Abstract
Purpose
We evaluated the survival of patients with muscle invasive bladder cancer undergoing radical cystectomy without neoadjuvant chemotherapy to confirm the utility of existing clinical tools to identify low risk patients who could be treated with radical cystectomy alone and a high risk group most likely to benefit from neoadjuvant chemotherapy.
Materials and Methods
We identified patients with muscle invasive bladder cancer who underwent radical cystectomy without neoadjuvant chemotherapy at our institution between 2000 and 2010. Patients were considered high risk based on the clinical presence of hydroureteronephrosis, cT3b-T4a disease, and/or histological evidence of lymphovascular invasion, micropapillary or neuroendocrine features on transurethral resection. We evaluated survival (disease specific, progression-free and overall) and rate of pathological up staging. An independent cohort of patients from another institution was used to confirm our findings.
Results
We identified 98 high risk and 199 low risk patients eligible for analysis. High risk patients exhibited decreased 5-year overall survival (47.0% vs 64.8%) and decreased disease specific (64.3% vs 83.5%) and progression-free (62.0% vs 84.1%) survival probabilities compared to low risk patients (p <0.001). Survival outcomes were confirmed in the validation subset. On final pathology 49.2% of low risk patients had disease up staged.
Conclusions
The 5-year disease specific survival of low risk patients was greater than 80%, supporting the distinction of high risk and low risk muscle invasive bladder cancer. The presence of high risk features identifies patients with a poor prognosis who are most likely to benefit from neoadjuvant chemotherapy, while many of those with low risk disease can undergo surgery up front with good expectations and avoid chemotherapy associated toxicity.
Keywords: urinary bladder neoplasms, cystectomy, neoadjuvant therapy, risk, outcomes assessment
Radical cystectomy with pelvic lymph node dissection is the gold standard for muscle invasive bladder cancer. Advances in surgical technique and perioperative care have improved outcomes after RC, but many patients experience relapse after surgery, likely from micrometastasis present at RC.1,2 The Intergroup trial (SWOG [Southwest Oncology Group]-8710) reported that patients randomized to 3 cycles of neoadjuvant methotrexate, vinblastine, doxorubicin and cisplatin followed by RC had an improved median survival of 77 months vs 46 months with RC alone.3
Although there was a clear survival benefit in the entire cohort that received NAC, the most dramatic improvement in median survival from 24 to 65 months was seen in patients with T3 or greater disease. These data have been borne out in meta-analyses showing that cisplatin based NAC combinations significantly improve overall survival, translating into an approximate 6% risk reduction of death at 5 years.4,5 However, this comes with some cost. SWOG-8710 reported grade 3 and 4 toxicity in 35% and 37% of patients, respectively, most of which was related to granulocytopenia and mucositis.3 The International Collaboration of Trialists reported similar grade 3 and 4 hematological toxicity in comparable patients treated with cisplatin, methotrexate and vinblastine.4
If NAC always provided a clear survival advantage or was free of serious toxicity, the decision to treat all eligible patients would be simple. However, since multimodal therapy provides only modest benefit with notable toxicity and, at present, we cannot reliably identify likely responders, it is challenging to decide on the appropriate course of therapy.3,4 Rather than recommending NAC for all patients with MIBC, we actively adopted a risk adapted approach to select individuals with high risk features for NAC, and in doing so, spared the morbidity and expense for those with low risk disease for whom RC alone should be curative. In the last 3 decades our approach to the clinical staging and risk assessment of MIBC was based on previous studies from our institution examining adjuvant chemotherapy in patients with BC,6 and has evolved beyond AJCC (American Joint Committee on Cancer) criteria7 to incorporate 3-D imaging, EUA and TUR to identify a 3-D palpable mass (cT3b) or local invasion to adjacent viscera (cT4a), the presence of hydroureteronephrosis, and histological evidence of LVI, micropapillary, and/or small cell features to meet our definition of high risk MIBC. Our published data revealed a 30% to 40% incidence of occult lymph node metastasis in the HR population, while the historical risk in those classified as LR was less than 10%.8,9 In this study we defined clinical LR MIBC with outcomes comparable to those of organ confined MIBC when treated with RC alone, and validated the results in an independent subset from another institution.
Materials and Methods
On approval from the MDACC internal review board, all patients who underwent RC without neoadjuvant chemotherapy for confirmed MIBC between January 2000 and December 2010 were identified. Patients dying of surgical complications and those with incomplete followup were excluded from the study. Patients were classified as HR or LR based on the presence of identifiable preoperative HR features (hydroureteronephrosis, cT3b-T4a disease [eg palpable 3-D mass on EUA, prostate stromal invasion], LVI or VH on TUR). VH was defined as histological evidence of neuroendocrine or micropapillary disease on biopsy.
Comprehensive clinical, pre-RC laboratory and final pathological data were collected, and extensively reviewed to ensure completeness and accuracy. As is standard practice at MDACC, all outside pathological and radio-graphic data were independently rereviewed, and all patients underwent repeat staging TUR and EUA before radical cystectomy. RC was performed with intent to cure, and included extended bilateral pelvic lymph node dissection with en bloc RC and urinary diversion. Tumor staging was based on the AJCC Cancer Staging Manual.7
Outcomes of interest included survival (OS, DSS and PFS) and rate of pathological up staging. Kaplan-Meier methods were used to calculate OS. Multivariable competing risks regression analysis, based on the model by Fine and Gray,10 was used to calculate cause specific mortality and DSS and PFS probabilities, using death from nonBC as the competing variable. Followup was defined as time from RC to death or last known followup (OS and DSS), or to documented date of disease recurrence (PFS). Pearson chi-square analysis was used to identify variables differing between HR and LR patients. Independent predictors of pathological up staging were identified using multivariable logistic regression analysis. For the validation subset (USC) patients were grouped as high or low risk based on MDACC criteria, and identical survival analyses were performed as previously described. All analyses were performed using STATA® version 12 and p ≤0.05 was considered significant.
Results
Of 1,285 patients who underwent RC at MDACC between 2000 and 2010, a total of 98 HR and 199 LR eligible patients who underwent radical cystectomy without NAC were identified. Median followup was 40.6 months (IQR 19.3, 72.2) for the entire cohort and 56.7 months (IQR 29.7, 91.7) for those alive at the time of analysis. Demographic and clinical characteristics for the entire cohort and clinical characteristics of the HR cohort are listed in tables 1 and 2, respectively. A total of 36 (36.7%) HR and 37 (18.6%) LR patients died of BC (p = 0.001). On final pathology 26 (26.5%) HR cases were down staged to pT2N0 or less with no HR features (VH or LVI) present while 98 (49.2%) LR cases were reclassified to HR (pT3 or greater, node positive disease and/or HR histological features), including 83 (84.7%) pT3 or greater and 29 (29.6%) with positive nodes (table 3). On multivariable analysis including all preclinical variables, the sole predictor of pathological up staging in the LR cohort was a preoperative hemoglobin level less than the lower limit of normal (OR 2.35, 95% CI 1.29, 4.30, p = 0.005).
Table 1. Baseline demographic and clinical characteristics of patients undergoing RC.
| Low Risk | High Risk | p Value | |||
|---|---|---|---|---|---|
| Median age (IQR) | 69.6 | (61.3, 74.8) | 72.6 | (63.1, 79.6) | 0.366 |
| No. gender (%): | 0.05 | ||||
| F | 22 | (11.1) | 19 | (19.4) | |
| M | 177 | (88.9) | 79 | (80.6) | |
| No. race (%): | 0.829 | ||||
| Caucasian | 180 | (90.5) | 86 | (87.0) | |
| African-American | 5 | (2.5) | 4 | (4.1) | |
| Hispanic | 13 | (6.5) | 7 | (7.1) | |
| Asian | 1 | (0.5) | 1 | (1.0) | |
| No. smoking history (%): | 0.557 | ||||
| Never | 43 | (21.9) | 23 | (23.5) | |
| Former | 115 | (58.7) | 61 | (62.2) | |
| Current | 38 | (19.4) | 14 | (14.3) | |
| No. body mass index (kg/m2) (%): | 0.150 | ||||
| Less than 25.0 | 47 | (24.9) | 38 | (35.5) | |
| 25.0–29.9 | 84 | (44.4) | 41 | (38.3) | |
| 30.0–34.9 | 43 | (22.8) | 17 | (15.9) | |
| 35.0 or Greater | 15 | (7.9) | 11 | (10.3) | |
| No. prior intravesical treatment (%) | 46 | (23.1) | 18 | (18.4) | 0.349 |
| No. concomitant carcinoma in situ (%) | 52 | (26.1) | 28 | (28.6) | 0.656 |
| No. clinical tumor stage (%): | <0.001 | ||||
| cT1 | 0 | (0) | 29 | (29.6) | |
| cT2 | 199 | (100.0) | 50 | (51.0) | |
| cT3b | 0 | 14 | (14.3) | ||
| cT4a | 0 | 5 | (5.1) | ||
| Median mos from TUR to RC (IQR) | 1.5 | (1.0, 2.4) | 1.4 | (0.9, 1.9) | 0.321 |
| No. preop hemoglobin (%): | 0.063 | ||||
| 12-16 (female) or 14-18 (male) | 104 | (52.3) | 42 | (42.0) | |
| Less than 12 (female) or less than 14 (male) | 95 | (47.7) | 58 | (59.2) | |
| Median pre-RC laboratory values (IQR): | |||||
| Glomerular filtration rate | 74.5 | (62, 83.5) | 69 | (50, 85) | 0.585 |
| Platelet count | 229 | (193, 277) | 248 | (210, 326) | 0.161 |
| Creatinine | 1.0 | (0.9, 1.3) | 1.0 | (0.9, 1.4) | 0.928 |
| Albumin | 4.1 | (4.0, 4.4) | 4.1 | (3.8, 4.4) | 0.530 |
| Calcium | 9.1 | (8.9, 9.4) | 9.1 | (8.8, 9.4) | 0.988 |
| Alkaline phosphatase | 77.5 | (66, 89) | 77 | (63, 97) | 0.960 |
| Lactate dehydrogenase | 447.5 | (397.5, 514) | 432 | (393, 505) | 0.461 |
| No. history of malignancy (%) | 22 | (11.1) | 18 | (18.4) | 0.090 |
Table 2. Baseline clinical characteristics of HR patients.
| No. (% of HR cohort) | |
|---|---|
| Hydroureteronephrosis | 39 (39.8) |
| LVI on TUR | 38 (38.8) |
| cT3 or cT4 disease | 20 (20.4) |
| Micropapillary on biopsy | 25 (25.5) |
| Total HR features: | |
| 1 | 77 (78.6) |
| 2 | 18 (18.4) |
| 3 | 3 (3.1) |
| Reason for not undergoing NAC: | |
| Comorbidity | 38 (38.8) |
| Physician choice | 31 (31.6) |
| Pt choice | 11 (11.2) |
| Unknown | 18 (18.4) |
Table 3. Final pathological data of patients with MIBC.
| Low Risk | High Risk | p Value | |||
|---|---|---|---|---|---|
| No. pathological tumor status (%): | 0.003 | ||||
| T0 | 24 | (12.1) | 2 | (2.0) | |
| Tis | 25 | (12.6) | 9 | (9.2) | |
| Ta | 5 | (2.5) | 6 | (6.1) | |
| T1 | 9 | (4.5) | 7 | (7.1) | |
| T2 | 53 | (26.6) | 16 | (16.3) | |
| T3 | 74 | (37.2) | 48 | (49.0) | |
| T4 | 9 | (4.5) | 10 | (10.2) | |
| No. pos lymph nodes (%): | 0.025 | ||||
| 0 | 158 | (79.4) | 64 | (65.3) | |
| 1–2 | 27 | (13.6) | 20 | (20.4) | |
| 3 or More | 14 | (7.0) | 14 | (14.3) | |
| Median lymph nodes examined (IQR) | 15 | (9, 24) | 15.5 | (9, 29) | 0.934 |
| No. extranodal extension in N+ (%) | 12 | (29.3) | 15 | (44.1) | 0.012 |
| No. pos surgical margin (%) | 5 | (2.5) | 5 | (5.1) | 0.245 |
| No. concomitant carcinoma in situ (%) | 98 | (49.3) | 54 | (55.1) | 0.342 |
| No. LVI (%) | 46 | (23.1) | 46 | (46.9) | <0.001 |
| Median cc estimated blood loss (IQR) | 975 | (600, 1,500) | 900 | (675, 1,300) | <0.001 |
| No. diversion (%): | 0.096 | ||||
| Ileal conduit | 107 | (53.4) | 62 | (63.9) | |
| Studer neobladder | 88 | (44.2) | 31 | (32.0) | |
| Indiana pouch | 4 | (2.0) | 4 | (4.1) | |
| Median mins surgical duration (IQR) | 405 | (329, 492) | 383 | (322, 477) | 0.445 |
| No. blood transfusion at surgery (%) | 108 | (54.3) | 68 | (69.4) | 0.013 |
OS, and DSS and PFS probabilities were each significantly decreased, and CSM was increased in the high vs low risk patient cohorts (table 4 and fig. 1). Patients with multiple HR features (20) demonstrated an increased risk of CSM compared to those with only 1 HR feature (78) (SHR 2.22, 95% CI 1.09, 4.51, p = 0.028). Survival based on disease reclassification after RC is shown in table 5 and figure 2. On multivariable analysis HR patients with organ confined disease and no HR features on final pathology had a similar CSM (SHR 1.16, 95% CI 0.31, 4.32, p = 0.829) and DSS probability as clinically defined LR patients who remained LR on final pathology. However, pathological up staging in LR patients was independently associated with an increase in CSM (SHR 3.55, 95% CI 1.61, 7.84, p = 0.002) and decreased DSS probability compared to LR patients who remained LR. However, importantly, HR patients who remained HR exhibited the lowest DSS probability compared to all other groups (p <0.001).
Table 4. Survival outcomes of patients with MIBC undergoing radical cystectomy without NAC based on preoperative high vs low risk classification.
| Low Risk | High Risk | |
|---|---|---|
| Overall survival OR (95% CI): | ||
| 1-Yr | 90.3 (85.2, 93.7) | 80.5 (71.2, 87.1) |
| 3-Yr | 75.0 (68.0, 80.7) | 56.3 (45.5, 65.6) |
| 5-Yr | 64.8 (56.7, 71.8) | 47.0 (35.8, 57.4) |
| % DSS probability:* | ||
| 1-Yr | 94.3 | 88.8 |
| 3-Yr | 85.9 | 73.7 |
| 5-Yr | 82.7 | 68.2 |
| % PFS probability:* | ||
| 1-Yr | 90.7 | 78.3 |
| 3-Yr | 86.2 | 69.0 |
| 5-Yr | 83.6 | 63.9 |
All values p <0.001.
Calculated using stepwise competing risks regression analysis with nonbladder cancer death as competing variable. Adjusted for age at surgery, gender, race, preoperative anemia, year of surgery and smoking history.
Figure 1.
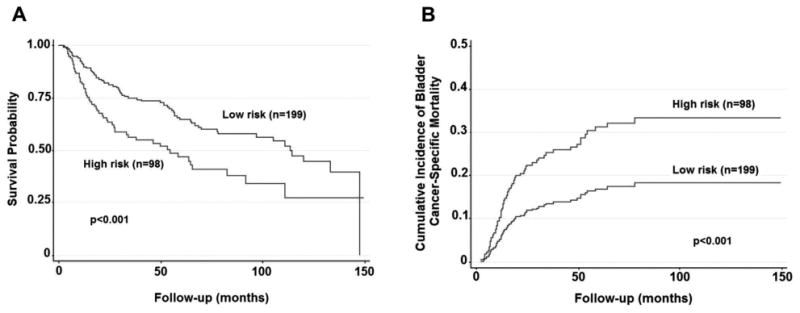
Survival of patients with MIBC who underwent radical cystectomy without NAC based on clinical high vs low risk (MDACC 2000 to 2010). A, Kaplan-Meier survival curve demonstrating overall survival. B, cumulative incidence of bladder cancer specific mortality, accounting for competing risk of nonbladder cancer death, and adjusted for age at surgery, gender, race, preoperative anemia, smoking history and year of surgery.
Table 5. Outcome data of patients with MIBC undergoing RC without NAC based on preoperative high vs low risk classification and final pathology.
| LR + Remained LR | LR but Up Staged | HR but Down Staged | HR + Remained HR | |
|---|---|---|---|---|
| No. pts | 101 | 98 | 26 | 72 |
| No. progression rate (%) | 11 (10.9) | 24 (24.5) | 6 (23.1) | 31 (43.1) |
| Overall survival OR (95% CI): | ||||
| 1-Yr | 96.9 (90.7, 99.0) | 83.7 (74.7, 89.7) | 100 | 73.5 (61.6, 82.2) |
| 3-Yr | 84.1 (74.6, 90.3) | 66.0 (55.4, 74.7) | 91.6 (70.5, 97.9) | 44.3 (32.4, 55.5) |
| 5-Yr | 72.2 (60.6, 80.9) | 57.8 (46.1, 67.7) | 85.1 (56.4, 95.1) | 35.0 (23.5, 46.7) |
| % DSS probability:* | ||||
| 1-Yr | 97.7 | 92.1 | 97.4 | 85.6 |
| 3-Yr | 93.7 | 79.4 | 92.8 | 64.7 |
| 5-Yr | 92.2 | 74.9 | 91.0 | 57.9 |
| % PFS probability:* | ||||
| 1-Yr | 94.6 | 87.0 | 88.1 | 74.1 |
| 3-Yr | 91.8 | 80.9 | 82.4 | 63.2 |
| 5-Yr | 90.3 | 77.6 | 79.3 | 56.6 |
Calculated using multivariable competing risks regression analysis with nonbladder cancer death as competing variable. Adjusted for age at surgery, gender, race, preoperative anemia, year of surgery, smoking history, total lymph nodes resected, margin status and adjuvant chemotherapy.
Figure 2.
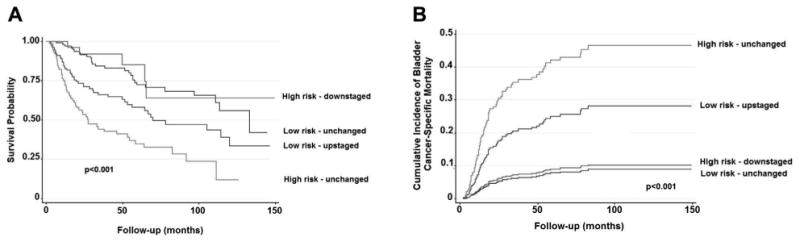
Survival of patients with MIBC who underwent radical cystectomy without NAC based on clinical high vs low risk and reclassification based on final pathology (MDACC 2000 to 2010). A, Kaplan-Meier analysis demonstrating overall survival. B, cumulative incidence of bladder cancer specific mortality, accounting for competing risk of nonbladder cancer death, and adjusted for age at surgery, gender, race, preoperative anemia, smoking history, history of intravesical therapy, margin status, total lymph nodes resected, adjuvant chemotherapy and year of surgery.
A total of 51 patients, 32 (16.2%) LR and 19 (19.4%) HR, received adjuvant chemotherapy, the majority of whom (98.0%) were high risk based on final pathology. Reasons for not receiving adjuvant chemotherapy included patient comorbidity (45%), physician choice (38%) or patient choice (16%). Overall, patients at MDACC who were selected for adjuvant chemotherapy had a decreased CSM (SHR 0.42, 95% CI 0.21, 0.87, p = 0.019). However, 5-year DSS probabilities were higher in HR patients who received adjuvant chemotherapy than for those who were observed, with 82.7% vs 67.3% (LR up staged to HR) and 73.3% vs 52.4% (HR remaining HR). A total of 37 (37.8%) HR and 35 (17.6%) LR patients experienced progression (p <0.001) at a median of 10.7 (95% CI 6.8, 17.8) and 9.1 (95% CI 5.6, 14.9) months, with a median time from RC to death of 18.2 (95% CI 13.5, 29.5) and 18.1 (95% CI 12.2, 27.4) months, respectively. The progression rate was lowest in LR cases that remained LR and highest in HR cases that remained HR on final pathology (table 5). Of those with progression 23 (65.7%) HR and 22 (64.7%) LR patients underwent salvage chemotherapy. There was no significant survival difference based on the delivery of salvage chemotherapy.
The MDACC criteria for preoperative high vs low risk disease were applied to an independent subset of patients who underwent RC without NAC at another institution (USC) during a 25-year period. This independent cohort consisted of 1,138 LR and 644 HR patients (tables 6 and 7). Consistent with our data, patients with pre-RC HR features demonstrated decreased OS and increased CSM compared to LR patients (fig. 3). Five-year OS, DSS and PFS probabilities were all significantly lower in patients with HR features present before RC compared to LR patients. Importantly, the percentage of HR patients with each pre-RC high risk feature and 5-year survival probabilities was similar between the MDACC and USC cohorts.
Table 6. Characteristics of patients with MIBC at USC (1983–2008) considered high risk before surgery.
| No. (% of HR cohort) | |
|---|---|
| Hydroureteronephrosis | 313 (48.6) |
| LVI on TUR | 292 (45.3) |
| cT3 or cT4 disease | 154 (23.9) |
| Micropapillary or small cell on biopsy | 19 (3.0) |
| Total HR features: | |
| 1 | 521 (80.9) |
| 2 | 112 (17.4) |
| 3 | 11 (1.7) |
Table 7. Survival of patients with MIBC at USC (1983–2008) based on the presence or absence of preoperative high risk features.
| Low Risk | High Risk | |
|---|---|---|
| Overall survival OR (95% CI): | ||
| 1-Yr | 90.1 (88.3, 91.9) | 85.3 (82.5, 88.1) |
| 3-Yr | 76.2 (73.6, 78.8) | 63.2 (59.2, 67.2) |
| 5-Yr | 67.9 (64.9, 70.9) | 52.4 (48.0, 56.8) |
| % DSS probability:* | ||
| 1-Yr | 95.0 | 90.7 |
| 3-Yr | 84.5 | 72.9 |
| 5-Yr | 79.8 | 65.6 |
| % PFS probability:* | ||
| 1-Yr | 89.3 | 80.7 |
| 3-Yr | 79.3 | 64.6 |
| 5-Yr | 75.8 | 59.3 |
All values p <0.001.
Calculated using multivariable competing risks regression analysis with non-bladder cancer death as competing variable.
Figure 3.
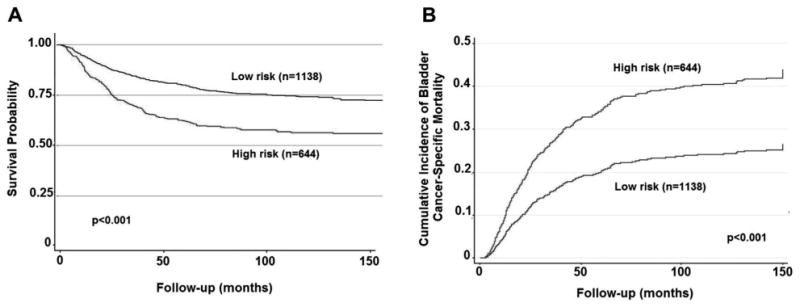
Survival of patients with MIBC who underwent radical cystectomy without NAC based on clinical high vs low risk (USC 1983 to 2008). A, Kaplan-Meier survival curve demonstrating overall survival. B, cumulative incidence of bladder cancer specific mortality, accounting for competing risk of noncancer death, and adjusted for age at surgery, gender, race, preoperative anemia, smoking history and year of surgery.
Discussion
While NAC has become the accepted standard of care for the treatment of MIBC on the basis of convincing level I evidence, contemporary studies show dramatic underuse of this approach, with less than 20% of patients receiving the recommended care.3,11 Possible explanations include the modest overall benefit in 5-year survival afforded by NAC to an unselected population with MIBC, since only 30% to 40% of cases are rendered pT1 or less and reliable identification of those who respond this dramatically is not yet feasible.4,5 Perioperative mortality rates are low, but morbidity is not insignificant and is unacceptable if there is no benefit to be gained from NAC.3,4 Not everyone seems to require NAC, as patients with organ confined MIBC, who account for up to 50% of those in contemporary surgical series, have approximately 80% durable DSS.1 The dilemma is articulated by the inadequacy of current clinical staging strategies that leave more than 50% of patients with extravesical disease clinically under staged. We face the quandary of offering NAC to every eligible patient with MIBC regardless of risk, or the challenge of improving clinical staging to accurately identify those patients with HR features likely to experience progression despite RC. A more selective approach to NAC should translate into a higher rate of prescribing such care to those most likely to benefit.
Numerous studies have highlighted the inaccuracy of clinical staging in BC with the incidence of pathological up staging ranging from 49% to 72% and patients with up staged disease more likely to die of BC.12–15 The pathological stage of those cases we designate as HR at MDACC is of particular concern, as those with LVI on TUR or cT3-cT4a disease had an 86% rate of nonorgan confined disease at RC. A pooled analysis of 778 patients who underwent RC at 4 different academic medical centers found pathological up staging in 42% with only 36% of patients considered to have clinical nonorgan confined disease (pT3N0 or greater, or pTxN+) at diagnosis.17 Most recently, in studying patients with suspected cT2 disease only, Canter et al found a 73% rate of up staging to pT3/T4 or pN+ with 5-year recurrence-free survival and disease specific survival of 56.5% and 59.5%, respectively.18 In comparison, 49% of our LR patient cohort was reclassified as HR at radical cystectomy with a DSS probability of approximately 75% at 5 years. Surprisingly we found that a preoperative hemoglobin less than the lower limit of normal was the only independent predictor of pathological up staging, which may indicate that baseline anemia may be a surrogate for occult aggressive disease in addition to other HR features, and has recently been described by other investigators as well.19
LVI is widely accepted as a HR feature for BC, as multiple studies have shown LVI to be associated with higher pathological stage, higher grade, positive surgical margins, and worse overall survival and recurrence-free survival.20–22 It is possible that our practice of repeat TUR before RC identifies more patients with LVI than was appreciated by review of the original pathology. As with LVI, multiple studies have demonstrated that the presence of hydroureteronephrosis or VH is associated with more advanced disease on final pathology as well as being an independent predictor of decreased DSS.23–26 In support of the inclusion of small cell histology in HR MIBC, we can look to our own experience. In a review of 46 patients who underwent RC for small cell BC, the 5-year DSS was 36%. However, in those patients treated with NAC, survival approached that of our LR group at 78%.27,28 This stark trend served as the basis of a phase 2 clinical trial of NAC in the setting of small cell BC.29 Due to the overlap of this phase 2 trial with the cohort used in the present study, there were no patients included in our present analysis with pre-RC small cell histology.
While there are inherent limitations of single center retrospective reviews, our results were confirmed using an independent cohort from another high volume institution. Not only did we find that HR patients identified from this independent cohort exhibited decreased survival and increased CSM compared to LR patients, we found that 5-year overall, and disease specific and progression-free survival probabilities were remarkably similar between the 2 institutions. These data indicate that this iteration of our risk stratification is consistent across institutions. Although we lack a control group of LR patients treated with NAC, given an 80% 5-year DSS and the appreciated survival benefits to NAC identified by randomized prospective trials, until we can prospectively identify likely responders to NAC, the advantage to the LR group would likely be trivial. We also did not present data showing that patients with HR features benefit from NAC as this is outside the scope of the current report.
Our objective was to define a clinical LR disease state with a disease specific survival after RC comparable to that of organ confined MIBC to justify our two-pronged approach to the management of MIBC (fig. 4). Our results indicate that carefully selected patients with low risk MIBC achieve a DSS probability of approximately 80% at 5 years after RC, which is comparable to those with pT2 disease. Notably if patients remain LR after RC, 5-year DSS probability is greater than 90%. The 5-year DSS probability of those with disease pathologically up staged or found to harbor HR histological features decreased significantly to approximately 75%, but was still far superior to HR patients who remained HR after RC.
Figure 4.
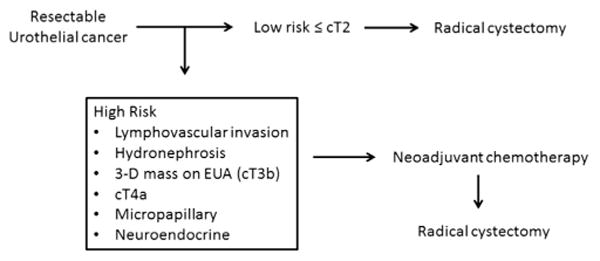
Neoadjuvant platform for clinical based staging and therapy for bladder cancer.
Our greatest concern is that despite a concerted effort to refine clinical staging, we still fail to identify a significant number of HR patients who may be candidates and might benefit from NAC. Our published experience provides assurance that this limitation does not necessarily compromise survival since we reported that adjuvant was as effective as neoadjuvant chemotherapy in the high risk setting.16 Indeed 31 of the 98 patients with disease pathologically up staged received adjuvant chemotherapy with 5-year DSS probabilities greater than 80%, indicating that adjuvant chemotherapy offers an acceptable 5-year DSS even with pathological up staging. However, this off protocol practice does not match the level of commitment to adjuvant chemotherapy that we anticipated from previous experience.16 Regardless, patients classified as LR before RC should be counseled that they may still need adjuvant chemotherapy given the high risk of pathological up staging.
Conclusions
The accumulation of experience at our institution and in the literature directed our current strategy of risk adapted NAC. Weighing the modest survival advantage with the potential toxicity led us to offer NAC to those patients at highest risk and most likely to benefit from treatment. Our results indicate that patients with MIBC without high risk features can achieve a DSS comparable to those with organ confined MIBC despite the fact that approximately 50% have disease under staged. Nonetheless, an important challenge remains to further refine clinicopathological assessment to limit clinical under staging and assure that HR individuals are identified for NAC.
Acknowledgments
Supported by the National Institutes of Health through MD Anderson Cancer Center Support Grant CA016672 and the Genitourinary GU SPORE in Bladder Cancer Grant P50CA091846.
Abbreviations and Acronyms
- 3-D
3-dimensional
- BC
bladder cancer
- CSM
cause specific mortality
- DSS
disease specific survival
- EUA
examination under anesthesia
- HR
high risk
- LR
low risk
- LVI
lymphovascular invasion
- MDACC
University of Texas M.D. Anderson Cancer Center
- MIBC
muscle invasive bladder cancer
- NAC
neoadjuvant chemotherapy
- OS
overall survival
- PFS
progression-free survival
- RC
radical cystectomy
- TUR
transurethral resection
- USC
University of Southern California
- VH
variant histology
References
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Hautmann RE, Gschwend JE, de Petriconi RC, et al. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol. 2006;176:486. doi: 10.1016/j.juro.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 4.Advanced Bladder Cancer Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361:1927. doi: 10.1016/s0140-6736(03)13580-5. [DOI] [PubMed] [Google Scholar]
- 5.Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol. 2004;171:561. doi: 10.1097/01.ju.0000090967.08622.33. [DOI] [PubMed] [Google Scholar]
- 6.Logothetis CJ, Johnson DE, Chong C, et al. Adjuvant cyclophosphamide, doxorubicin, and cisplatin chemotherapy for bladder cancer: an update. J Clin Oncol. 1988;6:1590. doi: 10.1200/JCO.1988.6.10.1590. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Boyd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th. New York: Springer; 2010. Urinary bladder; pp. 497–506. part IX. [Google Scholar]
- 8.Millikan R, Siefker-Radtke A, Grossman HB. Neoadjuvant chemotherapy for bladder cancer. Urol Oncol. 2003;21:464. doi: 10.1016/s1078-1439(03)00148-0. [DOI] [PubMed] [Google Scholar]
- 9.Wishnow KI, Levinson AK, Johnson DE, et al. Stage B (P2/3A/N0) transitional cell carcinoma of bladder highly curable by radical cystectomy. Urology. 1992;39:12. doi: 10.1016/0090-4295(92)90033-s. [DOI] [PubMed] [Google Scholar]
- 10.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496. [Google Scholar]
- 11.Raj GV, Karavadia S, Schlomer B, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011;117:276. doi: 10.1002/cncr.25429. [DOI] [PubMed] [Google Scholar]
- 12.Chang BS, Kim HL, Yang XJ, et al. Correlation between biopsy and radical cystectomy in assessing grade and depth of invasion in bladder urothelial carcinoma. Urology. 2001;57:1063. doi: 10.1016/s0090-4295(01)00998-0. [DOI] [PubMed] [Google Scholar]
- 13.Ficarra V, Dalpiaz O, Alrabi N, et al. Correlation between clinical and pathological staging in a series of radical cystectomies for bladder carcinoma. BJU Int. 2005;95:786. doi: 10.1111/j.1464-410X.2005.05401.x. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbeck BK, Miller DC, Dunn RL, et al. The effects of stage divergence on survival after radical cystectomy for urothelial cancer. Urol Oncol. 2005;23:77. doi: 10.1016/j.urolonc.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin S, Shephard J, Wallen E, et al. Comparison of the clinical and pathologic staging in patients undergoing radical cystectomy for bladder cancer. Int Braz J Urol. 2007;33:25. doi: 10.1590/s1677-55382007000100005. [DOI] [PubMed] [Google Scholar]
- 16.Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol. 2001;19:4005. doi: 10.1200/JCO.2001.19.20.4005. [DOI] [PubMed] [Google Scholar]
- 17.Shariat SF, Palapattu GS, Karakiewicz PI, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007;51:137. doi: 10.1016/j.eururo.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Canter D, Long C, Kutikov A, et al. Clinicopathological outcomes after radical cystectomy for clinical T2 urothelial carcinoma: further evidence to support the use of neoadjuvant chemotherapy. BJU Int. 2011;107:58. doi: 10.1111/j.1464-410X.2010.09442.x. [DOI] [PubMed] [Google Scholar]
- 19.Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst. 2013;105:499. doi: 10.1093/jnci/djt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lotan Y, Gupta A, Shariat SF, et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol. 2005;23:6533. doi: 10.1200/JCO.2005.05.516. [DOI] [PubMed] [Google Scholar]
- 21.Streeper NM, Simons CM, Konety BR, et al. The significance of lymphovascular invasion in transurethral resection of bladder tumour and cystectomy specimens on the survival of patients with urothelial bladder cancer. BJU Int. 2009;103:475. doi: 10.1111/j.1464-410X.2008.08011.x. [DOI] [PubMed] [Google Scholar]
- 22.Canter D, Guzzo T, Resnick M, et al. The presence of lymphovascular invasion in radical cystectomy specimens from patients with urothelial carcinoma portends a poor clinical prognosis. BJU Int. 2008;102:952. doi: 10.1111/j.1464-410X.2008.07732.x. [DOI] [PubMed] [Google Scholar]
- 23.Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer. 2007;110:62. doi: 10.1002/cncr.22756. [DOI] [PubMed] [Google Scholar]
- 24.Stimson CJ, Cookson MS, Barocas DA, et al. Preoperative hydronephrosis predicts extravesical and node positive disease in patients undergoing cystectomy for bladder cancer. J Urol. 2010;183:1732. doi: 10.1016/j.juro.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Chapman DM, Pohar KS, Gong MC, et al. Pre-operative hydronephrosis as an indicator of survival after radical cystectomy. Urol Oncol. 2009;27:491. doi: 10.1016/j.urolonc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Rogers CG, Palapattu GS, Shariat SF, et al. Clinical outcomes following radical cystectomy for primary nontransitional cell carcinoma of the bladder compared to transitional cell carcinoma of the bladder. J Urol. 2006;175:2048. doi: 10.1016/S0022-5347(06)00317-X. [DOI] [PubMed] [Google Scholar]
- 27.Siefker-Radtke AO, Dinney CP, Abrahams NA, et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol. 2004;172:481. doi: 10.1097/01.ju.0000132413.85866.fc. [DOI] [PubMed] [Google Scholar]
- 28.Lynch SP, Shen Y, Kamat A, et al. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur Urol. 2013;64:307. doi: 10.1016/j.eururo.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siefker-Radtke AO, Kamat AM, Grossman HB, et al. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J Clin Oncol. 2009;27:2592. doi: 10.1200/JCO.2008.19.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]


