Abstract
The presence of elevated HIV viral load within blood and genital secretions is a critical driver of transmission events. Long-term suppression of viral load to undetectable levels through the use of antiretroviral therapy is now standard practice for clinical management of HIV. Antiretroviral therapy therefore can play a key role as a means to curb HIV transmission. Results of a randomized clinical trial, in conjunction with several observational studies, have now confirmed that antiretroviral therapy markedly decreases HIV transmission risk. Mathematical models and population-based ecologic studies suggest that further expansion of antiretroviral coverage within current guidelines can play a major role in controlling the spread of HIV. Expansion of so-called “Treatment as Prevention” initiatives relies upon maximal uptake of the HIV continuum-of-care cascade to allow for successful identification of those not yet known to be HIV-infected, engagement of patients in appropriate care, and subsequently achieving sustained virologic suppression in patients with the use of antiretroviral therapy. Since 2010, the Joint United Nations AIDS (UNAIDS) program has called for the inclusion of antiretroviral treatment as a key pillar in the global strategy to control the spread of HIV infection. This has now been invigorated by the release of the World Health Organization’s 2013 Consolidated Antiretroviral Therapy Guidelines, recommending treatment to be offered to all HIV-infected individuals with CD4 cell counts below 500/mm3, and, regardless of CD4 cell count, to serodiscordant couples, TB and HBV co-infected individuals, pregnant women, and children below the age of 5 years.
Keywords: HIV care cascade, linkage to care, Seek and Treat, Treatment as Prevention
1. Introduction
The introduction of combination antiretroviral therapy (ART) has been associated with significant improvement in HIV-related morbidity and mortality [1, 2]. Long-term virologic suppression through the use of ART is now the primary goal of HIV therapy [3]. Potency of modern ART regimens has increased, associated with concomitant decrease in toxicities and simplification of the regimens with the increased availability of once-daily dosing and fixed dose combinations; as a result, HIV-infected individuals who are appropriately engaged in care are expected to see an expansion of their life expectancy in the order of four decades, approaching the overall longevity of the general population [4, 5].
Despite these advances, challenges exist in the global approach to the control of the HIV epidemic, which persists with an estimated 2.5 million individuals (range 2.2 – 2.8 million) thought to have newly acquired HIV in 2011 [6]. Prevention strategies have focused initially on behavioral interventions and condom use. Recent alternate biomedical prevention interventions have shown mixed results, with no benefit seen in trials involving treatment of genital ulcerative disease (i.e., herpes simplex virus) [7], mixed outcomes in the results of trials of vaginal microbicides [8, 9] and oral pre-exposure prophylaxis [10–12], and positive benefit in male circumcision trials [13]. Given the incontrovertible benefit of ART to HIV-infected individuals meeting treatment requirements, attention has focused on the potential secondary benefits that may be gained from expansion of ART treatment programs, with associated decreased risk of transmission in individuals accessing successful ART therapy. As described below, results of a randomized clinical trial, in conjunction with several observational studies, have now confirmed that antiretroviral therapy markedly decreases HIV transmission risk. Mathematical models and population-based ecologic studies suggest that further expansion of antiretroviral coverage within current guidelines can play a major role in controlling the spread of HIV. Maximizing programmatic identification of undiagnosed HIV-infected individuals and subsequent initiation of suppressive antiretroviral therapy has been the focus of so-called “Seek and Treat” or “Treatment as Prevention” initiatives.
2. Biologic plausibility for “Treatment as Prevention”
2.1 Mother to child transmission
Vertical transmission has long been linked to degree of maternal viral load [14, 15]. Use of suppressive combination antiretroviral therapy during pregnancy, delivery, and breastfeeding has been shown to significantly reduce the risk of vertical transmission, even in the context of a resource-limited sub-Saharan setting, where transmission occurred only in 1.1% of live-born infants [16]. In the developed world, vertical transmission is now uncommon. The transmission rate among HIV-infected women receiving ART in the United Kingdom and Ireland was 0.7% in the setting of vaginal delivery or planned caesarean section [17].
2.2 HIV viral load and sexual transmission
In a community-based study of 15,127 individuals in Rakai, Uganda, the relationship between HIV plasma viral load and risk of transmission was clearly evident; in a multivariate analysis there was an increase in the seroconversion rate ratio of 2.45 (95% confidence interval [CI] 1.85 – 3.26) for each log increase in viral load [18]. There was no evidence of transmission for the 51 individuals with plasma viral loads below 1500 copies/mL [18]. In a study of male-to-female transmission among 493 heterosexual couples in Thailand, each log increment of HIV viral load was associated with an odds ratio (OR) of 1.81 (95% CI 1.33 – 2.48) for transmission in a multivariate analysis [19]. No transmission events were documented for those with viral loads below 1000 copies/mL [19]. Similarly, in an analysis of 3381 serodiscordant couples (enrolled within the double-blind placebo-controlled randomized Partners in Prevention HSV/HIV Transmission trial of acyclovir for HIV transmission) with 5017 person-years of follow-up in Southern and East Africa [7], HIV transmission risk was 2.24 per 100 person-years in a log-linear relationship to log(10) of plasma viral load. Using this data, a mathematical model predicted a 50% reduction in transmission with a 0.70 log reduction in plasma viral load [20].
2.3 Antiretroviral therapy to reduce sexual transmission of HIV
Use of sustained ART therapy in the context of serodiscordant heterosexual relationships was associated with reduction in the risk of transmission soon after the introduction of combination regimens [21, 22]. Recent observational data continues to show a significant reduction in transmission risk with the use of ART. In the serodiscordant cohort nested within the Partners in Prevention study, use of ART was associated with a 92% reduction in transmission, from 2.24 (95% confidence interval [CI] 1.84 – 2.72)/100 person-years to 0.37 (95%CI 0.09 – 2.04)/100 person-years [23]. A meta-analysis evaluated 5,021 heterosexual couples and found an overall reduction in the rate of transmission for those receiving ART from 5.64 to 0.46 (95% CI 0.19 – 1.09)/100 person-years (a 92% reduction in risk of transmission) [24].
More recently, a randomized clinical trial (HPTN 052) evaluating the effect of early vs. deferred ART on transmission risk amongst serodiscordant couples has now been completed [25]. In this study, 1,763 couples (54% from Africa, 50% male HIV-infected partners) were randomized to receive immediate ART therapy (at a CD4 cell count of 350–500 cells/mm3) or deferred therapy initiated after CD4 decline or HIV symptom onset. Overall, 28 linked transmission events were noted, only one occurring in the early ART group (hazard ratio 0.04; 95% CI 0.01 – 0.27), a 96% reduction in transmission risk [25]. Although some observational studies [26] have not demonstrated similar benefits, the preponderance of evidence now supports a clear role for ART therapy in reducing HIV transmission.
3. Evaluation of Treatment as Prevention within British Columbia, Canada
3.1 Mathematical models
Mathematical models of the potential impact of ART on HIV incidence have been used extensively over the last decade in settings in the developed world, epidemics among men who have sex with men (MSM), and to assess the epidemic in sub-Saharan Africa [27]. These models are sensitive to the assumptions made in terms of treatment uptake, adherence, resistance to ART, and transmission risk, but serve to guide evaluation and potential interventions of Treatment as Prevention programs.
One of the early mathematical models was evaluated in British Columbia to assess the effects of potential expansion of antiretroviral coverage among those eligible for treatment, defined initially as a CD4 cell count < 200 cells/ μL [28]. In the semideterministic dynamic transmission model, increasing ART coverage from a baseline of 50% to 75% or 100% of those eligible under contemporary provincial treatment guidelines (with unchanged level of adherence) was predicted to be associated with a decrease in the annual incidence of HIV by 37% and 62%, respectively [28]. The model was updated to account for changing treatment guidelines, and assessed coverage for those deemed eligible for therapy on the basis of a CD4 cell count <350 cells/μL [29]. Expanding coverage to 75% of those deemed eligible for therapy was found to potentially avert 47% of new infections over 5 years [29]. Other models in the setting of developed-world epidemics have shown similar outcomes in some studies [30, 31] but not others [32, 33].
3.2 Ecologic studies
The effects of ART at a community and population level have been evaluated in British Columbia and in other settings (Table 1). The effect of ART on HIV incidence in a cohort of injection drug users within Vancouver’s Downtown Eastside neighborhood has been assessed [34] over the period of 1996–2007. A community viral load, calculated as the median measure of all viral load measurements for individuals with known HIV status was followed longitudinally (total 12,435 measures), and the impact on HIV incidence was evaluated while adjusting for risk behavior. Overall, the median community viral load concentration fell below 20,000 copies/mL after 1998 after the introduction of ART. Community viral load remained independently associated with time to HIV seroconversion (HR 3.32; 95%CI 1.82 – 6.08), but this was no longer statistically significant after median viral loads declined below 20,000 copies/mL [34]. Similar decreases in HIV incidence have been observed in the ALIVE cohort in Baltimore, Maryland, USA, where incidence decreased by 74% for every log decrease in community viral load [35]. In addition, there has been no evidence of behavioral disinhibition amongst a cohort of injection drug users in Vancouver where initiation of antiretroviral therapy was not associated with subsequent increased unprotected intercourse (aOR 0.93; 95%CI 0.61 – 1.40) or multiple sexual partnerships (aOR 0.93; 95%CI 0.61 – 1.40) in multivariate analysis [36]. This result is in keeping with findings from ART programs in sub-Saharan Africa [37], but contrasting results with increases in unprotected intercourse have been observed amongst certain MSM populations [38].
Table 1.
Ecologic studies of community viral load and treatment as prevention of new HIV infections.
| Setting | Time Period | Evaluation | Outcomes | Reference |
|---|---|---|---|---|
| Taiwan | 1984 – 2002 | National HIV surveillance data. Transmission rate estimated by use of exponential model. |
Transmission rate 0.391 new cases/prevalent cases pre-ART Transmission rate 0.184 new cases/prevalent cases post-HAART. Overall decrease 53%. |
[52] |
| Vancouver, British Columbia, Canada | 1996–2007 | Prospective cohorts of injection drug users. Median CVL*. Cox regression model to association with HIV incidence. |
CVL associated with time to HIV seroconversion (Hazard ratio 3.32 per log10 increase). After median viral load fell to <20,000 copies/mL, no statistical association with HIV incidence observed. |
[34] |
| San Francisco | 2004–2008 | HIV/AIDS public health surveillance for new diagnoses and calculated HIV incidence. Mean Community viral load. Poisson models for CVL and new HIV diagnoses. |
Significant decline in mean CVL 2004–2008 (p=0.037). Reduction in CVL associated with decrease in new HIV diagnoses (p=0.003). |
[53] |
| British Columbia, Canada | 1996–2009 | HAART† coverage from centralized registry. HIV public health surveillance for new diagnoses. Median CVL. Poisson models for CVL, ART coverage and new HIV diagnoses. |
547% increase in ART uptake. 52% decrease in new HIV diagnoses. For every 100 additional individuals on ART, new cases decreased by factor of 0.97. For every 1 log10 drop in CVL, new cases decreased by factor of 0.86. |
[39] |
| Hlabisa sub-district, KwaZulu-Natal, South Africa | 2004–2011 | Cohort of 16,667 HIV uninfected individuals. ART coverage and HIV prevalence within the surrounding community assessed, and rate of new seroconversions captured. |
As ART coverage within a community expands, risk of acquisition decreases: for communities with 30–40% ART penetration, risk of new infection dropped 38% compared to communities with <10% ART uptake. | [54] |
CVL = community viral load
ART = highly active antiretroviral therapy. Adapted from [55] with permission.
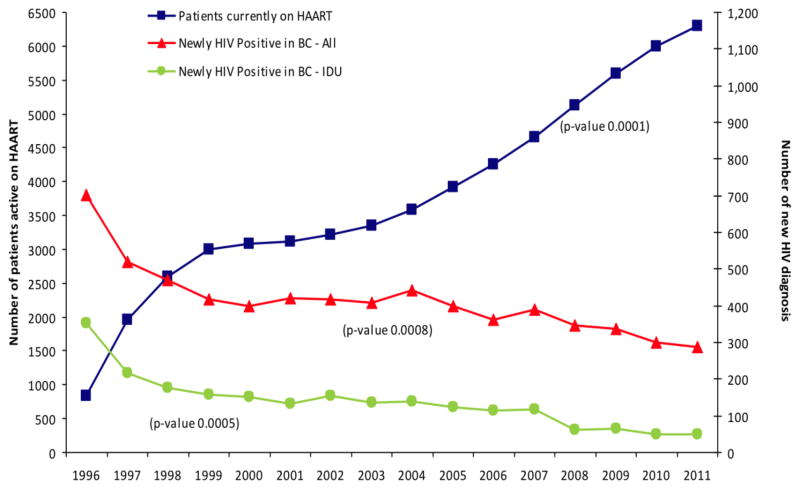
A population-based ecologic evaluation of ART expansion and decreasing new HIV diagnoses has been presented [39]. Antiretroviral therapy is provided free of charge to HIV-infected individuals within the Province of British Columbia, Canada, via a central Drug Treatment Program which maintains a population-based registry of individuals receiving ART. Between 1996 and 2012, the number of individuals receiving therapy increased 547% from 837 to approximately 7,700 individuals. During this time period, the proportion of individuals with viral load <500 copies/mL increased from below 10% to 50% (p <0.0001). Overall new diagnoses of HIV decreased from 702 to 248 cases per year between 1996 and 2012 (see Figure 1). The number of HIV diagnostic tests increased over this time period, and rates of syphilis, gonorrhea, and chlamydia increased, suggesting no changes in risk behavior [39]. The overall effectiveness of current regimens has resulted in improvement in viral load suppression, with the proportion of individuals achieving full virologic suppression with plasma viral load <50 copies/mL increasing from 64.7% in 2000 to 87.0% in 2008 [40]. In addition, the proportion of individuals with resistance to >2 drug classes has fallen to less than 2% of individuals currently enrolled in the program from 12% in 2000 [41]. These findings stand in contrast to other jurisdictions in Canada, where new diagnosis rates of HIV are either stable or increasing (Figure 2) [42].
Figure 1.
Number of active HAART participants and number of new HIV diagnoses per year in British Columbia, 1996–2011. Adapted from [39] with permission.
Figure 2.
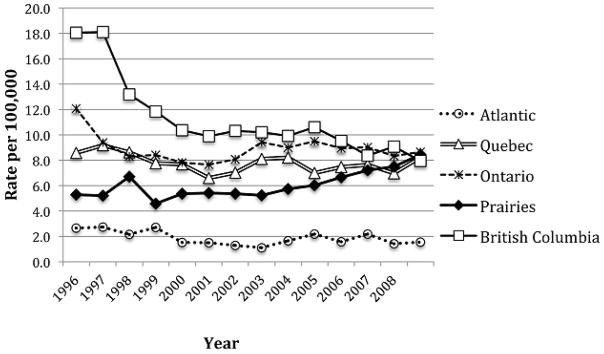
Rate of new HIV diagnoses by Region, Canada 1996–2008. From [42] with permission.
3.3 Cost effectiveness
In the British Columbia model, cost-savings accrue with infections averted over time; expanding ART access from 50% to 75%, of those meeting 2008 treatment guideline criteria, has been predicted to result in an eventual cost-savings of $900 million over 30 years [43]. Similarly, in an updated model in South Africa, expanding coverage to CD4 cell counts of 350 cells/mm3, 500 cells/mm3 or any CD4 level would avert infections and be cost-saving, with these benefits diminished if retention in care programs was diminished [44, 45]. Conversely, in models that have evaluated scenarios where treatment programs do not achieve full implementation or coverage, costs would, in fact, increase [46].
3.4 The continuum-of-care cascade
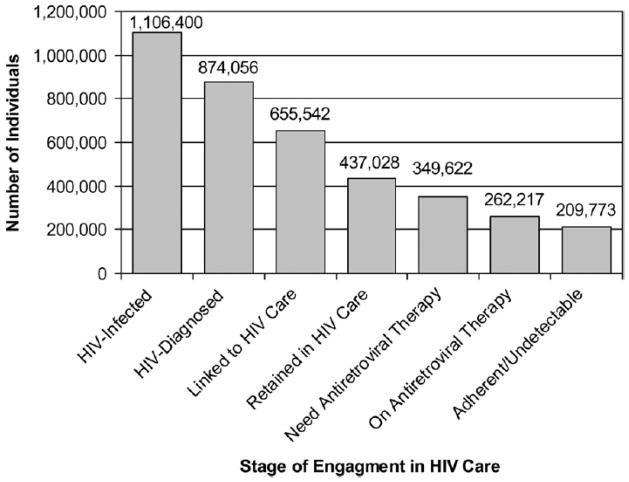
The HIV care cascade has been a seminal framework in which to evaluate potential gaps in HIV programs [47]. The care cascade evaluates the HIV treatment model from initial estimates of undiagnosed individuals living with HIV through proportions of individuals appropriately diagnosed, engaged and retained in care, and ultimately achieving sustained virologic suppression. Each aspect of this care cascade should be maximized if Treatment as Prevention programs are to succeed. Gardner et al. evaluated the care cascade for the United States as a whole, basing estimates on reported values for each step (Figure 3) [47]. Of the 1.2 million HIV-infected individuals in the United States, approximately 20% were unaware of their diagnosis, and ultimately only 19% of individuals were successfully retained and their viral load suppressed on ART. These estimates are remarkably similar to those obtained by the CDC using a number of U.S. national surveillance instruments, with 28% of all HIV-infected individuals’ viral loads fully suppressed on ART [48]. A similar HIV care cascade has been generated for British Columbia, evaluating estimated undiagnosed cases within the province, those engaged and retained in care, those adherent to medications, and those with fully suppressed viral loads [49]. In this model, virologic suppression was defined as having a sustained undetectable plasma viral load (<50 copies/mL for period 1999 – 2009). Overall, only 14% of individuals were thought to be undiagnosed in 2009, compared to 47.1% in 1996, and 32.1% (range 26.8 – 37.3%) had a fully suppressed viral load in 2009[49].
Figure 3.
Spectrum of engagement in care, United States. From Gardner et al [47]
4. Conclusion
Antiretroviral therapy can reduce individual-level morbidity and mortality for those affected by HIV, and has been demonstrated to significantly reduce transmission risk, particularly amongst heterosexual serodiscordant couples. At a population level, expansion of successful ART programs translates into decreased community burden of viremic individuals, with concomitant decrease in transmission and decreased incidence of HIV. Potential barriers may include the impact of acute HIV infections as a cause of ongoing transmission, efficacy of ART for prevention benefits within MSM communities, and risks of concomitant behavioral disinhibition.
At present, the overall proportion of individuals receiving ART is low due to high proportions of undiagnosed individuals and incomplete virologic suppression in those known to be HIV-infected. Strategies to improve engagement at each step of the care cascade represent critical interventions to maximize the individual and societal impact of ART and therefore deliver on the promise of HIV Treatment as Prevention. Since 2010, the Joint United Nations AIDS (UNAIDS) program has called for the inclusion of antiretroviral treatment as a key pillar in the global strategy to control the spread of HIV infection [50]. This has now been invigorated by the release of the World Health Organization’s 2013 Consolidated Antiretroviral Therapy Guidelines, calling for treatment to be offered to all HIV-infected individuals with CD4 cell counts below 500/mm3, and, regardless of CD4 cell count, to serodiscordant couples, TB and HBV co-infected individuals, pregnant women, and children below the age of 5 years [51]. The stage is therefore set for the global community to implement Treatment as Prevention as a highly cost-effective opportunity to achieve the dual goal of halting HIV/AIDS-related morbidity and mortality and curbing the spread of HIV.
Footnotes
- Dr. Mark Hull receives support from the U.S. National Institute on Drug Abuse (grant number R01DA031043-01). He has served on speakers bureau or advisory boards of Merck, Janssen, Vertex, Bristol-Myers-Squibb, ViiV, Pfizer.
- Dr. Julio Montaner is supported by the British Columbia Ministry of Health; through an Avant-Garde Award (No. 1DP1DA026182) from the National Institute on Drug Abuse (NIDA), at the U.S. National Institutes of Health (NIH); and through a Knowledge Translation Award from the Canadian Institutes of Health Research (CIHR). He has also received financial support from the International AIDS Society, United Nations AIDS Program, World Health Organization, National Institutes of Health Research-Office of AIDS Research, National Institute of Allergy & Infectious Diseases, The United States President’s Emergency Plan for AIDS Relief (PEPfAR), Bill & Melinda Gates Foundation, French National Agency for Research on AIDS & Viral Hepatitis (ANRS), the Public Health Agency of Canada, the University of British Columbia, Simon Fraser University, Providence Health Care, and Vancouver Coastal Health Authority. He has received grants from Abbott, Biolytical, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
References
- 1.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–4. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 4.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa F, Lodwick RK, Smith CJ, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26:335–43. doi: 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- 6.UNAIDS. UNAIDS report on the global AIDS epidemic. 2012 [Google Scholar]
- 7.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. New Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. New Engl J Med. 2008;359:463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 10.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. New Engl J Med. 2012;367:411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 14.Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. New Engl J Med. 1996;335:1621–9. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 15.Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. New Engl J Med. 1999;341:385–93. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. New Engl J Med. 2010;362:2282–94. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend CL, Cortina-Borja M, Peckham CS, et al. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22:973–81. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 18.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. New Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 19.Tovanabutra S, Robison V, Wongtrakul J, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J Acquir Immune Defic Syndr. 2002;29:275–83. doi: 10.1097/00126334-200203010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PloS One. 2010;5:e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castilla J, Del Romero J, Hernando V, et al. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;40:96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan PS, Kayetinkore K, Chomba E. Reduction of HIV transmission risk and high risk sex while prescribed ART: results from discordant couples in Rwanda and Zambia. Abstract52bLB. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. [Google Scholar]
- 23.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attia S, Egger M, Muller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Ge Z, Luo J, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr. 2010;55:232–8. doi: 10.1097/QAI.0b013e3181e9b6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams BG, Lima V, Gouws E. Modelling the impact of antiretroviral therapy on the epidemic of HIV. Curr HIV Res. 2011;9:367–82. doi: 10.2174/157016211798038533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lima VD, Johnston K, Hogg RS, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis. 2008;198:59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 29.Lima VD, Hogg RS, Montaner JS. Expanding HAART treatment to all currently eligible individuals under the 2008 IAS-USA Guidelines in British Columbia, Canada. PloS One. 2010;5:e10991. doi: 10.1371/journal.pone.0010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlebois ED, Das M, Porco TC, et al. The effect of expanded antiretroviral treatment strategies on the HIV epidemic among men who have sex with men in San Francisco. Clin Infect Dis. 2011;52:1046–9. doi: 10.1093/cid/cir085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorensen SW, Sansom SL, Brooks JT, et al. A mathematical model of comprehensive test-and-treat services and HIV incidence among men who have sex with men in the United States. PloS One. 2012;7:e29098. doi: 10.1371/journal.pone.0029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287:650–4. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 33.Walensky RP, Paltiel AD, Losina E, et al. Test and treat DC: forecasting the impact of a comprehensive HIV strategy in Washington DC. Clin Infect Dis. 2010;51:392–400. doi: 10.1086/655130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk G, Galai N, Astemborski J, et al. Decline in community viral load strongly associated with declining HIV incidence among IDU. 2011. Abstract 484. 18th Conference on Retroviruses and Opportunistic Infections; 27 February–2; March; Boston, Massachusetts. [Google Scholar]
- 36.Marshall BD, Milloy MJ, Kerr T, et al. No evidence of increased sexual risk behaviour after initiating antiretroviral therapy among people who inject drugs. AIDS. 2010;24:2271–8. doi: 10.1097/QAD.0b013e32833dd101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkatesh KK, de Bruyn G, Lurie MN, et al. Decreased sexual risk behavior in the era of HAART among HIV-infected urban and rural South Africans attending primary care clinics. AIDS. 2010;24:2687–96. doi: 10.1097/QAD.0b013e32833e78d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips AN, Cambiano V, Nakagawa F, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PloS One. 2013;8:e55312. doi: 10.1371/journal.pone.0055312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill VS, Lima VD, Zhang W, et al. Improved virological outcomes in British Columbia concomitant with decreasing incidence of HIV type 1 drug resistance detection. Clin Infect Dis. 2010;50:98–105. doi: 10.1086/648729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Serna A, Lima VD, Montaner JS, et al. “Test-and-treat” strategy for control of HIV and AIDS can lead to a decrease, not an increase, of multidrug-resistant viruses. Clin Infect Dis. 2013;57:478–9. doi: 10.1093/cid/cit257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogg RS, Heath K, Lima VD, et al. Disparities in the burden of HIV/AIDS in Canada. PloS One. 2012;7:e47260. doi: 10.1371/journal.pone.0047260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston KM, Levy AR, Lima VD, et al. Expanding access to HAART: a cost-effective approach for treating and preventing HIV. AIDS. 2010;24:1929–35. doi: 10.1097/QAD.0b013e32833af85d. [DOI] [PubMed] [Google Scholar]
- 44.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 45.Granich R, Kahn JG, Bennett R, et al. Expanding ART for treatment and prevention of HIV in South Africa: estimated cost and cost-effectiveness 2011–2050. PloS One. 2012;7:e30216. doi: 10.1371/journal.pone.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by ‘test and treat’ in hyperendemic settings. AIDS. 2010;24:729–35. doi: 10.1097/QAD.0b013e32833433fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vital signs: HIV prevention through care and treatment--United States. MMWR. 2011;60:1618–23. [PubMed] [Google Scholar]
- 49.Nosyk B, Montaner J, Colley G, et al. Estimating the cascade of HIV care longitudinally in British Columbia: 1996–2009. Abstracts and proceedings, 3rd Annual International Treatment as Prevention Workshop; Vancouver, Canada. April 22–25, 2013. [Google Scholar]
- 50.UNAIDS. [Accessed July 10, 2013.];Treatment 2015. Available at http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/JC2484_treatment-2015_en.pdf.
- 51.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2013 Jun 26; [PubMed] [Google Scholar]
- 52.Fang CT, Hsu HM, Twu SJ, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004;190:879–85. doi: 10.1086/422601. [DOI] [PubMed] [Google Scholar]
- 53.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PloS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanser F, Barnighausen T, Grapsa E, et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–71. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hull MW, Montaner J. Antiretroviral therapy: a key component of a comprehensive HIV prevention strategy. Curr HIV/AIDS Rep. 2011;8:85–93. doi: 10.1007/s11904-011-0076-6. [DOI] [PubMed] [Google Scholar]