Abstract
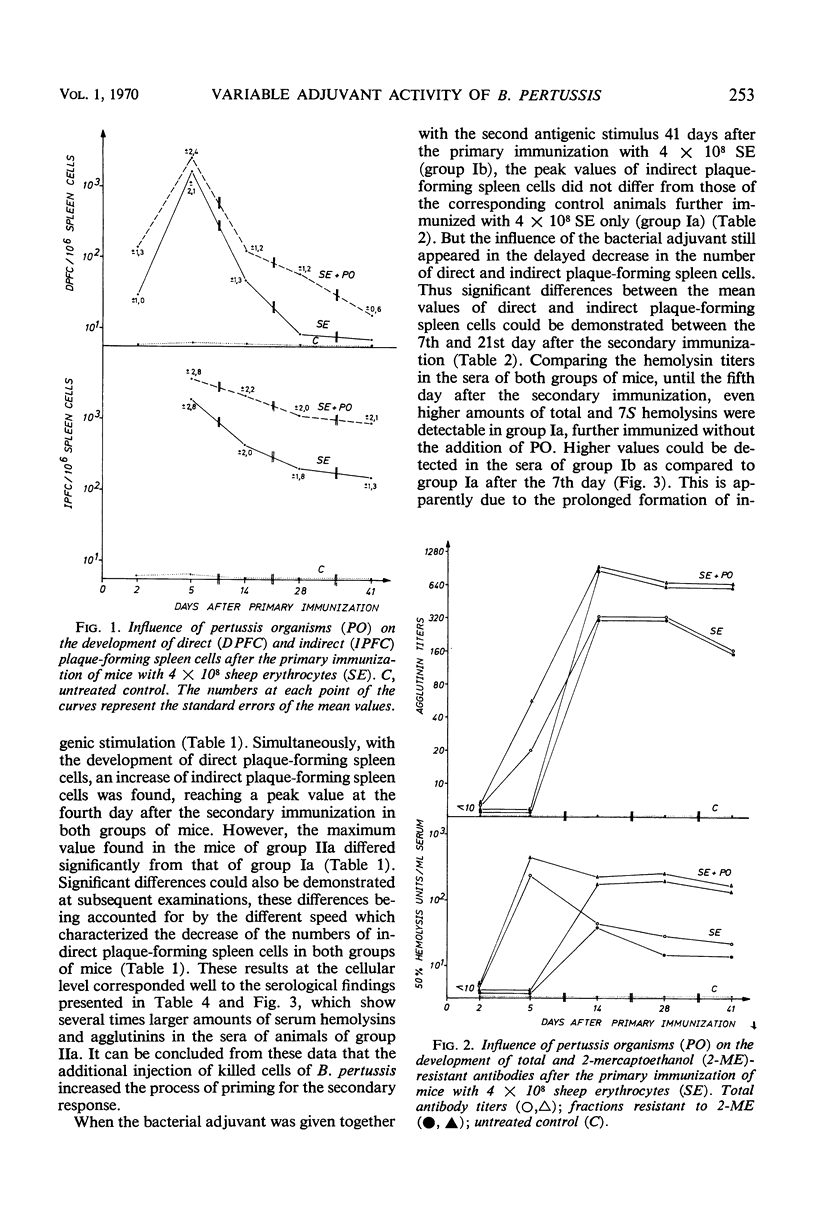
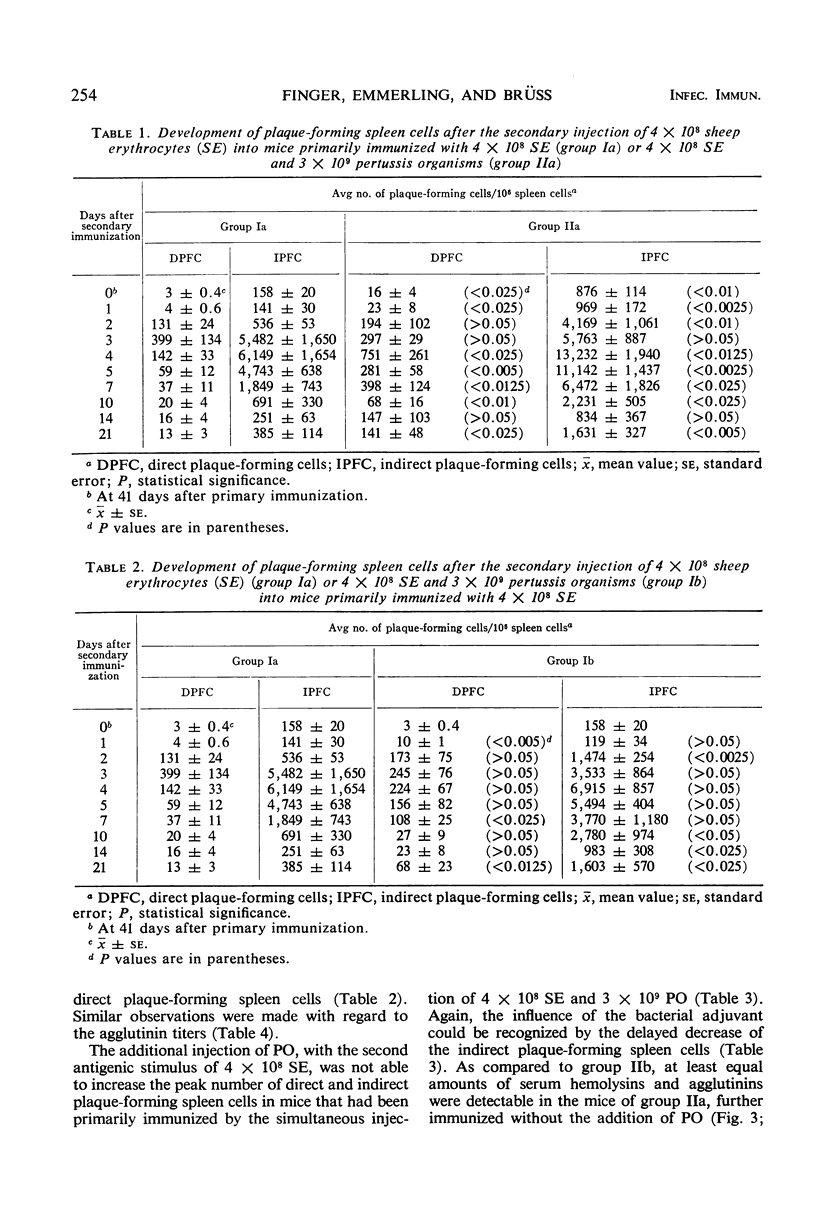
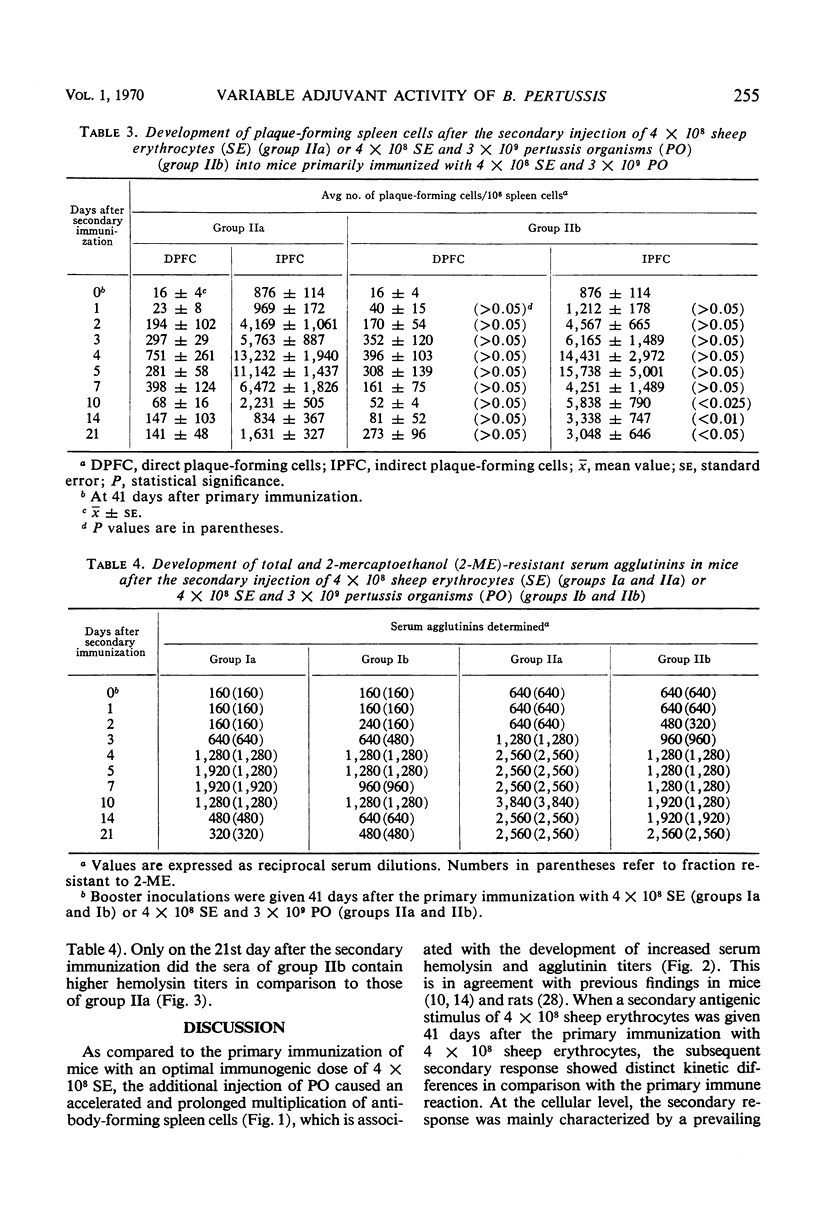
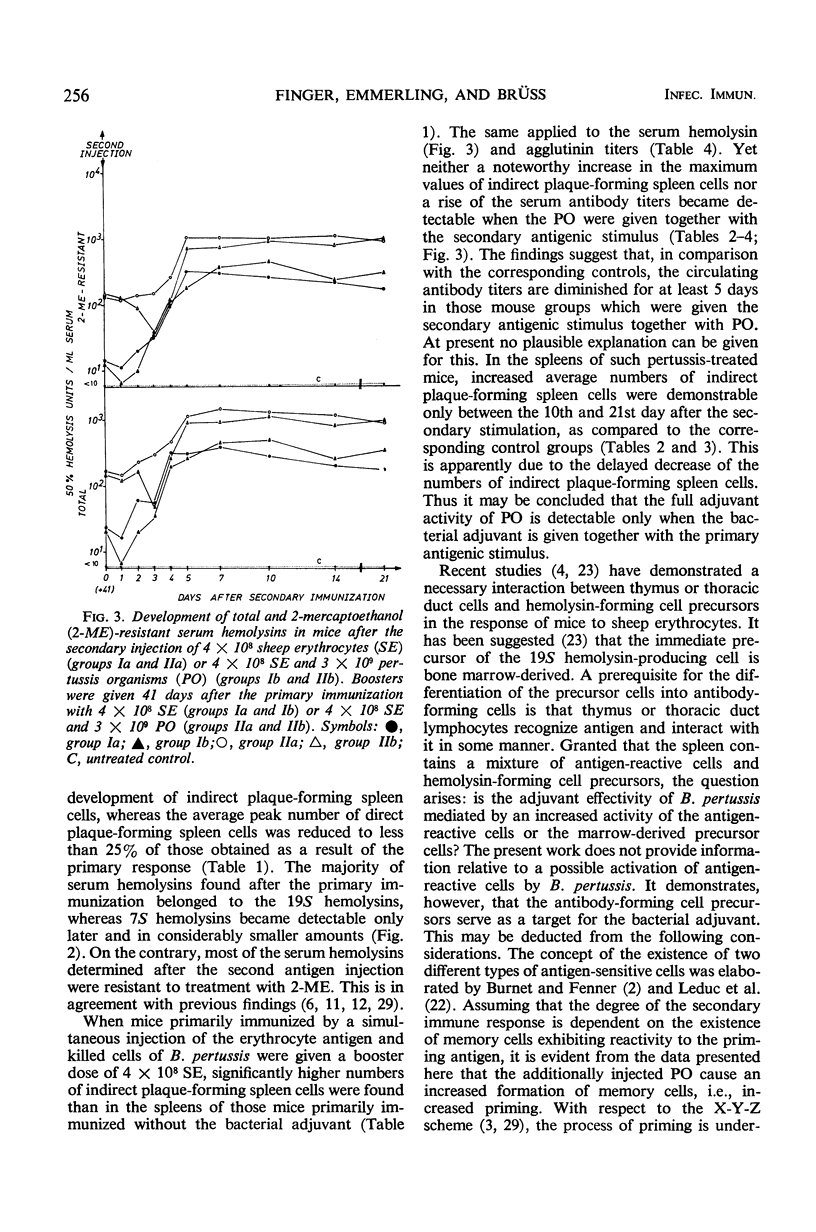
We carried out a study on the adjuvant effect of Bordetella pertussis vaccine on the primary and secondary immune responses of the mouse to sheep erythrocytes, quantitating antibody-producing spleen cells and serum antibody. The simultaneous injection of sheep erythrocytes and B. pertussis, when compared to immunization with sheep red blood cells alone, resulted in an increased and prolonged multiplication of antibody-forming spleen cells. The adjuvant effect was also documented by increased production of serum hemolysins and agglutinins. Further, B. pertussis enhanced the priming effect of the antigen for the secondary response. However, when the bacterial adjuvant was given together with a second antigenic stimulus 41 days after the primary immunization, the peak values of direct and indirect plaque-forming spleen cells did not differ from the corresponding control animals further inoculated with sheep erythrocytes alone. Nonetheless, the influence of the bacterial adjuvant was still expressed by the delayed decrease of the numbers of plaque-forming spleen cells. On the basis of the X-Y-Z scheme it is suggested that B. pertussis cells as adjuvant enhance the multiplication of antigen-sensitive X cells or affect the initial stages of differentiation of these cells. This effect of the pertussis vaccine can be distinguished from a general proliferative action on other cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beneke G., Finger H., Emmerling P. Einfluss von Bordetella pertussis auf das lymphatische Gewebe von Mäsuen. II. Proliferiernde Zellsysteme in der Milz nach Injektion vonBoredtelle pertussis. Z Med Mikrobiol Immunol. 1968;154(3):178–195. [PubMed] [Google Scholar]
- Byers V. S., Sercarz E. E. The X-Y-Z scheme of immunocyte maturation. IV. The exhaustion of memory cells. J Exp Med. 1968 Feb 1;127(2):307–325. doi: 10.1084/jem.127.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Immunocompetence of transferred thymus-marrow cell combinations. J Immunol. 1966 Dec;97(6):828–832. [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Dissociation of human serum macroglobulins. Science. 1957 Mar 29;125(3248):600–601. doi: 10.1126/science.125.3248.600. [DOI] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Brüss E. Enfluss von Bordetella pertussis auf das lymphatische Gewebe von Mäusen. V. Der Einfluss von Bordetella pertussis auf die Präparation des lymphoretikulären Gewebes für immunologische Zweitreaktion. Z Med Mikrobiol Immunol. 1969;155(1):48–64. [PubMed] [Google Scholar]
- FLEMING D. S., GREENBERG L., BEITH E. M. The use of combined antigens in the immunization of infants. Can Med Assoc J. 1948 Aug;59(2):101–105. [PMC free article] [PubMed] [Google Scholar]
- Finger H. Die Bedeutung bakterieller Adjuvantien für die Ausbildung der anaphylaktischen Schockbereitschaft bei Maus und Ratte II. Die Unterdrückung der anaphylaktischen Schockbereitschaft bei Maus und Ratte. Z Hyg Infektionskr. 1965;151(3):270–290. [PubMed] [Google Scholar]
- Finger H., Emmerling P. Agarqualität und Diäthylaminoäthyl-Dextran als kritische Komponenten der LHG-Technik. Z Immunitatsforsch Allerg Klin Immunol. 1968 Aug;136(2):145–157. [PubMed] [Google Scholar]
- Finger H., Emmerling P., Brüss E. Das immunologische Gedächtnis der Maus nach Cyclophosphamid-Behandlung. Z Immunitatsforsch Allerg Klin Immunol. 1969 Jun;138(2):191–206. [PubMed] [Google Scholar]
- Finger H., Emmerling P., Brüss E. The influence of Bordetella pertussis on the preparation of mouse spleens for the secondary immune response. Can J Microbiol. 1969 Jul;15(7):814–816. doi: 10.1139/m69-143. [DOI] [PubMed] [Google Scholar]
- Finger H., Emmerling P., Offenhammer A. Increased primary immune response and priming in mice to subimmunogenic doses of sheep erythrocytes by Bordetella pertussis. Experientia. 1969 Aug 15;25(8):866–867. doi: 10.1007/BF01897926. [DOI] [PubMed] [Google Scholar]
- Finger H., Emmerling P., Schmidt H. Accelerated and prolongated multiplication of antibody-forming spleen cells by Bordetella pertussis in mice immunized with sheep red blood cells. Experientia. 1967 Jul 15;23(7):591–592. doi: 10.1007/BF02137991. [DOI] [PubMed] [Google Scholar]
- Finger H., Emmerling P., Tusch H., Bredt W. Einfluss von Bordetta pertussis auf das lymphatische Gewebe von Mäusen. 3. Die beeinflussung der Kinetik der Antikörperbildung gegen Schaferythrozyten durch Bordetella pertussis. Z Immunitatsforsch Allerg Klin Immunol. 1968 Aug-Sep;136(3):268–284. [PubMed] [Google Scholar]
- Finger H. Zur Frage nach einem einheitlichen Wirkungsprinzip immunologischer Adjuvantien. Arch Hyg Bakteriol. 1965 Dec;149(7):732–746. [PubMed] [Google Scholar]
- Hege J. S., Cole L. J. Antibody plaque-forming cells: kinetics of primary and secondary responses. J Immunol. 1966 Apr;96(4):559–569. [PubMed] [Google Scholar]
- KIND L. S. Relationship of the anaphylaxis sensitizing and adjuvant properties of Hemophilus pertussis vaccine. J Immunol. 1957 Sep;79(3):238–242. [PubMed] [Google Scholar]
- LEDUC E. H., COONS A. H., CONNOLLY J. M. Studies on antibody production. II. The primary and secondary responses in the popliteal lymph node of the rabbit. J Exp Med. 1955 Jul 1;102(1):61–72. doi: 10.1084/jem.102.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNOZ J. Comparison of Bordetella pertussis cells and Freund's adjuvant with respect to their antibody inducing and anaphylactogenic properties. J Immunol. 1963 Jan;90:132–139. [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J., Bergman R. K. Histamine-sensitizing factors from microbial agents, with special reference to Bordetella pertussis. Bacteriol Rev. 1968 Jun;32(2):103–126. doi: 10.1128/br.32.2.103-126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- Rowley D. A., Fitch F. W., Mosier D. E., Solliday S., Coppleson L. W., Brown B. W. The rate of division of antibody-forming cells during the early primary immune response. J Exp Med. 1968 May 1;127(5):983–1002. doi: 10.1084/jem.127.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzl J., Jílek M. Number of antibody-forming cells in primary and secondary reactions after administration of antigen. Nature. 1967 Dec 23;216(5121):1233–1235. doi: 10.1038/2161233a0. [DOI] [PubMed] [Google Scholar]
- TALIAFERRO W. H., TALIAFERRO L. G. The dynamics of hemolysin formation in intact and splenectomized rabbits. J Infect Dis. 1950 Jul-Aug;87(1):37–62. doi: 10.1093/infdis/87.1.37. [DOI] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]



