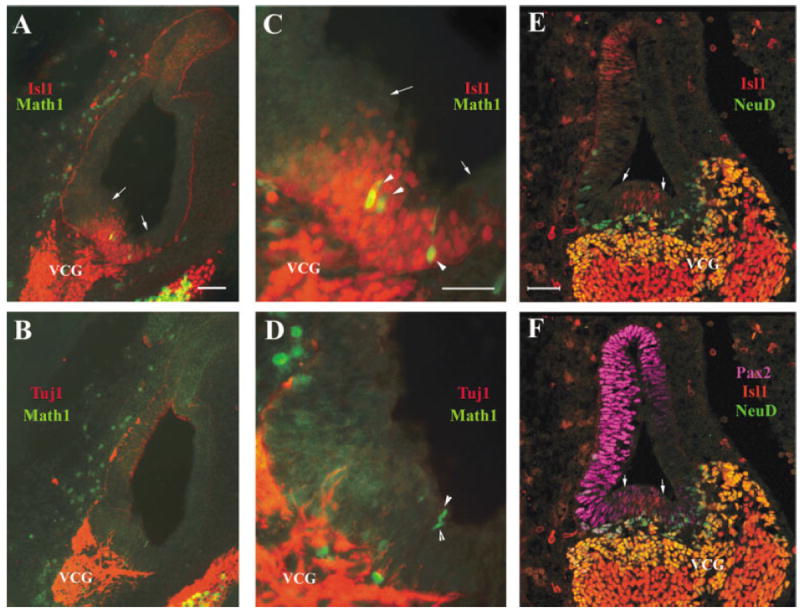
To confirm the expression of Isl1 in the sensory lineage of the inner ear, we further studied its expression at later stages. At E12.5, hair cells in the vestibule have started to differentiate, as seen by the expression of a hair cell cytoplasmic marker myosin VIIa in the sensory epithelia (Fig. 5A) where Tuj1-positive vestibular nerve fibers end (Fig. 5D). Isl1 is expressed in the sensory regions of the vestibular epithelia at this stage (Fig. 5B,C), including hair cells (Fig. 5B,C, arrowheads indicate Isl1+-hair cell nuclei) and supporting cells and/or hair cell precursors that have yet to express myosin VIIa (Fig. 5B,C). It is noticeable that the Isl1 signal is weaker in newly differentiated hair cells compared with surrounding supporting cells and/or hair cell precursor cells, indicating that Isl1 starts to be down-regulated as hair cell differentiation initiates.
Fig. 5.
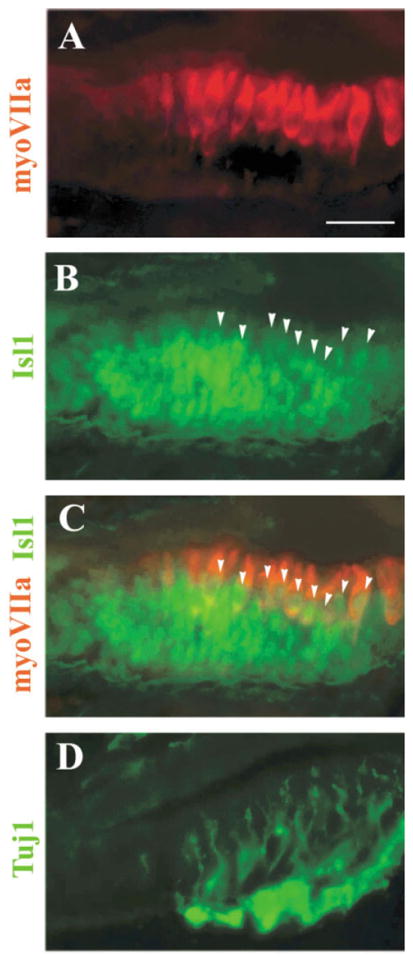
Islet1 (Isl1) expression in the nascent vestibular sensory organs. A–D: Adjacent sections for the utricle were stained for a hair cell marker myosin VIIa (A), Isl1 (B), neuronal β-tubulin (D, Tuj1), or double stained for myosin VIIa (C, red, cytoplasmic staining) and Isl1 (C, green, nuclear staining). Arrowheads in B,C indicate Isl1 and myosin VIIa double-positive cells. Scale bar = 50 μm in A (applies to A–D).
The cochlear duct has formed as an outpocketing (Morsli et al., 1998) from the ventral medial region of the otocyst by E12.5. In the cochlear epithelium, the expression of both the Isl1 protein (Fig. 6A) and mRNA (Fig. 6B) demarcates distinctly the ventral portion of the cochlear epithelium (Fig. 6A,B, between the two arrowheads). By this stage, the cochlear and vestibular ganglion neurons have segregated into their corresponding discrete regions in the developing inner ear. The expression of Isl1 still persists in the neuronal lineage as shown in Figure 6 in the cochlear ganglion neurons (Fig. 6A,B, SG), which also express neuronal β-tubulin (Fig. 6C, SG).
Fig. 6.
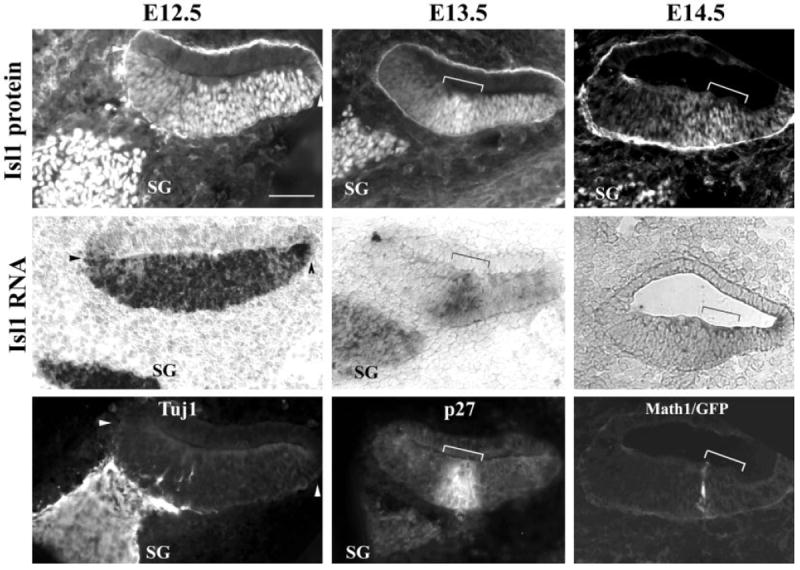
Islet1 (Isl1) expression in the cochlea. A–I: Adjacent cochlear sections from embryonic day (E) 12.5 (A–C), E13.5 (D–F), and E14.5 (G–I) stained for Isl1 antibody (A,D,G), Isl1 mRNA (B,E,H), neuronal β-tubulin antibody (C, Tuj1), p27Kip1 antibody (F), or with Math1/GFP signal (I). Arrowheads in A–C mark the boundaries of the ventral cochlear epithelium expressing Isl1 at E12.5. Brackets (D–I) indicate the primordial organ of Corti before terminal differentiation. SG, spiral ganglion neurons. Medial is at the left of the images. GFP, green fluorescent protein. Scale bar = 50 μm in A (applies to A–I).
By E13.5, the cells within the sensory primordium of the cochlea exit the cell cycle to form a distinct zone of non-proliferating cells demarcated precisely by the onset of the cyclin-dependent kinase inhibitor p27Kip1 in the cochlea (Chen and Segil, 1999). Subsequently, starting at E14.5, hair cells and supporting cells of the organ of Corti arise strictly within the zone of nonproliferating cells or the p27Kip1-expressing domain (Chen and Segil, 1999). In the cochlear duct, the expression of Isl1 at E13.5 is down-regulated at the medial region of the ventral portion, while relatively higher expression of Isl1 protein and mRNA was seen in a distinct zone of cells (Fig. 6D,E, bracket). This zone of cells with relative high expression of Isl1 (Fig. 6D,E, bracket) coincides with the expression domain of p27Kip1 (Fig. 6F, bracket) that marks the primordial organ of Corti before terminal differentiation (Chen and Segil, 1999). In contrast to the differential regulation of Isl1 in the cochlear duct, the expression of Isl1 in SG neurons is maintained (Fig. 6E, SG).
Differential expression of Islet1 in the sensory and neuronal lineages of the inner ear
Hair cell differentiation starts around E14.5 in the cochlea as indicated by the expression of Math1 (Chen et al., 2002) or Math1/GFP in the sensory primordium (Fig. 6I). The expression levels of Isl1 in the ventral cochlear duct are further reduced. Only weak signals of Isl1 are detected in the sensory primordium at this time (Fig. 6G,H, brackets). Math1/GFP+-hair cells are seen arising at the medial border of the sensory primordium (Fig. 6I, labeled cells at the medial border of the bracket).
Once initiated, hair cell differentiation in the primordial organ of Corti continues along the medial-to-lateral and basal-to-apical axes of the cochlear duct by E165, the organ of Corti from the basal to middle regions of the cochlear duct has been patterned into its mature sensory arrays composed of hair cells (Fig. 7A, red) and supporting cells (Sher, 1971). By this time, Isl1 is not detectable in the differentiated cochlear epithelium (Fig. 7A, green) but is sustained in SG neurons (Fig. 7A, SG, green). In the vestibular sensory organs, the expression of Isl1 is also down-regulated and is not detectable in either the hair cell layer (Fig. 7B,C, brackets) or the supporting cell layer (Fig. 7B,C, arrowheads).
Fig. 7.
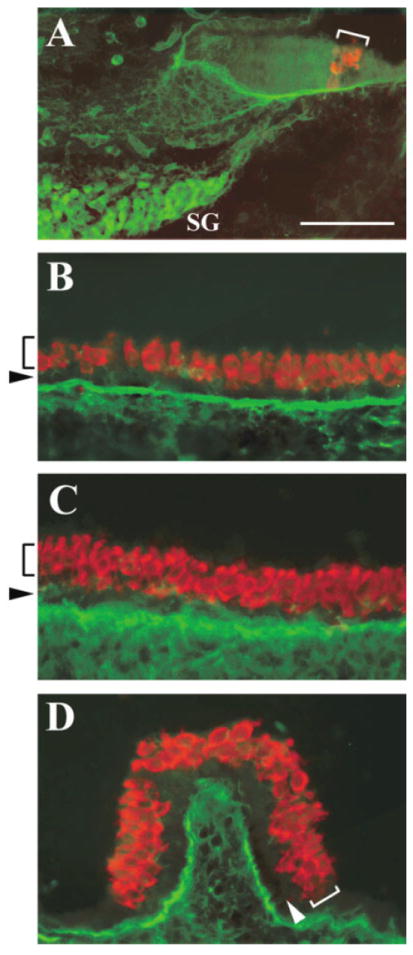
Differential expression of Islet1 (Isl1) in the sensory and neuronal cells of the inner ear. A–D: Cross-sections of the cochlea (A), saccule (B), utricle (C), and crista (D) at embryonic day 16.5 double stained for Isl1 (A, green) and Math1/GFP (A, red) antibodies, or Isl1 (B–D, green) and myosin VIIa (B–D, red) antibodies. The brackets indicate the organ of Corti in the cochlea (A), or the hair cell layer in the vestibular sensory organs (B–D). The arrows indicate the supporting cell layer in the vestibular sensory organs (B–D). Isl1 is absent in the sensory organs but persists in SG neurons. SG, spiral ganglion neurons; GFP, green fluorescent protein. Scale bar = 50 μm in A (applies to A–D).
In summary, Isl1 is expressed initially in the NeuroD-expressing neuronal lineage and in the population of cells giving rise to the sensory lineage of the inner ear. Subsequently, it is maintained in the neuronal lineage but down-regulated in the sensory lineage upon initiation of hair cell differentiation.
The expression of Isl1 in both the sensory and neuronal lineages suggested that the two lineages might derive from common progenitors, or that common pathways regulate the expression of a subset of genes, including Isl1, in both lineages. The expression of Isl1 revealed an early and common step in the development of both the neuronal and sensory lineages. A recent study reported a similar expression pattern of a chicken HD gene in the developing chicken inner ear (Stone et al., 2003), suggesting the involvement of conserved common molecular mechanisms in the development of both lineages in vertebrate ears.
However, the timing and requirement for Isl1 in the development of the neuronal and sensory lineages may be different. In the neuronal lineage, Isl1 is expressed in the neuroblast precursors and newly delaminated neuroblast cells that express NeuroD or cells with a restricted cell fate of becoming vestibular and cochlear ganglion neurons. As the neuronal differentiation progresses, NeuroD is down-regulated while Isl1 is maintained in the lineage. Therefore, the role of Isl1 in the inner ear neuronal lineage development is likely in executing precisely the cell type-specific terminal differentiation for vestibular and cochlear ganglion neurons in coordination with other factors, such as the bHLH gene NeuroD, and in subsequent maintenance of the terminally differentiated neurons. In the epithelial lineages, Isl1 is initially expressed in the entire ventral region of the cochlear epithelium at E12.5 (Fig. 6A,B), followed by a more restricted expression in the cochlear epithelium (Fig. 6D,E). The expression of Isl1 precedes that of p27Kip1 and Math1 in the cochlea. Interestingly, we have found that several other genes, such as Tac1 (Bilkei-Gorzo et al., 2002), Nr2f2 (Jonk et al., 1994), and Frzb (Hoang et al., 1996; Leyns et al., 1997; Wang et al., 1997), have a similar initial expression pattern in the cochlea epithelium, distinctively in the entire ventral portion of the cochlear epithelium at E12.5 (data not shown). At later stages, the expression of these genes becomes more restricted and is limited to different regions of the cochlear epithelium (data not shown). Studies of members of the bone morphogenetic protein family (Morsli et al., 1998) and the Delta/Notch pathway (Adam et al., 1998; Morrison et al., 1999; Zheng et al., 2000) suggest that these genes may also share such a common expression in the newly formed cochlea at E12.5. It is possible that cells in the ventral region of the cochlea all have a similar molecular composition with an equal competency at E12.5, and that subsequent molecular events lead to the restriction of these cells to only become a certain cell type within the cochlear epithelium. It is conceivable that Isl1 might function with genes upstream of Math1 in specification of the ventral cochlear fate at E12.5 or before the formation of the cochlear duct at E11.5, as suggested by the expression of Isl1 in the ventral region of the cochlea at E12.5 (Fig. 6) or in the ventral region of the otocyst at E11.5 (Fig. 4), respectively. It is worth noting that the introduction of the Math1 gene in the ventral region of the cochlear duct leads to ectopic production of hair cells in postnatal rodents (Zheng and Gao, 2000; Shuo et al., 2003; Kawamoto et al., 2003), indicating that, although these cells have differentiated, they still maintain a residual competency to become hair cells given proper signals.
Together, our data indicates that Isl1 is expressed in both the neuronal and the sensory lineages of the inner ear. The expression of Isl1 in the inner ear raises the possibility that it plays a role in the development of inner ear sensory/neuronal lineages, such as in specifying the inner ear-specific neuronal/sensory competency in coordination with bHLH genes. In addition, involvement of multiple LIM-HD genes might also contribute to the specification of different subtype of cells. In vertebrates, pairs of related LIM-HD genes typically exhibiting an overlapping profile of expression and function at sequential steps during development to diversify subtype cell fates (Thaler et al., 2004). In particular, the LIM-HD gene Islet2 (Isl2), structurally related to Isl1, might also contribute to the machinery governing the sequential or concomitant developmental events for the determination of cell types within the inner ear. Conditional inactivation of Isl1 or Isl1 and its homolog Isl2 may prevent early lethality of mice in the absence of Isl1 (Pfaff et al., 1996) and allow its functional study in the development of the neuronal and sensory lineages of the inner ear. Characterization of cellular context-dependent factors in the cochlea essential for hair cell induction, and the combination of neurogenic and cellular context-dependent factors (e.g., Isl1) might greatly potentiate induction of hair cells targeted for therapeutic applications.
Acknowledgments
We thank Paul Chen, Juan Llamas, and Welly Makmura for animal care; Seung-Jong Yoo, Jeffrey Saeks, and Connie Tang for technical assistance in cryosection preparation; Jane E. Johnson for Math1/GFP animals; Christine Petit and A. El-Amraoui for myosin VIIa antibody; Frank Middleton for Affymetrix microarray analysis; Matthew Holley for communications on expressions of otocyst-specific genes; and the Developmental Studies Hybridoma Bank for Isl1 antibody.
Grant sponsor: National Institutes of Health; Grant number: EY013426; Grant number: DC04709; Grant number: DC004189; Grant number: DC005213.
LITERATURE CITED
- Adam J, Myat A, Le Rous I, Eddison M, Henrique D, Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Allan DW, Thor S. Together at last: bHLH and LIM-HD regulators cooperate to specify motor neurons. Neuron. 2003;38:675–677. doi: 10.1016/s0896-6273(03)00329-5. [DOI] [PubMed] [Google Scholar]
- Bachy I, Vernier P, Retaux S. The LIM-homeodomain gene family in the developing Xenopus brain: conservation and divergences with the mouse related to the evolution of the forebrain. J Neurosci. 2001;21:7620–7629. doi: 10.1523/JNEUROSCI.21-19-07620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Zimmer A. Diminished anxiety- and depression-related behaviors in mice with selective deletion of the Tac1 gene. J Neurosci. 2002;22:10046–10052. doi: 10.1523/JNEUROSCI.22-22-10046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J, Goodyear RJ, Richardson GP. Sensory organ development in the inner ear: molecular and cellular mechanisms. Br Med Bull. 2002;63:39–57. doi: 10.1093/bmb/63.1.39. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Fekete D, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Barald KF, Lomax MI. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Development of the auditory system. New York: Springer; 1997. pp. 80–145. [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WQ. Hair cell development in higher vertebrates. Curr Top Dev Biol. 2003;57:293–319. doi: 10.1016/s0070-2153(03)57010-7. [DOI] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of ngn1 and math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci U S A. 1995;92:9815–9819. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Hoang B, Moos M, Jr, Vukicevic S, Luyten FP. Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. J Biol Chem. 1996;271:26131–26137. doi: 10.1074/jbc.271.42.26131. [DOI] [PubMed] [Google Scholar]
- Jonk LJ, de Jonge ME, Pals CE, Wissink S, Vervaart JM, Schoorlemmer J, Kruijer W. Cloning and expression during development of three murine members of the COUP family of nuclear orphan receptors. Mech Dev. 1994;47:81–97. doi: 10.1016/0925-4773(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW, Bianchi LM. Development and neuronal innervation of the organ of Corti. In: Willott JF, editor. Handbook of mouse auditory research. New York: CRC; 2001. pp. 137–156. [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. see comments. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam CR, Gridley NT, Kelley MW. Expression of Math1 and HES5 in the cochlea of wildtype and Jag2 mutant mice. J Assoc Res Otolaryngol. 2000;1:161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol. 2002;442:378–391. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- Lee JE. Basic helix-loop-helix genes in neural development. Curr Opin Neurobiol. 1997a;7:13–20. doi: 10.1016/s0959-4388(97)80115-8. [DOI] [PubMed] [Google Scholar]
- Lee JE. NeuroD and neurogenesis. Dev Neurosci. 1997b;19:27–32. doi: 10.1159/000111182. [DOI] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. Synchronization of neurogenesis and motor neuron specification by direct coupling of bHLH and homeodomain transcription factors. Neuron. 2003;38:731–745. doi: 10.1016/s0896-6273(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific β-tubulin isotype during chick embryogenesis. Cell Motil Cytoskel. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Lewis J. Neurogenic genes and vertebrate neurogenesis. Curr Opin Neurobiol. 1996;6:3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu M, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai M. Essential role of Beta2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of Corti. Proc Natl Acad Sci U S A. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, dela Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–144. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A, Hodgette C, Gossler A, Hrabe de Angelis M, Lewis J. Expression of Delta1 and Serrat1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84:169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Riley BB. Genes controlling the development of the zebrafish inner ear and hair cells. Curr Top Dev Biol. 2003;57:357–388. doi: 10.1016/s0070-2153(03)57012-0. [DOI] [PubMed] [Google Scholar]
- Romand R, Varela-Nieto I. Development of auditory and vestibular system-3: molecular development of the inner ear. Curr Top Dev Biol. 2003;57:1–481. [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220(Suppl):1–44. [PubMed] [Google Scholar]
- Sahly I, El-Amraoui A, Abitbol M, Petit C, Dufier JL. Expression of myosin VIIa during mouse embryogenesis. Anat Embryol (Berl) 1997;196:159–170. doi: 10.1007/s004290050088. [DOI] [PubMed] [Google Scholar]
- Sher AE. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol Suppl. 1971;285:1–77. [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Stone JS, Shang JL, Tomarev S. Expression of Prox1 defines regions of the avian otocyst that give rise to sensory or neural cells. J Comp Neurol. 2003;460:487–502. doi: 10.1002/cne.10662. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Koo SJ, Kania A, Lettier K, Andrews S, Cox C, Jessell TM, Praff SL. A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron. 2004;41:337–350. doi: 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- Theiler K. The house mouse: atlas of embryonic development. New York: Springer-Verlag; 1989. [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Yang Z, Ding K, Pan L, Deng M, Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–60. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]


