Introduction
The first recorded application of skin substitutes (xenografts) dates back to the 15th century BC. as mentioned in the Papyrus of Ebers.1 In 1981 Yannas and Burke 2-3 developed artificial skin substitutes, a dermal regeneration template composed of a bilayer of temporary silicone epidermis and a porous collagen-chondroitin 6-sulfate fibrillar dermis, for the treatment of acute extensive burns. Eventually, upper limb surgeons expanded the use of these skin substitutes to cover areas as small as the fingertips.4
Soft tissue repair of the upper extremity has traditionally followed the hierarchy of the reconstructive ladder that progresses from simple methods of wound closure such as the application of skin grafts to more sophisticated methods of free tissue transfer. Over the past two decades, technological innovations, especially in the field of tissue engineering, have introduced other viable alternatives for provision of skin coverage, remarkably biosynthetic materials such as dermal skin substitutes. When dermal skin substitutes are used in conjunction with a skin graft, the resulting skin is of better quality, thickness and pliability than reconstructions using only split-thickness skin grafts (STSG).5 Furthermore, dermal substitutes can be used in the hand to cover critical structures such as tendons devoid of paratenon, cartilage without perichondrium and bone without periosteum. The full biointegration of dermal substitutes requires a well-vascularized wound bed that is clear of infection. Thus in potentially contaminated devascularized wounds, autologous tissue transfer is more appropriate. However there are numerous clinical situations in which dermal skin substitutes would offer the upper limb surgeon an additional reconstructive option in solving difficult problems where local tissue is damaged or not available. The terms ‘dermal skin substitutes’ is commonly used to refer to a wide range of products that are currently available in the market.6 In this article we will use the terms dermal skin substitutes, dermal substitutes, dermal matrices and dermal regeneration template interchangeably to refer to several types of skin substitutes that have been commonly reported in the soft tissue reconstructions of the upper extremity.
Histological properties of dermal skin substitutes
Dermal skin substitutes are a heterogeneous group of wound coverage materials that aid in wound closure and replace some of the functions of the skin, either temporarily or permanently, depending on the product characteristics.7 They provide several biological and physiological properties of human dermis that allow and/or promote new tissue growth and optimize the conditions for healing.
These biodegradable materials are composed of a bilayer of collagen and glycosaminoglycan covered by a temporary epidermal substitute.8 The porous matrix applied to the wound bed acts as a scaffold for the in-growth of the host's fibroblasts, endothelial cells and endogenous collagen that subsequently vascularizes and creates a distinct layer called the ‘neodermis’.9 Contrary to skin grafts, dermal matrices are independent of imbibition or inosculation for immediate viability. Additionally, they invoke minimal inflammatory or immunologic response owing to their acellular composition.8. Moiemen et al. described four distinct histological phases of integration of one type of dermal substitutes (Table 1.), based on histological specimens obtained from sites of soft tissue repaired with dermal substitutes.9 In another study by the same group analyzed specimens obtained more than 2 years from sites reconstructed with dermal skin substitutes. They found that the newly formed collagen and elastin consists of an abnormal morphology that does not resemble normal skin collagen. Yet, they reported good clinical outcomes in terms of decreased scarring, skin pliability and acceptable patient satisfaction. Additionally, for the first time they reported the presence of nerve fibers within the reticular (deep) dermis.10 This suggests that a gradual regeneration process of nerve endings occur within the dermal regeneration template, a finding that correlated with improvement of sensory function among patients involved in their study. On the other hand, adnexal structures such as sweat glands or hair follicles have not been observed to grow within the newly formed dermis.10 Finally, the authors concluded that beyond a 2 year period, the dermal matrix is completely replaced by native collagen and no long-term residual template matrix can be found within the examined specimens, a property that keeps with the biodegradable nature of these dermal substitutes.
Table 1.
Four stages of dermal skin substitutes integration within host tissue as described by Moeimen et al.(Reconstructive surgery with Integra dermal regeneration template: histologic study, clinical evaluation, and current practice. Plast Reconstr Surg. 2006 Jun;117(7 Suppl):160S-174S.)
| Histological Stage | Duration | Sequence of events |
|---|---|---|
| Imbibition | Minutes | Dermal substitutes start to adhere to wound bed. |
| Fibroblast Migration | Day 7 | Fibroblasts start collagen secretion. |
| Neovascularisation | Day 14 | Neovascularization (formation of new blood vessels). |
| Remodeling and maturation | Day 28 | Host collagen replaces the dermal collagen matrix. |
Application of dermal substitutes
The mainstay of management of upper extremity wounds is centered on the adequate surgical debridement of wounds followed by early soft tissue coverage and mobilization. Following debridement, dermal skin substitutes can be used to cover the resultant defect. Although the composition of different dermal regeneration templates is slightly different, their method of application and uses are principally similar. Nevertheless, one must always follow the manufacturer's instructions for each type of dermal regeneration template used.
The first step requires the assessment of wound vascularity. The amount of vascularized tissue for the successful incorporation of dermal substitutes is not well defined.11 Whether the wound is eligible to be covered by a dermal matrix or not is therefore left to the surgeon's judgment and experience. Irradiated wounds or wounds with extensive devitalized tissue are not suitable for coverage with dermal skin substitutes. Another important factor for the successful take of dermal matrices includes is the presence or absence of infection. Heimbach et al. reported a 26% less take in infected versus non-infected sites.12 Moreover, Shirley and colleagues reported a case of a fatal toxic shock syndrome where the dermal substitute grafted site was colonized with Methicillin Resistant Staphylococcus Aureus (MRSA).13 For these reasons it is strongly recommended that clear bacteriology cultures of the wounds should be obtained prior to the application of dermal substitutes.
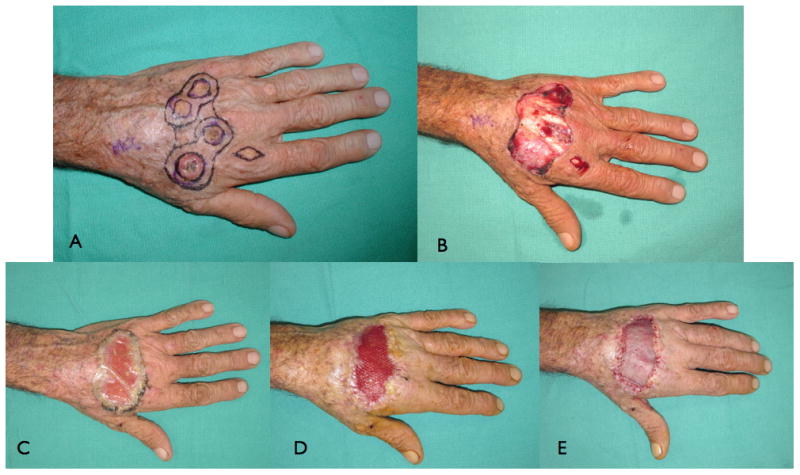
Once the wound is deemed suitable for coverage, the dermal matrix is applied and secured along the rim of the defect in a similar fashion to a skin graft. The dermal matrix should be applied as adherent as possible to the wound bed. Any fluid collection, air bubbles or folding of the template could intervene between the inner surface of the dermal matrix and the wound bed, and consequently may result in graft failure. Some surgeons may mesh dermal substitutes similar to skin grafts to increase the surface area covered by the dermal substitutes and allow fluid extravasation e.g. hematoma or seroma. However, we advocate the use of un-meshed dermal substitutes whenever possible to fill the void, which decrease the possibility of recurrent skin contractures and improve the final aesthetic appearance of the grafted site (Fig. 1A-F). Dermal regeneration templates that are currently available require either single staged or two staged reconstructions. The single stage procedure applies the dermal matrix covered with a skin graft in one procedure, whereas the latter involves the application of the dermal matrix followed by coverage with a skin graft after 5-7 days later to allow for vascularization of the regeneration template. Typically a STSG setting of 0.011 to 0.015 inch is used to cover the dermal matrix. Although the successful take of full-thickness (FTSG) skin grafts over dermal substitutes has been reported in small-sized wounds over the digits, the greater metabolic demand of FTSG make them prone to ischemia and necrosis and are therefore discouraged.14
Figure 1.
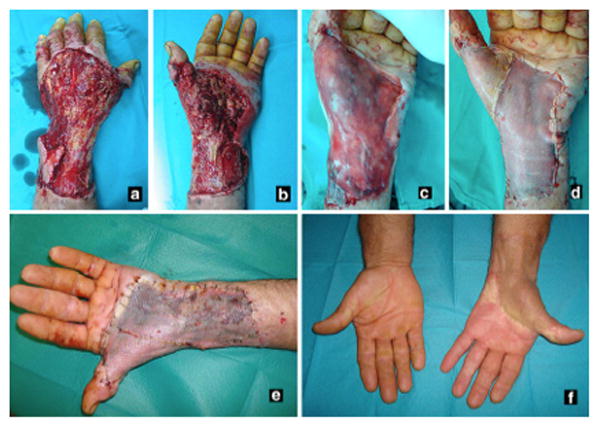
Showing reconstruction of an extensive avulsion injury of the upper extremity with dermal skin substitutes. The dermal matrix should be applied on a vascular bed after wound debridement and secured to wound edges (a-c). A sheet of single stage dermal matrix was applied to the wound and covered directly with a skin graft in one procedure (d). Complete take of the composite dermal substitute and skin graft 1 week after surgery (e), and good postoperative results at 2-year follow-up. However, when using a 2 staged dermal substitutes, place the regeneration template with the silicone side up. After 2-3 weeks the silicone layer is peeled off and covered with a skin graft (not shown in this image). (From Demiri E, Papaconstantinou A, Dioyssiou A et al. Reconstruction of skin avulsion injuries of the upper extremity with integra® dermal regeneration template and skin grafts in a single-stage procedure. Arch Orthop Traum Su. Epub Aug 2013; with permission.)
Following the application of dermal substitutes, simple non-adhesive dressings are used to cover the wound site. Negative pressure wound therapy/dressing (NPWT) has been used to stabilize dermal substitutes to prevent shearing forces and minimize development of hematoma and seroma. It has also been postulated that the application of NPWT may improve take of composite dermal substitutes and skin grafts by stimulating angiogenesis, increasing cell proliferation and enhancing vascularization of the regeneration template. However, the current evidence derived from a randomized control trial and histological studies does not support this assumption.15-16
Clinical indications and outcomes of dermal skin substitutes
Dermal skin substitutes have been commonly used in acute and chronic reconstructive burn surgery. The Food and Drug Administration (FDA) more recently extended the indications of dermal skin substitutes to include non-burn-related, traumatic, and chronic extremity wounds.17 Furthermore these medical devices have been proposed as part of the surgeon's reconstructive ladder. (Fig 2).5 However, the role of dermal substitutes in upper limb reconstruction is still evolving. Apart from burn surgery there is no clear guidance on the exact indications of these medical devices for several reasons. First, wounds of the upper extremity have various etiologies and patients often have different co-morbidities. Second, the current available evidence is mostly comprised of non-comparative retrospective case series and individual surgeons' experiences. Therefore, evaluating the effectiveness of dermal substitutes and their long-term functional outcomes in the overall wound healing of the extremity is difficult to interpret.
Figure 2.

Modification of the reconstructive ladder incorporating dermal skin substitutes. (Adapted from Janis JE, Kwon RK, Attinger CE. The new reconstructive ladder: modifications to the traditional model. Plast Reconstr Surg. 2011 Jan;127 Suppl 1:205S-212S; with permission.)
Another area of concern is cost associated with dermal substitutes. Aside from clinical effectiveness, costs need to be evaluated in order to comprehensively assess the benefits of a new treatment. Within the current global economic uncertainties, the high initial purchase cost of dermal substitutes may prove a disincentive to healthcare commissioners and purchasing bodies that may limit their availability to surgeons and patients. This is particularly true in developing countries, whereas economically privileged countries are more likely to afford the use dermal skin substitutes. To date there is a single cost analysis study that compared the total costs involved with dermal substitutes reconstructions versus total costs of skin graft only reconstruction for treatment of small-sized burns. Though the total costs among dermal substitutes group culminated higher expenses per patient (2218 Euros), this was not significantly higher than the skin graft only group (1703 Euros).18 Interestingly, indirect health-related costs such as length of hospital stay and overheads were found to be the most important factors influencing the total cost of treatment. The decision to use dermal substitutes may therefore be a clinical one. A major limitation of this study was the limited follow-up period of 12 months. The costs of further reconstructive surgeries or revisions were not considered. Thus it is imperative that further cost-effectiveness studies with longer follow-up are conducted to justify the use of dermal substitutes and help in resource allocation. Lastly, surgeon's experience and patient preferences should also be considered in the final decision-making process. Nonetheless, dermal skin substitutes have been employed in all of the following with various degrees of success.
Burn injuries
The upper extremity and especially the hands are amongst the most commonly affected areas of the body with burn injuries. Burns of these ‘special areas’ are considered severe, because even a small wound may cause profound functional disability. Additionally, in regions with requirements of elasticity, pliability and mobility such as the axilla, elbow, wrist and hand regions, it is important to replace burnt areas with skin of similar characteristics to the native tissue to preserve the range of motion and consequently the limb function. Current indications of dermal skin substitutes include both acute (e.g. deep partial or full thickness burns, Figure 3 & 4) and chronic burns (e.g. hypertrophic scars and scar contractures). Autologus skin grafts have been traditionally employed for the provision of skin coverage following debridement of burns. However, the resultant aesthetic disfigurement, development of adherent scars and recurrent scar contractures may require revision surgeries to enhance aesthetics and restore the function and mobility of the affected parts.
Figure 3.
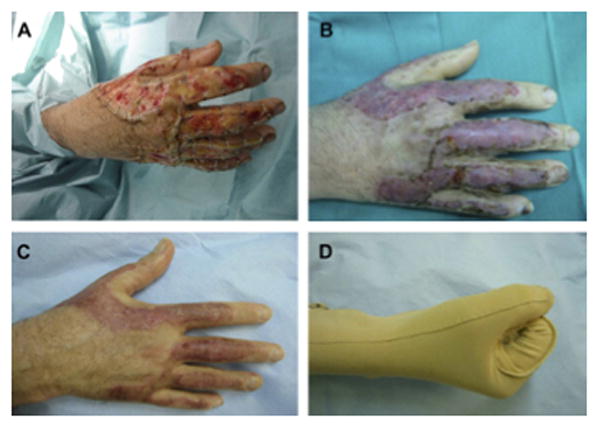
A Full-thickness burn of the dorsum of the hand and fingers (A-B). Two weeks post split-thickness skin graft over neodermis and elastic compression therapy (C-D). (From Cuadra A, Correa G, Roa R, et al. Functional results of burned hands treated with Integra. J Plast Reconstr Aesthet Surg. 2012 Feb; 65 (2): 228-34; with permission.)
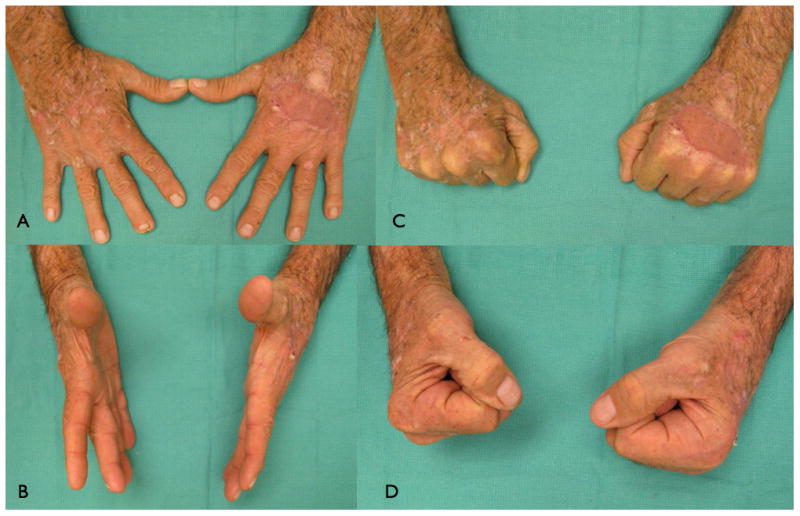
Figure 4.
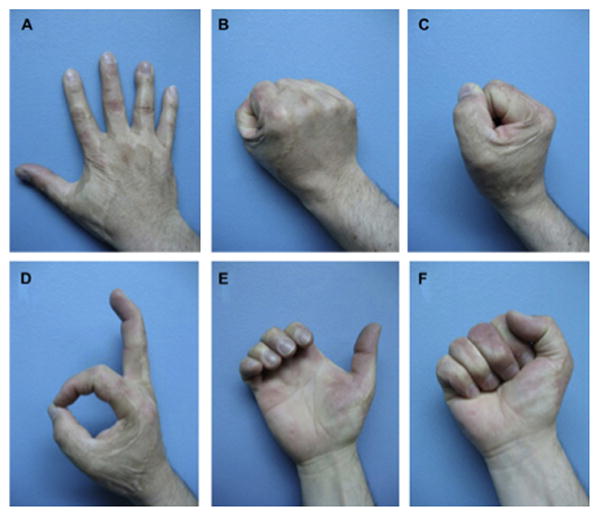
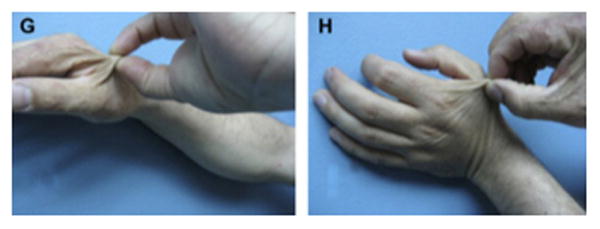
A six-year follow-up after dermal substitute reconstruction of the hand of patient in figure 3 demonstrating a good range of motion (A-F). Pliability of the skin in the un-burned hand (G) compared to pliability of the skin in the burned hand treated with skin substitute (H). (From Cuadra A, Correa G, Roa R, et al. Functional results of burned hands treated with Integra. J Plast Reconstr Aesthet Surg. 2012 Feb; 65 (2): 228-34; with permission)
A prospective controlled trial of patients with bilateral acute full-thickness burns located on the dorsum of the hand was conducted to evaluate the effectiveness of dermal substitutes in treating such a common injury.19 Intra-individual comparison was performed by treating one hand with composite dermal substitutes and skin grafts (intervention), and the contralateral hand was treated using the traditional method of burn excision and skin grafting (control). A total of 18 patients were included and a single staged dermal regeneration template was used in this study. Assessors were blinded to the intervention and control groups. Outcomes measures are wound site evaluation of dermal substitutes and skin graft take, need for re-grafting, skin elasticity assessed by Vancouver Burn Skin Score, and range of motion assessed by measuring the Finger-Tip-Palmar- Crease-Distance and Finger-Nail-Table-Distance of the index to small fingers. The results of this study showed no significant difference between the two groups regarding dermal substitute or skin graft take or the need for re-grafting. However hands treated with the dermal regeneration template were superior to skin grafted only wounds in skin elasticity and active range of motion. The use of a single staged regeneration template allows early institution of physical therapy once the composite graft is deemed stable, a process that usually takes 5-7 days. However if a two staged dermal regeneration template is used, the hand could be immobilized for up to 2 weeks before a skin graft can be applied to the dermal matrix, which may increase risk of joints stiffness. Several other reports have showed similar results using dermal substitutes for treating acute and chronic burns on the hands and digits.20-23
Contracture release and scar resurfacing using dermal substitutes have also been reported in the upper extremity such as in skin contractures around the axilla and elbow joint. Skin contractures of these regions are notoriously difficult to treat and often result in severe restriction of movement that prevent patients from performing functions of daily living such as the ability to eat, shower or drive independently. Conventionally, the treatment of these skin contractures included either scar lengthening procedures by the means of multiple Z-plasties or by scar excision and resurfacing with skin grafts, fasciocutaneous flaps or recruitment of adjacent skin after a period of tissue expansion. A multicenter study of 13 study centers in the United States, France, Germany and the United Kingdom was conducted to evaluated the outcomes of contracture release procedures incorporating a dermal regeneration template for 89 consecutive patients who underwent a total of 127 contracture releases.24 Thirty-nine of the treated contractures were located at the axilla and elbow regions. Postoperatively, the most common observed complication was wound infection followed by fluid collection underneath the regeneration template such as a seroma or hematoma. In regards to recurrence of skin contractures, this was not observed during the duration of follow-up-period of the study that extended for 11 months. Physician ratings of contracture release outcomes in range of motion or function were rated as good to excellent in 75% of the cases. Patient reported outcomes showed that 82% of the patients were satisfied with postoperative range of motion, aesthetic appearance and pain relief. Despite these encouraging results, the findings of this study should be interpreted with caution due to the relatively short postoperative follow-up period that can be considered as a limitation, as wounds may take up to 24 months to form mature scars or recurrence of contracture.
Traumatic Injuries
Traumatic high-energy shearing forces cause disruption of tissue planes that often result in skin avulsions and degloving injuries. The advantages of early wound coverage are well recognized by minimizing infection and preventing tissue desiccation as well as allowing patients' early rehabilitation and mobilization. Based on wound characteristics and structures affected, these injury patterns are conventionally treated by debridement of devitalized tissues followed by provision of adequate soft tissue coverage. Wounds with exposed tendons and bones are not suitable for skin graft coverage. Regional or distant flap transfers can be appropriate alternatives; however, co-existence of multiple injuries or substantial patients' morbidity may preclude patients from undergoing lengthy flap procedures. Dermal skin substitutes can be thought in these circumstances either as a temporary or a permanent method of wound coverage (Fig. 1&5).
Figure 5.
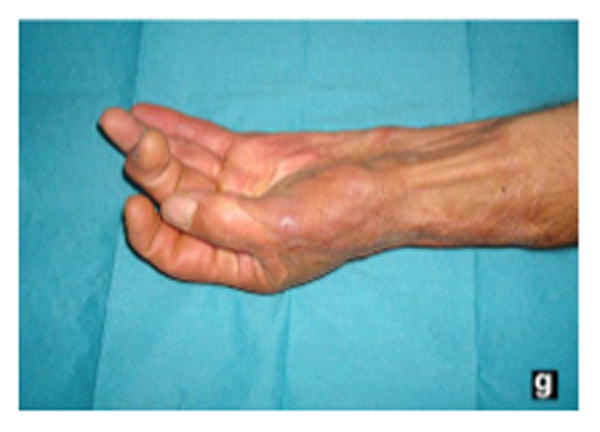
A 2-year follow-up of the patient in figure 1, demonstrating good pinch grip of the thumb. (From Demiri E, Papaconstantinou A, Dioyssiou A et al. Reconstruction of skin avulsion injuries of the upper extremity with Integra® dermal regeneration template and skin grafts in a single-stage procedure. Arch Orthop Traum Su. Epub Jan 2013; with permission)
The ultimate goal of any reconstructive procedure is the resumption of normal activities of the affected limb as fast as possible to avoid joints stiffness and disuse atrophy. For soft tissue reconstruction, most of published work have assessed the success of dermal substitutes based on their ability of successful integration and provision of definitive closure of the defect. However, optimizing hand function following complex injury requires a stable and durable soft tissue coverage that can withstand secondary surgeries to address underlying deformities. Thus far, the possibility of performing secondary corrective surgery at a site that has been previously reconstructed with dermal substitutes has not been investigated. Furthermore, despite the good skin elasticity and pliability of wound sites reconstructed with dermal substitutes, these biodegradable material do not replace like with like. Tissue replaced with dermal skin substitutes lack essential components of normal skin such as sweat gland and hair follicles. Additionally, restoration of sensibility is suboptimal. These are all important considerations especially when reconstructing tissue over the palm of the hand or volar surface of the digits where the hand function can be greatly affected if replaced with tissue of a less quality or that do not resemble normal skin characteristics. Local flaps if available are considered the treatment of choice to repair small to medium sized soft tissue defects. For larger defects the wound extent and the need for functional repair should be addressed first. The comparative effectiveness of dermal substitutes and skin graft to flap surgery has yet to be shown. However in an isolated soft tissue defect whether the reconstruction should be performed using dermal skin substitutes or autologous tissue transfer, this should be evaluated on a case-by-case basis (Table 2) after taking into account a myriad of considerations including structures affected, patient's co-morbidities, patient's preference, surgeon's skills and length of limb immobilization.
Table 2. Summary of different features of soft tissue reconstructive techniques of the upper extremity.
| Method of reconstruction | Different characteristics of various methods of soft-tissue reconstruction | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coverage of bare tendons, bone & joints† | Size of defect coverage | Skin quality | Sensory recovery* | Number of procedures required+ | Mean duration of postoperative limb immobilization required | Level of difficulty | Donor-site deformity | Cost | |
| Split-thickness skin graft (STSG) | No | Small to large sized defects | Average | No | Single | 7-10 days | Easy to perform | Yes | $ |
| Full thickness skin graft (FTSG) | No | Small defects | Good | No | Single | 7-10 days | Easy to perform | Yes (minimal scarring) | $ |
| Dermal Substitutes | Yes | Small to large sized defects | Good-Very good | Suboptimal recovery | One to two procedures depending on the use of a single or two-stage dermal substitute | 7-21 days‡ | Easy to perform. However there is a learning curve to perform satisfactorily | No | $$$$ |
| Local Flaps | Yes | Small defects | Excellent | Yes | One or two procedures depends on type of flap used | 7 days | Average difficulty | Yes (minimal scarring) | $ |
| Pedicled and regional Flaps | Yes | Small to large sized defects | Very good | No | Two or more | 21 days | Average difficulty | Yes | $$ |
| Microvascular free flaps | Yes | Large sized defects | Very good | No | Usually single but may require revision procedures | 7 days | Difficult, requires adequate expertise and training to achieve good results | Yes (muscle flaps> fasciocutaneous flaps) | $$$ |
Tendons, bones and cartilage without paratenon, periosteum and perichondrium.
Not including specialized sensation of the palm of the hand
Not including secondary or revision surgery.
Single stage dermal substitutes requires shorter period of limb immobilization.
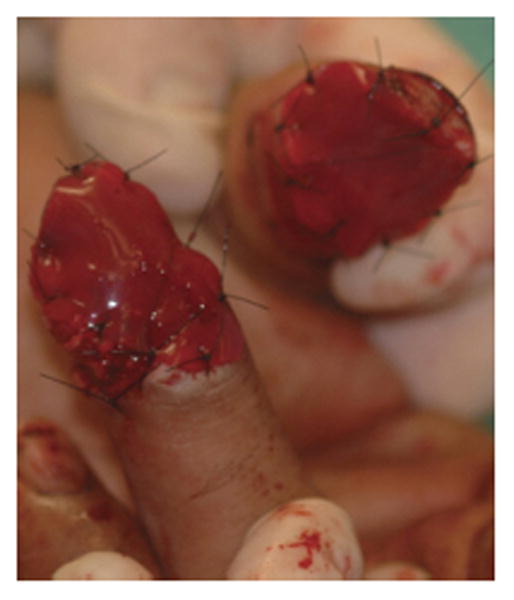
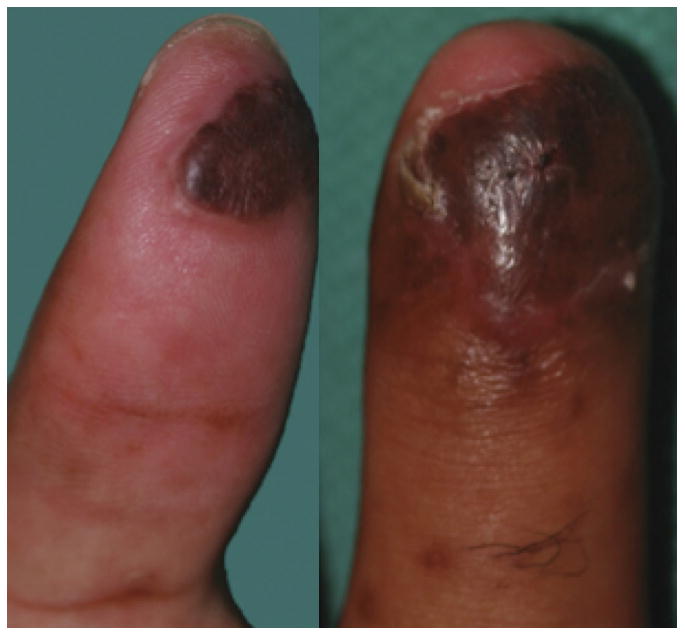
Only a few studies and case reports have been published so far where surgeons utilized dermal skin substitutes for immediate wound coverage after traumatic injuries25-26. Graham et al. reported 5 patients with severe degloving injuries of the upper extremity who were treated with dermal skin substitutes.27 The surface area of reconstructed areas ranged from 50-1000 cm2 and 90% of the patients have sustained underlying open fractures. Soft tissue reconstruction of these defects was achieved by means of composite dermal substitutes and split-thickness skin grafts. The wounds were first debrided at the same day of injury then were covered with a negative pressure therapy temporarily. After the wounds were deemed viable and clear of infection, dermal substitutes were applied at a median of 14 days and skin grafts were placed at a median of 27 days post injury, respectively. At a 5 months follow-up, all patients had complete skin graft take and no contractures or other graft-related complications were noted that required revision or limited return to normal function. In another series Taras et al.28 treated 21 traumatic finger injuries resulting in soft-tissue loss with exposed underlying bone, joints, hardware, and tendons with dermal skin substitutes and full-thickness skin grafts (Fig. 6 A-C). All patients received two operations that were spaced 3 weeks apart. The first procedure required wound debridement and application of the skin substitute followed by a second procedure that covered the wound with dermal skin substitute and full-thickness skin grafts. Patients were followed up for 12 months. The wounds reconstructed ranged from 1cm2-24cm2 whilst the largest single area covered with dermal substitutes was 12 cm2. Complete graft-take was reported in 16 (80%) digits and partial graft loss was reported in 4 (20%) digits. However no further treatment was required and the majority of patients were able to return back to their employment. Dermal regeneration templates have also been used in a ‘stacked’ fashion to increase the thickness and durability of coverage over exposed bone and tendons.29 One should be cautious employing these different applications of dermal substitutes that may considerably increase the duration of treatment and immobilization of the hand that may result in further problems such as hand stiffness.
Figures 6.
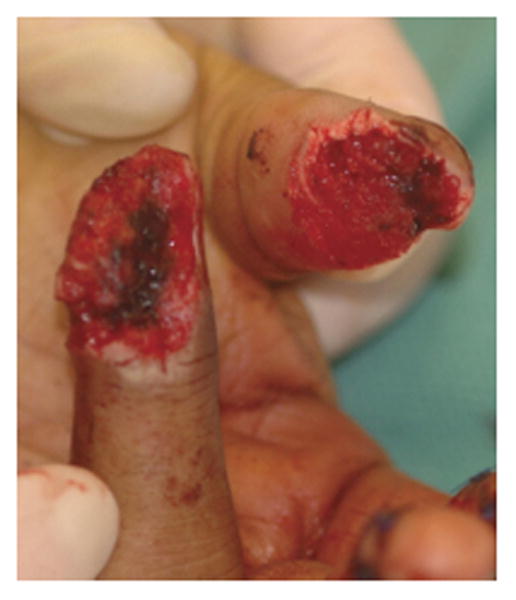
A-C. Demonstrating an amputation injury of the thumb and ring fingers successfully covered by dermal regeneration template. (From Taras JS, Sapienza A, Roach JB, et al. Acellular dermal regeneration template for soft tissue reconstruction of the digits. Journ Hand Surg. 2010 Mar; 35 (3): 415-21; with permission.)
Other potential indications of dermal substitutes
Soft-tissue reconstruction after tumor resection
Tumor resection and wide local excision may leave patients with defects requiring complex reconstructive surgery. The reconstructive approach following tumor resection differs greatly from the traditional approach of soft-tissue reconstruction resulting from other mentioned etiologies, for several reasons. First, the treatment of suspected benign tumors (e.g. pyogenic granuloma or a soft-tissue lipoma) is usually achieved by a direct excision of the lesion and skin closure. However in suspected malignant tumors such as basal cell carcinoma (BCC), squamous cell carcinoma (SCC) or malignant melanoma (MM), a resection margin of healthy adjacent tissue is usually required that would necessitates some form of a soft-tissue reconstruction to close the resultant defect. Second, different types of tumors require different treatment and without formal histopathology results, it is difficult for the surgeon to predict the actual size and extent of the final defect that need to be reconstructed.
Conventionally, skin grafts or locoregional flaps have been used to cover defects following tumor resection in the hand and upper extremity. However, skin grafts are prone to contraction, leave a contour defect and are less robust, especially when used to cover previously irradiated wound beds. Locoregional flaps offer superior results than skin grafts because they provide tissue of similar texture, color and thickness. Yet, the disadvantage of reconstructing soft tissue defects following tumor excision with local or regional flaps is the limited ability to monitor disease recurrence of the reconstructed area.
Dermal skin substitutes may be a reasonable alternative for soft-tissue reconstruction following tumor resection. They can act as a temporizing reconstruction whilst awaiting formal histology. They do not burn any reconstructive bridges and may also be the only treatment needed (Fig 7& 8). We find this application of dermal substitutes particularly useful in elderly patients and patients deemed of a higher anesthetic risk. Chalmers and colleagues successfully treated 5 patients using dermal skin substitutes and skin grafts following excision of SCC and MM from the digits.30 A 100% graft take and full range of motion was achieved in the majority of patients. Only a few case reports are currently available that discuss the role of dermal substitutes to reconstruct skin defects following tumors resection.31-33
Figure 7.
A skin defect resulting in exposed tendons following Mohs surgery to remove squamous cell carcinoma on the dorsum of the hand (A-B). The defect primarily covered with dermal skin substitute (C). Two weeks later, a sheet of split-thickness skin graft was applied (D-E).
Figure 8.
One-month follow-up, demonstrating a good range of motion of patient in figure 7.
Soft-tissue reconstruction after radial forearm flap harvest
Although the radial forearm flap is regarded as a workhorse flap in reconstructive surgery, the aesthetic and functional morbidity of the donor-site remains a considerable concern. Donor-site repair of radial forearm flap has been usually achieved by either split-thickness or full thickness skin grafts. These methods may result in complications such as tendon exposure, decreased sensation, functional disability (including limited hand mobility and decreased strength), and poor aesthetic appearance. Harvesting a suprafascial radial forearm flaps34-37 may help to minimize these complications, but not every surgeon is well versed with this technique. To date there is no consensus on the best way of closing radial forearm flap defects. Several authors have used dermal substitutes to cover radial forearm defects with satisfactory results (Fig. 9).35-36 Murray et al. repaired radial forearm free flap donor-sites in 29 patients using composite dermal substitutes and ultra-thin split-thickness skin grafts.38 Full healing was achieved within 4–6 weeks with negligible donor-site complications, excellent aesthetics, and minimal scar contracture. Gravvanis and colleagues applied the same technique as Murray in 6 patients following suprafacial radial forearm free flap harvest.39 After 24 days, donor-site demonstrated complete wound healing. After 9 months, all patients had normal range of motion of the wrist and the fingers, normal power grip, and power pinch. Finally, Medina used a two-staged novel approach to improve aesthetics of radial forearm free flap donor-sites using dermal substitutes. In the first stage, the dermal matrix was implanted in the forearm for 2 weeks.40 In the second stage, skin flaps are raised superficial to the dermal matrix then the radial forearm tissue along with dermal matrix are harvested on the vascular pedicle (as a prefabricated flap). The forearm skin flaps are then reflected back to cover the defect.
Figure 9.
A soft-tissue defect following radial forearm free flap covered with a single stage Matriderm and split-thickness skin graft (A-C). At 12 month follow up skin cover demonstrating good pliability and normal range of motion of the hand. (From Haslik W, Kamolz LP, Mann F, et al. Management of Full-thickness skin defects in the hand and wrist region: first long-term experiences with the dermal matrix matriderm. J Plast Reconstr Aesthet Surg. 2010 Feb: 63(2): 360-64; with permission)
Soft-tissue reconstruction after excision of Dupuytren contracture
Dupuytren disease is a fibroproliferative disorder of unclear etiology that often results in shortening and thickening of the palmar fascia, leading to permanent and irreversible flexion contracture of the digits. In severe or recurrent disease, a dermofasciectomy may be required. Dermofasciectomy involves the removal of diseased palmar fascia (cord and nodule) and overlying affected skin, a full-thickness skin graft is usually required to cover the resultant defect. Alternatively, a skin substitute without a skin graft can be placed over these defects that would eventually heal by epithelization process and spare the patient from creating a donor site. So far the use of dermal matrices with Dupuytren disease has been limited to a single case report. Ellis reported a patient with severe recurrent Dupuytren contracture of the palm extending to the small finger that underwent prior failed needle aponeurectomy and palmar fasciectomy and resulted in severe flexion contracture and functional loss of the digit.41 Following excision of the cord and skin, a defect measuring 14cm2 was resurfaced with a dermal regeneration template. After 2-year follow-up period the patient demonstrated a substantial improvement of range of motion of the finger and no signs of recurrence of the disease. Although this technique may sound promising, further studies on histological evidence regarding disease progression and recurrence is required in order to support a wider application of this treatment modality.
Summary
Tissue repair with dermal skin substitutes appears to be a useful tool in reconstructive surgery. The immediate skin coverage achieved using dermal substitutes consists a reconstructive option for managing injuries of the upper extremity that minimizes donor-site morbidity and provides final acceptable functional and aesthetic outcomes. However, one must always be conscious of publication bias in which favorable results tend to be more reported and published. Furthermore, as hand surgeons develop innovative approaches to solve upper extremity problems, and the indications of dermal skin substitutes continue to evolve, more comparative prospective trials are needed to evaluate the long-term benefits of dermal skin substitutes in the context of reconstructive surgery.
Figure 10.
Key points.
The provision of skin coverage for the upper extremity presents surgeons with several challenges. The special functional and physiological requirements of the integument such as mobility and sensibility need special consideration when performing soft issue reconstructions of the upper limb.
Adhering to the concept of the reconstructive ladder, soft tissue reconstruction of the upper extremity have treatment options that range from the application of skin grafts to reconstruction with microsurgical free tissue transfer. Dermal skin substitutes have been applied recently as part of the reconstructive ladder to expand the reconstructive options.
Common clinical indications of dermal skin substitutes in the upper extremity includemanagement of burns and soft-tissue coverage of traumatic injuries with exposed tendons, joints and bones. Other clinical uses may involve resurfacing of wounds following tumor resection, excision of Dupuytren cords and repair of congenital hand deformities (e.g. skin coverage following syndactyly release).
Common associated complications of dermal skin substitutes are infection, hematoma, graft failure and the need for multiple procedures. One should consider whether the cost of dermal skin substitute justified its use and the stiffness of the hand that results from the required long period of immobility to assure vascularization.
Synopsis.
Dermal skin substitutes are a group of biologically engineered material composed of collagen and glycosaminoglycans that are devoid of cellular structures. These biodegradable materials act as an artificial dermis to promote neo-vascularization and neo-dermis formation. Following wound epithelization after covering the dermis with thin skin graft, the dermal regeneration template is completley re-absorbed. Originally developed to replace skin in burn injuries where there is shortage of skin donor sites, the use of dermal skin substitutes has been expanded to other reconstructive problems requiring soft tissue coverage. Despite the well-recognized advantages of dermal substitutes, their engraftment rate, associated complications such as infection, hematoma and seroma formation as well as the need for meticulous postoperative wound care and cost should be carefully evaluated when selecting this treatment option over more established methods of soft tissue coverage such as tissue transfer. In this article we review the indications, advantages and limitations of dermal skin substitutes for soft tissue reconstruction of the upper-extremity.
Acknowledgments
Supported in part by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and National Institute on Aging (R01 AR062066) and from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (2R01 AR047328-06) and a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) (to Dr. Kevin C. Chung). Dr Singhal has received a fellowship grant from Indian Council of Medical Research, New Delhi, India to study various applications of skin substitutes.
Footnotes
Disclosure: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
References
- 1.Halim Ahmad Sukari, Khoo Teng Lye, Jumaat Shah, et al. Biologic and synthetic skin substitutes: An overview. Indian J Plast Surg. 2010 Sep;43(Suppl):S23–S28. doi: 10.4103/0970-0358.70712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke JF, Yannas IV, et al. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981 Oct;194(4):413–428. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yannas IV, Burke JF, Orgill DP, Skrabut EM. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982;215:174–6. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- 4.Jacoby SM, Bachoura A, Chen NC, Shin EK, Katolik LI. One-stage Integra coverage for fingertip injuries. Hand. 2013:1–5. doi: 10.1007/s11552-013-9513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janis JE, Kwon RK, Attinger CE. The new reconstructive ladder: modifications to the traditional model. Plast Reconstr Surg. 2011 Jan;127(Suppl 1):205S–212S. doi: 10.1097/PRS.0b013e318201271c. [DOI] [PubMed] [Google Scholar]
- 6.Límová M. Active wound coverings: bioengineered skin and dermal substitutes. Surg Clin North Am. 2010 Dec;90(6):1237–55. doi: 10.1016/j.suc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20:493–508. doi: 10.1097/01.ASW.0000288217.83128.f3. [DOI] [PubMed] [Google Scholar]
- 8.Lou RB, Hickerson WL. The use of skin substitutes in hand burns. Hand Clin. 2009 Nov;25(4):497–509. doi: 10.1016/j.hcl.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Moiemen NS, Vlachou E, Staiano JJ, et al. Reconstructive surgery with Integra dermal regeneration template: histologic study, clinical evaluation, and current practice. Plast Reconstr Surg. 2006 Jun;117(7 Suppl):160S–174S. doi: 10.1097/01.prs.0000222609.40461.68. [DOI] [PubMed] [Google Scholar]
- 10.Moiemen N, Yarrow J, Hodgson E, et al. Long-term clinical and histological analysis of Integra dermal regeneration template. Plast Reconstr Surg. 2011;127(3):1149–54. doi: 10.1097/PRS.0b013e31820436e3. [DOI] [PubMed] [Google Scholar]
- 11.Iorio ML, Shuck J, Attinger CE. Wound healing in the upper and lower extremities: a systematic review on the use of acellular dermal matrices. Plast Reconstr Surg. 2012 Nov;130(5 Suppl 2):232S–41S. doi: 10.1097/PRS.0b013e3182615703. [DOI] [PubMed] [Google Scholar]
- 12.Heimbach D, Luterman A, Burke J, Cram A, Herndon D, Hunt J, et al. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg. 1988;208:313–320. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirley R, Teare L, Dziewulski P, Frame J, Navsaria H, Myers S. A fatal case of toxic shock syndrome associated with skin substitute. Burns. 2010 Sep;36(6):e96–8. doi: 10.1016/j.burns.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo M. The use of Integra in hand and upper extremity surgery. J Hand Surg Am. 2012 Mar;37(3):583–6. doi: 10.1016/j.jhsa.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Moiemen NS, Yarrow J, Kamel D, Kearns D, Mendonca D. Topical negative pressure therapy: does it accelerate neovascularisation within the dermal regeneration template, Integra? A prospective histological in vivo study. Burns. 2010 Sep;36(6):764–8. doi: 10.1016/j.burns.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Bloemen MC, van der Wal MB, Verhaegen PD, Nieuwenhuis MK, van Baar ME, van Zuijlen PP, Middelkoop E. Clinical effectiveness of dermal substitution in burns by topical negative pressure: a multicenter randomized controlled trial. Wound Repair Regen. 2012 Nov-Dec;20(6):797–805. doi: 10.1111/j.1524-475X.2012.00845.x. [DOI] [PubMed] [Google Scholar]
- 17. [Last accessed 12, December 2013]; www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices.
- 18.Hop MJ, Bloemen MC, van Baar ME, Nieuwenhuis MK, van Zuijlen PP, et al. TOPSKIN Study Group. Cost study of dermal substitutes and topical negative pressure in the surgical treatment of burns. Burns. 2013 Sep 12; doi: 10.1016/j.burns.2013.08.025. pii: S0305-4179(13)00264-7 (In press) [DOI] [PubMed] [Google Scholar]
- 19.Ryssel H, Germann G, Kloeters O, et al. Dermal substitution with Matriderm® in burns on the dorsum of the hand. Burns. 2010;36(8):1248–53. doi: 10.1016/j.burns.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Dantzer E, Queruel P, Salinier L, et al. Dermal regeneration template for deep hand burns: clinical utility for both early grafting and reconstructive surgery. Br J Plast Surg. 2003 Dec;56(8):764–74. doi: 10.1016/s0007-1226(03)00366-7. [DOI] [PubMed] [Google Scholar]
- 21.Cuadra A, Correa G, Roa R, Piñeros JL, et al. Functional results of burned hands treated with Integra. J Plast Reconstr Aesthet Surg. 2012 Feb;65(2):228–34. doi: 10.1016/j.bjps.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Haslik W, Kamolz LP, Manna F, et al. Management of full-thickness skin defects in the hand and wrist region: first long-term experiences with the dermal matrix Matriderm. J Plast Reconstr Aesthet Surg. 2010 Feb;63(2):360–4. doi: 10.1016/j.bjps.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Hudson DA, Renshaw A. An algorithm for the release of burn contractures of the extremities. Burns. 2006 Sep;32(6):663–8. doi: 10.1016/j.burns.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Frame JD, Still J, Lakhel-LeCoadou A, et al. Use of dermal regeneration template in contracture release procedures: a multicenter evaluation. Plast Reconstr Surg. 2004 Apr 15;113(5):1330–8. doi: 10.1097/01.prs.0000111883.93604.85. [DOI] [PubMed] [Google Scholar]
- 25.Katrana F, Kostopoulos E, Delia G, et al. Reanimation of thumb extension after upper extremity degloving injury treated with Integra. J Hand Surg Eur Vol. 2008 Dec;33(6):800–2. doi: 10.1177/1753193408096021. [DOI] [PubMed] [Google Scholar]
- 26.Wolter TP, Noah EM, Pallua N. The use of Integra in an upper extremity avulsion injury. Br J Plast Surg. 2005 Apr;58(3):416–8. doi: 10.1016/j.bjps.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Graham GP, Helmer SD, Haan JM, Khandelwal A. The use of Integra® Dermal Regeneration Template in the reconstruction of traumatic degloving injuries. J Burn Care Res. 2013 Mar-Apr;34(2):261–6. doi: 10.1097/BCR.0b013e3182853eaf. [DOI] [PubMed] [Google Scholar]
- 28.Taras JS, Sapienza A, Roach JB, et al. Acellular dermal regeneration template for soft tissue reconstruction of the digits. J Hand Surg Am. 2010 Mar;35(3):415–21. doi: 10.1016/j.jhsa.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Helgeson MD, Potter BK, Evans KN, et al. Bioartificial dermal substitute: a preliminary report on its use for the management of complex combat-related soft tissue wounds. J Orthop Trauma. 2007 Jul;21(6):394–9. doi: 10.1097/BOT.0b013e318070c028. [DOI] [PubMed] [Google Scholar]
- 30.Chalmers RL, Smock E, Geh JL. Experience of Integra(®) in cancer reconstructive surgery. J Plast Reconstr Aesthet Surg. 2010 Dec;63(12):2081–90. doi: 10.1016/j.bjps.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Pauchot J, Elkhyat A, Rolin G, Mac S, et al. Dermal equivalents in oncology: benefit of one-stage procedure. Dermatol Surg. 2013 Jan;39(1 Pt 1):43–50. doi: 10.1111/dsu.12044. [DOI] [PubMed] [Google Scholar]
- 32.Smock ED, Barabas AG, Geh JLC. Reconstruction of a thumb defect with Integra following wide local excision of a subungual melanoma. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2010;63(1):e36–7. doi: 10.1016/j.bjps.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Carothers JT, Brigman BE, Rizzo M. Stacking of a dermal regeneration template for reconstruction of a soft-tissue defect after tumor excision from the palm of the hand: a case report. The Journal of hand surgery. 2005;30(6):1322–6. doi: 10.1016/j.jhsa.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Chang SC, Miller G, Halbert CF, et al. Limiting donor site morbidity by suprafascial dissection of the radial forearm flap. Microsurgery. 1996;17(3):136–40. doi: 10.1002/(SICI)1098-2752(1996)17:3<136::AID-MICR7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Avery CM, Pereira J, Brown AE. Suprafascial dissection of the radial forearm flap and donor site morbidity. Int J Oral Maxillofac Surg. 2001 Feb;30(1):37–41. doi: 10.1054/ijom.2000.0016. [DOI] [PubMed] [Google Scholar]
- 36.Peña I, de Villalaín L, García E, et al. Use of autologous skin equivalents with artificial dermal matrix (integra) in donor site coverage in radial forearm free flaps: preliminary cases. J Oral Maxillofac Surg. 2012 Oct;70(10):2453–8. doi: 10.1016/j.joms.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Rowe NM, Morris L, Delacure MD. Acellular dermal composite allografts for reconstruction of the radial forearm donor site. Ann Plast Surg. 2006 Sep;57(3):305–11. doi: 10.1097/01.sap.0000221622.41450.60. [DOI] [PubMed] [Google Scholar]
- 38.Murray RC, Gordin EA, Saigal K, et al. Reconstruction of the radial forearm free flap donor site using integra artificial dermis. Microsurgery. 2011 Feb;31(2):104–8. doi: 10.1002/micr.20833. [DOI] [PubMed] [Google Scholar]
- 39.Gravvanis AI, Tsoutsos DA, Iconomou T, et al. The use of integra artificial dermis to minimize donor-site morbidity after suprafascial dissection of the radial forearm flap. Microsurgery. 2007;27(7):583–7. doi: 10.1002/micr.20406. [DOI] [PubMed] [Google Scholar]
- 40.Medina CR, Patel SA, Ridge JA, et al. Improvement of the radial forearm flap donor defect by prelamination with human acellular dermal matrix. Plast Reconstr Surg. 2011 May;127(5):1993–6. doi: 10.1097/PRS.0b013e31820cf427. [DOI] [PubMed] [Google Scholar]
- 41.Ellis CV, Kulber DA. Acellular dermal matrices in hand reconstruction. Plast Reconstr Surg. 2012 Nov;130(5 Suppl 2):256S–69S. doi: 10.1097/PRS.0b013e318265a5cf. [DOI] [PubMed] [Google Scholar]


