Abstract
Objective
The external knee adduction moment (EAM) is often considered a surrogate measure of the distribution of loads across the tibiofemoral joint during walking. This study was undertaken to quantify the relationship between the EAM and directly measured medial tibiofemoral contact forces (Fmed) in a sample of subjects across a spectrum of activities.
Methods
The EAM for 9 patients who underwent total knee replacement was calculated using inverse dynamics analysis, while telemetric implants provided Fmed for multiple repetitions of 10 activities, including walking, stair negotiation, sit-to-stand activities, and squatting. The effects of the factors “subject” and “activity” on the relationships between Fmed and EAM were quantified using mixed-effects regression analyses in terms of the root mean square error (RMSE) and the slope of the regression.
Results
Across subjects and activities a good correlation between peak EAM and Fmed values was observed, with an overall R2 value of 0.88. However, the slope of the linear regressions varied between subjects by up to a factor of 2. At peak EAM and Fmed, the RMSE of the regression across all subjects was 35% body weight (%BW), while the maximum error was 127 %BW.
Conclusion
The relationship between EAM and Fmed is generally good but varies considerably across subjects and activities. These findings emphasize the limitation of relying solely on the EAM to infer medial joint loading when excessive directed cocontraction of muscles exists and call for further investigations into the soft tissue–related mechanisms that modulate the internal forces at the knee.
The knee is required to transmit multiples of body weight across the joint, even during normal activities of daily living (1,2). Excessive joint contact forces, in particular loading conditions under which the mechanical forces are transferred disproportionally across the tibiofemoral joint, are considered to be a driving factor of osteoarthritis (OA) (3). As a result of the disease, the structural integrity and functionality of the joint are compromised, and pain and loss of overall function and mobility often ensue (4). Understanding the conditions under which high forces are transferred primarily through one compartment of the knee is therefore a key prerequisite for the identification of patients at risk of early onset or accelerated progression of OA, and is essential for planning and evaluating therapeutic approaches that aim to specifically alter the mechanics of tibiofemoral articulation (5).
Despite the importance of joint loading conditions in the context of OA initiation and progression, accurate noninvasive methods for direct assessment of the compartmental loading within the human knee are currently not available. As a result, indirect measures, including the external knee adduction moment (EAM), are often used as surrogate measures of the load distribution in the frontal plane, since these can be readily derived using gait analysis and inverse dynamics techniques (6). Importantly, there is strong evidence suggesting that the EAM is a predictor of progression of knee OA (7–9). There is also evidence that the EAM can be modified by surgical procedures such as high tibial osteotomy (5,10). Noninvasive approaches to reduce the EAM have included orthoses, footwear interventions (11,12), gait modifications such as increased lateral trunk lean (13,14), “medial thrust gait” (15), and active changes in foot progression angle (16,17). While those studies indicate that the EAM can indeed be manipulated and reduced, their efficacy varies considerably (18), possibly because a reduction in the EAM may not be consistently linked to a reduction in the medial joint contact force, but rather to a change in the ratio of the medial to the total axial tibiofemoral contact force (MR) (19).
Recently, telemetric implants have enabled the knee joint contact forces to be measured directly (20). Such implants have allowed an understanding of the effect of variable-stiffness shoes (11), nordic walking poles (15), and valgus braces (21) for reducing the medial tibiofemoral contact force (Fmed) in vivo. Furthermore, first investigations into the relationship between the EAM and the in vivo measured Fmed found a good correlation between the 2 measures (22). However, although a correlation between EAM and Fmed was demonstrated by manipulating shoe stiffness (11), reducing EAM by modifying gait patterns did not appear to have a consistent impact on Fmed (23). Importantly, those studies were performed during walking activities in a single subject only, and it therefore remains unclear whether these initial findings are consistent across different activities or between subjects. It is indeed conceivable that variations in muscle activation patterns, resulting from either different locomotor requirements, variations in individual soft tissue competence, or differences in neuromuscular control (24), might influence the manner in which the external moments are balanced by active and passive soft tissue forces between activities and subjects (25).
Although the muscle forces are known to be key determinants of the internal forces transmitted at the knee (26), and are thus probably key modulators of the relationship between external moments and internal joint contact forces (27), their specific influence remains elusive due to a lack of methods for their direct determination. Direct in vivo measurements of the joint contact forces, however, have already found the MR to be activity dependent (28,29), highlighting the need to further elucidate the relationship between EAM and Fmed across a spectrum of activities. An improved understanding of the conditions under which the EAM might serve as a suitable measure of the internal loading conditions within the joint could allow improved clinical decision making based on accessible noninvasive measures. The objective of the present study was therefore to quantify the relationship between the EAM and directly measured Fmed, as well as MR, in a sample of subjects across a spectrum of activities.
PATIENTS AND METHODS
Subjects and activities
Gait analysis was performed on 9 patients (6 men and 3 women) who underwent total knee replacement (TKR) (mean ± SD age 70 ± 5 years, body mass 90.5 ± 12.6 kg, and height 1.72 ± 0.04 meters). Patients were assessed a mean ± SD of 26 ± 13 months after surgery, and had a mechanical axis angle ranging from 4.5° valgus to 7° varus. Each subject had previously been implanted with a telemetric knee implant that was based on a commercially available design (Innex; Zimmer) (20) and that allowed the noninvasive transmission of internal joint contact force data (∼100 Hz, 3 forces + 3 moments that acted on the tibial tray). The telemetric implants were used to record in vivo tibiofemoral forces during the following activities: walking, stair climbing (20-cm step height and 26-cm step run), stair descending, sit-to-stand (seat height adjusted to 90° knee flexion during sitting), stand-to-sit, 3 variants of squatting, one-legged stance, and a transfer of the subject's body weight from one leg to the other in a standing posture. While maintaining the reference position of their feet established for the neutral squat (approximately shoulder-width apart), subjects were asked to squeeze their knees together (valgus squat) or push their knees apart (varus squat). All subjects completed the activities in a single session, following a standardized protocol with the same order of activities, with sufficient time allowed to rest between activities to prevent fatigue. Data for the squat variants could not be obtained for 3 patients (K6L, K7L, and K9L) because of the finite amount of time available for the measurement session. Six subjects completed all 10 activities. On average, 5 repetitions of each activity were free from data errors and suitable for analysis. All subjects provided written informed consent to participate in the procedures, and the study was approved by the local ethics committee.
Subject-specific anatomy
Subject-specific musculoskeletal models of each subject's lower limb bones and muscles were derived from postoperative full-leg computed tomography scans (1-mm slice thickness). A reference muscle geometry, based on the Visible Human data set (26,30), was then adapted to each subject using the techniques described by Trepczynski and coworkers (26). These anatomical models included bone surfaces, implant surfaces, and anatomical landmarks, which were used to quantify the bony geometry and to consistently define local coordinate systems for describing segment as well as 3-dimensional (3-D) implant positions and orientations. Segment circumferences were each collected at 2 locations in order to approximate the segment mass distribution using geometric relationships, and hence the inertial parameters of the segments (30).
Determination of the external moments
During each activity, the external loads were measured using two 6 degrees of freedom force plates (AMTI), while the 3-D kinematics of each subject's lower limbs were measured using reflective markers attached to the skin, tracked at 120 Hz using a 10-camera motion capture system (Vicon). Six markers were placed on the pelvis, 8 on each thigh and shank, and 4 on each foot (31), for determining skeletal kinematics, as described in detail previously (26). Subject-specific skeletal anatomy was fitted to dynamic functional joint axes and centers (31,32), using a global optimization approach. The segment and joint kinematics, as well as the ground reaction forces and inertial parameters, were then used as input to an inverse dynamics approach to yield the 3-D intersegmental resultant forces and moments (external moments) throughout the lower limb. Although the 3-D external moments were calculated for each joint, only the EAM and the external knee flexion moment (EFM) were considered.
Relationship between the external moments and the internal forces
The medial and lateral components of the axial tibiofemoral joint contact force (Fmed, Flat) were computed based on the total axial force and the internal knee adduction moment (IAM) measured by the telemetric implants, together with the distance between the medial and lateral contact points. Decomposition of the tibiofemoral contact force was based on the assumption that negligible friction existed in the frontal plane, with contact fixed at the lowest points on the tibial inlay. Laboratory tests showed this decomposition to be accurate within 3% as long as the in vivo measured axial force was >1,000N (21). As a result, only those sections of the activity cycles for which the in vivo axial force was >1,000N were considered in the analyses. Relevant movement phases of the activities were defined as the phase from heel strike to toe-off for walking and stair negotiation, as the phases during which the knee flexion changed for squatting and rising from a chair and sitting, or as the phase where only the leg of interest had ground contact during a one-legged stance. The relative time (peak time point as a fraction of the activity cycle), knee flexion, and MR were determined at both peaks. The MR of the axial tibiofemoral force was computed as:
Relationships between Fmed and EAM across subjects and activities
Exploratory analyses suggested that the relationship between Fmed and EAM was generally linear, but MR and EAM appeared to be related in a nonlinear fashion. Therefore, to assess whether subjects and activities were important factors for the relationship between external moments and internal forces, linear and nonlinear regression modeling techniques considering either Fmed or MR as the dependent variable were used.
The factor “subject” was assessed using linear mixed-effects model analysis performed on all activities, including a total of 430 data sets across all 10 subjects. Random effects were modeled using random slopes and random intercepts for the crossed grouping variables “subject” and “activity,” to assess whether and how the regressions linking Fmed and EAM varied across subjects. Analyses focused on the relationship between the peak EAM and the in vivo measured Fmed at the same time point.
Three additional linear mixed-effects models were evaluated for investigating the factor “activity,” the relationship between the external moments and internal forces throughout the entire loaded phases across activities. These models considered either the signed EFM or its absolute value (23):
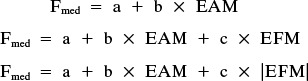 |
where a, b, and c are free regression parameters.
These analyses used the same 430 data sets but were based on all frames rather than peak values per activity alone. Random effects were again considered but with the focus on evaluating whether and how the regressions linking Fmed and EAM varied across activities.
Interaction of EAM and mechanical axis angle
For assessing the relationships between peak Fmed and EAM, an additional model included the mechanical axis angle, a measure of static limb alignment, as a covariate to determine whether the interaction of EAM and mechanical axis angle explained significant variance in the data.
Relationship between the MR and the EAM
Nonlinear regression was used to quantify the relationship between the MR and EAM. Initial analyses demonstrated that an arctangent function appropriately estimated the data, since MR appeared to asymptotically approach extreme values (0 = lateral and 1 = complete medial loading). The nonlinear relationship was described as:
In order to characterize the MR–EAM relationship, a first fit was performed over all frames for all subjects and activities. To further explore the role of alignment, 2 additional groups were compared. Group 1 (valgus group) included subjects with a mechanical axis angle less than the median value, while group 2 (varus group) included those with a mechanical axis angle greater than the median for all 9 subjects.
Role of the soft tissues in balancing the external moments
Finally, to better understand the role of the soft tissues in balancing the external moments, the relationship between the EAM and the IAM, resulting from the MR, was analyzed using linear regression over the relevant movement phases. The difference between the in vivo measured tibiofemoral contact force and the intersegmental resultant force at the knee (from the inverse dynamics analysis) was determined at the instant of peak Fmed to further quantify subject-specific and activity-specific strategies for balancing the external moments through the soft tissues.
Evaluation of significant effects
Analyses were performed using the R software package (2013; R Core Team). The mixed-effects models were evaluated using the lme4 package. Visual inspection of residual plots did not reveal any obvious deviation from normality or homoscedasticity. R2 is reported as a measure of the strength of association, the fitted parameters, and also the root mean square error (RMSE) of the predictions. A correlation was considered good, moderate, or poor if R2 was equal to or greater than 0.75, R2 was less than 0.75 but greater than 0.5, and R2 was equal to or less than 0.5, respectively. P values for the mixed-effects models were obtained by likelihood ratio tests of the model with the effect in question against the model without the effect in question. P values less than 0.05 were considered significant.
RESULTS
Peak external moments versus internal forces
Analysis of the peak EAM and peak Fmed across the spectrum of activities revealed that those with a one-legged stance phase had generally larger EAMs and medial forces than activities where both feet remained on the ground. The knee flexion angles at either peak EAM or peak Fmed varied substantially across activities (Table 1). During activities with loading on one leg, peak EAM and peak Fmed occurred at similar, smaller knee flexion angles, while during movements with double limb support, peak loading typically occurred at >50° flexion. The knee flexion angles at peak Fmed were generally higher than at peak EAM. Overall, peak EAM ranged from 0–7.5% body weight × height (%BW × Ht) while Fmed varied between 55 and 359 %BW. Across all subjects and activities, EAM and Fmed at the time of peak EAM had a good correlation (R2 = 0.88), with an RMSE of 35 %BW (P < 0.01). However, the slope of the regression varied considerably across subjects (Figure 1), with values ranging from 24 to 46 1/height. Across all activities and subjects, the interaction of EAM with mechanical axis angle was not found to have a significant effect on the relationship between Fmed and EAM (P > 0.05). A substantial but variable contribution of the subject-specific soft tissue forces to the total axial compressive force at peak Fmed revealed fractions ranging from a mean ± SD of 0.63 ± 0.09 up to 0.79 ± 0.06 for the 9 individuals (Table 2).
Table 1.
Peak EAM and peak Fmed values, their timing (fraction of the respective movement cycle), and the corresponding knee flexion angles for each activity*
| Activity | Peak EAM [%BW × Ht] | Peak Fmed [%BW] | Relative time at peak EAM | Relative time at peak Fmed | Knee flexion at peak EAM [degrees] | Knee flexion at peak Fmed [degrees] | Published peak EAM data [%BW × Ht] (ref.) |
|---|---|---|---|---|---|---|---|
| Walking | 3.05 ± 0.99 | 195.68 ± 35.81 | 0.29 ± 0.17 | 0.55 ± 0.25 | 20 ± 5 | 16 ± 7 | 3.46 (36), 2.91 (37), 3.0 (50), 3.9 (42) |
| Stair climbing | 2.97 ± 1.08 | 214.54 ± 41.55 | 0.38 ± 0.14 | 0.45 ± 0.23 | 41 ± 9 | 41 ± 11 | 1.52(8), 3.13 (38), 4.69 (38) |
| Stair descending | 4.47 ± 1.38 | 236.63 ± 43.59 | 0.35 ± 0.22 | 0.35 ± 0.22 | 36 ± 14 | 37 ± 17 | 3.4 (34), 4.67 (39) |
| Sit-to-stand | 0.79 ± 0.37 | 121.50 ± 31.20 | 0.56 ± 0.23 | 0.33 ± 0.28 | 46 ± 25 | 71 ± 29 | 1.15 (40) |
| Stand-to-sit | 1.38 ± 0.56 | 137.11 ± 30.73 | 0.60 ± 0.19 | 0.71 ± 0.11 | 62 ± 21 | 73 ± 16 | – |
| Squat | 1.06 ± 0.57 | 123.30 ± 34.71 | 0.47 ± 0.37 | 0.42 ± 0.28 | 53 ± 24 | 76 ± 25 | – |
| Squat varus | 1.34 ± 0.64 | 140.29 ± 30.15 | 0.47 ± 0.33 | 0.47 ± 0.22 | 58 ± 23 | 88 ± 11 | – |
| Squat valgus | 0.78 ± 0.49 | 105.43 ± 29.91 | 0.37 ± 0.29 | 0.38 ± 0.19 | 69 ± 26 | 86 ± 13 | – |
| Weight transfer | 3.25 ± 1.05 | 197.73 ± 30.72 | 0.33 ± 0.16 | 0.40 ± 0.21 | 10 ± 6 | 8 ± 5 | – |
| One-legged stance | 3.44 ± 1.46 | 214.40 ± 56.61 | 0.54 ± 0.27 | 0.70 ± 0.26 | 14 ± 5 | 14 ± 6 | 2.86–3.94 (42) |
Values are the mean ± SD. EAM = external knee adduction moment; Fmed = medial tibiofemoral contact force; %BW × Ht = % body weight × height.
Figure 1.
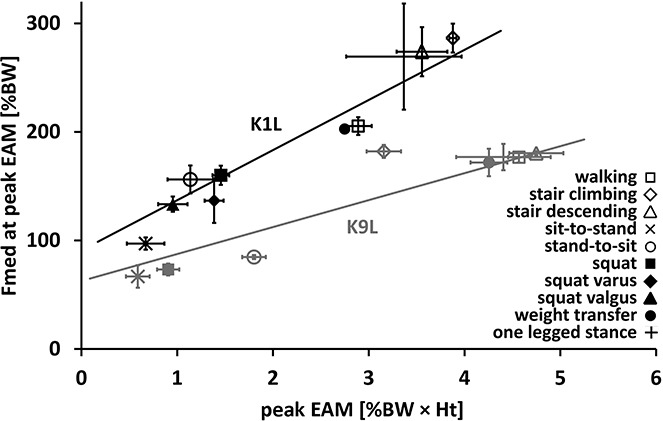
Relationship between peak external knee adduction moment (EAM), measured as % body weight × height (%BW × Ht) and medial tibiofemoral contact force (Fmed), measured as %BW, at the same time point for all activities and trials for 2 representative subjects (K1L and K9L). Symbols represent the mean; vertical and horizontal lines show the SD.
Table 2.
Peak Fmed, corresponding axial soft-tissue force, and the ratio of axial soft-tissue force to the total axial knee contact force for each subject over all activities*
| Subject | Peak Fmed [%BW] | Fsoft-tissue at peak Fmed [%BW] | Ratio of Fsoft-tissue to Ftotal at peak Fmed |
|---|---|---|---|
| K1L | 218 ± 65 | 275 ± 44 | 0.79 ± 0.06 |
| K2L | 192 ± 60 | 218 ± 55 | 0.74 ± 0.08 |
| K3R | 149 ± 46 | 173 ± 37 | 0.69 ± 0.10 |
| K4R | 132 ± 45 | 179 ± 36 | 0.72 ± 0.06 |
| K5R | 170 ± 43 | 235 ± 71 | 0.76 ± 0.11 |
| K6L | 175 ± 26 | 260 ± 69 | 0.76 ± 0.07 |
| K7L | 231 ± 74 | 221 ± 32 | 0.73 ± 0.05 |
| K8L | 177 ± 46 | 212 ± 37 | 0.75 ± 0.10 |
| K9L | 150 ± 53 | 124 ± 42 | 0.63 ± 0.09 |
Values are the mean ± SD. Fmed = medial tibiofemoral contact force; %BW = % body weight; Fsoft-tissue = axial soft-tissue force; Ftotal= total axial knee contact force.
External moments versus internal forces throughout stance
When the entire stance phase of all activities was considered, EAM significantly predicted Fmed (P < 0.01), and these parameters were also found to correlate well, producing an overall R2 of 0.87 and average RMSE of 29 %BW. R2 increased by ∼4% when either EFM or the absolute value of the EFM were additionally considered, while the average RMSE decreased to 26 %BW compared to the model that included only the EAM. Analysis of the random effects per activity demonstrated that the EAM had a larger influence on Fmed for those activities with loading primarily on one limb (Table 3). In contrast, Fmed was most sensitive to the EFM for activities where both limbs were loaded.
Table 3.
Comparison of the fixed effects and random effects of 3 different models for predicting Fmed based on the EAM alone or using combinations of the EAM and the EFM*
| Fmed = a + b × EAM | Fmed = a + b × EAM + c × EFM | Fmed = a + b × EAM + c × |EFM| | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a [%BW] | b [1/height] | RMSE [%BW] | a [%BW] | b [1/height] | c [1/height] | RMSE [%BW] | a [%BW] | b [1/height] | c [1/height] | RMSE [%BW] | |
| Fixed effects | 80 | 34 | 67 | 32 | 1 | 64 | 33 | 5 | |||
| Random effects | |||||||||||
| Activity | |||||||||||
| Walking | 4 | 3 | 31 | 18 | 5 | −13 | 30 | 26 | 3 | −12 | 31 |
| Stair climbing | 25 | 1 | 34 | 31 | 4 | −1 | 35 | 31 | 3 | −3 | 34 |
| Stair descending | −7 | 5 | 34 | 13 | 4 | −2 | 33 | 10 | 5 | −5 | 33 |
| Sit-to-stand | 5 | −18 | 32 | −14 | −10 | 8 | 22 | −18 | −8 | 6 | 19 |
| Stand-to-sit | 1 | −3 | 29 | −3 | −13 | 8 | 23 | −2 | −12 | 5 | 23 |
| Squat | −1 | 3 | 23 | −13 | 0 | 6 | 20 | −16 | 1 | 3 | 19 |
| Squat varus | −1 | 2 | 18 | −12 | 1 | 8 | 14 | −18 | 2 | 6 | 13 |
| Squat valgus | −8 | −8 | 18 | −21 | −10 | 7 | 15 | −26 | −9 | 4 | 13 |
| Weight transfer | −7 | 7 | 31 | −2 | 8 | −6 | 29 | 5 | 7 | −3 | 30 |
| One-legged stance | −11 | 10 | 43 | 1 | 11 | −17 | 36 | 6 | 8 | −1 | 40 |
Errors in the model predictions are reported as the root mean square error (RMSE). Fmed = medial tibiofemoral contact force; a, b, c, = free regression parameters; EAM = external knee adduction moment; EFM = external knee flexion moment; %BW = % body weight.
The nonlinear regression of MR as a function of EAM across all subjects and activities (Figure 2) estimated the parameters of the arctangent function to be b1 = 44.73 and b2 = 0.26 (P < 0.001) and had an RMSE of 0.08 (∼11% of mean peak MR). Subjects with a more valgus knee alignment tended to exhibit MR and EAM values in the lowest range (Figure 2) and subjects with a more varus mechanical axis angle had values in the range of the maximum MR and EAM values (Figure 2). However, the general characteristics of the curves fitted for the valgus and varus mechanical axis angle groups were similar (for the valgus group, b1 = 38.49 and b2 = 0.26 and for the varus group, b1 = 49.03 and b2 = 0.2; all P < 0.001).
Figure 2.
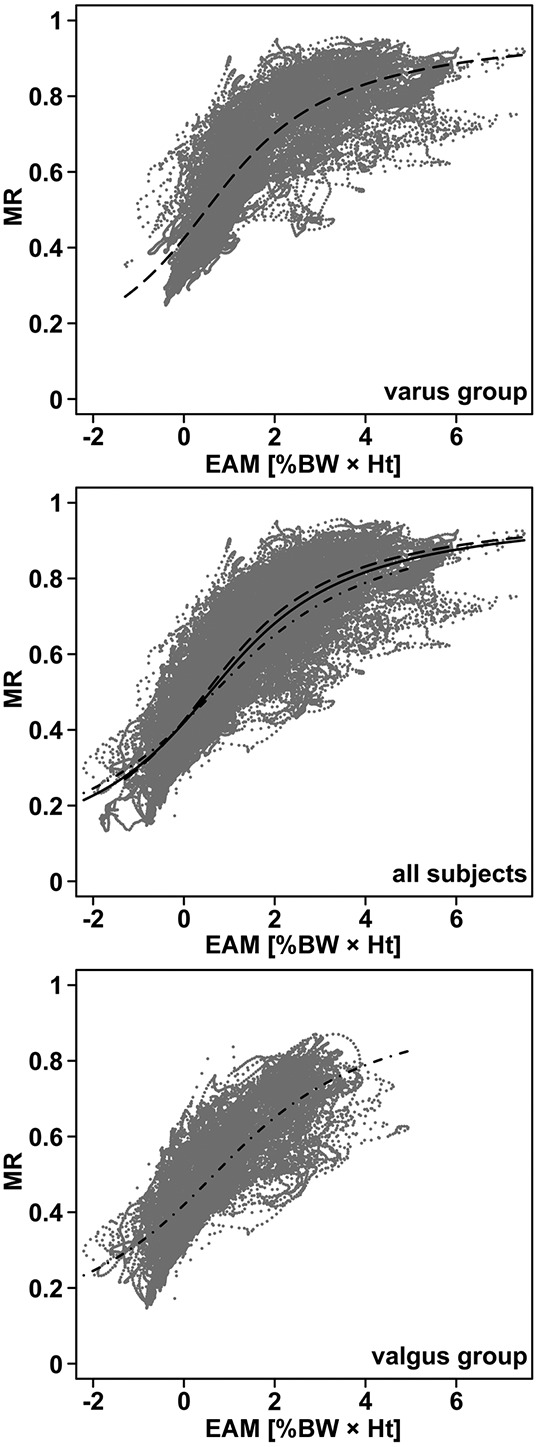
Ratio of the medial to the total axial tibiofemoral contact force (MR) plotted against the external knee adduction moment (EAM) across all activities (gray dots). Top, Data for the 4 subjects with a mechanical axis angle greater than the median (varus group). Middle, Data points and fit across all subjects. Bottom, Data for the 4 subjects with a mechanical axis angle smaller than the median (valgus group). The nonlinear arctangent fits are shown as a solid line (for all subjects), broken lines (for the varus group), and broken and dotted lines (for the valgus group). %BWHt = % body weight × height.
External moments versus internal contact moments
There was a good correlation between the IAM and EAM across all subjects and activities, with an R2 of 0.90. For walking and stair climbing, the slope of the regression was 0.65 and 0.62 respectively, while the intercept was very small, with values of 0.034 and 0.007 %BW × Ht.
General loading profile
For activities with a mostly one-legged stance, the EAM and Fmed graphs were similar (Figure 3). Peak values tended to occur at similar time points during stair negotiation (Table 1). During the sit-to-stand and stand-to-sit activities, peak Fmed tended to occur at larger knee flexion angles than peak EAM. Generally, during activities with a mostly two-legged stance, a relationship between EAM and Fmed was less obvious, as both variables reached smaller values and showed less prominent peaks than during a one-legged stance.
Figure 3.
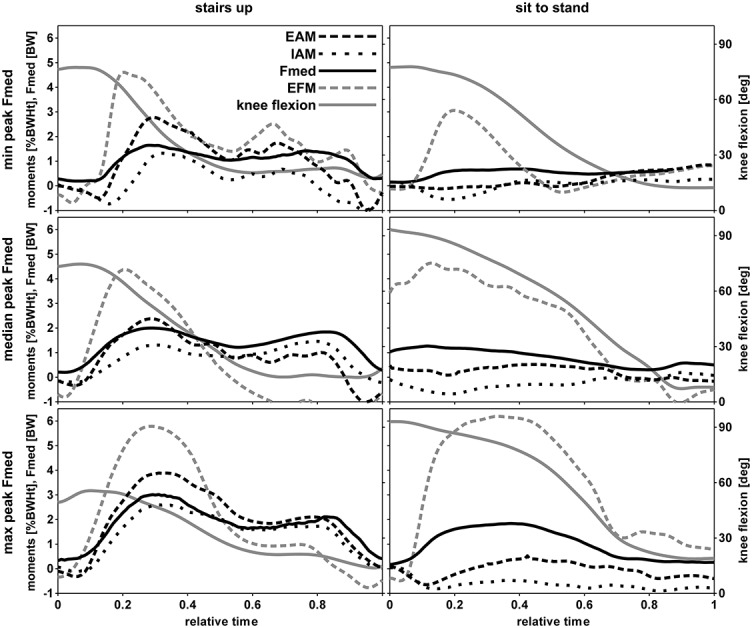
External knee adduction moment (EAM), internal knee adduction moment (IAM), medial tibiofemoral contact force (Fmed), external knee flexion moment (EFM), and knee flexion angle for representative trials of stair climbing and sit-to-stand activities. For each activity, the 3 trials representing the minimum (min), median, and maximum (max) peak Fmed value, determined from all subjects, are shown. %BWHt = % body weight × height.
Load modulation in high flexion
During squatting, peak Fmed occurred at knee flexion angles ranging from a mean ± SD of 76 ± 25° for a normal squat to 88 ± 11° for a varus squat (Table 1). Compared to the conditions during walking and stair negotiation, EAM at peak Fmed across the 3 different squat variants was small and ranged from −1.29 %BW × Ht to 1.88 %BW × Ht. However, some subjects were able to modify the EAM at peak Fmed by >1 %BW × Ht. In one subject, the mean MR at peak Fmed was even doubled from 0.25 during valgus squat to 0.51 during the varus variant. The mean ± SD MR at peak Fmed across the 3 squat variants was 0.15 ± 0.07.
DISCUSSION
Using the combined power of precise gait analysis procedures and direct measurements of in vivo forces within the knee, this study provides unprecedented insight into the conditions under which the more readily accessible external moments are related to the actual contact forces and their distribution across the joint. By assessing these biomechanical relationships in 9 subjects over 10 different activities, we found a good correlation between peak EAM and the corresponding Fmed, with an overall RMSE of Fmed regression of 35 %BW. However, the maximum error was 127 %BW, which compares to a peak Fmed value of ∼300 %BW, indicating that the relationship varies considerably across subjects and activities.
The wide spectrum of activities and the intersubject variability examined in this study cover a large range of both parameters to facilitate a more complete analysis of the relationship between peak EAM and Fmed. While previous studies of the role of mechanics in OA disease progression identified the EAM as an important factor, they often concentrated on walking only (7,9). We found that the largest Fmed values occurred during the functionally important stair negotiation activities, with knee flexion angles more than twice as large as those during walking (Table 1). Our finding thus corroborates previous research that found particularly challenging contact mechanics during stair negotiation also at the patellofemoral joint (33), providing supporting evidence for the notion that stair negotiation, although less frequent than walking, presents considerable challenges to knee function in elderly individuals (16,34) but might also offer increased sensitivity for differentiating treatment effects (35). Although only a few studies considered frontal plane knee mechanics during stair negotiation, the EAMs determined in our cohort and their relation to the conditions during walking and chair-rise activities were consistent with previously published results (Table 1) (8,36–42).
By examining variants of the squat activity, we demonstrated that subjects are able to modify the medial–lateral load distribution, and that such modifications are not limited to activities when the knee is more extended. This expands upon previous in vivo studies that have documented the influence of nonsurgical interventions on Fmed (21) and corroborates the notion that specific kinematic strategies used by patients may indeed be able to modify the MR at the knee. The relationships between EAM and Fmed described here therefore provide essential knowledge for analyses aiming to consider a wider spectrum of activities to investigate the role of mechanics in OA disease initiation and progression (43).
In particular, for such activities where peak Fmed forces occurred in more flexed positions, it seems likely that the EFM contributes substantially to Fmed (23). However, evaluation of activities with high knee flexion has shown that the inclusion of the EFM reduces the RMSE of Fmed predictions primarily during sit-to-stand-to-sit and squat activities (where both legs are on the ground and the EAM is relatively small), while the error in stair negotiation activities remained virtually unchanged, despite significant knee flexion. This indicates that a sufficiently large EAM has a dominant influence on medial knee loading across a large range of knee flexion. While the regression models that included both the EAM and EFM consistently explained the largest variation in Fmed, consistent with previous work investigating walking (23), a considerable improvement in prediction accuracy would necessitate additional consideration of the EFM during sit-to-stand-to-sit and squatting activities.
The contact forces transferred at the knee are mainly a result of the action of the soft tissues (26,44). Mean ± SD compressive soft tissue forces across our spectrum of activities varied substantially between subjects and ranged from 124 ± 42 to 275 ± 44 %BW, contributing to the overall axial tibiofemoral contact forces by over 60% (Table 2). The variable magnitude of the contact forces at the knee, resulting from the different soft tissue forces, might at least partially explain the large variability in clinical response to changes in EAM often found in intervention studies (45). Even the smallest medial contact forces observed during the valgus squat reached values >100 %BW, with values as high as 236 %BW observed on the medial condyle alone during stair descent (Table 1), thus underlining the importance of the soft tissues in modulating the joint contact forces, but in particular, the load distribution across the condyles of the knee.
Across all subjects, we observed a good correlation between the IAM and the EAM. Given the small intercept of the IAM versus EAM regression for both walking and stair climbing, the slope provides a good estimate of the fraction of the EAM supported by an uneven medial–lateral tibiofemoral contact force distribution. Here, we found that almost two-thirds of the EAM was balanced by the contact forces alone. This finding is of particular interest for musculoskeletal modeling approaches that aim to estimate the contact forces at the knee (27,30,44), and which often assume that the EAM is completely balanced by muscle forces. Our measurements, however, indicate that such an assumption might result in a considerable overestimation of the knee contact forces when a too large proportion of the moment was actively balanced by muscle forces. The IAM-to-EAM ratio of almost 2:3 determined for walking in 9 subjects differs considerably from the results of a previous study in a single subject (22), which demonstrated an IAM-to-EAM ratio of 1:10. It remains unclear how much such differences stem from different implant designs and surgical techniques.
We found a good correlation between the peak EAM and the corresponding Fmed across a peak EAM range of 0–7.5 %BW × Ht. However, the large subject-specific variation of regression slopes, differing by a factor of more than 2, indicates that the individual modulation of external loads to produce the joint contact forces through the action of the soft tissues can differ considerably. As a result, the estimation of changes in Fmed, based on changes in EAM alone, is likely to exhibit limited accuracy under conditions in which excessive, directed cocontraction of muscles exists (19). This may help to explain why interventions shown to reduce the KAM have not generally resulted in altered disease progression (19,45,46).
While this study has a number of strengths, including, e.g., the unique cohort of subjects undertaking a spectrum of activities, as well as the ability to directly measure the tibiofemoral contact forces in vivo, there are also certain limitations to be considered. Whether results obtained in subjects with TKR apply to native knees remains unknown. Also, while it is unlikely that a study with a comparable size cohort will be performed, the number of measured subjects remains small. In order to maximize the number of subjects available, we opted against further standardization of parameters such as limb alignment (44). While passive knee laxity, which plays a role in TKR function and MR at the knee, is considered to be a critical factor for the development and progression of OA (47), we acknowledge that knee joint laxity was not characterized in detail. The combination of measures of frontal and transverse plane laxity (48), together with more detailed computer modeling approaches to systematically investigate and thus better understand the interactions between passive stability and the active soft tissues (26) in balancing the forces across the knee, is therefore a target for future research.
Although many studies have considered the EAM in the context of providing early, nonsurgical interventions to address function and symptoms in knee OA (7,11–13,49,50), the subjects considered here underwent TKR for the treatment of end-stage OA. However, all subjects here had good clinical function and were able to master all activities of our test protocol. Moreover, with respect to variables such as average walking speed, and the magnitude and timing of the peak EAM data, our results are well within the range of data published previously (5,16,34,38–41), including the limited data that exists on in vivo tibiofemoral loading (1,11,13,15,22,29).
This study establishes a robust relationship between peak EAM and Fmed. Moreover, our results on the variation of the relationship between the two measures provides critical, previously unavailable information for interpreting the EAM in the many studies that have no direct access to the contact forces and their distribution transmitted across the knee joint. The findings of this study call for more detailed investigations into the soft tissue–related mechanisms that modulate the internal forces at the knee, but also indicate that EAM should be used only cautiously as a surrogate measure for Fmed under conditions when excessive, directed muscle coactivation could be anticipated.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Heller had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Trepczynski, Kutzner, Bergmann, Taylor, Heller.
Acquisition of data. Trepczynski, Kutzner.
Analysis and interpretation of data. Trepczynski, Kutzner, Bergmann, Taylor, Heller.
ROLE OF THE STUDY SPONSOR
Zimmer GmbH had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Zimmer GmbH.
Acknowledgments
We would like to thank Dr. M. T. Sanchez-Santos (Oxford NIHR Musculoskeletal Biomedical Research Unit, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK) for expert advice and support with the statistical analyses.
REFERENCES
- 1.D'Lima DD, Patil S, Steklov N, Slamin JE, Colwell CW., Jr Tibial forces measured in vivo after total knee arthroplasty. J Arthroplasty. 2006;21:255–62. doi: 10.1016/j.arth.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, et al. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech. 2010;43:2164–73. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013;21:10–5. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–73. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 5.Prodromos CC, Andriacchi TP, Galante JO. A relationship between gait and clinical changes following high tibial osteotomy. J Bone Joint Surg Am. 1985;67:1188–94. [PubMed] [Google Scholar]
- 6.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–9. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 7.Bennell KL, Bowles KA, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70:1770–4. doi: 10.1136/ard.2010.147082. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman KR, Hughes C, Morrey BF, Morrey M, An KN. Gait characteristics of patients with knee osteoarthritis. J Biomech. 2001;34:907–15. doi: 10.1016/s0021-9290(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki T. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–22. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birmingham TB, Giffin JR, Chesworth BM, Bryant DM, Litchfield RB, Willits K, et al. Medial opening wedge high tibial osteotomy: a prospective cohort study of gait, radiographic, and patient-reported outcomes. Arthritis Rheum. 2009;61:648–57. doi: 10.1002/art.24466. [DOI] [PubMed] [Google Scholar]
- 11.Erhart JC, Dyrby CO, D'Lima DD, Colwell CW, Andriacchi TP. Changes in in vivo knee loading with a variable-stiffness intervention shoe correlate with changes in the knee adduction moment. J Orthop Res. 2010;28:1548–53. doi: 10.1002/jor.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinman RS, Bowles KA, Metcalf BB, Wrigley TV, Bennell KL. Lateral wedge insoles for medial knee osteoarthritis: effects on lower limb frontal plane biomechanics. Clin Biomech (Bristol, Avon) 2012;27:27–33. doi: 10.1016/j.clinbiomech.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Mundermann A, Asay JL, Mundermann L, Andriacchi TP. Implications of increased medio-lateral trunk sway for ambulatory mechanics. J Biomech. 2008;41:165–70. doi: 10.1016/j.jbiomech.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Simic M, Hunt MA, Bennell KL, Hinman RS, Wrigley TV. Trunk lean gait modification and knee joint load in people with medial knee osteoarthritis: the effect of varying trunk lean angles. Arthritis Care Res (Hoboken) 2012;64:1545–53. doi: 10.1002/acr.21724. [DOI] [PubMed] [Google Scholar]
- 15.Fregly BJ, D'Lima DD, Colwell CW., Jr Effective gait patterns for offloading the medial compartment of the knee. J Orthop Res. 2009;27:1016–21. doi: 10.1002/jor.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo M, Axe MJ, Manal K. The influence of foot progression angle on the knee adduction moment during walking and stair climbing in pain free individuals with knee osteoarthritis. Gait Posture. 2007;26:436–41. doi: 10.1016/j.gaitpost.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Shull PB, Shultz R, Silder A, Dragoo JL, Besier TF, Cutkosky MR, et al. Toe-in gait reduces the first peak knee adduction moment in patients with medial compartment knee osteoarthritis. J Biomech. 2013;46:122–8. doi: 10.1016/j.jbiomech.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Birmingham T. Implications of clinical biomechanics research on rehabilitation for OA. Osteoarthritis Cartilage. 2012;20(Suppl 1):S1. [abstract] [Google Scholar]
- 19.Andriacchi TP. Valgus alignment and lateral compartment knee osteoarthritis: a biomechanical paradox or new insight into knee osteoarthritis? Arthritis Rheum. 2013;65:310–3. doi: 10.1002/art.37724. [editorial] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinlein B, Graichen F, Bender A, Rohlmann A, Bergmann G. Design, calibration and pre-clinical testing of an instrumented tibial tray. J Biomech. 2007;40(Suppl 1):S4–10. doi: 10.1016/j.jbiomech.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Kutzner I, Kuther S, Heinlein B, Dymke J, Bender A, Halder AM, et al. The effect of valgus braces on medial compartment load of the knee joint–in vivo load measurements in three subjects. J Biomech. 2011;44:1354–60. doi: 10.1016/j.jbiomech.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Zhao D, Banks SA, Mitchell KH, D'Lima DD, Colwell CW, Jr, Fregly BJ. Correlation between the knee adduction torque and medial contact force for a variety of gait patterns. J Orthop Res. 2007;25:789–97. doi: 10.1002/jor.20379. [DOI] [PubMed] [Google Scholar]
- 23.Walter JP, D'Lima DD, Colwell CW, Jr, Fregly BJ. Decreased knee adduction moment does not guarantee decreased medial contact force during gait. J Orthop Res. 2010;28:1348–54. doi: 10.1002/jor.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh NB, Arampatzis A, Duda G, Heller MO, Taylor WR. Effect of fatigue on force fluctuations in knee extensors in young adults. Philos Trans A Math Phys Eng Sci. 2010;368:2783–98. doi: 10.1098/rsta.2010.0091. [DOI] [PubMed] [Google Scholar]
- 25.Heller MO, Konig C, Graichen H, Hinterwimmer S, Ehrig RM, Duda GN, et al. A new model to predict in vivo human knee kinematics under physiological-like muscle activation. J Biomech. 2007;40(Suppl 1):S45–53. doi: 10.1016/j.jbiomech.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Trepczynski A, Kutzner I, Kornaropoulos E, Taylor WR, Duda GN, Bergmann G, et al. Patellofemoral joint contact forces during activities with high knee flexion. J Orthop Res. 2012;30:408–15. doi: 10.1002/jor.21540. [DOI] [PubMed] [Google Scholar]
- 27.Pandy MG, Andriacchi TP. Muscle and joint function in human locomotion. Annu Rev Biomed Eng. 2010;12:401–33. doi: 10.1146/annurev-bioeng-070909-105259. [DOI] [PubMed] [Google Scholar]
- 28.Halder A, Kutzner I, Graichen F, Heinlein B, Beier A, Bergmann G. Influence of limb alignment on mediolateral loading in total knee replacement: in vivo measurements in five patients. J Bone Joint Surg Am. 2012;94:1023–9. doi: 10.2106/JBJS.K.00927. [DOI] [PubMed] [Google Scholar]
- 29.Varadarajan KM, Moynihan AL, D'Lima D, Colwell CW, Li G. In vivo contact kinematics and contact forces of the knee after total knee arthroplasty during dynamic weight-bearing activities. J Biomech. 2008;41:2159–68. doi: 10.1016/j.jbiomech.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heller MO, Bergmann G, Deuretzbacher G, Durselen L, Pohl M, Claes L, et al. Musculo-skeletal loading conditions at the hip during walking and stair climbing. J Biomech. 2001;34:883–93. doi: 10.1016/s0021-9290(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 31.Taylor WR, Kornaropoulos EI, Duda GN, Kratzenstein S, Ehrig RM, Arampatzis A, et al. Repeatability and reproducibility of OSSCA, a functional approach for assessing the kinematics of the lower limb. Gait Posture. 2010;32:231–6. doi: 10.1016/j.gaitpost.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Heller MO, Kratzenstein S, Ehrig RM, Wassilew G, Duda GN, Taylor WR. The weighted optimal common shape technique improves identification of the hip joint center of rotation in vivo. J Orthop Res. 2011;29:1470–5. doi: 10.1002/jor.21426. [DOI] [PubMed] [Google Scholar]
- 33.Goudakos IG, Konig C, Schottle PB, Taylor WR, Singh NB, Roberts I, et al. Stair climbing results in more challenging patellofemoral contact mechanics and kinematics than walking at early knee flexion under physiological-like quadriceps loading. J Biomech. 2009;42:2590–6. doi: 10.1016/j.jbiomech.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Sacco IC, Trombini-Souza F, Butugan MK, Passaro AC, Arnone AC, Fuller R. Joint loading decreased by inexpensive and minimalist footwear in elderly women with knee osteoarthritis during stair descent. Arthritis Care Res (Hoboken) 2012;64:368–74. doi: 10.1002/acr.20690. [DOI] [PubMed] [Google Scholar]
- 35.Konig C, Sharenkov A, Matziolis G, Taylor WR, Perka C, Duda GN, et al. Joint line elevation in revision TKA leads to increased patellofemoral contact forces. J Orthop Res. 2010;28:1–5. doi: 10.1002/jor.20952. [DOI] [PubMed] [Google Scholar]
- 36.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20:101–7. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 37.McClelland JA, Webster KE, Feller JA, Menz HB. Knee kinetics during walking at different speeds in people who have undergone total knee replacement. Gait Posture. 2010;32:205–10. doi: 10.1016/j.gaitpost.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Mandeville D, Osternig LR, Lantz BA, Mohler CG, Chou LS. The effect of total knee replacement on the knee varus angle and moment during walking and stair ascent. Clin Biomech (Bristol, Avon) 2008;23:1053–8. doi: 10.1016/j.clinbiomech.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Luepongsak N, Amin S, Krebs DE, McGibbon CA, Felson D. The contribution of type of daily activity to loading across the hip and knee joints in the elderly. Osteoarthritis Cartilage. 2002;10:353–9. doi: 10.1053/joca.2000.0511. [DOI] [PubMed] [Google Scholar]
- 40.Amin S, Luepongsak N, McGibbon CA, LaValley MP, Krebs DE, Felson DT. Knee adduction moment and development of chronic knee pain in elders. Arthritis Rheum. 2004;51:371–6. doi: 10.1002/art.20396. [DOI] [PubMed] [Google Scholar]
- 41.Takacs J, Hunt MA. The effect of contralateral pelvic drop and trunk lean on frontal plane knee biomechanics during single limb standing. J Biomech. 2012;45:2791–6. doi: 10.1016/j.jbiomech.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 42.Lim BW, Kemp G, Metcalf B, Wrigley TV, Bennell KL, Crossley KM, et al. The association of quadriceps strength with the knee adduction moment in medial knee osteoarthritis. Arthritis Rheum. 2009;61:451–8. doi: 10.1002/art.24278. [DOI] [PubMed] [Google Scholar]
- 43.Maly MR, Robbins SM, Stratford PW, Birmingham TB, Callaghan JP. Cumulative knee adductor load distinguishes between healthy and osteoarthritic knees–a proof of principle study. Gait Posture. 2013;37:396–401. doi: 10.1016/j.gaitpost.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Heller MO, Taylor WR, Perka C, Duda GN. The influence of alignment on the musculo-skeletal loading conditions at the knee. Langenbecks Arch Surg. 2003;388:291–7. doi: 10.1007/s00423-003-0406-2. [DOI] [PubMed] [Google Scholar]
- 45.Pham T, Maillefert JF, Hudry C, Kieffert P, Bourgeois P, Lechevalier D, et al. Laterally elevated wedged insoles in the treatment of medial knee osteoarthritis: a two-year prospective randomized controlled study. Osteoarthritis Cartilage. 2004;12:46–55. doi: 10.1016/j.joca.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Bennell KL, Bowles KA, Payne C, Cicuttini F, Williamson E, Forbes A, et al. Lateral wedge insoles for medial knee osteoarthritis: 12 month randomised controlled trial. BMJ. 2011;342:d2912. doi: 10.1136/bmj.d2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma L, Lou C, Felson DT, Dunlop DD, Kirwan-Mellis G, Hayes KW, et al. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42:861–70. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 48.Moewis P, Wolterbeek N, Diederichs G, Valstar E, Heller MO, Taylor WR. The quality of bone surfaces may govern the use of model based fluoroscopy in the determination of joint laxity. Med Eng Phys. 2012;34:1427–32. doi: 10.1016/j.medengphy.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Hunt MA, Birmingham TB, Bryant D, Jones I, Giffin JR, Jenkyn TR, et al. Lateral trunk lean explains variation in dynamic knee joint load in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:591–9. doi: 10.1016/j.joca.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Moyer RF, Birmingham TB, Chesworth BM, Kean CO, Giffin JR. Alignment, body mass and their interaction on dynamic knee joint load in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2010;18:888–93. doi: 10.1016/j.joca.2010.03.017. [DOI] [PubMed] [Google Scholar]


