Abstract
Objective
Interstitial lung disease (ILD) is the leading cause of death in patients with systemic sclerosis (SSc; scleroderma). Although SSc-related ILD is more common and severe in African Americans than in Caucasians, little is known about factors underlying this significant health disparity. The aim of this study was to examine the role that low expression of caveolin-1 might play in susceptibility to ILD among African Americans.
Methods
Assays of monocyte migration toward stromal cell–derived factor 1 (SDF-1) were performed using monocytes from Caucasian and African American healthy donors and patients with SSc. For fibrocyte differentiation studies, total peripheral blood mono-nuclear cells were incubated on fibronectin-coated plates. Protein expression was evaluated by immuno-histochemistry and Western blotting.
Results
Monocytes from healthy African American donors and those from patients with SSc had low caveolin-1 levels, enhanced migration toward the CXCR4 ligand SDF-1, and enhanced differentiation to fibrocytes. Enhanced migration and differentiation of monocytes from African Americans and patients with SSc appeared to be attributable to the lack of caveolin-1, because restoring caveolin-1 function using a caveolin-1 scaffolding domain peptide inhibited these processes. Although they differed from monocytes from Caucasians, monocytes from both African Americans and patients with SSc were not identical, because SSc monocytes showed major increases from baseline in ERK, JNK, p38, and Smad2/3 activation, while monocytes from African Americans showed only limited ERK activation and no activation of JNK, p38, or Smad2/3. In contrast, SDF-1 exposure caused no additional ERK activation in SSc monocytes but did cause significant additional activation in monocytes from African Americans.
Conclusion
African Americans may be predisposed to SSc-related ILD due to low baseline caveolin-1 levels in their monocytes, potentially affecting signaling, migration, and fibrocyte differentiation. The monocytes of African Americans may lack caveolin-1 due to high levels of transforming growth factor β in their blood.
The prevalence of lung involvement in patients with systemic sclerosis (SSc; scleroderma) is >70 % (1), and lung disease is the primary cause of morbidity and mortality among these patients (2,3). Several studies (4–11) have revealed highly significant data demonstrating that SSc is more severe in African American individuals than in Caucasian individuals in terms of increased prevalence, earlier age at disease onset, increased risk of lung involvement, increased probability of having the more severe diffuse form of SSc, and increased mortality. In particular, African American patients with SSc also have significantly decreased lung function, including reductions in forced vital capacity and diffusing capacity for carbon monoxide. Such differences are not explained by socioeconomic status, access to health care, or autoantibody status (12).
Although the focus on African American patients with SSc has been considerable, very few studies have addressed the underlying differences between healthy African American and Caucasian individuals that might result in a predisposition in the African American population toward SSc and ILD. Racial differences in the expression and function of various profibrotic and antifibrotic cytokines have been reported (12). Bogatkevich and colleagues observed that bronchoalveolar lavage (BAL) fluid from healthy African American individuals contains much higher levels of hepatocyte growth factor (HGF) and several other cytokines (insulin-like growth factor binding protein, osteoprotegerin, stem cell factor, thrombopoietin, and vascular endothelial growth factor) compared with BAL fluid from healthy Caucasians, and that lung fibroblasts from healthy African Americans are much less responsive to added HGF than are fibroblasts from healthy Caucasians (13). In another study, serum levels of the profibrotic cytokine transforming growth factor β (TGFβ) were twice as high in healthy African Americans as in healthy Caucasians (14).
Caveolin-1, a protein associated with plasma membrane invaginations known as caveolae and with other cellular membranes, is a promising therapeutic target in patients with ILD, including patients with SSc-related ILD. Caveolin-1 binds to and thereby inhibits the function of kinases in several major signaling families including protein kinase C, MAPK, Src, and protein G (15–18) and regulates cell signaling and cell functions induced by the major profibrotic cytokine TGFβ (19–21). There are multiple points of intersection between TGFβ and caveolin-1 signaling. For example, TGFβ inhibits caveolin-1 expression in a variety of cell types including fibroblasts (lung and skin) and monocytes (22–24), suggesting that the TGFβ-rich milieu in the blood and tissue of patients with SSc may lead to reduced caveolin-1 expression in fibroblasts and monocytes and thereby to an altered behavior of these cells that promotes fibrosis. Caveolin-1 also modulates TGFβ signaling in dermal fibroblasts by inhibiting Smad3 phosphorylation and its translocation to the nucleus (24) and via its effects on the endocytosis of TGFβ ligand–receptor complexes. TGFβ receptors sort to both caveolin-1–rich lipid rafts and early endosomes (25,26). The internalization of TGFβ receptors is dependent on the function of caveolin-1 in lipid rafts and clathrin in early endosomes. Early endosomal internalization increases TGFβ signaling, while caveolin-1–dependent internalization leads to receptor degradation, thereby inhibiting TGFβ signaling (27).
We and other investigators have demonstrated that caveolin-1 is deficient in the lung tissue of patients with SSc and those with idiopathic pulmonary fibrosis and in cells isolated from the lung tissue and blood of these patients, including fibroblasts, monocytes, and neutrophils (19,28,29). Similarly, caveolin-1 is deficient in mice in which ILD has been induced with bleomycin or irradiation (28,30). Caveolin-1–null mice spontaneously exhibit thickened, hypercellular alveolar walls and increased extracellular matrix deposition (31,32). The effects of caveolin-1 deficiency in cells and in animals can be reversed by using either the adenovirus encoding full-length caveolin-1 or the caveolin-1 scaffolding domain (CSD) peptide (19,28). When this 20–amino acid domain of caveolin-1 is synthesized in fusion with the antennapedia internalization sequence, it can enter cells and inhibit kinases much like full-length caveolin-1 (33,34). Thus, the central role that caveolin-1 plays in regulating inflammatory cell infiltration and fibrosis in the model of bleomycin-induced ILD has been demonstrated using the CSD peptide to reverse the deficiency of caveolin-1 in several cell types and thereby inhibit disease progression (28,29).
In the course of studies of the role of caveolin-1 in the regulation of cell function and signaling in SSc, we made the unexpected and striking observation that many of the alterations in function and signaling observed in monocytes from patients with SSc were also observed in monocytes from healthy African Americans. Herein we have characterized several similarities and differences in monocyte function and signaling in monocytes from patients with SSc and healthy African American individuals. These similarities may contribute to the predisposition of African Americans to SSc and ILD.
Patients and Methods
Blood donors
Under a protocol approved by the Institutional Review Board for Human Research for a Rheumatology Research Repository, patients with SSc-related ILD were recruited from the Scleroderma Clinic at the Medical University of South Carolina. All patients fulfilled the American College of Rheumatology criteria for SSc (35) and had evidence of SSc-related ILD (29).
Patients with SSc were classified as having either limited cutaneous SSc or diffuse cutaneous SSc, according to the criteria proposed by LeRoy et al (36). Disease duration was determined based on the time when the first non–Raynaud's phenomenon symptoms were documented. The criteria used to define visceral involvement are described below. Demographic data for the patients with SSc and the healthy control donors are available from the corresponding author.
Pulmonary involvement was defined as the demonstration of abnormalities on high-resolution computed tomography (ground-glass changes and/or fibrosis), pulmonary hypertension based on right heart catheterization, restrictive changes on pulmonary function testing, or reduced diffusing capacity for carbon monoxide. Gastrointestinal involvement was defined as a history of gastroesophageal reflux disease based on either subjective and/or objective findings. Some patients had symptoms of reflux requiring treatment with a proton pump inhibitor and/or H2 antagonist; others had abnormal motility documented by esophageal manometry or findings of esophagitis on upper endoscopy. Cardiac involvement was defined as echocardiographic evidence of left ventricular diastolic dysfunction, a pericardial effusion, elevated peak right ventricular systolic pressure, and right ventricular and/or right atrial dilatation; the presence of conduction abnormalities on a 12-lead electrocardiogram was also considered sufficient. Renal involvement was defined as a history of rapidly progressive renal failure.
Antinuclear antibodies (ANAs) and anticentromere antibodies were determined by immunofluorescence analysis using HEp-2 cells as the substrate. Anti–Scl-70 (topoisomerase I) antibodies were determined by enzyme immunoassay.
Peripheral blood mononuclear cell (PBMC) and monocyte isolation
PBMCs were isolated using standard methods (29), by centrifugation on density 1.083 Histopaque cushions. Monocytes were then isolated from the PBMCs by immunodepletion using a Dynal Monocyte Negative Isolation Kit (Invitrogen), resulting in a cell population of ~95% Mac-1 antibody–positive monocytes (29).
Peptide treatments
The CSD peptide (amino acids 82–101 of caveolin-1; DGIWKASFTTFTVTKYWFYR) was synthesized as a fusion peptide to the C-terminus of an antennapedia internalization sequence (RQIKIWFQNRRMKWKK). The antennapedia internalization sequence alone was used as control peptide and showed no effect on cell behavior when compared with no added peptide. When treating cells with peptides, stock solutions of peptides (10 mM in 100% dimethyl sulfoxide) were diluted to the indicated final concentrations.
Monocyte migration assays
Monocyte migration assays were performed as previously described (37). Briefly, SDF-1 (100 ng/ml in RPMI 1640/1% bovine serum albumin [BSA]) was placed into the lower wells of Neuro Probe Multiwell Chemotaxis Chambers fitted with polycarbonate filters (pore size 5 μm). Twenty-five microliters of cell suspension (1 × 106 cells/ml) with or without TGFβ pretreatment (45 minutes, 10 ng/ml in RPMI 1640 with 1% BSA) was placed in the upper wells. Peptides at a final concentration of 0.1 μM were added to cells before they were placed in the upper chamber. After incubation for 2.5 hours at 37°C in a 5% CO2 incubator, filters were removed, fixed, and stained with DAPI (Invitrogen). Cells on the underside of the membrane were photographed and counted in 6 high-power fields per filter.
Monocyte signaling/Western blotting
Levels of caveolin-1 and CXCR4, baseline levels and activation of ERK, JNK, p38, and Smad2/3, and levels of TGFβ receptor type I (TGFβRI) and TGFβRII were determined by Western blotting of sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample buffer extracts of freshly isolated monocytes. To determine the effects of CSD peptide, monocytes isolated as described above were cultured overnight in 6-well tissue culture plates (2 × 106 cells/well) in RPMI 1640/20% fetal calf serum (FCS). Attached cells were then treated for 3 hours with fresh medium (RPMI 1640/1% BSA) supplemented with 0.1 μM CSD peptide or control peptide. To determine the effects of SDF-1 (200 ng per ml), cells were incubated for the indicated number of minutes. In both cases, cells were next washed twice with phosphate buffered saline and then extracted with sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample buffer. Western blotting was performed using the indicated antibodies.
Immunocytochemical analysis
Images were obtained using a Leica DMI 4000B fluorescence microscope. To detect F-actin and CXCR4 or TGFβRI and TGFβRII in monocytes, cells isolated as described above were cultured overnight in 6-well tissue culture plates (1 × 105 cells/well) on coverslips in RPMI 1640/20% FCS. For F-actin and CXCR4, cells attached to coverslips were further treated for 3 hours with RPMI 1640/1% BSA supplemented with either 0.1 μM CSD peptide or control peptide. Cells were then fixed and permeabilized, labeled with fluorescein isothiocyanate–conjugated phalloidin (Sigma-Aldrich) to detect F-actin, labeled with appropriate primary and secondary antibodies, and counterstained with the nuclear stain DAPI.
To detect type I collagen and α-smooth muscle actin (α-SMA), fibrocytes differentiated on coverslips in the presence of CSD peptide or control peptide as described above were fixed and permeabilized, labeled with appropriate primary and secondary antibodies, and counterstained with DAPI.
Monocyte-to-fibrocyte differentiation
Monocyte-to-fibrocyte differentiation was performed as previously described (38,39), with minor modifications. Briefly, PBMCs were isolated as described above from 40 ml of peripheral blood and resuspended at 1 × 107/ml in Dulbecco's modified Eagle's medium/20% FCS supplemented with HEPES (1M at 1:50; Lonza), L-glutamine (200 mM at 1:500; Lonza), and antibiotic/antimycotic solution (1:100; Corning). Two milliliters of PBMC suspension per well was placed in wells of 6-well fibronectin-coated (39,40) plates. On day 2, peptides (CSD peptide or control peptide) were added to the cultures at a final concentration of 0.1 μM. On day 5, the medium was changed to fresh peptide-containing medium. Changing the medium caused the removal of unattached cells (including most of the T cells and B cells) from the cultures. Phase-contrast images were acquired on day 15, and fibrocytes were quantified in terms of elongated, spindle-shaped cells per 10 × field. For immunocytochemistry, the same methods were used, except that the wells contained fibronectin-coated coverslips.
Antibodies and chemokines
Rabbit anti–caveolin-1 (sc-894), rabbit anti-CXCR4 (sc-9046), rabbit anti-TGFβRI (sc-9048), rabbit anti-TGFβRII (sc-17792), and goat anti– phospho-JNK (sc-12882) were obtained from Santa Cruz Biotechnology. Rabbit anti–ERK-1/2 (no. 9102), mouse monoclonal anti–phospho–ERK-1/2 (no. 9106, clone E10), rabbit anti-JNK (no. 9252), rabbit anti-p38 (no. 9212), rabbit anti– phospho-p38 (no. 9211), rabbit anti-Smad2/3 (no. 3102), and rabbit anti–phospho-Smad2/3 (no. 3101) were from Cell Signaling Technology. Mouse monoclonal anti–α-SMA antibody (no. A2547, clone 1A4) was obtained from Sigma. Mouse monoclonal anti-GAPDH (catalog no. MAB374, clone 6C5) was obtained from Millipore. A rabbit antibody against the human Cola1(I) C-terminal propeptide was prepared in our laboratory. The chemokine SDF-1 was obtained from Pepro-Tech (no. 300-28B).
Statistical analysis
Immunoreactive polypeptides on Western blots and immunofluorescent labeling of cells in immunocytochemistry experiments were quantified by densitometry, using ImageJ version 1.32 software (National Institutes of Health). The resulting data were analyzed using Student's t-test.
Results
Low caveolin-1 levels in monocytes from African Americans
To evaluate the potential role of caveolin-1 in the predisposition of African Americans to SSc, we compared caveolin-1 levels in monocytes derived from Caucasians, African Americans, and patients with SSc. The level of caveolin-1 in monocytes from African Americans was only 40% the level in monocytes from Caucasians (P < 0.01), i.e., almost as low as in SSc monocytes (Figure 1A). Interestingly, caveolin-1 levels in monocytes from African American and Caucasian patients with SSc were not statistically significantly different.
Figure 1.
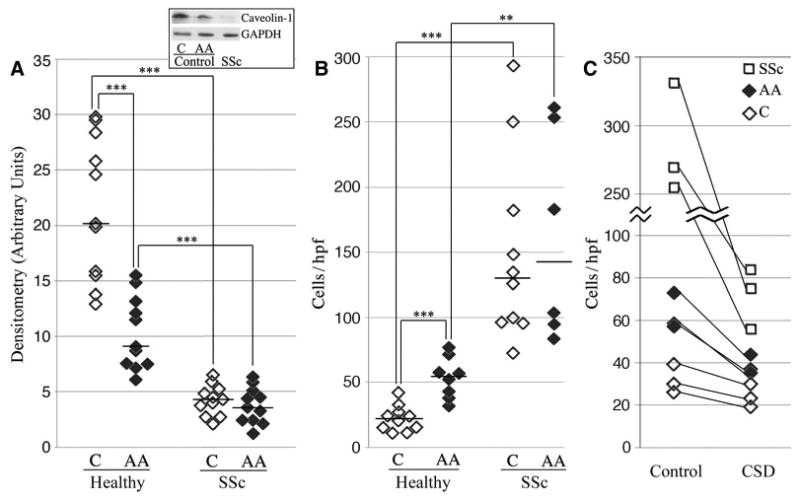
Low expression of caveolin-1 in the monocytes of healthy African Americans results in enhanced migration toward stromal cell–derived factor 1 (SDF-1). Monocytes obtained from Caucasian (C) and African American (AA) healthy donors and patients with systemic sclerosis (SSc) were characterized in terms of their expression of caveolin-1 and their migration toward SDF-1. A, Caveolin-1 levels (normalized to GAPDH loading control) as assessed by densitometry. Boxed area shows a Western blot (50 μg total protein per lane) from a typical experiment using monocyte extracts from the indicated sources. B, Monocyte migration toward SDF-1. In A and B, each data point represents a single subject; horizontal lines show the mean. C, Inhibition of monocyte migration by the caveolin-1 scaffolding domain (CSD) peptide. The lines connect the data points for each subject. ** = P < 0.01; *** = P < 0.001. hpf = high-power field.
Migration of monocytes from African Americans
Given the causal relationship between low caveolin-1 levels and enhanced migration toward SDF-1 in SSc monocytes (37) and the low caveolin-1 levels in monocytes from African Americans, we evaluated the ability of monocytes from African Americans to migrate toward SDF-1 (Figure 1B). As predicted from their reduced expression of caveolin-1, the migration of monocytes from African Americans toward SDF-1 was enhanced 2.5-fold compared with monocytes from Caucasians (P < 0.001). Treatment with CSD peptide almost completely blocked the enhanced migration of monocytes from African Americans and patients with SSc (Figure 1C), corroborating the functional significance of low caveolin-1 levels in monocytes from African Americans. Despite the difference in migration between monocytes from African Americans and monocytes from Caucasians, as with caveolin-1 expression, there was no significant difference in migration between monocytes from African American and Caucasian patients with SSc (Figure 1B). These data strongly support the concept that African Americans may be predisposed to the development of SSc because their monocytes share features with SSc monocytes.
Cytoskeleton activation and CXCR4 overexpression in monocytes from patients with SSc and monocytes from African Americans
Given that SSc monocytes are hypermigratory and deficient in caveolin-1 (29,37), and that monocyte migration toward SDF-1 requires organization of the cortical F-actin cytoskeleton and the expression of CXCR4, we compared F-actin and CXCR4 expression in monocytes from Caucasians, African Americans, and patients with SSc, with and without CSD peptide treatment, using phalloidin to detect F-actin and specific antibodies to detect CXCR4.
Phalloidin staining was punctate in monocytes from both Caucasians and African Americans but was more intense in those from African Americans (Figure 2A). In SSc monocytes, phalloidin staining was even more intense, and in addition to the punctate staining, cortical staining was extremely intense (Figure 2A). Although the level of CXCR4 staining was low in monocytes from Caucasians, the level was much higher in monocytes from African Americans and higher still in SSc monocytes (Figure 2A). In all cases, treatment with CSD peptide greatly decreased the levels of staining for F-actin and CXCR4.
Figure 2.
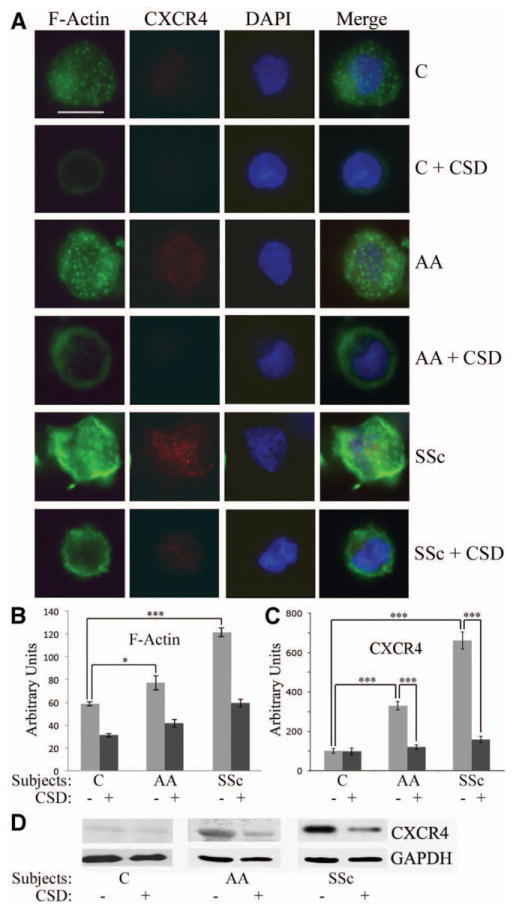
The CSD peptide reverses the enhanced expression of F-actin and CXCR4 observed in monocytes from African Americans and patients with SSc. Monocytes were isolated from the blood of healthy Caucasians and African Americans, and from patients with SSc. One portion was attached to coverslips, treated with CSD or control peptide, stained for F-actin and CXCR4, and counterstained with DAPI. Another portion was treated with CSD or control peptide and extracted for Western blotting. A, Representative images showing F-actin and CXCR4 expression in cells obtained from healthy Caucasians and African Americans and from patients with SSc (n = 4 donors in each group), with and without CSD peptide treatment. Staining for F-actin and CXCR4 was enhanced in monocytes from healthy African Americans and from patients with SSc, and treatment with CSD decreased this staining. Bar = 5 μm. B and C, Densitometric quantification of staining for F-actin (B) and CXCR4 (C). Values are the mean ± SEM (n = 10 cells in each group). * = P < 0.05; *** = P < 0.001. D, Representative Western blot showing enhanced CXCR4 expression in monocytes from African Americans and patients with SSc that was reversed by CSD treatment. The level of CXCR4 in Caucasian subjects treated with control peptide was set at 100 arbitrary units. See Figure 1 for definitions.
F-actin expression was quantified by densitometry (Figure 2B), and CXCR4 staining data were quantified (Figure 2C) and validated by Western blotting (Figure 2D), confirming the up-regulation of CXCR4 in monocytes from African Americans and patients with SSc and the reversal of this increase by CSD peptide. These results suggested that the relative lack of caveolin-1 in monocytes from healthy African Americans and those from patients with SSc leads to the reorganization of the cytoskeleton and overexpression of CXCR4, which in turn results in hypermigration toward SDF-1.
ERK hyperactivation in monocytes from African Americans
We previously demonstrated that ERK, JNK, and p38 are highly phosphorylated in monocytes from patients with SSc compared with monocytes from Caucasians (29). When monocytes from African Americans were compared with monocytes from Caucasians, ERK hyperactivation but not JNK or p38 hyperactivation was observed in the monocytes of African Americans (Figure 3A). CSD peptide treatment inhibited ERK phosphorylation in monocytes from both Caucasians and African Americans but particularly in those from African Americans (Figures 3B and C).
Figure 3.
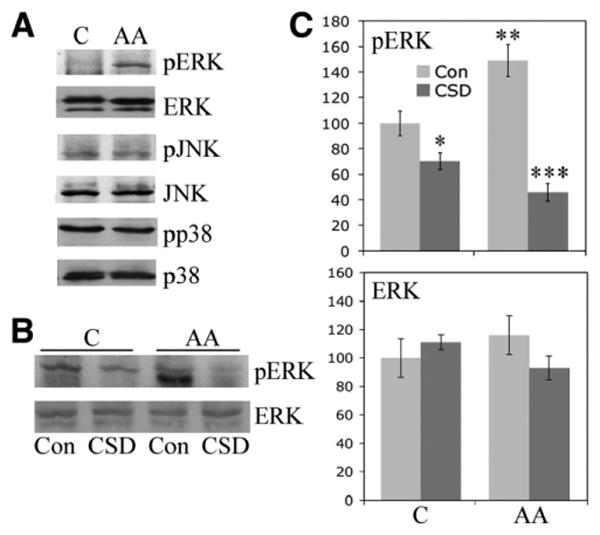
Enhanced ERK signaling in monocytes from African Americans. Monocytes were isolated from the blood of healthy Caucasian and African American donors and cultured on 6-well plates. The expression and activation of the indicated signaling molecules were evaluated by Western blotting (50 μg total protein per lane). A, Representative set of Western blots showing hyperactivation of ERK, but not JNK or p38, in monocytes from African Americans compared with those from Caucasians. Similar results were obtained in 3 experiments performed with cells from different subjects. B, Representative Western blot showing that CSD peptide reverses the enhanced activation of ERK in monocytes from African Americans incubated with control (Con) peptide. C, Quantification of the results shown in B, as determined by densitometry. The levels of pERK and ERK in Caucasian subjects treated with control peptide were set at 100 arbitrary units. Values are the mean ± SEM of 3 independent experiments using cells from different subjects. * = P < 0.05 versus monocytes from Caucasians treated with control peptide; ** = P < 0.01 versus monocytes from Caucasians treated with control peptide; *** = P < 0.001 versus monocytes from African Americans treated with control peptide. See Figure 1 for other definitions.
In addition, we examined ERK activation downstream from the binding of SDF-1 to CXCR4. SDF-1 activated ERK in monocytes from both Caucasians and African Americans; however, the activation was enhanced in monocytes from African Americans (additional information is available from the corresponding author). Although a 1-minute exposure to SDF-1 enhanced ERK activation similarly in monocytes from Caucasians and African Americans, 2-minute and 5-minute exposures enhanced ERK activation 2–3-fold more in monocytes from African Americans (P < 0.001). SDF-1 did not increase the already high level of ERK activation in SSc monocytes.
TGFβ function and signaling
To determine the roles that TGFβ might play in the functional differences between monocytes from Caucasians and monocytes from African Americans, we first examined the effect of TGFβ on monocyte migration toward SDF-1. TGFβ treatment increased the migration of monocytes from both Caucasians and African Americans by a similar ratio (~2.5-fold) (Figure 4A). In all cases, CSD treatment strongly inhibited migration. When we studied canonical TGFβ signaling by examining the expression and activation of Smad2/3, we observed little Smad2/3 expression or activation in monocytes from either Caucasian or African Americans; however, the expression and activation of Smad2/3 in SSc monocytes increased significantly (Figures 4B and C). It was noteworthy that the increase in total Smad2/3 was 2-fold greater than the increase in activated Smad2/3 (P < 0.001). To the best of our knowledge, this is the first observation of Smad expression in monocytes.
Figure 4.
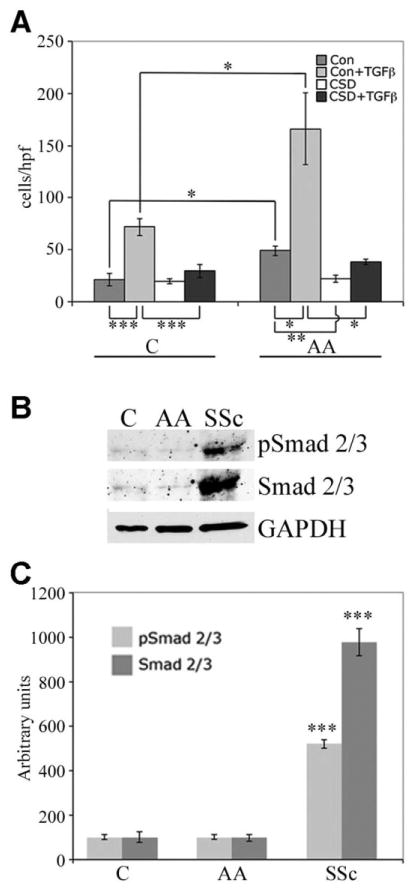
Transforming growth factor β (TGFβ) function and signaling and Smad2/3 expression and activation in monocytes from Caucasians, African Americans, and patients with SSc. A, Monocyte migration toward SDF-1. Monocytes from healthy Caucasian and African American donors were treated with CSD peptide or control peptide, with or without TGFβ. * = P < 0.05; ** = P < 0.01; *** = P < 0.001. B, Representative Western blots showing similar expression and activation in monocytes from healthy Caucasian and African American donors compared with extremely elevated expression and activation in SSc monocytes. C, Quantification of the results shown in B, as determined by densitometry. The levels of Smad2/3 and pSmad2/3 in Caucasian subjects were set at 100 arbitrary units. Bars show the mean ± SEM of 4 independent experiments performed with cells from different subjects. *** = P < 0.001 versus the same parameter in healthy Caucasians and African Americans. See Figure 1 for other definitions.
To further compare TGFβ signaling in monocytes from Caucasians and those from African Americans, we examined TGFβRI and TGFβRII levels. Western blot analyses and immunostaining of isolated monocytes revealed that TGFβRI was expressed at higher levels in monocytes from African Americans than in monocytes from Caucasians and at still higher levels in SSc monocytes. In contrast, TGFβRII was undetectable in monocytes from both Caucasians and African Americans but was present at high levels in SSc monocytes. CSD treatment significantly decreased the levels of TGFβRI and TGFβRII in all monocytes in which they were expressed (additional information is available from the corresponding author).
Enhanced monocyte-to-fibrocyte differentiation in African American patients with SSc
Besides migration into damaged tissue, another critical function for monocytes in fibrotic diseases of the lung and other organs is differentiation to fibrocytes. In turn, fibrocytes differentiate into fibroblasts and secrete cytokines that activate resident fibroblasts. Monocyte-to-fibrocyte differentiation is routinely performed using total PBMCs as the starting population.
Therefore, as a first step in comparing monocyte-to-fibrocyte differentiation in Caucasians, African Americans, and patients with SSc, we compared the starting populations. Remarkable differences in PBMC concentrations were observed in the blood of Caucasians, African Americans, and patients with SSc (Figure 5). Although PBMC concentrations were higher in the blood of both African Americans and patients with SSc compared with the concentration in the blood of Caucasians, the levels in African American patients with SSc were far higher than those in Caucasian patients with SSc (Figure 5). Unlike monocyte migration, which was similar in Caucasian and African American patients with SSc, the PBMC concentration in the blood of African American patients with SSc was far higher than that in the blood of Caucasian patients with SSc (Figure 5).
Figure 5.
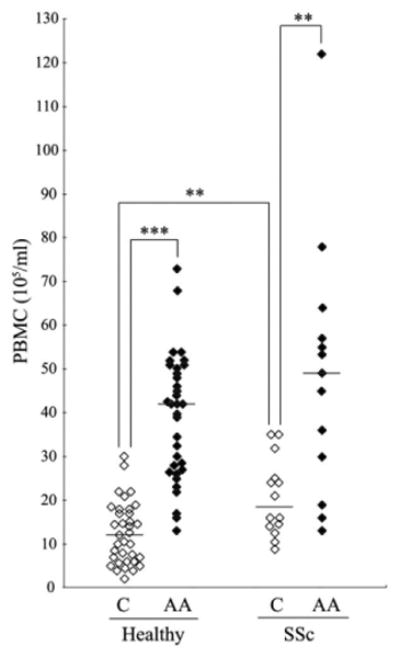
Peripheral blood mononuclear cell (PBMC) levels in blood samples from healthy Caucasian and African American donors and Caucasian and African American patients with SSc, as determined by hemocytometry. Each data point represents a single subject; horizontal lines show the mean. ** = P < 0.01; *** = P < 0.001. See Figure 1 for other definitions.
Fibrocyte differentiation was quantified in terms of the number of PBMCs that take on a spindle-shaped morphology in culture. In cultures that started with a similar number of PBMCs, fibrocyte differentiation was enhanced ~2.5-fold among monocytes from African Americans and ~3.5-fold among SSc monocytes compared with monocytes from Caucasians (Figures 6A and B). In monocytes obtained from all of the groups, differentiation was inhibited >50% by CSD peptide treatment (Figure 6C). To the best of our knowledge, this is the first demonstration that fibrocyte differentiation is enhanced in SSc monocytes as well as in monocytes from African Americans. When the expression of the differentiation markers type I collagen and α-SMA was examined (Figure 6D), collagen was observed in all fibrocytes, while α-SMA was preferentially observed in fibrocytes from both African Americans and patients with SSc. CSD peptide treatment blocked α-SMA expression but did not affect collagen expression.
Figure 6.
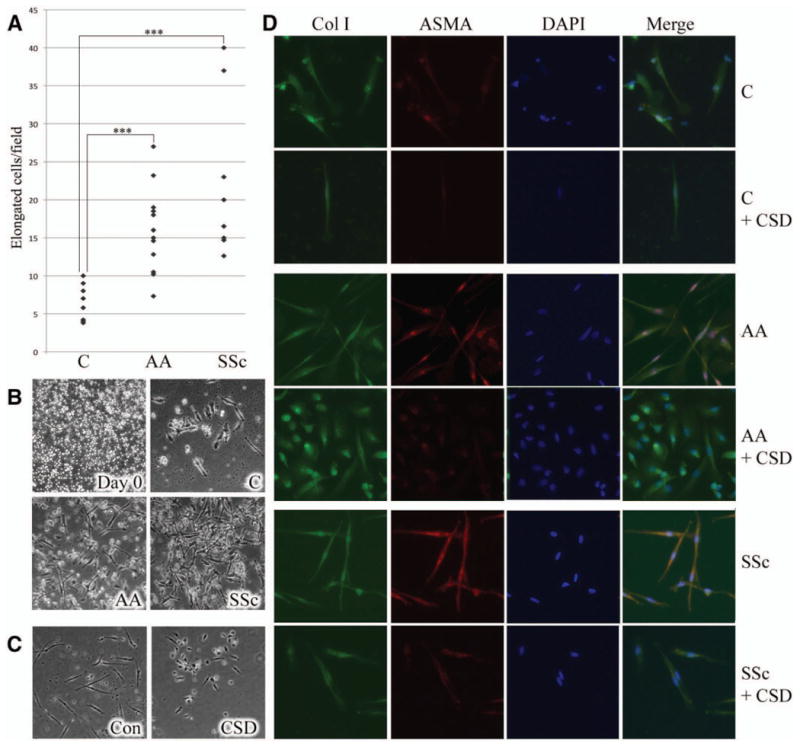
Enhanced monocyte-to-fibrocyte differentiation in healthy Caucasian and African American donors and in patients with SSc. A, Fibrocyte differentiation as quantified by the number of peripheral blood mononuclear cells (PBMCs) that acquire a spindle-shaped morphology in culture. Each data point represents a single subject. *** = P < 0.001. B, Representative image showing a typical PBMC starting population (day 0) (top left) and representative fields showing fibrocyte differentiation in PBMCs from healthy Caucasians (top right) and African Americans (bottom left) and patients with SSc (bottom right) after 15 days of culture. C, Inhibition of fibrocyte differentiation by CSD peptide. PBMCs from Caucasian donors were treated with control (Con) peptide or CSD peptide during 15 days of culture. Similar inhibition of fibrocyte differentiation by CSD was observed in 5 experiments performed with PBMCs from different healthy Caucasian and African American donors and patients with SSc. D, Fibrocyte phenotypes. Fibrocytes from healthy Caucasian and African American donors and patients with SSc were stained with antibodies against type I collagen (Col I) and α-smooth muscle actin (α-SMA) and counterstained with DAPI. Note that α-SMA staining was more prominent in fibrocytes from African Americans and patients with SSc compared with that in fibrocytes from Caucasians and was inhibited by CSD treatment. Similar results were obtained in 3 experiments performed with PBMCs from different Caucasian and African American donors and patients with SSc. Original magnification × 200. See Figure 1 for other definitions.
Discussion
It is well established that SSc-related ILD is more prevalent and more severe in African Americans (29) than in Caucasians and it has been proposed that, in addition to genetics and serologic determinants, this may be attributable to a combination of socioeconomic, behavioral, and environmental factors (12). However, prior studies from our group as well as more recent studies by Steen and colleagues suggest that neither socioeconomic status, access to health care, nor autoantibody status can account for this health disparity (41–43). The concept that healthy African Americans may be predisposed to the development of SSc has received little study. Here, we provide a wide range of data on monocyte migration, monocyte differentiation to fibrocytes, and the regulation of these processes, which indicate that monocytes from healthy African Americans exhibit a variety of features observed in SSc monocytes that may contribute to the pathogenesis of SSc.
At the cellular level, monocytes from African Americans show enhanced migration toward SDF-1 compared with monocytes from Caucasians, but their migration is not as enhanced as that of SSc monocytes. Similarly, monocytes from African Americans show enhanced fibrocyte differentiation compared with those from Caucasians, but the differentiation is not as enhanced as that of SSc monocytes. Monocytes from African Americans, like those from patients with SSc (29), are deficient in caveolin-1 but, again, are not as deficient as SSc monocytes. The enhanced migration and differentiation of monocytes from African Americans and those from patients with SSc appear to be attributable to their lack of caveolin-1, as evidenced by the fact that when cells are treated with CSD peptide to restore caveolin-1 function, their enhanced migration and differentiation are blocked. The serum level of TGFβ in healthy African Americans was observed to be twice as high as that in healthy Caucasians (14), and in the present study, we demonstrated increased TGFβRI levels in monocytes from African Americans while TGFβRII was increased only in SSc monocytes. Because we showed that TGFβ knocks down caveolin-1 expression in monocytes (29), it is a reasonable hypothesis that the deficiency in caveolin-1 expression in monocytes from African Americans is attributable to the high level of TGFβ in the blood of these healthy individuals.
To further study the mechanism of migration, we examined the expression of F-actin and the SDF-1 receptor CXCR4. The expression of these molecules was enhanced in monocytes from African Americans compared with those from Caucasians, but this increase was not as great as that in SSc monocytes. The enhanced expression of F-actin and CXCR4 was blocked by CSD peptide.
To study the phenotype of fibrocytes, we examined their expression of type I collagen and α-SMA. Although type I collagen levels were similar in the fibrocytes of Caucasians, African Americans, and patients with SSc, α-SMA expression was enhanced in fibrocytes from African Americans compared with those from Caucasians, although not to the level observed in SSc fibrocytes. CSD peptide did not affect type I collagen expression but did block the enhanced expression of CXCR4. It remains to be determined why CSD peptide did not block the expression of type I collagen. Two reasonable, nonexclusive hypotheses are as follows: 1) the level of CSD peptide that cells received was insufficient to inhibit type I collagen expression; and 2) type I collagen expression is a very early step in fibrocyte differentiation and therefore is difficult to block.
To expand studies on signaling in monocytes, we also examined the expression and activation of both MAPK and Smad2/3. We previously observed enhanced activation of ERK, JNK, and p38 in SSc monocytes (29). In contrast, we did not observe activation of JNK or p38 in monocytes from African Americans and only limited ERK activation. However, when monocytes were treated with SDF-1, extraordinarily high activation of ERK was observed in monocytes from African Americans compared with those from Caucasians, while no activation was seen in SSc monocytes beyond the high baseline level of ERK activation observed in the absence of SDF-1. Although massive Smad2/3 expression and activation were observed in SSc monocytes, no difference in Smad2/3 expression and activation was observed between monocytes from African Americans and those from Caucasians.
Besides SDF-1 activation of ERK an additional parameter was observed that was enhanced more in monocytes from African Americans than in SSc monocytes, namely, the number of PBMCs per milliliter of blood. Interestingly, this parameter was increased in African American patients with SSc compared with Caucasian patients with SSc, even though no difference was observed between African American patients with SSc and Caucasian patients with SSc in any other parameter examined (monocyte migration toward SDF-1, caveolin-1 expression). We did not detect any significant difference in the ratio between Caucasians, African Americans, and patients with SSc among the cell types that comprise PBMCs.
In summary, most of the parameters that we examined show a graded response with a significant difference between the monocytes of healthy African Americans and healthy Caucasians and a still greater difference between the monocytes of patients with SSc and those of healthy Caucasians. These parameters include caveolin-1 expression, monocyte migration, F-actin expression, CXCR4 expression, ERK activation, TGFβRI expression, fibrocyte differentiation, and expression of α-SMA in differentiated fibrocytes. However, certain parameters follow other patterns. Although JNK (29), p38 (29), and Smad2/3 activation and TGFβRII overexpression are observed in SSc monocytes, they are not observed in the monocytes of African Americans. Monocytes from healthy African Americans are more activated than monocytes from patients with SSc in terms of the number of PBMCs per milliliter of blood and in the response of these monocytes to SDF-1 as read out in terms of ERK activation. Thus, monocytes from African Americans are not simply equivalent to partially activated SSc monocytes.
It is interesting to speculate whether these differences between healthy persons and patients with SSc occur in other diseases and are affected by treatments received by patients with SSc. We expect that in any disease of the lung or other tissue in which monocytes are exposed to profibrotic and proinflammatory cytokines, caveolin-1 levels will be decreased, leading to the altered cell behaviors described here. Indeed, we previously reported that TGFβ or tumor necrosis factor α treatment strongly decreased caveolin-1 levels in monocytes (29). This viewpoint is supported by our observations that caveolin-1 levels are low in the monocytes of patients with asthma (44), leading to enhanced migration of these cells (data not shown). The effect of treatment is a complicated question in the study of any disease in which specimens from humans are used. Again, we expect that if the treatment affects a patient's cytokines, it will affect his or her monocyte caveolin-1 level and downstream functions. Nevertheless, this is an open question deserving further study.
We propose that healthy African American individuals are predisposed to SSc because activation of their monocytes primes them for a heightened response to a variety of potential insults, including various pathogens, medical conditions, and environmental factors. Similarly, monocyte activation may predispose otherwise healthy African American individuals to a variety of other fibrotic diseases known to be more prevalent and more severe in African Americans, including hypertension, focal glomerulosclerosis, diabetic nephropathy, end-stage renal disease, keloids, uterine leiomyomata, sarcoidosis, and glaucoma (12).
Acknowledgments
Supported by grants from the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS] grant P60-AR-049459, Multidisciplinary Clinical Research Center, to Dr. Silver; National Heart, Lung, and Blood Institute grant R01-HL-73718 and National Center for Complementary and Alternative Medicine grant R21-AT-004450 to Dr. Hoffman; NIAMS grant R01-AR-062078, R03-AR-056767, and K01-AR-054143 to Dr. Tourkina; and National Center for Research Resources [NCCR] Construction grant C06-RR-0154550), the US Department of Defense (grant USARMY/USAMRAA W81XWH-11-1-0508 to Dr. Silver), and the South Carolina Clinical and Translational Research Institute (SCTR Pilot Project grant to Dr. Hoffman). Dr. Hoffman's work was also supported as a coinvestigator on NIH/NCRR grants P20-RR-016434, P20-RR-016434-09S2, and P20-RR-021949, and by a grant from the Leducq Foundation. Dr. Tourkina is recipient of a grant and a Marta Max Award from the Scleroderma Foundation.
Footnotes
Author Contributions: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Tourkina had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Reese, Silver, Hoffman, Tourkina.
Acquisition of data. Reese, Perry, Heywood, Visconti, Lee, Hatfield, Silver, Tourkina.
Analysis and interpretation of data. Reese, Bonner, Visconti, Hatfield, Silver, Hoffman, Tourkina.
References
- 1.Atamas SP, White B. Cytokine regulation of pulmonary fibrosis in scleroderma. Cytokine Growth Factor Rev. 2003;14:537–50. doi: 10.1016/s1359-6101(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 2.Altman RD, Medsger TA, Jr, Bloch DA, Michel BA. Predictors of survival in systemic sclerosis (scleroderma) Arthritis Rheum. 1991;34:403–13. doi: 10.1002/art.1780340405. [DOI] [PubMed] [Google Scholar]
- 3.Silver RM. Interstitial lung disease of systemic sclerosis. Int Rev Immunol. 1995;12:281–91. doi: 10.3109/08830189509056718. [DOI] [PubMed] [Google Scholar]
- 4.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–54. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 5.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–55. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 6.Steen VD, Conte C, Owens GR, Medsger TA., Jr Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37:1283–9. doi: 10.1002/art.1780370903. [DOI] [PubMed] [Google Scholar]
- 7.Laing TJ, Gillespie BW, Toth MB, Mayes MD, Gallavan RH, Jr, Burns CJ, et al. Racial differences in scleroderma among women in Michigan. Arthritis Rheum. 1997;40:734–42. doi: 10.1002/art.1780400421. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan E, Furst DE. Systemic sclerosis mortality in the United States: 1979-1998. Eur J Epidemiol. 2005;20:855–61. doi: 10.1007/s10654-005-2210-5. [DOI] [PubMed] [Google Scholar]
- 9.McNearney TA, Reveille JD, Fischbach M, Friedman AW, Lisse JR, Goel N, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemographic, and behavioral factors. Arthritis Rheum. 2007;57:318–26. doi: 10.1002/art.22532. [DOI] [PubMed] [Google Scholar]
- 10.Nietert PJ, Mitchell HC, Bolster MB, Shaftman SR, Tilley BC, Silver RM. Racial variation in clinical and immunological manifestations of systemic sclerosis. J Rheumatol. 2006;33:263–8. [PubMed] [Google Scholar]
- 11.Beall AD, Nietert PJ, Taylor MH, Mitchell HC, Shaftman SR, Silver RM, et al. Ethnic disparities among patients with pulmonary hypertension associated with systemic sclerosis. J Rheumatol. 2007;34:1277–82. [PubMed] [Google Scholar]
- 12.Silver RM, Bogatkevich G, Tourkina E, Nietert PJ, Hoffman S. Racial differences between blacks and whites with systemic sclerosis. Curr Opin Rheumatol. 2012;24:642–8. doi: 10.1097/BOR.0b013e328356d9dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogatkevich GS, Ludwicka-Bradley A, Highland KB, Hant F, Nietert PJ, Singleton CB, et al. Down-regulation of collagen and connective tissue growth factor expression with hepatocyte growth factor in lung fibroblasts from white scleroderma patients via two signaling pathways. Arthritis Rheum. 2007;56:3468–77. doi: 10.1002/art.22874. [DOI] [PubMed] [Google Scholar]
- 14.August P, Suthanthiran M. Transforming growth factor – and progression of renal disease. Kidney Int Suppl. 2003;87:S99–104. doi: 10.1046/j.1523-1755.64.s87.15.x. [DOI] [PubMed] [Google Scholar]
- 15.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain: implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–33. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 16.Oka N, Yamamoto M, Schwencke C, Kawabe J, Ebina T, Ohno S, et al. Caveolin interaction with protein kinase C: isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem. 1997;272:33416–21. doi: 10.1074/jbc.272.52.33416. [DOI] [PubMed] [Google Scholar]
- 17.Rybin VO, Xu X, Steinberg SF. Activated protein kinase C isoforms target to cardiomyocyte caveolae: stimulation of local protein phosphorylation. Circ Res. 1999;84:980–8. doi: 10.1161/01.res.84.9.980. [DOI] [PubMed] [Google Scholar]
- 18.Tourkina E, Gooz P, Pannu J, Bonner M, Scholz D, Hacker S, et al. Opposing effects of protein kinase Cα and protein kinase Cεe on collagen expression by human lung fibroblasts are mediated via MEK/ERK and caveolin-1 signaling. J Biol Chem. 2005;280:13879–87. doi: 10.1074/jbc.M412551200. [DOI] [PubMed] [Google Scholar]
- 19.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-β/SMAD signaling through an interaction with the TGF-β type I receptor. J Biol Chem. 2001;276:6727–38. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 21.Le Saux CJ, Teeters K, Miyasato SK, Hoffmann PR, Bollt O, Douet V, et al. Down-regulation of caveolin-1, an inhibitor of transforming growth factor-β signaling, in acute allergen-induced airway remodeling. J Biol Chem. 2008;283:5760–8. doi: 10.1074/jbc.M701572200. [DOI] [PubMed] [Google Scholar]
- 22.Yan SR, Fumagalli L, Berton G. Activation of SRC family kinases in human neutrophils: evidence that p58C-FGR and p53/56LYN redistributed to a Triton X-100-insoluble cytoskeletal fraction, also enriched in the caveolar protein caveolin, display an enhanced kinase activity. FEBS Lett. 1996;380:198–203. doi: 10.1016/0014-5793(96)00029-4. [DOI] [PubMed] [Google Scholar]
- 23.Uhal BD. Apoptosis in lung fibrosis and repair. Chest. 2002;122:293S–8S. doi: 10.1378/chest.122.6_suppl.293s. [DOI] [PubMed] [Google Scholar]
- 24.Trojanowska M. Noncanonical transforming growth factor β signaling in scleroderma fibrosis. Curr Opin Rheumatol. 2009;21:623–9. doi: 10.1097/BOR.0b013e32833038ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-β receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2008;20:713–9. doi: 10.1097/bor.0b013e3283103d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 27.Del Galdo F, Sotgia F, de Almeida CJ, Jasmin JF, Musick M, Lisanti MP, et al. Decreased expression of caveolin-1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008;58:2854–65. doi: 10.1002/art.23791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tourkina E, Richard M, Gooz P, Bonner M, Pannu J, Harley R, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–61. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 29.Tourkina E, Richard M, Oates J, Hofbauer A, Bonner M, Gooz P, et al. Caveolin-1 regulates leucocyte behaviour in fibrotic lung disease. Ann Rheum Dis. 2010;69:1220–6. doi: 10.1136/ard.2009.117580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasper M, Reimann T, Hempel U, Wenzel KW, Bierhaus A, Schuh D, et al. Loss of caveolin expression in type I pneumocytes as an indicator of subcellular alterations during lung fibrogenesis. Histochem Cell Biol. 1998;109:41–8. doi: 10.1007/s004180050200. [DOI] [PubMed] [Google Scholar]
- 31.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 32.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 33.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–7. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 34.Bernatchez PN, Bauer PM, Yu J, Prendergast JS, He P, Sessa WC. Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proc Natl Acad Sci U S A. 2005;102:761–6. doi: 10.1073/pnas.0407224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 36.LeRoy EC, Black C, Fleishmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets, and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 37.Tourkina E, Bonner M, Oates J, Hofbauer A, Richard M, Znoyko S, et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair. 2011;4:15. doi: 10.1186/1755-1536-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol. 2009;86:1111–8. doi: 10.1189/jlb.0309132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–8. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 40.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–18. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nietert PJ, Silver RM. Patterns of hospital admissions and emergency room visits among patients with scleroderma in South Carolina, USA. J Rheumatol. 2003;30:1238–43. [PubMed] [Google Scholar]
- 42.Nietert PJ, Silver RM, Mitchell HC, Shaftman SR, Tilley BC. Demographic and clinical factors associated with in-hospital death among patients with systemic sclerosis. J Rheumatol. 2005;32:1888–92. [PubMed] [Google Scholar]
- 43.Steen V, Domsic RT, Lucas M, Fertig N, Medsger TA., Jr A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum. 2012;64:2986–94. doi: 10.1002/art.34482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bains SN, Tourkina E, Atkinson C, Joseph K, Tholanikunnel B, Chu HW, et al. Loss of caveolin-1 from bronchial epithelial cells and monocytes in human subjects with asthma. Allergy. 2012;67:1601–4. doi: 10.1111/all.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]


