Abstract
The planar cell polarity (PCP) pathway is conserved throughout evolution, but it mediates distinct developmental processes. In Drosophila, members of the PCP pathway localize in a polarized fashion to specify the cellular polarity within the plane of the epithelium, perpendicular to the apicobasal axis of the cell. In Xenopus and zebrafish, several homologs of the components of the fly PCP pathway control convergent extension. We have shown previously that mammalian PCP homologs regulate both cell polarity and polarized extension in the cochlea in the mouse. Here we show, using mice with null mutations in two mammalian Dishevelled homologs, Dvl1 and Dvl2, that during neurulation a homologous mammalian PCP pathway regulates concomitant lengthening and narrowing of the neural plate, a morphogenetic process defined as convergent extension. Dvl2 genetically interacts with Loop-tail, a point mutation in the mammalian PCP gene Vangl2, during neurulation. By generating Dvl2 BAC (bacterial artificial chromosome) transgenes and introducing different domain deletions and a point mutation identical to the dsh1 allele in fly, we further demonstrated a high degree of conservation between Dvl function in mammalian convergent extension and the PCP pathway in fly. In the neuroepithelium of neurulating embryos, Dvl2 shows DEP domain-dependent membrane localization, a pre-requisite for its involvement in convergent extension. Intriguing, the Loop-tail mutation that disrupts both convergent extension in the neuroepithelium and PCP in the cochlea does not disrupt Dvl2 membrane distribution in the neuroepithelium, in contrast to its drastic effect on Dvl2 localization in the cochlea. These results are discussed in light of recent models on PCP and convergent extension.
Keywords: Mouse, Planar cell polarity, Convergent extension, Neurulation
INTRODUCTION
Many of the conserved genetic pathways and cell biological mechanisms required during eukaryotic development have been uncovered over the past few decades, leading to a basic understanding of fundamental processes required during embryogenesis. During evolution, these conserved pathways and mechanisms are adapted and modified according to the demands of the evolved body plan. Two examples of conserved pathways are the Wnt and planar cell polarity (PCP) pathways, which share some common components (Cadigan and Nusse, 1997; Keller, 2002; Mlodzik, 2002; Myers et al., 2002; Tree et al., 2002a; Veeman et al., 2003a; Wallingford et al., 2002). The Wnt pathway was first defined in Drosophila, where it is required for the anteroposterior (AP) polarity of the body segments and cell fate determination (Cadigan and Nusse, 1997). In Xenopus, the Wnt pathway participates in axis determination and dorsoventral (DV) patterning (Cadigan and Nusse, 1997). In mammals, an expanded pathway has diverse effects on cell proliferation and patterning during gastrulation and organogenesis (Huelsken and Birchmeier, 2001; Wang and Wynshaw-Boris, 2004; Yamaguchi, 2001). Similarly, the conserved PCP pathway was first uncovered in Drosophila, where it regulates the polarity of a cell within the plane of the epithelium, perpendicular to the apicobasal axis of the cell. Manifestations of this polarity includes uniformly oriented parallel arrays of cellular extensions called trichomes on the epithelial cells of the wing and precisely coordinated orientation of ommatidial units of the compound eye (Strutt, 2002; Tree et al., 2002a; Veeman et al., 2003a). In Xenopus and zebrafish, an homologous pathway regulates convergent extension, a coordinated extension of the AP axis with concomitant narrowing of the mediolateral (ML) axis (Carreira-Barbosa et al., 2003; Darken et al., 2002; Goto and Keller, 2002; Keller, 2002; Kinoshita et al., 2003; Park and Moon, 2002; Takeuchi et al., 2003; Veeman et al., 2003b; Wallingford et al., 2002; Wallingford et al., 2000). During convergent extension in these animals, several PCP proteins have been shown to control the polarity of lamellipodial protrusions that drive cell intercalation (Jessen et al., 2002; Wallingford et al., 2000). In zebrafish, the PCP pathway also appears to regulate convergent extension, at least in part, through determining the orientation of cell division (Gong et al., 2004).
The multifunctional protein Dishevelled (Dsh in fly, XDsh in Xenopus, and Dvl1, Dvl2 and Dvl3 in mammals) is involved in both the canonical Wnt signaling pathway as well as the PCP pathway (Mlodzik, 2002; Strutt, 2002; Tree et al., 2002a; Veeman et al., 2003a; Wallingford et al., 2002; Wallingford and Habas, 2005). In the canonical Wnt pathway, Dsh is important in transmitting a signal initiated from Wnt binding to its receptor Frizzled and co-receptor Arrow/Lrp5/6 on the cell surface. As a consequence, the cytoplasmic protein Armadillo/β-catenin becomes stabilized and is transported into the nucleus to activate transcription (He et al., 2004; Wang and Wynshaw-Boris, 2004). In the PCP pathway in fly, Dsh participates with a different set of proteins such as Strabismus (Stbm), Prickle (Pk), Diego (Dgo) and Flamingo (Fmi) (Das et al., 2004; Jenny et al., 2005; Strutt, 2002; Tree et al., 2002a; Veeman et al., 2003a). Although the precise mechanisms through which Dsh determine PCP is not well defined, an asymmetric plasma membrane localization of Dsh and the other PCP members appears to be both required for and dependent upon proper establishment of the PCP pathway in the fly (Axelrod, 2001; Bastock et al., 2003; Das et al., 2004; Jenny et al., 2003; Jenny et al., 2005; Rawls and Wolff, 2003; Strutt et al., 2002; Strutt, 2002; Tree et al., 2002a; Tree et al., 2002b; Veeman et al., 2003a).
Recent genetic studies have also provided evidence that a putative PCP pathway exists in mammals (Curtin et al., 2003; Hamblet et al., 2002; Kibar et al., 2001; Montcouquiol et al., 2003; Murdoch et al., 2001; Wang et al., 2005; Wang et al., 2006). Loop tail (Lp) is a point mutation of Ltap or Vangl2, a homolog of the fly PCP component Stbm (Kibar et al., 2001; Montcouquiol et al., 2003; Murdoch et al., 2001), while Crash and Spin cycle (Crsh, Scy) are distinct mutations of Celsr1, a homolog of the PCP pathway member fmi (Curtin et al., 2003). Frizzled 3 (Fzd3) and frizzled 6 (Fzd6) encode two of the Frizzled receptors in mammals (Wang et al., 2006). Lp, Crsh, Scy, Fzd3−/−; Fzd6−/− and Dvl1−/−; Dvl2−/− mutants result in misorientation of stereocilia of sensory hair cells in the cochlea. The uniform orientation of the stereocilia on hair cells in the cochlea has been proposed to be a manifestation of PCP (Lewis and Davies, 2002). Indeed, we found that a transgenic Dvl2-EGFP protein capable of rescuing the stereocilia orientation defects in Dvl1−/−; Dvl2−/− mutants displayed asymmetric plasma membrane localization that is disrupted in Lp/Lp embryos (Wang et al., 2005). Others reported a similar Vangl2-dependent asymmetric localization of Fzd3 and Fzd6 in the sensory hair cells, utricles and cristae (Wang et al., 2006), suggesting a conserved PCP pathway as the underlying mechanism in coordinating stereocilia orientation. In addition, Lp, Crsh, Scy, Fzd3−/−; Fzd6−/− and Dvl1−/−; Dvl2−/− mutants all result in a unique severe neural tube closure defect in which the entire neural tube from mid-brain to tail fails to close, a congenital defect termed craniorachischisis in humans (Curtin et al., 2003; Hamblet et al., 2002; Kibar et al., 2001; Murdoch et al., 2001; Wang et al., 2006). The cause of craniorachischisis in these mutants has been inferred to be failure of convergent extension, because, in Xenopus, overexpression of wild-type or mutant forms of XDsh and Stbm (which blocks convergent extension) usually results in similar neural tube closure defects (Copp et al., 2003; Darken et al., 2002; Goto and Keller, 2002; Ueno and Greene, 2003; Wallingford and Harland, 2002). However, the developmental mechanisms disrupted by the loss of conserved mammalian PCP pathway members during neurulation have not been investigated. There is no experimental evidence that the neural tube defect in these PCP mutant mice results from convergent extension defects, and if so, it is not clear how PCP regulates convergent extension in mammals.
In this study, we provide evidence that during neurulation, Dvl1 and Dvl2 are required for concomitant lengthening and narrowing of neural plate, a morphogenetic process that resembles convergent extension. Using a Dvl2 BAC transgene expressing a functional Dvl2-EGFP protein, we found that subcellular Dvl2 localization in neuroepithelium is consistent with its function in convergent extension. Intriguingly, although Lp genetically interacts with Dvl genes during neurulation and in cochlea development, Lp appears to have no effect on Dvl2 localization in the neuroepithelium, in contrast to its drastic effect on Dvl2 distribution in the cochlea (Wang et al., 2005). We found that part of the N-terminal DIX domain, which is essential for Dvl2 function in the Wnt pathway (Capelluto et al., 2002), was largely dispensable for its function during neurulation or recruitment to the plasma membrane. By contrast, the C-terminal DEP domain, which is solely required for Dsh/Dvl function in PCP/convergent extension in flies and Xenopus (Axelrod et al., 1998; Boutros et al., 1998; Rothbacher et al., 2000; Wallingford and Harland, 2001; Wallingford et al., 2000), was essential for Dvl2 function in neurulation and for plasma membrane localization. To further test whether we can genetically distinguish PCP and convergent extension, we produced a Dvl2 BAC transgene carrying a point mutation identical to the dsh1 allele in fly, which specifically abolishes the PCP pathway. Our analysis of the mutant transgene suggests stringent conservation of Dvl function in convergent extension and PCP.
MATERIALS AND METHODS
Length-to-width ratio (LWR) measurement in neurulating embryos
Embryos derived from appropriate crosses were dissected at E8.5 and yolk sacs were removed for PCR genotyping. Embryos were fixed in 4% paraformaldehyde at 4°C overnight and stored in PBS. After counting somite numbers, embryos were transferred into an empty dish and allowed to extend naturally. Embryos were photographed with a Spot CCD camera mounted on a Leica MZFL III dissection microscope. Images of both dorsal and ventral view of each embryo were acquired and imported into ImageProPlus, and length-to-width ratio measurements of the trunk were made as follows. On each embryo, a line was drawn along the body edge from the base of the allantoic bud to the base of the headfold. The average of the traces on the left and right sides was used for the length measurement, the average distance between these two traces was determined by ImageProPlus and used for the width measurement. Averages of the LWR derived from ventral and dorsal view image were taken as the final LWR of the embryo. At least three embryos of each genotype at each somite stage were measured. Statistical analysis was performed by using the Mann-Whitney U-test through GraphPad InStat software.
Generating Dvl2-EGFP and mutant ΔDIX-EGFP, ΔDEP-EGFP and dsh1-EGFP BAC transgenes
A BAC clone containing the Dvl2 genomic sequence, derived from a PCR screen of a BAC library from Genome Systems, was characterized for genomic integrity and potential rearrangement by using 11 overlapping PCR reactions that covered the entire Dvl2 genomic region. The existence of at least two flanking genes on the 3′ and 5′ ends were confirmed by designing specific PCR primers based on the sequence information in the mouse genome database. Modifications of the BAC, including insertion of two LoxP sites and EGFP-coding sequence in-frame to the last codon of Dvl2 were performed as described (Lee et al., 2001). Dvl2-EGFP1 had an EGFP cassette fused in-frame to the last codon of Dvl2 and a LoxP site inserted into intron 2 for genotyping purpose (see Mouse strains and genotyping), whereas Dvl2-EGFP2 contained an additional LoxP site inserted in the 3′ flanking gene Acadvl (acyl-Coenzyme A dehydrogenase, very long chain). Using BAC recombineering techniques, ΔDIX-EGFP and ΔDEP-EGFP deletion mutant transgenes were generated by removing sequence encoding amino acids 67-159 and amino acids 442 to 736, respectively. dsh1-EGFP point mutation was generated by replacing AAG with ATG to introduce a K to M substitution at amino acid 446. Modified BACs were purified and pronuclear injection was performed as described to create transgenic mice (Lee et al., 2001).
Mouse strains and genotyping
Mice carrying Lp mutation was obtained from the Jackson Laboratory (Bar Harbor, ME) and genotyped as described (Murdoch et al., 2001). Genotyping of the targeted Dvl1 and Dvl2 alleles was performed as described (Hamblet et al., 2002). To differentiate the Dvl2-EGFP BAC transgene from the endogenous wild-type Dvl2, a pair of primers flanking intron 2 were used (E2F, TGCTTCAATGGAAGGGTTGTC; E3R, TTCT-GTCCGAGACTCATGGG) for PCR (94°C for 20 seconds, 56°C for 20 seconds, 72°C for 30 seconds; 35 cycles). Endogenous wild-type Dvl2 allele gave rise to a PCR product of 370 bp, while the Dvl2-EGFP or dsh1-EGFP transgenes, owing to the insertion of the 34 bp LoxP site in intero 2, gave rise to a PCR product of 404 bp. The targeted Dvl2-null allele would not be amplified in this PCR reaction. The same PCR was used to genotype for ΔDEP-EGFP and dsh1-EGFP transgenes. To genotype and differentiate ΔDIX-EGFP transgenes from the endogenous Dvl2 and Dvl2 null alleles, a three primer PCR genotyping strategy was used (I1F, TTGCTCACTGAGTGCCTCTTGC; E3R, TTCTGTCCGAGACTCATGGG; R3, GCCATGTTCACTGCTGTCTCTC).
Confocal microscopy
To visualize native GFP signal from Dvl2-EGFP or dsh1-EGFP mice, embryos or cochleas of appropriate stages were dissected and briefly fixed in 4% paraformaldehyde at 4°C for 1 hour. Embryos were then counterstained with Alexa Fluor 568 phalloidin (A-12380, Molecular Probe) according to the manufacturer’s protocol. For antibody staining, embryos were fixed in 4% paraformaldehyde at 4°C overnight. After blocking in PBS + 5% normal goat serum, embryos were stained with an anti-GFP antibody (A-6455, Molecular Probe, 1:1000) and a donkey anti-rabbit IgG antibody (A-21206, Molecular Probe, 1:1000). Embryos were counterstained with Alexa Fluor 568 phalloidin. For analyzing planar cell polarity of the organ of Corti, whole-mount organs of Corti were stained with rhodamine or fluoroscein-conjugated phalloidin. Image was acquired using an Olympus FV 1000 confocal scanning microscope.
RESULTS
Dvl1−/−; Dvl2−/− mutants disrupt concomitant lengthening and narrowing of neural plate
As previously reported, 2-3% of Dvl2−/− single mutants display spina bifida, while Dvl1−/−; Dvl2−/− mutants display craniorachischisis (Hamblet et al., 2002). Dvl2−/−; Dvl3−/− double mutants also display craniorachischisis (J.W., N.S.H. and A.W.-B., unpublished). However, Dvl1−/− (Lijam et al., 1997) and Dvl3−/− single mutants or Dvl1−/−; Dvl3−/− double mutants (J.W. and A.W.-B., unpublished) do not display neural tube defects. These findings indicate that Dvl2 is the most important mammalian Dvl gene for neural tube closure and is sufficient by itself for normal neural tube closure. By contrast, Dvl1 and Dvl3 are not sufficient by themselves for normal neural tube closure, but contribute significantly when Dvl2 is completely missing.
In an effort to determine the cause of the craniorachischisis defect in Dvl1−/−; Dvl2−/− mutants, we performed in situ hybridization to determine dorsoventral (DV) patterning as defective DV patterning in the spinal cord is known to cause severe neural tube closure defects (Copp et al., 2003; Ybot-Gonzalez et al., 2002). We found normal dorsal expression of Wnt1, Pax3 and Engrailed2 and ventral expression of sonic hedgehog (shh) in the spinal cord of Dvl1−/−; Dvl2−/− mutants (data not shown). These results suggest that the failure of neural tube closure in Dvl1−/−; Dvl2−/− mutants is not due to abnormal DV patterning. Furthermore, the normal expression of En2, a known Wnt target gene, suggests that the canonical Wnt pathway was not globally disrupted in Dvl1−/−; Dvl2−/− mutants.
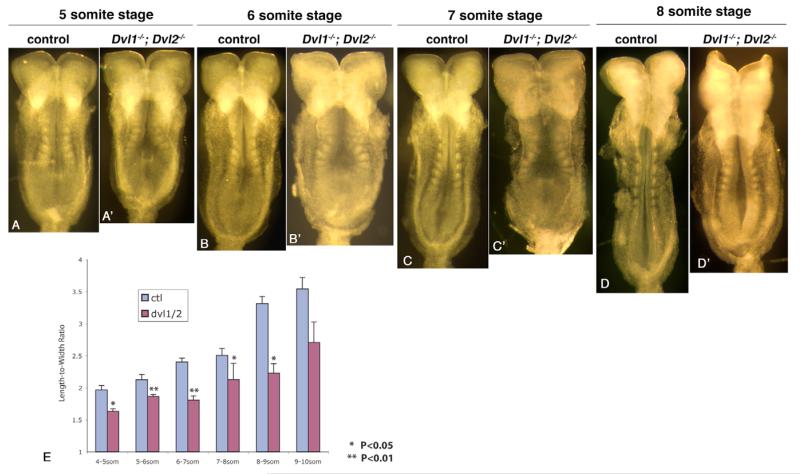
Recent studies in Xenopus have indicated an essential role of XDsh-mediated convergent extension morphogenetic movement in neural tube closure (Wallingford and Harland, 2002). In Xenopus, convergent extension results in coordinated elongation of the AP axis and narrowing of the ML axis, and is reflected as a continuous increase of the length-to-width ratio (LWR) during neurulation (Wallingford et al., 2002; Wallingford and Harland, 2002). To determine whether Dvl1−/−; Dvl2−/− mutants display defects consistent with a disruption of convergent extension, we adopted the methods used in Xenopus (Wallingford and Harland, 2002) and measured the LWR of neurulating mouse embryos (Fig. 1; Materials and methods). In control embryos, we found that the LWR continuously increased during neurulation (Fig. 1E). However, when we systematically measured Dvl1−/−; Dvl2−/− mutant embryos, we found consistent, significant reduction of LWR from the earliest time point where we could perform this analysis (Fig. 1E). More strikingly, from the five- to seven-somite stages, immediately before the first apposition of neural folds at closure 1 at the 7-somite stage in control embryos (Fig. 1C) (Ybot-Gonzalez et al., 2002), there was virtually no increase of the LWR in Dvl1−/−; Dvl2−/− mutant embryos.
Fig. 1. Dvl1−/−; Dvl2−/− mutants displayed reduced length-to-width ratio during neurulation.
(A-D) Five- to eight-somite stage Dvl1−/−; Dvl2+/− or Dvl1−/−; Dvl2+/+ control embryos. (A′-D′) Dvl1−/−; Dvl2−/− mutants of equivalent somite stages. Floor plates are expanded in Dvl1−/−; Dvl2−/− mutants. (E) Length-to-width ratio (LWR) changes of control and Dvl1−/−; Dvl2−/− mutant embryos at different somite stage during neurulation. Each point represents the average of at least three separate embryos. The error bars represent standard deviation. *P<0.05; **P<0.01.
This result reveals that, like Xenopus, convergent extension, which is defined as concomitant lengthening and narrowing of a tissue (Wallingford et al., 2002), occurs during mouse neurulation and is affected in Dvl1−/−; Dvl2−/− mutants prior to the time at which neural tube closure defects are observed. As suggested in Xenopus (Wallingford and Harland, 2002), the resulting wider neural plate in Dvl1−/−; Dvl2−/− mutants may also be incompatible with normal neural tube closure in the mouse.
Null (Pinson et al., 2000) and hypomorphic (Kokubu et al., 2004) alleles of the Wnt co-receptor Lrp6 result in the less severe neural tube defects exencephaly or spina bifida, implicating the canonical Wnt pathway in neural tube closure. In addition, the canonical Wnt pathway is crucial for the elongation of the AP axis through, at least in part, activating brachyury (T) expression (Yamaguchi et al., 1999). Therefore, we analyzed the expression of T to confirm that the reduced LWR was not due to the loss of function of the canonical Wnt pathway. In the early headfold stage, T is expressed in the notochord, node and primitive streak. In Wnt3a-null mice, T expression was reported to be lost in the anterior primitive streak. The loss of T expression was interpreted as the cause of axis shortening in Wnt3a−/− mutants (Yamaguchi et al., 1999). However, in Dvl1−/−; Dvl2−/− mutants, T expression in the anterior primitive streak appeared to be normal, suggesting that the reduced LWR was not due to a failure of the canonical Wnt pathway (Fig. 2A,B) and that reduced dose of Dvl in Dvl1−/−; Dvl2−/− mutants does not cause global loss of function of Wnt signaling.
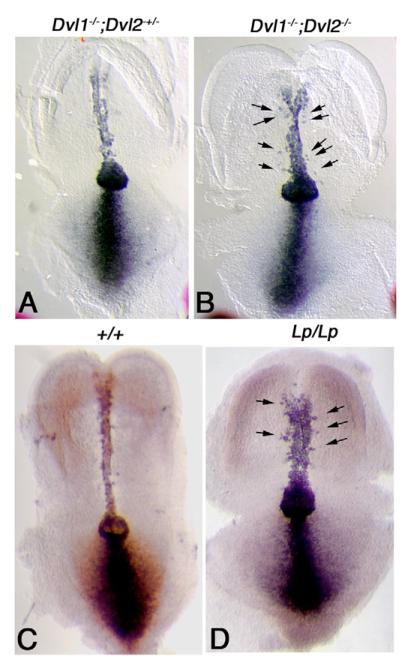
Fig. 2. In situ hybridization analysis of Brachyury (T) in early headfold stage Dvl1−/−; Dvl2−/− and Lp/Lp mutant embryos.
(A) T, a known downstream target gene of the canonical Wnt pathway, was expressed in the notochord, node and primitive streak in control embryos (Dvl1−/−; Dvl2+/−). (B) In Dvl1−/−; Dvl2−/− mutants, T expression in the node and primitive streak was maintained, suggesting normal activity of the Wnt pathway. However, T expression in the notochord was abnormally widened. T-positive cells were also observed outside of the notochord (arrows), consistent with a disruption of cell movement towards the central midline. Compared with wild-type littermates (C), T expression was also widened in the notochord of Lp/Lp embryos (D). T-positive cells were also observed outside of the notochord (D, arrows).
In contrast to the primitive streak, we observed a different pattern of T staining in the notochord. In the wild-type embryos, the notochord was slender and there were no T-positive cells outside of the notochord (Fig. 2A). In the Dvl1−/−; Dvl2−/− mutants, however, the notochord was wider, and there were also a few T-positive cells outside of the notochord (Fig. 2B). As lengthening of the notochord in the mouse was argued to be driven by convergent extension-like cell movements in the absence of cell division (Beddington, 1994), the different pattern of T staining in the Dvl1−/−; Dvl2−/− mutants suggests a reduction of PCP-dependent convergence of the notochord cells towards midline.
Lp/Lp embryos also display disruption of convergent extension
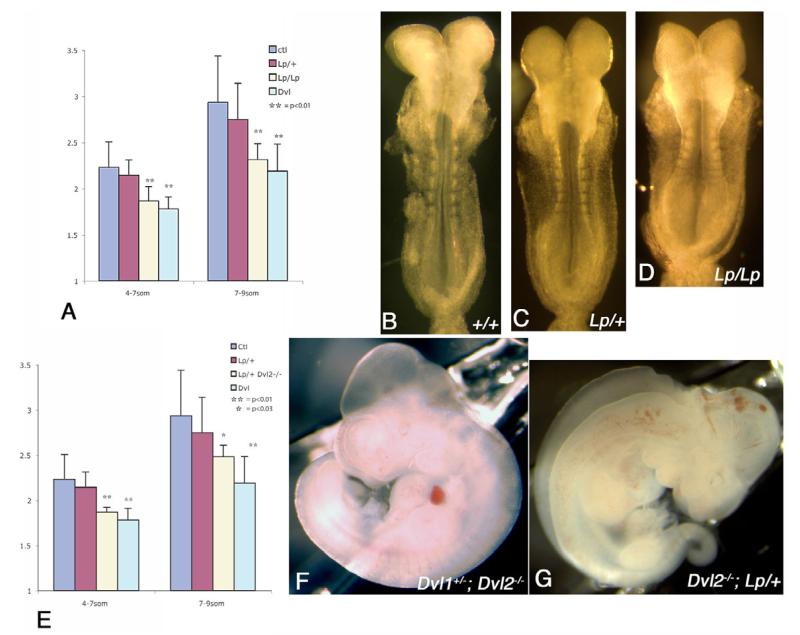
To test the validity of this hypothesis, we examined Lp mutant that harbors a loss-of-function mutation in the mouse PCP gene Vangl2 (Kibar et al., 2003; Kibar et al., 2001; Murdoch et al., 2001). When we analyzed T expression in the Lp/Lp mouse embryos, we observed a similar broadening of the notochord, as well as stranding of T-positive cells outside of the notochord, further evidence that these defects could be due to a disruption of convergent extension (Fig. 2C,D). Furthermore, when we determined the LWR in neurulating Lp/Lp mutant embryos, we found that, similar to Dvl1−/−; Dvl2−/− mutants, Lp/Lp embryos displayed a significant reduction of the LWR prior to closure 1 (Fig. 3A). Lp/+ mutants display kinky or looped tails but nonetheless manage to close their neural tubes in most cases, and displayed a moderate reduction of LWR. Accompanying the reduced LWR, Lp/+ embryos displayed delayed closure of neural tube: their neural tubes often remained unfused at the 8- to 9-somite stage, while in the same stage wild-type embryos, neural tube has fused extensively along the AP axis (Fig. 3B,C). These results provide further evidence that the mammalian PCP pathway regulates convergent extension to effect neural tube closure in the mouse.
Fig. 3. Genetic interaction between Dvl2 and Lp and reduced length-to-width ratio (LWR) in Lp/Lp and Dvl2−/−; Lp/+ embryos during neurulation.
(A) Similar to Dvl1−/−; Dvl2−/− embryos, Lp/Lp mutants displayed significant reduction of LWR during neurulation, while Lp/+ embryos displayed intermediate reduction. At the eight-somite stage, some of the neural folds were already apposed and started to fuse at the dorsal midline in wild-type embryos (B). Parallel to the reduction of LWR, however, the neural folds were still open in Lp/+ embryos (C) and widely apart in Lp/Lp embryos (D) of the same somite stage. Similar to both Dvl1−/−; Dvl2−/− and Lp/Lp mutants, all Dvl2−/−; Lp/+ embryos also displayed significant reduction of LWR during neurulation (E). From a cross between Dvl1+/−; Dvl2+/−; Lp/+ and Dvl2−/− mice, all progeny that were Dvl2−/− or Dvl1+/−; Dvl2−/− (F) displayed normal neural tube closure at E10.5. By contrast, all Dvl1+/−; Dvl2−/−; Lp/+ and Dvl2−/−; Lp/+ embryos displayed craniorachischisis (G). The error bars represent s.d.
Dvl2 and Lp genetically interact during neurulation
As Dsh and Stbm interact with each other and both are involved in the PCP in fly and convergent extension in Xenopus and zebrafish (Darken et al., 2002; Goto and Keller, 2002; Park and Moon, 2002; Wallingford and Harland, 2002; Wallingford et al., 2000), we determined whether Dvl and Lp genetically interact during mouse neurulation. We crossed Dvl1+/−; Dvl2+/−; Lp/+ triple heterozygotes with Dvl2−/− homozygotes, and genotyped the surviving offspring (Table 1). Out of 48 pups that survived, 50% of the expected Dvl2−/− homozygotes were recovered (expected six, recovered three), consistent with our previous observation that 50% of Dvl2−/− mutants die at birth because of heart defects (Hamblet et al., 2002). Reducing the dose of Dvl1 by half did not affect the viability of Dvl2−/− mutants as we were still able to recover 50% of the expected Dvl1+/−; Dvl2−/− mutants (Table 1). However, reducing the dose of Lp by half had much more deleterious effect on the viability of Dvl2−/− mutants, as we recovered no neonates that were Dvl2−/−; Lp/+, either with or without additional Dvl1 mutation.
Table 1. Neonates derived from genetic cross between Dvl1+/−; Dvl2+/−; Lp/+ female and Dvl2−/− male mice.
|
Dvl1+/−; Dvl2+/−; Lp/+ × Dvl2−/− |
||||||||
|---|---|---|---|---|---|---|---|---|
| Dvl1+/+; | Dvl1+/−; | Dvl1+/+; | Dvl1+/−; | Dvl1+/+; | Dvl1+/−; | Dvl1+/+; | Dvl1+/−; | |
| Genotype | Dvl2+/−; Lp/+ | Dvl2+/−; Lp/+ | Dvl2+/−; +/+ | Dvl2+/−; +/+ | Dvl2−/−; Lp/+ | Dvl2−/−; Lp/+ | Dvl2−/−; +/+ | Dvl2−/−; +/+ |
| Observed (48 total) | 14 | 10 | 6 | 12 | 0 | 0 | 3 | 3 |
| Expected (48 total) | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
When we dissected the embryos from this mating at midgestation stages, we found that Dvl1+/−; Dvl2−/− and Dvl2−/− mutants had closed neural tubes. By contrast, all Dvl1+/−; Dvl2−/−; Lp/+ and Dvl2−/−; Lp/+ showed craniorachischisis, a phenocopy of the Dvl1−/−; Dvl2−/− and Lp/Lp mutants (Fig. 3F,G). This result indicates that Dvl2 and Lp genetically interact and suggests they function together in the mouse to promote convergent extension during neurulation. Consistent with this hypothesis, Dvl2−/−; Lp/+ embryos displayed a severe reduction of the LWR prior to closure 1, resembling Dvl1−/−; Dvl2−/− and Lp/Lp mutants (Fig. 3E).
Association of neural tube closure defect with PCP defect in the cochlea
In addition to the neural tube closure defect, we recently found that Dvl1−/−; Dvl2−/− mutants and Dvl2−/−; Lp/+ also display disruption of the uniform stereociliary bundle orientation in the cochlea (Wang et al., 2005), a manifestation of mammalian PCP (Klein and Mlodzik, 2005; Lewis and Davies, 2002). The association of these two phenotypes has also been reported in two other mammalian PCP mutants identified so far, Lp and Celsr, strongly implying that a conserved PCP pathway regulates both hair cell polarity and neural tube closure.
We further found that both defects can be modified by genetic background variation. Although both defects were close to 100% penetrant in an inbred 129 SvEv background, Dvl1−/−; Dvl2−/− mutants showed incompletely penetrant neural tube closure defects in a mixed 129 SvEv/C57B6 background. When F1 Dvl1+/−; Dvl2+/− mice, derived from an intercross between inbred 129 Dvl1−/−; Dvl2+/− mice and wild-type C57B6 mice, were back-crossed to 129 Dvl1−/−; Dvl2+/− mice, 13% of the expected F2 Dvl1−/−; Dvl2−/− mutants survived to adults (out of 557 weaning age progeny genotyped, 86 Dvl1−/−; Dvl2−/− expected, 11 recovered), indicating successful neural tube closure during embryogenesis. This result suggests that multiple dominant modifiers from C57B6 background act together to rescue this phenotype. Interestingly, in all Dvl1−/−; Dvl2−/− mutants, either recovered at E18.5 or as adults, where the neural tube defects were rescued, the stereociliary bundles in cochlea also displayed normal polarity (data not shown), while all E18.5 Dvl1−/−; Dvl2−/− mutants that showed craniorachischisis also displayed stereociliary polarity defects (n=10) (see Wang et al., 2005). The strict association between neural tube closure and stereociliary polarity in Dvl1−/−; Dvl2−/− mutants indicates that both phenotypes can be modified by genetic background variation to the same extent, further arguing a highly conserved genetic circuitry underlies both mammalian convergent extension and PCP.
Plasma membrane distribution of Dvl2 and its dependence on the DEP domain during neurulation
During fly wing development, it has been reported that Dsh proteins are localized asymmetrically on the plasma membrane (Axelrod, 2001; Bastock et al., 2003). This asymmetric membrane distribution is both dependent upon and important for the proper establishment of planar cell polarity (Axelrod, 2001; Bastock et al., 2003). We recently generated a Dvl2 bacterial artificial chromosome (BAC) transgene that contains an EGFP-coding sequence inserted in-frame to the last codon of Dvl2 (Wang et al., 2005) and found that transgenic Dvl2-EGFP protein displayed a Vangl2-dependent polarized membrane distribution in the organ of Corti. Furthermore, the transgene was also able to rescue the cochlea elongation and stereocilia orientation defects in Dvl1−/−; Dvl2−/− mutants (Wang et al., 2005), indicating that the Dvl2 BAC transgene can fully substitute for the endogenous Dvl2 function during inner ear development.
We further found that this Dvl2 BAC transgene, as well as an additional Dvl2 BAC transgene (Dvl2-EGFP2, Fig. 4A) that contains two flanking LoxP sites, was also able to rescue Dvl1−/−; Dvl2−/− and Dvl2−/−; Lp/+ mutants to fertile adults, suggesting that the encoded Dvl2-EGFP fusion protein could also substitute for the endogenous Dvl2 during neurulation (Fig. 4B). Consistent results were acquired from all six independent lines derived from the two transgenes, suggesting BAC transgenic approach is a reliable method to produce transgenic protein capable of replacing endogenous Dvl2, presumably owing to its ability to recapitulate more consistently the endogenous gene expression pattern and level (Lee et al., 2001).
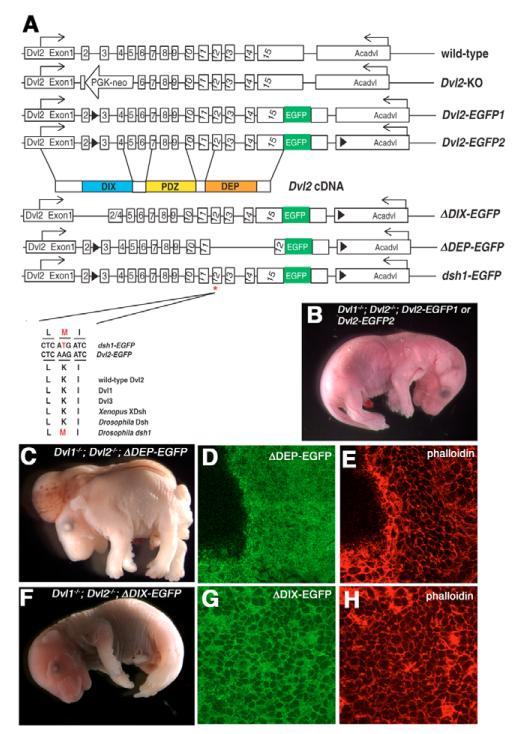
Fig. 4. Domain requirement of Dvl2 for its involvement in convergent extension and membrane distribution during neurulation.
(A) Schematic diagram of the genomic structure of the endogenous wild-type Dvl2 allele, the targeted Dvl2 null allele (Dvl2 KO), the two wild-type Dvl2 BAC transgenes, Dvl2-EGFP1 and Dvl2-EGFP2, and three mutant Dvl2 BAC transgenes: ΔDIX-EGFP, ΔDEP-EGFP and dsh1-EGFP. Although Dvl2-EGFP1 had an EGFP cassette fused in-frame to the last codon of Dvl2 and a LoxP site inserted into intron 2 for genotyping purpose (see Materials and methods), Dvl2-EGFP2 contained an additional LoxP site inserted in the 3′ flanking gene Acadvl (acylcoenzyme A dehydrogenase, very long chain). ΔDIX-EGFP and ΔDEP-EGFP deletion mutant transgenes were generated by removing sequence encoding amino acids 67-159 and 442-736, respectively. dsh1-EGFP point mutation was generated by replacing AAG with ATG to introduce a K to M substitution at amino acid 446. Lower panel showed sequence alignment of the wild-type Dvl2-EGFP and the modified dsh1-EGFP BAC transgenes, as well as endogenous mouse Dvl1, Dvl2, Dvl3, Xenopus XDsh, Drosophila Dsh and the dsh1 mutation identified in fly. Although Dvl2-EGFP1, Dvl2-EGFP2 (B) and ΔDIX-EGFP (F) can fully rescue the neural tube closure defects in Dvl1−/−; Dvl2−/− mutants, ΔDEP-EGFP transgenes completely failed to rescue the neural tube defects in Dvl1−/−; Dvl2−/− mutants (C). Confocal analysis indicated that although ΔDIX-EGFP was primarily localized to the membrane (G,H), ΔDEP-EGFP was evenly distributed in the cytoplasm (D,E).
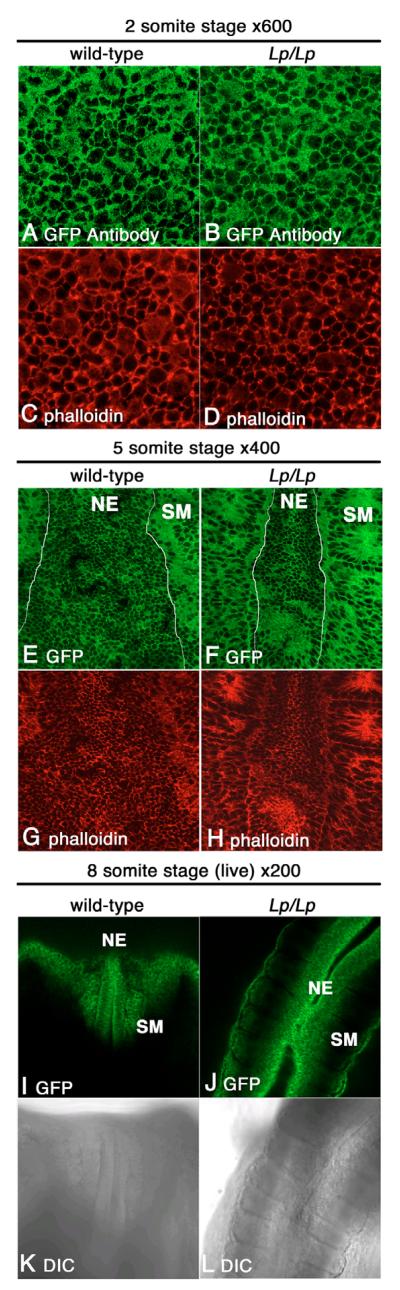
We investigated whether the Dvl2 protein localized in a polarized fashion during mouse neurulation using these Dvl2 BAC transgenes. In neurulating embryos of different somite stages, we observed the Dvl2-EGFP transgenic protein primarily localized to the plasma membrane in the neuroepithelium (Fig. 5A,E,I). The more diffuse apparent distribution of Dvl2-EGFP and F-actin in some cells in Fig. 5A-D was the result of capture of a single focal plane immediately underneath the apical plasma membrane, presumably reflecting a close association of Dvl2-EGFP to the apical plasma member. When the entire series of z stacks were examined (data not shown), it was clear that both Dvl and actin were localized to the lateral plasma membrane as individual optical sections are scanned more basally through the cell. Nevertheless, we could not find any evidence for an asymmetric pattern of Dvl2-EGFP distribution on the plane of the neuroepithelium (Fig. 5A,E,I; data not shown). Furthermore, the Lp mutation did not affect the membrane localization of Dvl2-EGFP as we observed the same membrane distribution pattern in Lp/Lp mutants (Fig. 5B,F,J; data not shown). Consistent results were observed in multiple transgenic lines using confocal microscopy of Dvl2-EGFP from live (Fig. 5I,J) and briefly fixed embryos (Fig. 5E,F), or from permanently fixed embryos stained with an anti-GFP antibody (Fig. 5A,B). In addition, although manipulating the activity of a number of PCP homologs in Xenopus and zebrafish could alter the cell orientation and elongation along the ML axis (Jessen et al., 2002; Veeman et al., 2003b; Wallingford et al., 2000), our analysis did not reveal obvious differences of cell polarity in the neuroepithelium of wild-type and Lp/Lp embryos (compare Fig. 5E,F with 5G,H). We note that in Xenopus, ML cell polarity in the mesoderm undergoing convergent extension is easy to observe because as the cells intercalate, they align and elongate mediolaterally (Shih and Keller, 1992). By contrast, neural cells elongate only episodically during convergent extension in Xenopus, so at any given time only a few cells will be elongated and appear mediolaterally polarized (Elul et al., 1997). Thus, it may be more difficult to assess mediolateral polarity in neural cells with static confocal imaging.
Fig. 5. Dvl2-EGFP was primarily localized to the membrane in the neuroepithelium during neurulation.
Single optical sections from laser-scanning confocal microscopy of neuroepithelium from neurulating embryos showed that, in the wild-type background, Dvl2-EGFP was primarily localized to the plasma membrane in the neuroepithelium (A,E,I). Consistent results were observed in wild-type and Lp/Lp mutant two-somite stage embryos stained with an anti-EGFP antibody (A,B) or through direct visualization of Dvl2-GFP signal from briefly fixed five-somite stage embryo (E,F) or live eight-somite stage embryos in culture (I,J). The apparently diffuse cytoplasmic distribution of Dvl2-EGFP and actin in some cells in A,B was due to scanning of the focal plane immediately underneath the apical plasma membrane during confocal laser microscopy. Wild-type and mutant embryos from the two- and five-somite stage were also stained with Phalloidin (C,D,G,H) to label F-actin. When the entire series of z stacks were examined (data not shown), it was clear that both Dvl and actin are localized to the lateral plasma membrane as individual optical sections are scanned more basally through the cell. Curiously, Dvl2-EGFP and actin distribution also appeared to be more diffuse in the somatic mesoderm at the five-somite stage (E-H). Overall Dvl2-EGFP distribution in the neuroepithelium was not altered in the Lp/Lp mutants (B,D,F,H,J,L; data not shown). (K,L) DIC images of live wild-type (K) or Lp/Lp mutant (L) embryos in culture. NE, neuroepithelium; SM, somatic mesoderm.
Two of the conserved domains in Dsh/Dvl, DIX and DEP, play different roles in the Wnt pathway and the PCP/convergent extension (Axelrod et al., 1998; Boutros et al., 1998; Rothbacher et al., 2000; Wallingford and Habas, 2005; Wallingford et al., 2000). In Xenopus, mediolateral intercalation in the mesoderm requires the C-terminal DEP domain but not the N-terminal DIX domain of Xdsh (Wallingford and Harland, 2001; Wallingford et al., 2000). To confirm that mammalian neurulation requires the same domain of Dvl2, we generated mutant Dvl2 BAC transgenes identical to the ΔDEP mutant in Xenopus (ΔDEP-EGFP, Fig. 4A) (Rothbacher et al., 2000; Wallingford and Harland, 2001; Wallingford and Harland, 2002; Wallingford et al., 2000). When crossed into Dvl1−/−; Dvl2−/− background, ΔDEP-EGFP completely failed to rescue the neurulation defects (Fig. 4C; eight Dvl1−/−; Dvl2−/−;ΔDEP-EGFP expected, six recovered, all of which displayed craniorachischisis). By contrast, a ΔDIX-EGFP transgene, in which part of the DIX domain was deleted, could still fully rescue the neurulation defect in Dvl1−/−; Dvl2−/− mutants (Fig. 4F; 12 Dvl1−/−; Dvl2−/−;ΔDIX-EGFP expected, 15 recovered, all of which displayed normal neural tube closure). As the ΔDIX-EGFP transgene removed a VKEEIS motif in the N-terminal region that is essential for Dvl2 function in the canonical Wnt pathway (Capelluto et al., 2002), this result also confirmed that the neural tube closure defect in Dvl1−/−; Dvl2−/− mutants was not due to the loss of function of Wnt signaling.
When we investigated the distribution of the mutant Dvl2 protein during neurulation, we found that although ΔDIX-EGFP was still primarily membrane localized (Fig. 4G), indistinguishable from wild-type Dvl2-EGFP, ΔDEP-EGFP was evenly distributed in the cytoplasm (Fig. 4D). This result is consistent with existing literature and confirmed the requirement of the DEP domain in targeting Dvl2 to the plasma membrane during mammalian neurulation.
A point mutation identical to the fly dsh1 allele disrupted the ability of Dvl2-BAC to rescue the neurulation defect in Dvl1−/−; Dvl2−/−
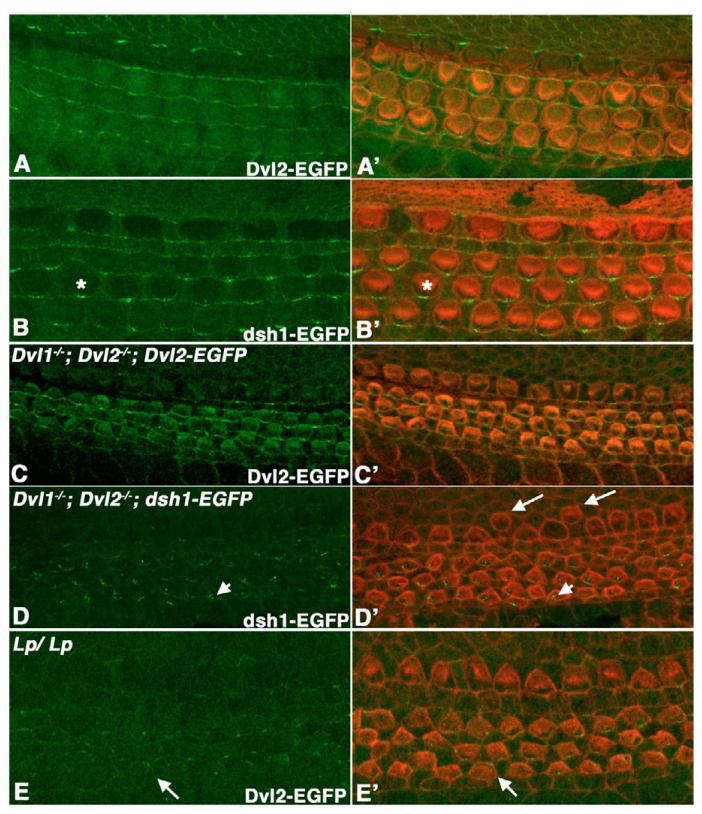
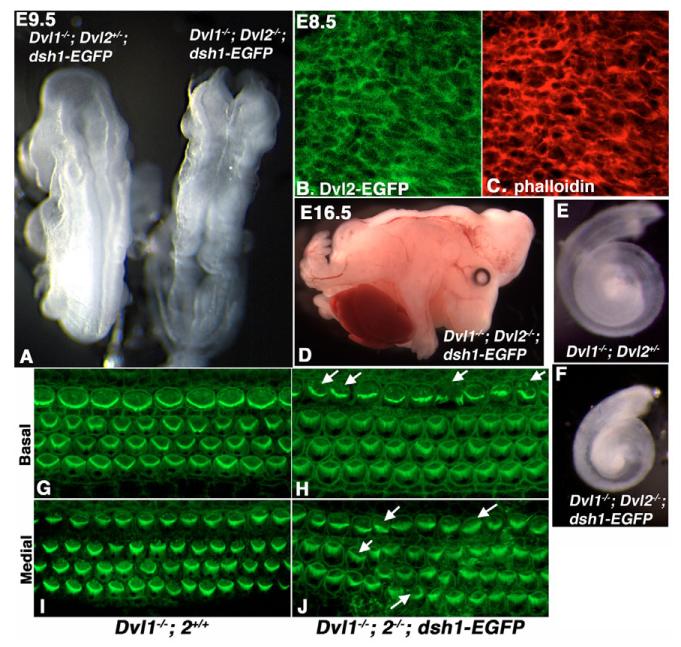
The result above suggested that DEP-dependent plasma membrane localization of Dvl2-EGFP could be a pre-requirement for its involvement in neural convergent extension and neural tube closure. However, there were differences in Dvl2-EGFP localization in the neuroepithelium and that in the organ of Corti, where Dvl2-EGFP showed an asymmetric membrane distribution that was disrupted in Lp/Lp mutants, reminiscent of fly PCP establishment (Fig. 6E) (see Wang et al., 2005). We wondered whether this could be due to a difference in how Dvl proteins function during convergent extension and PCP establishment. To further address this issue, we tested whether the point mutation dsh1 identified in fly, which specifically abolished the PCP (Axelrod et al., 1998; Boutros et al., 1998), might affect convergent extension in mammals. The dsh1 mutation results in a K to M missense mutation in the C-terminal DEP domain (Axelrod et al., 1998; Boutros et al., 1998). Using BAC recombineering, we introduced an identical mutation into Dvl2-EGFP BAC (Fig. 4A) and produced transgenic mice. All Dvl1−/−; Dvl2−/−; dsh1-EGFP embryos recovered at E9.5 or later displayed craniorachischisis (Fig. 7A,D, eight expected, six recovered, all of which displayed craniorachischisis), suggesting that the dsh1 mutation completely disrupted the ability of Dvl2 to function during convergent extension. In addition, consistent with our proposal that convergent extension underlies cochlea elongation (Wang et al., 2005), we observed a shorter and wider cochlea in Dvl1−/−; Dvl2−/−; dsh1-EGFP embryos (Fig. 6E,F). However, in either wild-type or Dvl1−/−; Dvl2−/− background, this mutation did not appear to appreciably affect the membrane localization of Dvl2-EGFP (Fig. 7B,C) in neuroepithelium during neurulation.
Fig. 6. Localization of Dvl2-EGFP and dsh1-EGFP in the organ of Corti.
(A-E) Confocal scanning at the apical surface of hair cells in whole-mount organ of Corti at E18.5, showing the native Dvl2-EGFP (A,C,E) or dsh1-EGFP (B,D) signal. (A′-E′) are the overlay of Dvl2-GFP (green, A′,C′,E′) or dsh1-EGFP (green, B′,D′) and hair cell membrane and stereociliary bundles (red) outlined with phalloidin staining. At E18.5 in an otherwise wild-type background, dsh1-EGFP localization along the distal membrane appeared to be more punctate (asterisk in B and B′) when compared with wild-type Dvl2-EGFP (A,A′). By contrast, in Dvl1−/−; Dvl2−/− background at E16.5, dsh1-EGFP localization on the membrane was mostly lost and accumulation in the cytoplasm was observed (arrowheads in D,D′). Dvl1−/−; Dvl2−/−; dsh1-EGFP mutants also display mis-alignment of inner hair cells (arrows in D′). Although wildtype Dvl2-EGFP could maintain an even distribution along the distal membrane in Dvl1−/−; Dvl2−/− background at E16.5 (C,C′), its membrane localization was reduced and no longer restricted to the distal side (arrows in E,E′) in Lp/Lp mutants at this stage.
Fig. 7. dsh1-EGFP failed to rescue the craniorachischisis, cochlear elongation and stereociliary polarity phenotypes in Dvl1−/−; Dvl2−/− embryos.
(A,D) Dvl1−/−; Dvl2−/−; dsh1-EGFP embryos (right in A) displayed craniorachischisis at E9.5 and E16.5, suggesting that dsh1-EGFP was not able to compensate for the convergent extension defect in neurulation Dvl1−/−; Dvl2−/− mutants. (B) Confocal analysis indicated that dsh1-EGFP was primarily localized to the plasma membrane in the neuroepithelium. (C) Confocal analysis of phalloidin staining. (E,F) Cochlea recovered at p0 from Dvl1−/−; Dvl2+/− and Dvl1−/−; Dvl2−/−; dsh1-EGFP mice, indicating that dsh1-EGFP also failed to rescue the cochlea elongation defect in Dvl1−/−; Dvl2−/− mutants that was also thought to be due to disruption of convergent extension. (G,I) Confocal scan at either the base (G) or medial region (I) showing uniform stereociliary orientation in the organ of Corti of a p0 Dvl1−/−; Dvl2+/+ mouse. (H,J) However, in the organ of Corti of a Dvl1−/−; Dvl2−/−; dsh1-EGFP mouse, the stereociliary bundles were mis-oriented (arrows) in some hair cells. Misalignment in the outer hair cells could also be observed.
To determine whether dsh1 also affects the PCP establishment in mammals, we analyzed the stereociliary bundle orientation in the cochlea. In P0 (postnatal day 0) control pups, the stereociliary bundles of sensory hair cells in the cochlea showed uniform orientation (Fig. 7G,I). By contrast, in a P0 Dvl1−/−; Dvl2−/−; dsh1-EGFP cochlea, we observed a disruption of the uniform stereocilia orientation and a less recognizable form of stereocilia (Fig. 7H,J), resembling the Dvl1−/−; Dvl2−/− mutants. Occasional mis-alignment of inner hair cells, which has also been found in Dvl1−/−; Dvl2−/− and Dvl2−/−; Lp/+ mutants (Wang et al., 2005), was also observed (Fig. 7J and Fig. 5D′, arrows).
In fly wing development, the dsh1 mutation was thought to disrupt PCP establishment through abolishing DSH-EGFP localization to the plasma membrane (Axelrod, 2001). To investigate how the dsh1 mutation might disrupt PCP in mammals, we examined its distribution during stereociliary bundle formation. In E16.5 Dvl1−/−; Dvl2−/−; Dvl2-EGFP embryos, before stereociliary polarity could be detected, wild-type Dvl2-EGFP was already localized to the distal membrane (Fig. 6C,C′), similar to that in the more mature hair cells at E18.5 (Fig. 6A,A′). However, in E16.5 Dvl1−/−; Dvl2−/−; dsh1-EGFP embryos, most dsh1-EGFP lost its membrane localization (Fig. 6D,D′, arrowheads) and formed aggregates in the apical cytoplasm, suggesting that the dsh1 mutation affected Dvl function in mammalian PCP establishment through interfering with its localization to the membrane. Intriguingly, the dsh1-EGFP aggregates were not evenly distributed throughout the apical cytoplasm but rather restricted primarily to the distal side of hair cells. Furthermore, the cytoplasmic dsh1-EGFP distribution was largely rescued by increasing the dose of wild-type Dvl: in phenotypically wild-type E18.5 Dvl1−/−; Dvl2+/−; dsh1-EGFP cochlea, dsh1-EGFP protein was primarily localized to membrane at the distal side of hair cells (Fig. 6B,B′), although its localization appeared to be somewhat punctate when compared with wild-type Dvl2-EGFP (Fig. 6A,A′). Similar differences were also noticed at E16.5 (data not shown). Presumably, the presence of wild-type Dvl3 and Dvl2 protein may account for the partial rescue of dsh1-EGFP localization defects.
It is interesting to note that the Lp mutation also affected Dvl2 membrane localization but in a different fashion: in E16.5 Lp/Lp mutants, Dvl2-EGFP displayed randomized and reduced apical membrane localization but rarely formed aggregates in the apical cytoplasm (Fig. 6E). Nevertheless, both the randomized membrane distribution of Dvl2-EGFP in Lp/Lp mutants and the loss of membrane distribution of dsh1-EGFP in Dvl1−/−; Dvl2−/− mutants correlated with disruption of the uniform stereociliary polarity during later development (Montcouquiol et al., 2003; Wang et al., 2005). Collectively, these results demonstrated the importance of polarized Dvl2 membrane localization in mammalian PCP establishment in hair cells, but not in neuroepithelium during neurulation.
DISCUSSION
Recent studies have revealed that convergent extension in Xenopus and zebrafish requires several proteins whose homologs are involved in establishing PCP in fly, suggesting that a mechanism highly similar to the PCP pathway regulates convergent extension (Mlodzik, 2002; Myers et al., 2002; Tada et al., 2002; Veeman et al., 2003a). However, significant differences have also been observed that may represent evolutionary adaptation in each pathway for the distinct downstream cellular and developmental process (Mlodzik, 2002; Tada et al., 2002; Veeman et al., 2003a). Our results further demonstrate that convergent extension in the neuroepithelium and PCP co-exist in the mouse and may have co-evolved by using the same set of core PCP proteins.
Using LWR measurements and T in situ of neurulating Dvl1−/−; Dvl2−/− embryos, we demonstrate an essential function of mammalian Dvl genes in a coordinated lengthening of the AP axis and narrowing of the ML axis, a morphogenetic process closely resembling convergent extension in Xenopus. By contrast, although Dsh/Dvl are also essential in the canonical Wnt pathway, our in situ analysis on Dvl1−/−; Dvl2−/− embryos did not reveal global loss of function of Wnt signaling during neurulation, presumably because the remaining Dvl3 activity is sufficient to compensate for the loss of Dvl1 and 2 in Wnt signaling. These results are also consistent with our interpretation that the neural tube phenotype of the Dvl1−/−; Dvl2−/− mutant is not secondary to defects in the canonical Wnt pathway, but to defects in the PCP pathway. Further support for this interpretation derives from our findings that similar defects in LWR and T localization were also observed in Lp/Lp and Lp/+; Dvl2−/− mutants. Lp is a mutation of the Stbm homolog Vangl2, which is a core PCP pathway component but not part of the canonical Wnt pathway (Darken et al., 2002; Goto and Keller, 2002; Kibar et al., 2001; Murdoch et al., 2001). Finally, the fact that ΔDIX-EGFP BAC transgenes with a deletion of the VKEEIS motif essential for Dvl2 involvement in the canonical Wnt pathway (Capelluto et al., 2002) was still able to fully rescue the lethality of Dvl1−/−; Dvl2−/− mutants, while ΔDEP-EGFP and dsh1-EGFP transgenes completely failed to do so, indicated that the neural tube closure defect in Dvl1−/−; Dvl2−/− was not due to the loss of function of Wnt signaling, but solely to the disruption of the PCP pathway.
Our analyses of LWR suggest that neural plate undergoes coordinated lengthening and narrowing throughout neurulation, resembling convergent extension that occurs during Xenopus neural tube closure. Furthermore, three mouse mutants, Dvl1−/−; Dvl2−/−, Lp/Lp and Lp/+; Dvl2−/−, all displayed similar severe neural tube closure defects. In each case, significant reductions in LWR were found before the seven-somite stage, a crucial time of neurulation where elevating neural folds appose at dorsal midline. These findings suggest that the mammalian PCP pathway is the driving force behind this convergent extension-like morphogenesis process and that, as in the Xenopus, a disruption of this process is the cause rather than the result of neural tube closure defects. However, it is important to note that our data do not imply a similar type of cell movement is involved in mammalian neurulation. Although convergent extension in Xenopus is driven by cell intercalation (Elul and Keller, 2000; Elul et al., 1997; Shih and Keller, 1992; Wallingford and Harland, 2001), it can also be accomplished through directed migration or oriented cell division in zebrafish (Gong et al., 2004; Solnica-Krezel et al., 1995; Wallingford et al., 2002). The exact cell behavior behind mammalian neural convergent extension remains to be determined.
It has been argued that a highly conserved pathway analogous to the PCP pathway in fly regulates convergent extension in Xenopus and zebrafish. Our results extend and strengthen this notion in mammals by demonstrating that disruption of neural convergent extension and PCP establishment co-exist in three mouse mutants: Dvl1−/−; Dvl2−/−, Lp/Lp and Lp/+; Dvl2−/− (see also Wang et al., 2005). Based on the similar neural tube closure defect, we infer that mutations of the mammalian PCP homologs Celsr1 and Fzd3/6, which cause PCP defects in the ear, also lead to convergent extension defect during neurulation (Curtin et al., 2003; Wang et al., 2006). Our finding that the missense point mutation dsh1 identified in fly that specifically disrupts the PCP pathway (Axelrod et al., 1998; Boutros et al., 1998) also abolishes the function of Dvl2 to regulate convergent extension and stereociliary polarity suggested that Dsh/Dvl functions through very conserved mechanisms in convergent extension and establishment of PCP. This notion is also supported by that fact that genetic background variation modifies neural tube closure and stereociliary polarity defects to the same extent.
Using BAC transgenes that express Dvl2-EGFP fusion proteins capable of replacing endogenous Dvl2 function, we found that Dvl2-EGFP is primarily localized to the plasma membrane in neuroepithelium, a prerequisite for its involvement in convergent extension (Park et al., 2005). Similar to the findings in fly and Xenopus, we found that the C-terminal DEP domain was required for Dvl2-EGFP localization to the plasma membrane and its ability to mediate neural tube closure and presumably, convergent extension during neurulation. By contrast, although Vangl2 is known to bind to Dvl and the Lp mutation weakens the binding (Torban et al., 2004), we did not find any change of Dvl2-EGFP distribution in the neuroepithelium of neurulating Lp/Lp mutants, indicating that the membrane localization of Dvl2 in neuroepithelium is not crucially dependent upon Vangl2 function. This observation is in direct contrast to our previous findings in cochlea where Dvl2-EGFP displayed an Lp-dependent asymmetric membrane distribution that correlates with the localization and orientation of stereocilia, resembling the establishment of PCP in fly wing cells (Wang et al., 2005). Similarly, our current results with the dsh1-EGFP transgene also revealed a disparity between neuroepithelium undergoing convergent extension and sensory hair cells during PCP establishment. In fly, the dsh1 point mutation disrupts membrane localization of Dishevelled (Axelrod, 2001). Here, we found that the same point mutation abolished Dvl2-EGFP membrane localization in the cochlea sensory hair cells in Dvl1−/−; Dvl2−/− embryos and concomitantly, its ability to establish PCP. By contrast, although the dsh1 mutation also disrupted Dvl2-EGFP function in convergent extension during neurulation, we could not detect any significant change of dsh1-EGFP distribution in the neuroepithelium. Collectively, these data suggested that plasma membrane distribution of Dvl2-EGFP might be necessary for its involvement in convergent extension, but not sufficient. Like Dvl2, it has recently been shown that Fzd3 and Fzd6 display Lp-dependent asymmetric localization in the in the inner ear, but their distribution in the neuroepithelium has not been reported (Wang et al., 2006).
If Dvl proteins function in convergent extension and PCP through highly conserved mechanisms, then why would the Lp and dsh1 mutation have different effects on Dvl2-EGFP localization in neuroepithelium undergoing convergent extension and sensory hair cells undergoing PCP establishment? One possibility is that another Vangl homolog, Vangl1, codes for a protein that allows for the membrane localization of Dvl2 in neuroepithelium but not the inner ear. In zebrafish, Vangl1 and Vangl2 have largely non-overlapping patterns of expression but similar biochemical function (Jessen and Solnica-Krezel, 2004). Vangl1 expression has not been carefully examined in the developing mammalian neural tube or inner ear.
The second possibility is that in neuroepithelium undergoing convergent extension, Dvl2-EGFP is distributed at specific locations that depend upon Vangl2 and a functional PCP pathway, but our imaging techniques are not sensitive enough to recognize them. To resolve this, we are using different Cre transgenes and the floxed Dvl2-EGFP2 transgenes to generate chimeric embryos that consist of individual Dvl2-EGFP cells surrounded by non-GFP cells. Our preliminary results showed no polarized distribution of Dvl2-EGFP that could be correlated with either the AP or ML axis of the embryo. Similar studies in Xenopus also did not reveal consistent polarized membrane localization of Dsh in mesodermal cells undergoing convergent extension (Park et al., 2005).
A third possibility is that although Dvl functions similarly in PCP establishment and convergent extension, different cellular processes demand distinct modes of action. For example, during polarity establishment in the well-aligned sensory hair cells, polarized distribution of Dvl and other PCP components dictates the precise location of stereociliary assembly within the hair cells. By contrast, in cells undergoing dynamic cell rearrangement and intercalation, the localization of Dvl and other PCP components is relatively mobile to mediate temporary cytoskeleton assembly that underlies cell-cell adhesion and generates traction (Ulrich et al., 2005; Wallingford and Harland, 2002). Supporting evidence comes from the observation that in Xenopus, XDsh mutant that blocks convergent extension also leads to less stable membrane protrusions (Wallingford et al., 2000). This view is also consistent with the proposal that PCP signaling serves as a permissive factor in convergent extension, while the alignment and orientation of cell intercalation may be determined by the AP polarity (Ninomiya et al., 2004). In this scenario, membrane localization of Dvl in the neuroepithelium may represent only a steady state distribution that does not require the function of Vangl2, nor confer polarity.
To understand more thoroughly the function of the PCP pathway in mammals, it will be important to find out not only the similarities, but also the differences between PCP and convergent extension at the cellular and molecular level. In this regard, it is interesting to note that convergent extension in Xenopus and zebrafish also involves Wnt/Ca2+ signaling, a pathway that has not been implicated in PCP determination in fly but nonetheless requires the function of PCP proteins such as Dsh and Pk (Veeman et al., 2003a). Analyzing the function of the Wnt/Ca2+ pathway in the mouse may lead to a better understanding of how the core PCP proteins co-evolved to regulate both convergent extension and PCP determination.
Acknowledgments
We thank Ella Kothari and Jun Zhao in the UCSD Cancer Center Transgenic Core for transgenic service, and the UCSD/NINDS Neuroscience Core (NINDS P30 NS047101). This work is supported by a Pete Lopiccola Fellowship, a Clifford and Evelyn Cherry Fellowship and an AHA postdoctoral fellowship to J.W.; a Burroughs Wellcome Fund Career Award in the Biomedical Sciences to J.B.W.; and by grants from the National Institute of Health to J.B.W. (R01 GM074104), A.W.B. (R01 HD43173-02) and P.C. (R01 DC005213); and the Woodruff Foundation to P.C.
References
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Beddington RS. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. doi: 10.1242/dev.120.3.613. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419:726–729. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J. 2002;21:976–985. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- Elul T, Keller R. Monopolar protrusive activity: a new morphogenic cell behavior in the neural plate dependent on vertical interactions with the mesoderm in Xenopus. Dev. Biol. 2000;224:3–19. doi: 10.1006/dbio.2000.9746. [DOI] [PubMed] [Google Scholar]
- Elul T, Koehl MA, Keller R. Cellular mechanism underlying neural convergent extension in Xenopus laevis embryos. Dev. Biol. 1997;191:243–258. doi: 10.1006/dbio.1997.8711. [DOI] [PubMed] [Google Scholar]
- Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev. Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat. Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Solnica-Krezel L. Identification and developmental expression pattern of van gogh-like 1, a second zebrafish strabismus homologue. Gene Expr. Patterns. 2004;4:339–344. doi: 10.1016/j.modgep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Gauthier S, Lee SH, Vidal S, Gros P. Rescue of the neural tube defect of loop-tail mice by a BAC clone containing the Ltap gene. Genomics. 2003;82:397–400. doi: 10.1016/s0888-7543(03)00113-7. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Kokubu C, Heinzmann U, Kokubu T, Sakai N, Kubota T, Kawai M, Wahl MB, Galceran J, Grosschedl R, Ozono K, et al. Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development. 2004;131:5469–5480. doi: 10.1242/dev.01405. [DOI] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient escherichiacoli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Lewis J, Davies A. Planar cell polarity in the inner ear: how do hair cells acquire their oriented structure? J. Neurobiol. 2002;53:190–201. doi: 10.1002/neu.10124. [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum. Mol. Genet. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 2002;18:447–455. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–367. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- Park TJ, Gray RS, Sato A, Habas R, Wallingford JB. Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr. Biol. 2005;15:1039–1044. doi: 10.1016/j.cub.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Rawls AS, Wolff T. Strabismus requires Flamingo and Prickle function to regulate tissue polarity in the Drosophila eye. Development. 2003;130:1877–1887. doi: 10.1242/dev.00411. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–914. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Stemple DL, Driever W. Transparent things: cell fates and cell movements during early embryogenesis of zebrafish. BioEssays. 1995;17:931–939. doi: 10.1002/bies.950171106. [DOI] [PubMed] [Google Scholar]
- Strutt DI. The asymmetric subcellular localisation of components of the planar polarity pathway. Semin. Cell Dev. Biol. 2002;13:225–231. doi: 10.1016/s1084-9521(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Strutt D, Johnson R, Cooper K, Bray S. Asymmetric localization of frizzled and the determination of notch-dependent cell fate in the Drosophila eye. Curr. Biol. 2002;12:813–824. doi: 10.1016/s0960-9822(02)00841-2. [DOI] [PubMed] [Google Scholar]
- Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin. Cell Dev. Biol. 2002;13:251–260. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, Ueno N. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr. Biol. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Torban E, Wang HJ, Groulx N, Gros P. Independent mutations in mouse vangl2 that cause neural tube defects in looptail mice impair interaction with members of the dishevelled family. J. Biol. Chem. 2004;279:52703–52713. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin. Cell Dev. Biol. 2002a;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002b;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Ueno N, Greene ND. Planar cell polarity genes and neural tube closure. Birth Defects Res. C Embryo Today. 2003;69:318–324. doi: 10.1002/bdrc.10029. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003a;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 2003b;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development. 2001;128:2581–2592. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr. Opin. Genet. Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr. Biol. 2001;11:R713–R724. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Sonic hedgehog and the molecular regulation of mouse neural tube closure. Development. 2002;129:2507–2517. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]