Abstract
Cigarette smoking is highly addictive, and modern genetic research has identified robust genetic influences on nicotine dependence. An important step in translating these genetic findings is to identify the genetic factors affecting smoking cessation in order to enhance current smoking cessation treatments. We review the significance of variants in the nicotinic receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) in the prediction of smoking quantity, smoking cessation, and response to cessation medication in multiple studies of smoking cessation. Three common haplotypes (low-risk, intermediate-risk, and high-risk) in the CHRNA5-CHRNA3-CHRNB4 region are defined by rs16969968 and rs680244. The genetic variants in the CHRNA5-CHRNA3-CHRNB4 region that predict nicotine dependence also predict a later age of smoking cessation in a community-based sample. In a smoking cessation trial, these variants predict abstinence at end of treatment in individuals receiving placebo medication, but not amongst individuals receiving active medication. Pharmacological treatments moderate the genetic risk in affecting cessation success. These pharmacogenetic interactions have been reproduced by a recent meta-analysis of smoking cessation trials. The number needed to treat (NNT) is 4 for smokers with the high-risk haplotype, 7 for smokers with the intermediate-risk haplotype, and >1000 for smokers with the low-risk haplotype. The wide variation in NNT between smokers with different haplotypes supports the notion that personalized smoking cessation intervention based upon genotype could meaningfully increase the efficiency of such treatment. In summary, variants in the CHRNA5-CHRNA3-CHRNB4 region identify individuals at increased risk of cessation failure, and this increased risk can be ameliorated by cessation pharmacotherapy.
Keywords: Smoking cessation, personalized medicine, pharmacogenetics
1. Introduction
Cigarette smoking is a major global public health problem. Nicotine dependence is a classic addictive disorder with symptoms of craving, withdrawal syndrome, and heavy, uncontrollable use [1]. Nicotine dependence is also manifested by both quitting difficulty [2] and a high likelihood of lapses or relapses after a quit attempt [3–6]. Therefore, identification of the factors that contribute to smoking-cessation difficulties is a critical step in understanding the biology of nicotine dependence, enhancing prediction of prognosis and treatment outcomes, and informing more effective cessation treatments.
Genomic research can lead to personalized medicine [7]. Growing research in understanding human genomes and identifying specific genetic markers for a disease will not only lead to improved understanding of the biology underlying the disease, but also improved clinical care. In clinical practice, it is common for different patients to show different efficacy or side effects with the same medication regimen, such as tamoxifen for breast cancer risk reduction in patients with selected biomarkers [8]. Increasing evidence suggests that the risk/benefit ratio of the medication may vary with a person’s genetic makeup. The goal of personalized medicine is to tailor treatments for each patient to maximize benefits and minimize side effects.
2. Genetics of Nicotine Dependence
Multiple recent large-scale genetic meta-analyses based on tens of thousands of subjects of European descent confirm the association of 15q25.1 with smoking heaviness, defined by cigarettes per day [9–12], with the most robust associations being reported for rs16969968 and rs1051730, two highly correlated variants (p<5.57*10−72) (11). In the CHRNA5-A3-B4 region, at least two independent signals have been identified [10, 13]. The first signal tagged by rs16969968, a variant that results in an amino acid change in the α5 nicotinic cholinergic receptor (CHRNA5), alters nicotinic receptor conductance in vitro [14, 15]. A second, distinct signal tagged by rs680244 is associated with variability in CHRNA5 mRNA levels [16]. Individuals of European descent have one of the three common haplotypes in the region spanning CHRNA5 and the 3′ end of CHRNA3 [13], which can be defined by these two variants: rs16969968 and rs680244 [16]. These three haplotypes represent different risk levels of nicotine dependence: low-risk (H1, 21%), intermediate-risk (H2, 44%), and high-risk (H3, 36%) haplotypes.
3. Genetics of Smoking Cessation
The CHRNA5-CHRNA3-CHRNB4 variants have been less consistently associated with cessation outcomes than with smoking heaviness measures. Five studies show an association between the CHRNA5-CHRNA3-CHRNB4 region and successful smoking cessation [17–21]. All five found that the same genetic risk variants that contribute to smoking heaviness and nicotine dependence also predicted smoking cessation. Yet, other studies fail to confirm this association [22–24]. Uhl et al. [24], in a genome-wide association of three treatment cohorts, did not identify any nicotinic receptor genes as predictors of prospectively measured smoking cessation. One large genome-wide association meta-analysis that strongly supported the association between 15q25.1 and smoking heaviness reported a modest association with current versus former smoking as a measure of smoking cessation below genome-wide level of significance [11]. Variations in study design (with or without a placebo group), ascertainment, and definitions of smoking cessation (time to relapse, abstinence, or the contrast of former vs. current smoker) may explain these inconsistent findings.
3.1 CHRNA5-CHRNA3-CHRNB4 genetic variants predict age of smoking cessation
CHRNA5-CHRNA3-CHRNB4 haplotypes have been found to be associated with age of self-reported smoking cessation in a community-based sample [25]. Compared to the low-risk haplotype (H1), the high-risk haplotype (H3) was associated with a later quit age. The median age of smoking cessation was 57 years for those with haplotype H3, and 55 years for those with haplotypes H2 and H1. This study showed that the genetic variants in the chromosome 15q25 region that predict heavy smoking and nicotine dependence also predict a later age of smoking cessation in a large community-based sample. Those with the high-risk genetic variants quit later than those at low genetic risk, manifested as a 2-year delay in median quit age.
3.2 CHRNA5-CHRNA3-CHRNB4 genetic variants predict smoking relapse and response to medication
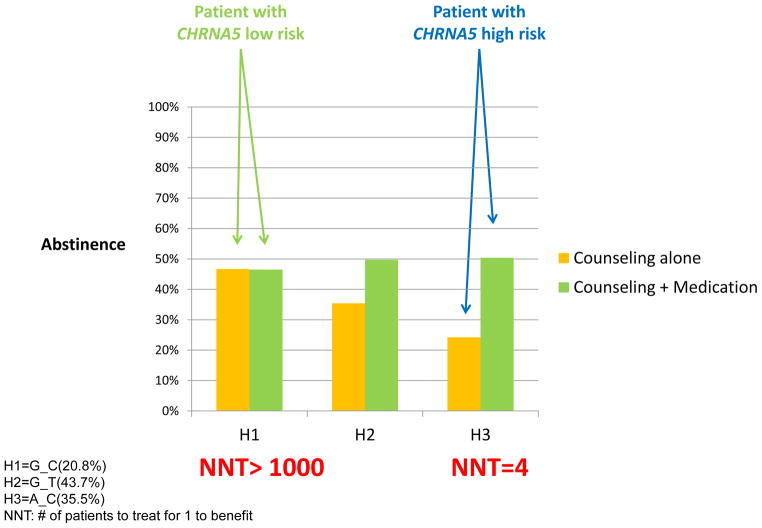
In a large-scale smoking cessation trial, smokers receiving cessation counseling with placebo medication, the high-risk haplotype (H3) that is associated with heavy smoking predicts failed abstinence in comparison to the low-risk haplotype (H1) [25]. Pharmacological treatment significantly increased the likelihood of abstinence in individuals with the high-risk haplotype (H3) but exerted little effects in individuals with the low-risk haplotype (H1). This is reflected by a significant interaction between treatment (placebo vs. active treatment) and haplotypes (Figure 1). Across the active pharmacologic treatment conditions, these genetic variants do not predict abstinence, and this reduced genetic effect with pharmacological treatments suggests that cessation treatments differ in effectiveness across the haplotypes and most strongly mitigate the genetic risks for cessation difficulty [26]. A recent meta-analysis of the Pharmacogenetics of Nicotine Addiction Treatment (PNAT) Consortium reported a similar pharmacogenetic interaction, in that patient responses to nicotine replacement therapy (NRT) were moderated by CHRNA5 genetic variants [27].
Figure 1.
CHRNA5 Predicts Cessation and Response to Medication: Number Needed to Treat (NNT) Varies with Haplotypes
H1=G_C(20.8%)
H2=G_T(43.7%)
H3=A_C(35.5%)
NNT: # of patients to treat for 1 to benefit
Medication efficacy is often represented by the number needed to treat (NNT) [25]. The NNT is 7 when computed across all individuals regardless of their haplotype status, supporting the established effect of pharmacotherapy. However, the NNT varies widely, depending on the individual’s haplotype. Based on their absolute risks, the NNT is 4 for smokers with the high-risk haplotype, 7 for smokers with the intermediate-risk haplotype, and >1000 for smokers with the low-risk haplotype. An NNT of 4 is an impressive finding, compared to the NNTs of many existing pharmacotherapies [28–30]. The wide variation in NNT between smokers with different haplotypes supports the notion that personalized smoking cessation intervention based upon genotype could meaningfully increase the efficiency of such treatment.
4. Conclusions
Multiple studies underscore the relation between the targeted haplotypes, nicotine dependence, and smoking cessation. There is a significant interaction between these CHRNA5-CHRNA3-CHRNB4 haplotypes and treatment on cessation success, and this reveals that cessation treatment effectiveness is modulated by the haplotypes. These findings strengthen the case for the development and rigorous testing of treatments that target patients with different genetic risk profiles based on the chromosome 15q25 region that includes the genes encoding the nicotinic receptor subunits. Those with the high/intermediate-risk haplotypes appear more biologically predisposed to having difficulty quitting without pharmacologic treatment, and this risk may be ameliorated by effective pharmacological treatment. Smoking cessation pharmacotherapy such as nicotine replacement therapy (NRT), bupropion, and varenicline are moderately effective, yet they do have side effects. Identifying genes related to responsiveness to pharmacologic treatment for nicotine addiction may lead to improved treatment algorithms that further the promise of personalized medicine [31].
Acknowledgments
The authors thank Sherri Fisher and Autumn Empson for their assistance in project coordination and editing/preparing the manuscript.
FUNDING SUPPORT
This research was supported by NIH grants P01 CA089392 (LJB), K02 DA021237 (LJB), and K08 DA030398 (LSC) from the National Institute on Drug Abuse, U01 HG004422 (LJB) from the National Human Genome Research Institute, and sub-award KL2 RR024994 (LSC) from the National Center for Research Resources.
Footnotes
DISCLOSURES
Dr. Bierut is an inventor on the patent “Markers for Addiction” (US 20070258898) covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. Dr. Chen declares no potential conflict of interest.
References
- 1.Breslau N, Johnson EO, Hiripi E, et al. Nicotine dependence in the United States: prevalence, trends, and smoking persistence. [see comment] Arch Gen Psychiatry. 2001;58:810–6. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- 2.Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health. 2000;90:1122–7. doi: 10.2105/ajph.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S555–70. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendricks PS, Prochaska JJ, Humfleet GL, et al. Evaluating the validities of different DSM-IV-based conceptual constructs of tobacco dependence. Addiction. 2008;103:1215–23. doi: 10.1111/j.1360-0443.2008.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozlowski LT, Porter CQ, Orleans CT, et al. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34:211–6. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 6.West R. Defining and assessing nicotine dependence in humans. In: Bock G, Goode J, editors. Understanding nicotine and tobacco addiction; Novartis Foundation Symposium, No 275; Chichester, UK: Wiley; 2005. pp. 36–58. [Google Scholar]
- 7.Green ED, Guyer MS. Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470:204–13. doi: 10.1038/nature09764. [DOI] [PubMed] [Google Scholar]
- 8.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2013 doi: 10.1200/JCO.2013.49.3122. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Liu JZ, Tozzi F, Waterworth DM, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–40. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saccone NL, Culverhouse RC, Schwantes-An TH, et al. Multiple independent loci at chromosome 15q25. 1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6(8):e1001053. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobacco and Genetics Consortium (TAG) Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–7. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–53. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss RB, Baker TB, Cannon DS, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4(7):e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bierut LJ, Stitzel JA, Wang JC, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–71. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol. 2011;79:119–25. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JC, Cruchaga C, Saccone NL, et al. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18:3125–35. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker TB, Weiss RB, Bolt D, et al. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11:785–96. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freathy RM, Ring SM, Shields B, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–7. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munafo MR, Johnstone EC, Walther D, et al. CHRNA3 rs1051730 genotype and short-term smoking cessation. Nicotine Tob Res. 2011;13:982–8. doi: 10.1093/ntr/ntr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarginson JE, Killen JD, Lazzeroni LC, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(3):275–84. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- 21.King DP, Paciga S, Pickering E, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2012;37:641–50. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breitling LP, Twardella D, Hoffmann MM, et al. Prospective association of dopamine-related polymorphisms with smoking cessation in general care. Pharmacogenomics. 2010;11:527–36. doi: 10.2217/pgs.10.1. [DOI] [PubMed] [Google Scholar]
- 23.Conti DV, Lee W, Li D, et al. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet. 2008;17:2834–48. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhl GR, Liu QR, Drgon T, et al. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–93. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LS, Baker TB, Piper ME, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry. 2012;169:735–42. doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berrettini WH, Wileyto EP, Epstein L, et al. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biol Psychiatry. 2007;61:111–8. doi: 10.1016/j.biopsych.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 27.Bergen AW, Javitz HS, Krasnow R, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics. 2013;23:94–103. doi: 10.1097/FPC.0b013e32835cdabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuzaki M, Yokoyama M, Saito Y, et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J. 2009;73:1283–90. doi: 10.1253/circj.cj-08-1197. [DOI] [PubMed] [Google Scholar]
- 29.Suissa S. Number needed to treat in COPD: exacerbations versus pneumonias. Thorax. 2013;68:540–3. doi: 10.1136/thoraxjnl-2012-202709. [DOI] [PubMed] [Google Scholar]
- 30.Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908–14. doi: 10.1016/S0140-6736(08)60416-X. [DOI] [PubMed] [Google Scholar]
- 31.Rutter JL. Symbiotic relationship of pharmacogenetics and drugs of abuse. AAPS J. 2006;8:E174–84. doi: 10.1208/aapsj080121. [DOI] [PMC free article] [PubMed] [Google Scholar]