Abstract
Objective
Psoriatic arthritis (PsA) is associated with HLA class I genes, in contrast to the association with HLA class II in rheumatoid arthritis (RA). Since IL-17+ cells are considered important mediators of synovial inflammation, we sought to determine whether IL-17–producing CD8+ T cells may be found in the joints of patients with PsA and whether these cells might contribute to the disease process.
Methods
Mononuclear cells from paired samples of synovial fluid (SF) and peripheral blood (PB) from patients with PsA or patients with RA were stimulated ex vivo, and CD4− T cells were examined by flow cytometry for cytokine expression, cytotoxic markers, and frequencies of γ/δ or mucosal-associated invariant T cells. Clinical measures of arthritis activity (C-reactive protein [CRP] level, erythrocyte sedimentation rate [ESR], Disease Activity Score in 28 joints [DAS28]) and power Doppler ultrasound (PDUS) scores for the presence of active synovitis in the aspirated knee were recorded and assessed for correlations with immunologic markers.
Results
Within the CD3+ T cell compartment, both IL-17+CD4− (predominantly CD8+) and IL-17+CD4+ T cells were significantly enhanced in the SF compared to the PB of patients with PsA (P = 0.0003 and P = 0.002, respectively; n = 21), whereas in patients with RA, only IL-17+CD4+ T cells were increased in the SF compared to the PB (P = 0.008; n = 14). The frequency of IL-17+CD4− T cells in PsA SF was positively correlated with the CRP level (r = 0.52, P = 0.01), ESR (r = 0.59, P = 0.004), and DAS28 (r = 0.52, P = 0.01), and was increased in patients with erosive disease (P < 0.05). In addition, the frequency of IL-17+CD4− T cells positively correlated with the PDUS score, a marker for active synovitis (r = 0.49, P = 0.04).
Conclusion
These results show, for the first time, that the PsA joint, but not the RA joint, is enriched for IL-17+CD8+ T cells. Moreover, the findings reveal that the levels of this T cell subset are correlated with disease activity measures and the radiographic erosion status after 2 years, suggesting a previously unrecognized contribution of these cells to the pathogenesis of PsA.
Psoriatic arthritis (PsA) is an inflammatory joint disease of unclear etiology that affects ∼10–30% of patients with the skin condition psoriasis (1). Although PsA, like rheumatoid arthritis (RA), can result in pain, loss of function, and damage of the joint, the disease is clinically, radiologically, and serologically distinct from RA (2–4). In addition, PsA and RA have different genetic associations with the major histocompatibility complex region that encodes HLA, in which RA is associated with HLA class II, whereas PsA is associated with HLA class I (5–7). These differences suggest that the immunopathologic mechanisms of these 2 diseases may also differ.
The association with HLA class I suggests that CD8+ T cells have a role in the pathogenesis of PsA. This is supported by observational data; patients with advanced human immunodeficiency virus (HIV) status and low CD4+ T cell counts may develop de novo or worsening PsA and/or psoriasis, whereas patients with CD4+ T cell–driven diseases such as RA have shown improvement at the onset of HIV infection (8,9). It has been suggested that the corresponding increase in memory CD8+ T cells, comprising up to 80% of the total T cell compartment in severe HIV infection, contributes to the development of PsA in this context (10).
Despite the suggestions that CD8+ T cells play an important role in the pathogenesis of PsA (11,12), most studies of T cell cytokine expression in PsA have focused on CD4+ T cells, particularly those expressing the proinflammatory cytokines interleukin-17A (IL-17A), interferon-γ (IFNγ), or tumor necrosis factor α (TNFα) (13–15). The proinflammatory cytokine IL-17 is of particular interest because of its potent osteoclastogenic activity and its ability to up-regulate matrix metalloproteinases and proinflammatory cytokines (IL-1β, IL-8, TNFα) (16). We previously showed that levels of synovial IL-17 messenger RNA (mRNA), in synergy with TNFα, are predictive of joint damage progression in RA (17), and that the percentage of synovial IL-17–producing CD4+ T cells is correlated with markers of disease activity and active synovitis in RA (18). IL-17+CD4+ T cells have been studied in patients with PsA (13,14,19,20); however, the role of IL-17+CD8+ T cells in the PsA joint is currently unknown.
Herein we present a detailed investigation of the presence of IL-17+ T cells and other cytokine-expressing T cells (CD4+ versus CD4− T cells) in the peripheral blood (PB) and synovial fluid (SF) of patients with PsA. Our findings show that IL-17+CD4− T cells are predominantly CD8+ cells, and their levels are significantly increased in the SF of patients with PsA. Moreover, the levels of these cells are significantly correlated with measures of disease activity, the erosion status assessed by radiography, and the presence of active synovitis assessed by power Doppler ultrasonography (PDUS). Our data suggest that IL-17–producing CD8+ T cells may constitute an as-yet-unrecognized pathogenic immune cell population in patients with PsA.
PATIENTS AND METHODS
Healthy controls and patients
Healthy control subjects were recruited from among university staff, hospital staff, and students. Patients with PsA and those with RA were recruited from the Guy's and St. Thomas' Foundation NHS Trust Rheumatology and Dermatology outpatient clinics. All subjects provided consent to donate PB samples, and where available, SF samples. Patients with PsA fulfilled the classification criteria of the Classification of Psoriatic Arthritis (CASPAR) Study Group (21), and patients with RA fulfilled the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA (22).
All patients underwent a clinical examination that included tender and swollen joint counts and the Disease Activity Score in 28 joints (DAS28) (23). Laboratory investigations included determination of the C-reactive protein (CRP) level and the erythrocyte sedimentation rate (ESR). For patients with PsA, radiographs of the hands and feet were obtained at the time of clinical assessment and in those with an arthritis duration of <2 years, followup radiographs were obtained after 2 years to assess the erosion status of the joints. Radiographs were read by an experienced clinician (BWK) who was blinded with regard to the study data. The presence of definite joint erosions, as evident on radiographs of the hands, feet (all subjects), and knees (when available), was used to define erosive (versus nonerosive) disease. Aspiration of SF samples was undertaken after the PDUS assessment. The demographic and clinical characteristics of the study subjects are summarized in Table 1. All subjects provided written informed consent, and ethics approval was granted by the Bromley Research Ethics Committee.
Table 1.
Demographic and clinical characteristics of the study subjects*
| Patients with PsA | Patients with RA | ||||
|---|---|---|---|---|---|
| All PsA (n = 33) | Paired PB/SF (n = 21) | All RA (n = 24) | Paired PB/SF (n = 14) | Healthy controls (n = 14) | |
| Female, no. (%) | 12 (36) | 6 (29) | 18 (75) | 10 (71) | 8 (57) |
| Age, mean ± SEM years | 45.1 ± 2.86 | 43.8 ± 3.73 | 54.3 ± 2.54 | 53.5 ± 3.20 | 43.8 ± 3.03 |
| Oligoarthritis, no. (%) | 25 (76) | 16 (76) | 0 (0) | 0 (0) | – |
| Disease duration, mean ± SEM years | 8.7 ± 1.43 | 7.3± 1.57 | 9.2 ± 1.73 | 12.2 ± 2.45 | – |
| Treatment, no. (%) | |||||
| Biologics | 8 (24) | 6 (29) | 4 (17) | 1 (7) | – |
| DMARDs | 12 (36) | 8 (38) | 17 (71) | 10 (71) | – |
| No treatment | 13 (39) | 7 (33) | 3 (12) | 3 (21) | – |
| DAS28 | |||||
| Mean ± SEM | 3.99 ± 0.21 | 3.87 ± 0.24 | 4.05 ± 0.32 | 4.79 ± 0.36 | – |
| Range | 1.6–6.6 | 1.4–6.9 | |||
| ESR, mean ± SEM mm/hour | 24.4 ± 4.42 | 29.9 ± 6.07 | 22.8 ± 2.86 | 23.4 ± 4.22 | – |
| CRP, mean ± SEM gm/dl | 31.8 ± 8.43 | 42.2 ± 12.0 | 13.0 ± 2.53 | 17.2 ± 4.10 | – |
| Erosive disease, no. (%) | 19 (57) | 13 (62) | 19 (80) | 12 (85) | – |
In total, 33 patients with psoriatic arthritis (PsA), 24 patients with rheumatoid arthritis (RA), and 14 healthy control subjects were recruited into the study. None of the patients had pure spondyloarthritis, arthritis mutilans, or distal interphalangeal joint arthritis. PB = peripheral blood; SF = synovial fluid; DMARDs = disease-modifying antirheumatic drugs; DAS28 = Disease Activity Score in 28 joints; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein.
Ex vivo cytokine staining
PB mononuclear cells (PBMCs) and SF mononuclear cells (SFMCs) were isolated by density-gradient centrifugation using lymphocyte separation medium (LSM 1077; PAA Laboratories). Cells were placed in culture medium (RPMI 1640 supplemented with 10% fetal bovine serum [FBS]) (batch F9665, lot 030M3399; Sigma-Aldrich) containing 1% penicillin/streptomycin and 1% l-glutamine (Gibco), followed by stimulation for 3 hours with phorbol myristate acetate (PMA; 50 ng/ml) and ionomycin (750 ng/ml) (both from Sigma-Aldrich) in the presence of GolgiStop (BD Biosciences), according to the manufacturer's instructions, at 37°C. Extracellular surface staining was performed at 4°C using the following antibodies: phycoerythrin (PE)–Cy7–conjugated anti-CD3, Pacific Blue–conjugated anti-CD8, allophycocyanin (APC)–Cy7–conjugated anti-CD14, fluorescein isothiocyanate (FITC)–conjugated anti-CD107a, Alexa Fluor 647–conjugated anti-CD161, FITC-conjugated anti–γ/δ T cell receptor (TCR), or PE-conjugated anti–Vα7.2 TCR (all from BioLegend). Thereafter, the cells were fixed with 2% paraformaldehyde and permeabilized with 0.5% saponin (Sigma-Aldrich). Intracellular cytokine staining was performed at 4°C with the following antibodies: Pacific Blue–conjugated anti-CD4, PerCP–Cy5.5–conjugated anti-CD4, FITC-conjugated anti–granzyme B, APC-conjugated anti-IFNγ, PerCP–Cy5.5–conjugated anti-IFNγ, Alexa Fluor 488–conjugated anti–IL-10, Alexa Fluor 488–conjugated anti–IL-17A, Alexa Fluor 647–conjugated anti–IL-17A, PE-conjugated anti–IL-17A, Alexa Fluor 647–conjugated anti–IL-21, PE-conjugated anti-perforin, APC-conjugated anti-TNFα (all from BioLegend), or PE-conjugated anti–IL-22 (R&D Systems).
All ex vivo cytokine stains were performed with freshly isolated cells. In addition, PBMCs and SFMCs were cryopreserved within 2 hours of isolation and stored in liquid nitrogen in culture medium supplemented with 50% FBS and 10% dimethyl sulfoxide. These samples were subsequently analyzed for the presence of cytotoxic molecules and mucosal-associated invariant T (MAIT) cells.
Flow cytometry
Ex vivo samples were acquired on a FACSCanto (BD Biosciences) and analyzed using FlowJo software (Tree Star). Viable single cells were identified based on their forward scatter area/forward scatter width profile, and T cells were gated by positive staining for CD3 and negative staining for CD14. Analysis of the CD4+ and CD4− T cell compartments was performed, and where indicated, analysis of the CD8+ T cell population was also performed.
PDUS image acquisition
PDUS examination of the joints was performed using a GE Logiq scanner (version 9; GE Healthcare) with a matrix array transducer at 5–14 MHz. Images recorded using PDUS were later analyzed in a blinded manner by 2 rheumatologists experienced in ultrasonography (NJG and TG). The knee joints were assessed at 5 points in the longitudinal and oblique planes at the suprapatellar pouch and the joint margins, and a mean PDUS score for the presence of active synovitis was calculated. Gain was reduced to the point where the signal in the cortical bone disappeared, to minimize background noise. PDUS images were graded using a semiquantitative scale, ranging from 0 to 3, where 0 = no signal, 1 = 1–2-pixel involvement, 2 = up to 50% pixel involvement, and 3 = ≥50% signal (18,24).
Statistical analysis
Results are expressed as the mean ± SEM for normally distributed data or as the median (interquartile range [IQR]) for nonparametric data. For paired PB and SF samples, Wilcoxon's matched pairs signed rank test or one-way analysis of variance was used (nonparametric data). Correlations were analyzed using Spearman's correlation coefficients for nonparametric data. In addition, linear regression analyses with line-of-best-fit were performed. All data were analyzed using Prism software (version 5; GraphPad). P values less than 0.05 were considered significant.
RESULTS
Increased SF levels of IL-17+CD4− T cells in patients with PsA, but not in patients with RA
Paired PBMCs and SFMCs from patients with PsA (n = 21) or patients with RA (n = 14) were stimulated ex vivo for 3 hours with PMA and ionomycin in the presence of GolgiStop, and the percentage of CD3+CD4+ and CD3+CD4− T cells expressing IL-17 was determined (Figures 1A and B). In patients with PsA, the percentage of IL-17+CD3+ T cells was significantly increased in the SF as compared to the PB (median 2.30%, IQR 1.2–3.69 versus 0.70%, IQR 0.56–0.85; P = 0.0004). An increased frequency of IL-17+ cells in the SF as compared to the PB was detected in both CD3+CD4− T cells (median 1.10%, IQR 0.36–2.19 versus 0.18%, IQR 0.09–0.32; P = 0.0003) and CD3+CD4+ T cells (median 1.47%, IQR 0.80–3.17 versus 0.71%, IQR 0.57–1.03; P = 0.002). In contrast, in patients with RA, the frequency of IL-17+ cells was increased in the SF as compared to the PB in the CD4+ T cell compartment only (median 1.27%, IQR 0.75–2.10 versus 0.63%, IQR 0.28–0.88; P = 0.008) and not in the CD4− T cell compartment (median 0.13%, IQR 0.06–0.28 versus 0.11%, IQR 0.06–0.21; P = 0.67).
Figure 1.
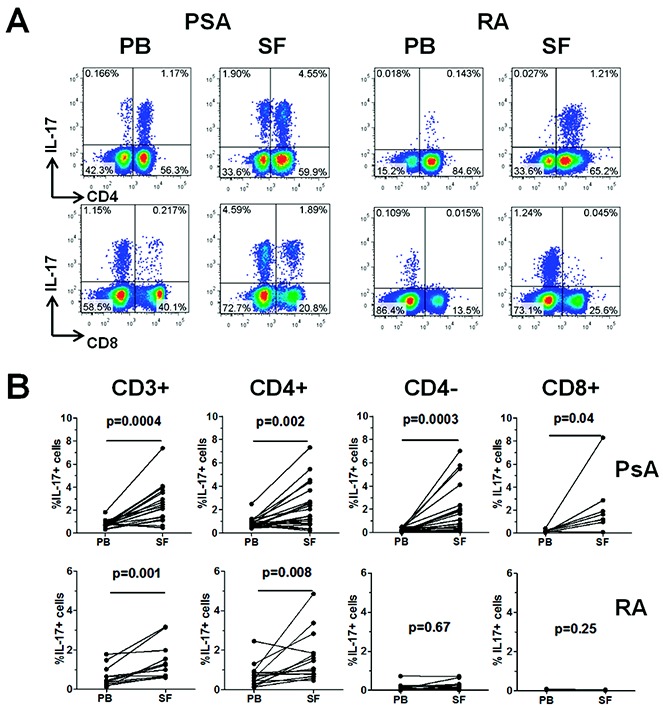
Frequency of interleukin-17 (IL-17)–expressing cells in CD3+, CD3+CD4+, CD3+CD4−, and CD3+CD8+ T cell populations in paired peripheral blood (PB) and synovial fluid (SF) samples from patients with psoriatic arthritis (PsA) or rheumatoid arthritis (RA). Mononuclear cells from paired PB and SF samples from patients with PsA (n = 21) and patients with RA (n = 14) were isolated and stimulated as described in Patients and Methods. A, Percentage of IL-17–expressing cells within CD4+ and CD4− T cells (top panels) and within CD8+ and CD8− T cells (bottom panels), as determined by flow cytometry in PB and SF from a representative patient with PsA and a representative patient with RA. B, Percentage of IL-17+ cells in total CD3+, CD4+, CD4−, or CD8+ T cells in paired PB and SF samples from patients with PsA and patients with RA. For the CD8+ subset, the percentage of IL-17+ cells was determined in paired samples from 8 PsA patients and 3 RA patients. Data were analyzed using Wilcoxon's matched pairs signed rank test. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.38376/abstract.
To clarify whether the IL-17+CD3+CD4− T cell subset was indeed also CD8+, we performed parallel analysis of IL-17 expression in CD4− T cells and CD8+ T cells in 11 samples (8 from patients with PsA and 3 from patients with RA). In the paired samples from patients with PsA, a similar increase in IL-17+ cells in the SF as compared to the PB was observed in both the CD4− and CD8+ T cell subsets (Figure 1B and Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38376/abstract).
Unlike some other PsA cohorts, in our cohort, the majority of patients, 76% (16 of 21), were classified as having oligoarthritis rather than polyarthritis. None of the patients had pure spondyloarthritis (SpA), arthritis mutilans, or distal interphalangeal joint arthritis. Subgroup analysis revealed that the increase in frequencies of IL-17+CD4− T cells and IL-17+CD4+ T cells in the SF as compared to the PB was of similar magnitude between individuals with oligoarthritis (P = 0.001 and P = 0.02, respectively) and those with polyarthritis (P = 0.06 and P = 0.06, respectively), although the difference did not reach statistical significance in the polyarthritis group due to its small sample size (see Supplementary Figure 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38376/abstract).
In addition, we sought to identify whether the IL-17+CD4− T cell population in the SF of patients with PsA is composed, in part, of CD161+ cells, MAIT cells, or γ/δ T cells, all of which have been demonstrated to contain IL-17–expressing cells (25–27). Cryopreserved PBMCs and SFMCs were stained for CD3, CD4, CD8, CD161, γ/δ TCR, Vα7.2 TCR, and IL-17. Similar to the above findings, the majority of IL-17+CD3+CD4− T cells were CD8+ (Figure 2A). Within the IL-17+CD3+CD4− T cell compartment, the majority of cells expressed CD161. However, fewer than 14% of the IL-17+CD3+CD4− non–γ/δ T cells expressed both CD161 and Vα7.2 TCR, the characteristic markers of MAIT cells (Figure 2A). Finally, very few cells in the IL-17+CD3+CD4− T cell compartment expressed γ/δ TCR (Figure 2A). Taken together, these results indicate that, although some IL-17+CD4− T cells in patients with PsA can be characterized as MAIT cells or γ/δ T cells, the majority comprise CD8+ and CD161+ T cells.
Figure 2.
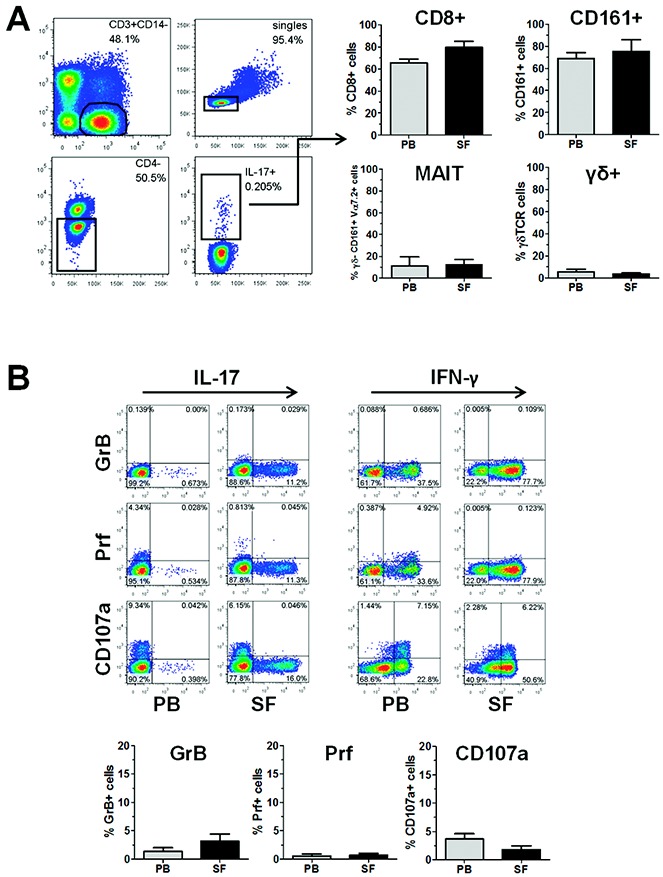
CD3+CD4− T cells in patients with psoriatic arthritis (PsA) are predominantly CD8+ T cells lacking markers associated with cytolytic activity. Cryopreserved mononuclear cells from paired peripheral blood (PB) and synovial fluid (SF) samples from patients with PsA were thawed and stimulated with phorbol myristate acetate and ionomycin in the presence of GolgiStop for 3 hours, and then stained for the expression of CD3, CD4, CD8, CD161, γ/δ T cell receptor (TCR), Vα7.2 TCR, granzyme B (GrB), perforin (Prf), and CD107a, along with interleukin-17 (IL-17). A, Left, Gating strategy to identify IL-17+CD3+CD4− T cells by flow cytometry. Representative results are shown. A, Right, Percentage of cells within the IL-17+CD3+CD4− T cell population that were either CD8+, CD161+, γ/δ−CD161+Vα7.2+ (mucosal-associated invariant T [MAIT] cells), or γ/δ+. B, Top, Coexpression of IL-17 or interferon-γ (IFNγ) and granzyme B, perforin, or CD107a in CD3+CD8+ T cells from PsA PB and SF, as determined by flow cytometry. Representative dot plots are shown. B, Bottom, Percentage of cells within the IL-17+ CD3+CD8+ T cell population that expressed granzyme B, perforin, or CD107a in PsA PB and SF. Results are the mean ± SEM of 4 samples. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.38376/abstract.
Lack of expression of cytotoxic function markers by IL-17+CD8+ T cells
We investigated whether the IL-17+CD8+ T cells identified in patients with PsA had features of classic cytotoxic lymphocytes, by analyzing the T cell expression of CD107a (a degranulation marker) and the cytotoxic proteins perforin and granzyme B (28). Cryopreserved PBMCs and SFMCs (n = 4 paired samples) were thawed, stimulated with PMA and iomomycin in the presence of GolgiStop, and stained for CD3, CD4, CD8, CD107a, perforin, granzyme B, IL-17, and IFNγ. Although a small, but distinct, population of IFNγ+CD8+ T cells in both the PB and SF samples from PsA patients expressed CD107a, perforin, and/or granzyme B, the IL-17+CD8+ subset of T cells, from either the PB or SF of PsA patients, showed very little expression of any of these markers (Figure 2B).
Characterization of other cytokine-expressing T cells in the PB and SF of patients with PsA
We assessed the T cell expression of other cytokines that have been previously associated with the development or regulation of inflammation in inflammatory skin and/or inflammatory joint diseases. In addition to the increase in IL-17+ T cells in PsA SF as compared to PB (as shown in Figure 1), within the CD3+CD4− T cell compartment (CD3+CD4− T cells [n = 13] or CD3+CD8+ T cells [n = 8]), we found a significantly increased frequency of IL-21+ cells in the PsA SF as compared to PB (median 1.19%, IQR 0.67–2.71 versus 0.62%, IQR 0.33–0.96; P = 0.02), but no other cytokines were differentially expressed in this T cell subset, although trends were observed for IFNγ and TNFα (Figure 3A). In contrast, within the CD4+ T cell compartment, we observed significantly increased frequencies of cytokine-expressing T cells in the PsA SF as compared to PB for all of the cytokines assessed (Figure 3B). Of note, we found no significant difference in the frequency of any cytokine-expressing PB T cells between PsA patients (n = 33) and healthy controls (n = 14) (results not shown).
Figure 3.
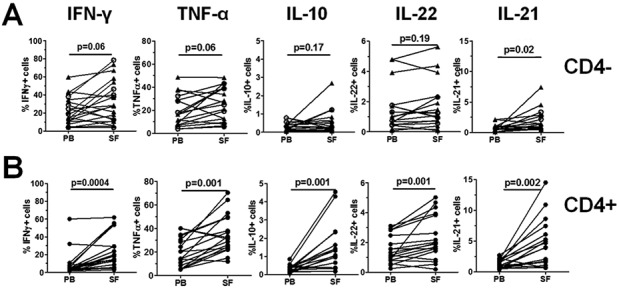
Frequencies of pro- and antiinflammatory cytokine-expressing T cells in paired peripheral blood (PB) and synovial fluid (SF) samples from patients with psoriatic arthritis (PsA). Mononuclear cells were isolated from paired samples of PsA PB and SF (n = 21), and then stimulated ex vivo with phorbol myristate acetate and ionomycin in the presence of GolgiStop and stained for expression of interferon-γ (IFNγ), tumor necrosis factor α (TNFα), interleukin-10 (IL-10), IL-22, and IL-21. Viable CD3+ T cells were gated and the percentages of cytokine-expressing cells were determined in A, CD3+CD4− T cells (triangles; n = 13) or CD3+CD8+ T cells (circles; n = 8), or in B, CD3+CD4+ T cells. Each symbol joined by a line represents a paired sample from a different patient. Data were analyzed using Wilcoxon's matched pairs signed rank test.
Positive correlation between frequency of SF IL-17+CD4− T cells, but not IL-17+CD4+ T cells, and clinical measures of disease activity in PsA
To gain an insight into the potential relevance of the different IL-17+ T cell subsets to disease pathogenesis, we assessed correlations of T cell subset frequencies with disease activity parameters. A strong positive correlation was observed between the frequency of SF IL-17+CD4− T cells (n = 21) and the CRP level (r = 0.52, P = 0.01), ESR (r = 0.59, P = 0.004), and DAS28 (r = 0.52, P = 0.01) in patients with PsA (Figure 4A). In contrast, no significant correlations were observed between the frequency of PsA SF IL-17+CD4+ T cells and any of the clinical parameters, although a trend toward a correlation with the ESR was observed (Figure 4B).
Figure 4.
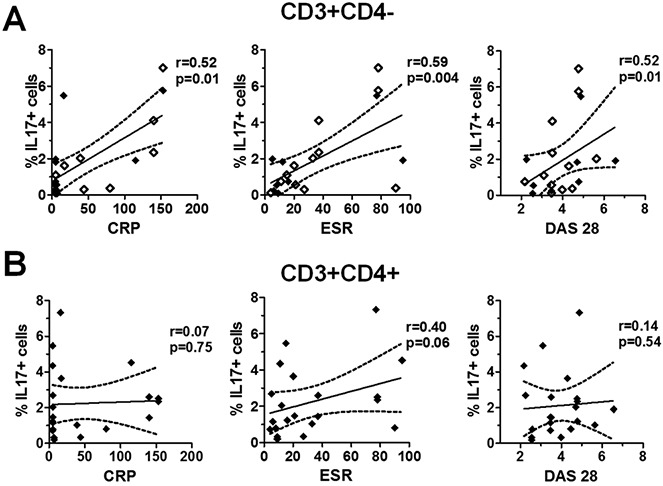
Correlation between the frequency of interleukin-17 (IL-17)–expressing CD4− and CD4+ T cells in psoriatic arthritis (PsA) synovial fluid (SF) and clinical parameters of disease. Clinical measures of disease activity (C-reactive protein [CRP] level, erythrocyte sedimentation rate [ESR], and Disease Activity Score in 28 joints [DAS28]) were determined at the time of PsA SF sampling. Mononuclear cells from the SF of patients with PsA (n = 21) were stimulated as described in Patients and Methods. The percentage of IL-17+ cells within the CD3+CD4− T cell population (including CD3+CD8+ cells [open diamonds]; n = 8) (A) and within the CD3+CD4+ T cell population (B) in PsA SF was plotted against the CRP level, ESR, and DAS28. Regression coefficients (solid lines) with 95% confidence intervals (broken lines) were calculated using Spearman's correlation coefficients for nonparametric data.
Correlation between frequency of SF IL-17+ CD4− T cells and the mean PDUS score for the presence of local synovitis and association with erosive disease status in PsA
PDUS images of the clinically swollen knee joints of patients with PsA (n = 17) were recorded prior to joint aspiration (representative images are shown in Figure 5A). PDUS images were scored by 2 independent assessors. Comparison of the scores between the 2 readers yielded an intraclass correlation coefficient of 0.822 (95% CI 0.66–0.91) and a weighted kappa value of 0.55 (95% CI 0.36–0.74), which is indicative of moderate-to-good agreement (29).
Figure 5.
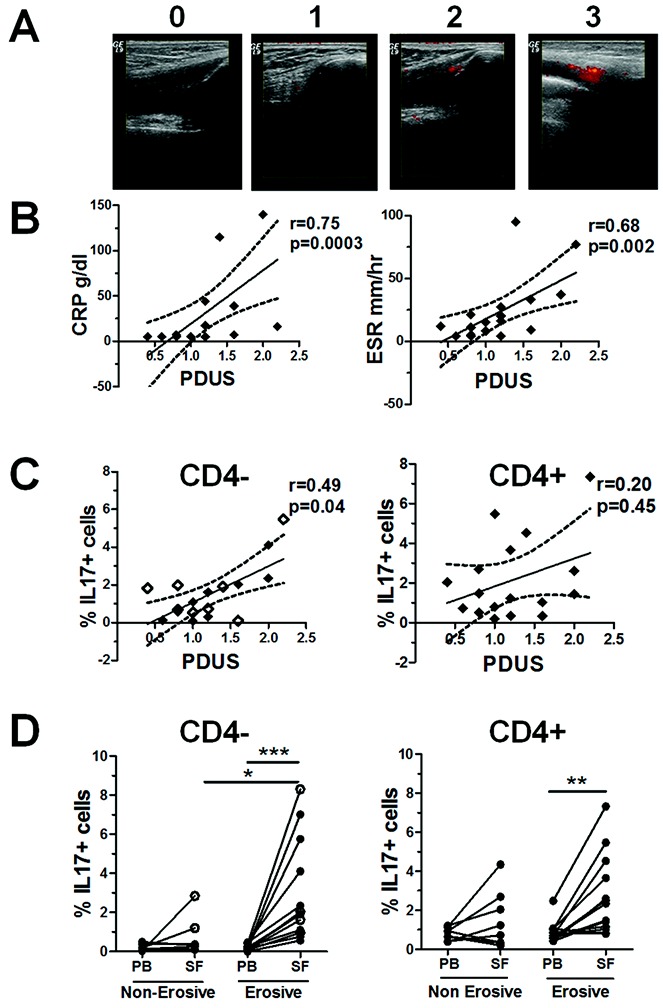
Correlation between the frequency of interleukin-17 (IL-17)–expressing CD4− T cells and the power Doppler ultrasound (PDUS) score for the presence of local synovitis in patients with psoriatic arthritis (PsA), and enrichment of this T cell subset in the joints of patients with erosive disease. A, Representative PDUS images illustrating the semiquantitative grading system for active synovitis in the knee joints of patients with PsA, where 0 = no signal, 1 = 1–2 pixels, 2 = <50% signal, and 3 = ≥50% signal. B, Correlations between measures of disease activity (the C-reactive protein level or erythrocyte sedimentation rate) and the mean PDUS score of the aspirated knee joints (n = 17). C, Correlations between the percentage of IL-17+ cells within CD3+CD4− T cells (including CD3+CD8+ T cells [open diamonds]; n = 8) or CD3+CD4+ T cells from PsA synovial fluid (SF) and the mean PDUS score in the same knee joint. In B and C, regression coefficients (solid lines) with 95% confidence intervals (broken lines) are shown for each plot. D, Percentage of IL-17+ cells within the CD3+CD4− T cell (including CD3+CD8+ cells [open circles]) and CD3+CD4+ T cell populations in paired samples of peripheral blood (PB) and SF from patients with erosive PsA (n = 13) compared to patients with nonerosive PsA (n = 8). Each symbol joined by a line represents a paired sample from a different patient. Data were analyzed using one-way analysis of variance for parametric or nonparametric data, where appropriate. ∗ = P < 0.05; ∗∗ = P < 0.01; ∗∗∗ = P < 0.001.
PDUS scores for the presence of active synovitis in the PsA knee joints were positively correlated with the CRP level (r = 0.75, P = 0.0003) and the ESR (r = 0.68, P = 0.002) (Figure 5B), thus reiterating their use as surrogate markers of inflammation. We observed a positive correlation between the frequency of SF IL-17+CD4− T cells, but not SF IL-17+CD4+ T cells, and the PDUS score of the aspirated knee joint (r = 0.49, P = 0.04 and r = 0.20, P = 0.45, respectively) (Figure 5C). In addition, the PDUS score was positively correlated with the frequency of SF IL-22+CD4− T cells (r = 0.58, P = 0.01), whereas this was not observed for SF IL-22+CD4+ T cells (results not shown). No correlations were found between the PDUS score and other cytokine-expressing CD4− or CD4+ T cells (results not shown).
Interestingly, the frequency of IL-17+CD4− T cells was significantly increased in the SF from PsA patients with erosive disease (n = 13) as compared to those with nonerosive disease (n = 8) (P < 0.05) (Figure 5D). Furthermore, in patients with erosive disease, the frequency of IL-17+ cells within both the CD4− and CD4+ T cell populations was significantly increased in the SF as compared to the PB (n = 13). This relationship was not observed for any of the other SF cytokine-expressing cells examined.
DISCUSSION
Our findings demonstrate that IL-17+CD4− T cells, consisting predominantly of CD8+ T cells, are present at increased levels in PsA SF as compared to paired samples of PsA PB and SF samples from patients with RA. In addition, the frequency of these SF IL-17+CD4− T cells is correlated with serologic, clinical, and imaging measures of PsA disease activity and erosive disease status. IL-17+CD8+ T cells may therefore represent a previously unrecognized T cell population involved in the pathogenesis of PsA.
IL-17+CD8+ T cells have not been previously investigated in PsA, but studies in psoriasis have shown that the numbers of these cells are increased in psoriatic skin plaques, when compared to healthy control skin, and this increased frequency is correlated with disease activity (30–34). Thus, in the same way that IL-17+CD8+ T cells have been shown to participate in skin inflammation in psoriasis, we propose that these cells are contributors to joint inflammation and progressive joint damage in PsA. These findings suggest that there is a potential similarity in immunopathologic characteristics between PsA and psoriasis, and indicate that there are differences compared to RA. The concept that PsA may be immunologically more similar to SpA than to RA has been recently proposed (35). In that review, the findings suggested that CD8+ T cells may potentiate the induction of inflammatory cytokines such as IL-1β, IFNγ, or TNFα. Our current findings suggest that there is a more direct mechanism for the pathogenic role of CD8+ T cells in PsA, through the expression of the proinflammatory and osteoclastogenic cytokine IL-17.
IL-17 is now considered to be a key cytokine in the pathogenesis of a number of autoimmune and autoinflammatory conditions in humans (16). As such, it is of interest that we found IL-17+ T cells to be particularly enriched in the SF of patients with erosive disease. Joint ultrasonography and PDUS are now used to detect joint inflammation (36). It is therefore noteworthy that the frequencies of SF IL-17+CD4− T cells, but not IL-17+CD4+ T cells, IFNγ+CD4− T cells, or TNFα+CD4− T cells, were positively correlated with the PDUS score for the presence of active synovitis in the aspirated joint in patients with PsA. These data further suggest that IL-17+CD4− T cells may be potentially important contributors to disease activity in PsA.
Additionally, we found that the PDUS score of the aspirated joint was positively correlated with the frequency of SF IL-22+CD4− T cells. IL-22, expressed by CD4+ and CD8+ T cells and natural killer cells, among others, has been implicated in the pathogenesis of psoriasis via its effects on keratinocyte proliferation and differentiation. The expression of IL-22 is increased at the mRNA level in psoriatic plaques, as compared to that in nonlesional skin (37). Interestingly, a significant proportion of PsA SF IL-22+CD4− T cells also expressed IL-17 (mean ± SEM 21.7 ± 6.04%) (results not shown), which may explain, in part, their positive correlation with the PDUS score. However, IL-22+CD4− T cell levels were not significantly increased in the PsA SF as compared to the PB, nor were they correlated with other disease activity measures or erosive status. Instead, IL-17+CD4− T cells were predominant correlates with disease activity markers, i.e., the CRP level, ESR, and DAS28.
Several groups have investigated the expression of IL-17 at sites of inflammation in CD4+ and CD8+ T cells, as well as in other cells, including γ/δ T cells, mast cells, neutrophils, and MAIT cells (26,27,38,39). IL-17+CD4+ T cells have been found in the lesional skin of patients with psoriasis (31,40,41), as well as in the joints of patients with RA (14,18,42,43), those with PsA (13,14), those with reactive arthritis (44), and those with ankylosing spondylitis (13,45). In a study by Raychaudhuri et al (46), an increase in the frequency of Th17 cells was observed in PsA SF as compared to PB. Moreover, those authors observed an enrichment of these cells in the synovial tissue and lesional skin of PsA patients (46). In contrast to our results, they reported that IL-17 expression was restricted to CD4+ T lymphocytes, which may be related to differences in the stimulation protocol. Raychaudhuri and colleagues used PMA–ionomycin stimulation for 24 hours, followed by 6 hours of stimulation with monensin before cytokine staining, whereas we stimulated cells for only 3 hours. It is possible that IL-17+CD8+ T cells might not be detected after a longer period of stimulation, due to IL-17 having already been secreted, or possibly due to cell death or exhaustion.
A previous study showed that mast cells were the predominant IL-17–expressing cells in RA synovial tissue, as determined by immunohistochemistry (47). Similar immunohistochemistry findings were observed in SpA synovial tissue, with results indicating that there were very few IL-17+CD3+ T cells, whereas IL-17+c-kit+ mast cells were enriched in the SpA synovium relative to the RA synovium, although these cells did not show signs of degranulation (39). The low detection rate of IL-17+ T cells in the tissue by immunohistochemistry may be partially attributable to rapid secretion of IL-17 by CD3+ cells. Previous studies of psoriatic skin samples demonstrated similarly that only IL-17+ mast cells could be identified by immunohistochemistry, but when the T cells were separated and stimulated in vitro, high levels of IL-17+CD8+ T cells and fewer IL-17+CD4+ T cells were detected by flow cytometry (33). These findings indicate that immunohistochemistry and flow cytometry may reveal different IL-17+ cell populations. Consistent with other studies (28,48), we found that the expression levels of CD107a, perforin, and granzyme B were attenuated in IL-17+CD8+ T cells compared to IFNγ+CD8+ T cells, indicating that these IL-17+CD8+ T cells do not have prototypical cytotoxic function and, rather, may be proinflammatory in nature.
The findings we present herein become more relevant in the context of anti–IL-17A antibody trials that are currently under way in patients with PsA. The first trial, assessing the efficacy and safety of secukinumab in patients with active PsA, revealed a modest improvement in patients as compared to controls, although the moderate results were thought to be due to the small cohort size (49). Further large trials are currently ongoing, and the results of these should be more conclusive regarding the pathogenic role of IL-17 in PsA.
Furthermore, recent genome-wide association studies in PsA have identified variants in the TRAF3IP2 gene (50), encoding Act-1 (NF-κB activator 1), a key mediator of IL-17 signaling, and in the RUNX3 gene (51), a transcription factor that promotes CD8+ T cell development in the thymus, as risk factors for disease development. It would be of interest to analyze future patients for these variants, to establish whether there is a link between these genotypes and IL-17+CD8+ T cells in PsA.
In conclusion, our data demonstrate, for the first time, that the frequency of IL-17+CD4− T cells is increased in PsA SF, and that these levels are correlated with blood, clinical, and imaging measures of disease activity. We did not detect these IL-17+CD4− T cells in RA SF. We show that IL-17+CD4− T cells comprise mainly CD8+ cells and lack classic cytolytic markers. In addition, SF IL-17+CD4− T cell levels are increased in PsA patients with erosive disease as compared to those with nonerosive disease. We propose that these cells represent a previously unrecognized population of immune cells that may represent a functionally important mechanism in the pathogenesis of PsA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Taams, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Menon, Gullick, Evans, Taams, Kirkham.
Acquisition of data. Menon, Walter, Rajasekhar, Garrood, Kirkham.
Analysis and interpretation of data. Menon, Gullick, Rajasekhar, Evans, Taams, Kirkham.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Figures.
REFERENCES
- 1.Christophers E. Psoriasis—epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26:314–20. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 2.Scott DL, Smith C, Kingsley G. Joint damage and disability in rheumatoid arthritis: an updated systematic review. Clin Exp Rheumatol. 2003;21:S20–7. [PubMed] [Google Scholar]
- 3.Courvoisier N, Dougados M, Cantagrel A, Goupille P, Meyer O, Sibilia J, et al. Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2008;10:R106. doi: 10.1186/ar2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003;42:1460–8. doi: 10.1093/rheumatology/keg384. [DOI] [PubMed] [Google Scholar]
- 5.Ho PY, Barton A, Worthington J, Plant D, Griffiths CE, Young HS, et al. Investigating the role of the HLA-Cw*06 and HLA-DRB1 genes in susceptibility to psoriatic arthritis: comparison with psoriasis and undifferentiated inflammatory arthritis. Ann Rheum Dis. 2008;67:677–82. doi: 10.1136/ard.2007.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eder L, Chandran V, Pellett F, Shanmugarajah S, Rosen CF, Bull SB, et al. Differential human leucocyte allele association between psoriasis and psoriatic arthritis: a family-based association study. Ann Rheum Dis. 2012;71:1361–5. doi: 10.1136/annrheumdis-2012-201308. [DOI] [PubMed] [Google Scholar]
- 7.Chandran V, Bull SB, Pellett FJ, Ayearst R, Rahman P, Gladman DD. Human leukocyte antigen alleles and susceptibility to psoriatic arthritis. Hum Immunol. 2013;74:1333–8. doi: 10.1016/j.humimm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Ouedraogo DD, Meyer O. Psoriatic arthritis in sub-Saharan Africa. Joint Bone Spine. 2012;79:17–9. doi: 10.1016/j.jbspin.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Furie RA. Effects of human immunodeficiency virus infection on the expression of rheumatic illness. Rheum Dis Clin North Am. 1991;17:177–88. [PubMed] [Google Scholar]
- 10.Morar N, Willis-Owen SA, Maurer T, Bunker CB. HIV-associated psoriasis: pathogenesis, clinical features, and management. Lancet Infect Dis. 2010;10:470–8. doi: 10.1016/S1473-3099(10)70101-8. [DOI] [PubMed] [Google Scholar]
- 11.Costello P, Bresnihan B, O'Farrelly C, FitzGerald O. Predominance of CD8+ T lymphocytes in psoriatic arthritis. J Rheumatol. 1999;26:1117–24. [PubMed] [Google Scholar]
- 12.Curran SA, FitzGerald OM, Costello PJ, Selby JM, Kane DJ, Bresnihan B, et al. Nucleotide sequencing of psoriatic arthritis tissue before and during methotrexate administration reveals a complex inflammatory T cell infiltrate with very few clones exhibiting features that suggest they drive the inflammatory process by recognizing autoantigens. J Immunol. 2004;172:1935–44. doi: 10.4049/jimmunol.172.3.1935. [DOI] [PubMed] [Google Scholar]
- 13.Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–17. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 14.Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–85. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 15.Yoo IS, Lee JH, Song ST, Kim JH, Lee HJ, Kang SW. T-helper 17 cells: the driving force of psoriasis and psoriatic arthritis. Int J Rheum Dis. 2012;15:531–7. doi: 10.1111/j.1756-185X.2012.01813.x. [DOI] [PubMed] [Google Scholar]
- 16.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 17.Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE Study cohort) Arthritis Rheum. 2006;54:1122–31. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 18.Gullick NJ, Evans HG, Church LD, Jayaraj DM, Filer A, Kirkham BW, et al. Linking power Doppler ultrasound to the presence of th17 cells in the rheumatoid arthritis joint. PLoS One. 2010;5:e12516. doi: 10.1371/journal.pone.0012516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veale DJ, Ritchlin C, FitzGerald O. Immunopathology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2):ii26–9. doi: 10.1136/ard.2004.031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celis R, Planell N, Fernandez-Sueiro JL, Sanmarti R, Ramirez J, Gonzalez-Alvaro I, et al. Synovial cytokine expression in psoriatic arthritis and associations with lymphoid neogenesis and clinical features. Arthritis Res Ther. 2012;14:R93. doi: 10.1186/ar3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, the CASPAR Study Group Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 22.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 23.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 24.Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug–induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761–73. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- 25.Maggi L, Santarlasci V, Capone M, Rossi MC, Querci V, Mazzoni A, et al. Distinctive features of classic and nonclassic (Th17 derived) human Th1 cells. Eur J Immunol. 2012;42:3180–8. doi: 10.1002/eji.201242648. [DOI] [PubMed] [Google Scholar]
- 26.Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–9. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 27.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Zhou Q, Yang WB, Xiong XZ, Du RH, Zhang JC. Pleural mesothelial cells promote expansion of IL-17-producing CD8+ T cells in tuberculous pleural effusion. J Clin Immunol. 2013;33:775–87. doi: 10.1007/s10875-012-9860-3. [DOI] [PubMed] [Google Scholar]
- 29.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 30.Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, et al. CD8+ T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J Invest Dermatol. 2013;133:973–9. doi: 10.1038/jid.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-γ: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–41. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009;86:435–43. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]
- 33.Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One. 2010;5:e14108. doi: 10.1371/journal.pone.0014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antiga E, Volpi W, Cardilicchia E, Maggi L, Fili L, Manuelli C, et al. Etanercept downregulates the Th17 pathway and decreases the IL-17+/IL-10+ cell ratio in patients with psoriasis vulgaris. J Clin Immunol. 2012;32:1221–32. doi: 10.1007/s10875-012-9716-x. [DOI] [PubMed] [Google Scholar]
- 35.Maeda S, Hayami Y, Naniwa T, Ueda R. The Th17/IL-23 axis and natural immunity in psoriatic arthritis. Int J Rheumatol. 2012;2012:539683. doi: 10.1155/2012/539683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum. 2007;57:116–24. doi: 10.1002/art.22461. [DOI] [PubMed] [Google Scholar]
- 37.Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–15. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenna TJ, Davidson SI, Duan R, Bradbury LA, McFarlane J, Smith M, et al. Enrichment of circulating interleukin-17–secreting interleukin-23 receptor–positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 2012;64:1420–9. doi: 10.1002/art.33507. [DOI] [PubMed] [Google Scholar]
- 39.Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Canete JD, et al. Interleukin-17–positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012;64:99–109. doi: 10.1002/art.33396. [DOI] [PubMed] [Google Scholar]
- 40.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–9. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 42.Van Hamburg JP, Corneth OB, Paulissen SM, Davelaar N, Asmawidjaja PS, Mus AM, et al. IL-17/Th17 mediated synovial inflammation is IL-22 independent. Ann Rheum Dis. 2013;72:1700–7. doi: 10.1136/annrheumdis-2012-202373. [DOI] [PubMed] [Google Scholar]
- 43.Evans HG, Gullick NJ, Kelly S, Pitzalis C, Lord GM, Kirkham BW, et al. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc Natl Acad Sci U S A. 2009;106:6232–7. doi: 10.1073/pnas.0808144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen H, Goodall JC, Gaston JS. Frequency and phenotype of T helper 17 cells in peripheral blood and synovial fluid of patients with reactive arthritis. J Rheumatol. 2010;37:2096–9. doi: 10.3899/jrheum.100146. [DOI] [PubMed] [Google Scholar]
- 45.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–56. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 46.Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL-17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem. 2012;359:419–29. doi: 10.1007/s11010-011-1036-6. [DOI] [PubMed] [Google Scholar]
- 47.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–40. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 48.Andersson J, Samarina A, Fink J, Rahman S, Grundstrom S. Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis. Infect Immun. 2007;75:5210–22. doi: 10.1128/IAI.00624-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349–56. doi: 10.1136/annrheumdis-2012-202646. [DOI] [PubMed] [Google Scholar]
- 50.Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet. 2010;42:996–9. doi: 10.1038/ng.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Apel M, Uebe S, Bowes J, Giardina E, Korendowych E, Juneblad K, et al. Variants in RUNX3 contribute to susceptibility to psoriatic arthritis, exhibiting further common ground with ankylosing spondylitis. Arthritis Rheum. 2013;65:1224–31. doi: 10.1002/art.37885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures.


