Abstract
Background
We evaluated whether addition of carotid ultrasound intima-media thickness (CIMT) measurements and risk categories of plaque help predict incident stroke and CVD in older adults.
Methods
Carotid ultrasound studies were recorded in the multicenter Cardiovascular Health Study (CHS). Cardiovascular disease (CVD) was defined as coronary heart disease plus heart failure plus stroke. Ten-year risk prediction Cox proportional hazards models for stroke and CVD were calculated using CHS-specific coefficients for Framingham Risk Score (FRS) factors. Categories of CIMT and CIMT plus plaque were added to FRS prediction models and categorical net reclassification improvement (NRI) and Harrell’s c-statistic were calculated.
Results
In 4,384 CHS participants (61% women, 14% black, baseline age 72 ± 5 yrs) without CVD at baseline, higher CIMT category and presence of plaque were both associated with higher incidence rates for stroke and CVD. Addition of CIMT improved ability of FRS-type risk models to discriminate cases from non-cases of incident stroke and CVD (NRI = 0.062, p=0.015 and NRI=0.027, p<0.001 respectively), with no further improvement by adding plaque. For both outcomes, NRI was driven by down-classifying those without incident disease. Although addition of plaque to CIMT did not result in a significant NRI for either outcome, it was significant among those without incident disease.
Conclusion
In older adults, addition of CIMT modestly improves 10-year risk prediction for stroke and CVD beyond a traditional risk factor model, mainly by down-classifying risk in those without stroke or CVD; addition of plaque to CIMT adds no statistical benefit in the overall cohort, although there is evidence of down-classification in those without events.
Background
The Framingham Risk Score (FRS) and other traditional cardiovascular disease (CVD) risk factors/algorithms have important predictive value for stroke and other CVD endpoints (1). Nonetheless, the majority of incident stroke and other CVD events occur in the low- and intermediate-risk groups characterized by these risk factor predictors. Previous reports have documented an association between carotid intima medial thickness (CIMT) and/or plaque with stroke, transient ischemic attacks (TIA), and other clinical manifestations of CVD (2-16).
Despite what is known regarding the importance of traditional CVD risk factors and measures of subclinical disease such as CIMT and plaque in predicting future stroke and other CVD events, there is a paucity of information regarding the relative prognostic value of adding carotid ultrasound measurement information to traditional risk factors in elderly individuals. Consequently, we evaluated, in a multicenter cohort of older adults without CVD at baseline, whether CIMT measurements and plaque could add incremental value to traditional risk factors in predicting the 10-year risk of incident stroke and CVD.
Methods
Study Population
The Cardiovascular Health Study (CHS) is a population-based prospective study of men and women aged 65 years or greater at baseline. The mean age of the study population at baseline was 72.8 ± 5.6 years. The overall study design for CHS has been previously published (17). Briefly, between 1989 and 1990, CHS enrolled 5,201 participants using Medicare eligibility lists in 4 communities: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. A second cohort of 687 black participants was recruited between 1992 and 1993. Participants included in this analysis had no evidence of coronary heart disease (CHD), heart failure (HF), or stroke at baseline. All participants underwent a baseline clinical examination which included history, physical examination, blood drawing, carotid ultrasound, and other tests.
Carotid Ultrasonography
Carotid arteries were evaluated at baseline using high-resolution B-mode ultrasonography (model SSA-270A ultrasound machine; Toshiba, Tustin, CA). The scanning protocol has been previously described in detail (3). The protocols for recording carotid ultrasound studies and measuring CIMT were the same for the scans performed in 1989-90 and 1992-93. Both examinations used on-site video tapes as well as direct image capture to a Macintosh II computer with the digital images and videotapes sent to the Ultrasound Reading Center for subsequent review and processing. The CHS protocol was such that, following imaging of the common carotid artery below the carotid artery bulb, images were acquired—with the ultrasound beam centered on the internal carotid artery (ICA) flow divider—from the anterolateral, lateral, and postero-lateral projections. Plaque measurements were made in either the proximal ICA or the bulb, whichever site had the largest wall protrusion. If a protrusion was not seen, then imaging was centered on the carotid bulb.
Quantitative measurements of CIMT were performed on one longitudinal image of the common carotid artery (CCA) and 3 longitudinal images of the ICA recorded from both the right and left carotid arteries. Measurements were performed on an image that was selected from a sequence of images replayed from a digital playback buffer. Frames that were free of motion—i.e., where the preceding and following images showed no motion—were selected. Although there was no attempt to select based on the cardiac cycle, subsequent review of the images has shown that this tended to be at end-diastole. A mouse-activated drawing tool was used to trace the boundaries of the lumen-intima and media-adventitia interfaces of the arterial wall. The distance between these 2 lines corresponded to the combined thickness of the intima and media (2,3). Maximal IMT of the CCA and ICA was calculated as the mean of the maximal IMT of the near and far walls from both left and right carotid arteries (4). The CIMT measure used in these analyses was the average of maximal CCA and ICA IMT as defined above after standardization—i.e., after subtraction of the mean and division by the standard deviation of the measurement. Focal plaque, when present, was included in the maximum IMT measurement. Gender-specific percentile categories were created using cutpoints at the 25th and 75th percentile of standardized CIMT for each gender.
Plaque was defined based on the presence of the greatest perceived protrusion of the carotid wall (specifically the intima-media thickness) in either the carotid bulb or proximal ICA. Three plaque categories were defined: no plaque, intermediate-risk, and high-risk, based on lesion surface, echogenicity, and texture characteristics (2). High-risk plaques were defined as having at least one of the following characteristics: irregular or ulcerated surface, echolucent, or heterogeneous in texture. Individuals with no plaque had lesion surface specified as smooth with lesion density and morphology both specified as “no lesion.” Any other combinations of lesion characteristics were defined as “intermediate-risk.”
Data on inter-sonographer and inter-reader variability for CCA and ICA far wall thickness and residual lumen have been previously published (3). The mean (± SD) maximal absolute inter-sonographer difference for the far wall CCA IMT measurement was 0.20±0.26 mm (R=0.52) and for the far wall ICA IMT was 0.65±0.69 mm (R=0.52). The mean (± SD) maximal absolute inter-reader differences in IMT measurements were lower for the inter-reader comparisons: 0.09±0.05 mm for the CCA IMT (R=0.91) and 0.41±0.57 (R=0.81) for the ICA IMT.
Cardiovascular Event Ascertainment
Methods used to assess CVD events including stroke and CHD in the CHS have been reported previously (18). Briefly, in CHS, potential clinical events were identified through: (1) clinic visits and surveillance calls by the field centers; (2) participant-initiated reports; and (3) secondary sources of events, including review of medical records and Medicare hospitalization data. The CHS Events Committee adjudicated CVD events by reviewing all pertinent data, including history, physical examination, chest radiography report, and medication use. CHD was defined as angina as well as nonfatal and fatal myocardial infarction, coronary artery bypass graft, or angioplasty. CVD was defined as a composite of nonfatal or fatal CHD, heart failure, or stroke during the follow-up period. Cause of death was adjudicated by the Events Committee. All deaths due to atherosclerotic CHD were captured in the CHS definition and all deaths due to atherosclerotic CHD or cerebrovascular disease were captured in the CVD definition. Individuals were censored at the earliest of the following: date of death, date of loss to follow-up, or 10 years.
Statistical Analyses
STATA 12 software was used for analyses. Baseline characteristics were summarized according to gender-specific categories of CIMT (<25th percentile, 25th-75th percentile, >75th percentile). Incidence rates of stroke and CVD were calculated per 1000 person-years as a function of CIMT percentile categories and plaque (absent, intermediate-risk, and high-risk). Cox proportional hazards models were used to determine—after adjusting for traditional FRS factors (age, gender, race, hypertension medications, systolic blood pressure, diabetes mellitus, total and HDL cholesterol, smoking)—the associations of CIMT categories and plaque categories with incident stroke and CVD within 10 years. Cox models were used to predict ten-year risk for stroke and CVD for a: (1) base model using the CHS-specific coefficients for traditional FRS factors, (2) CIMT model adding CIMT categories to the base model, and (3) full model adding plaque categories to the CIMT model. We also examined the full model using plaque (presence/absence) rather than plaque categories.
We assessed calibration by calculating likelihood ratio test p-values from the survival-adapted Hosmer-Lemeshow test. In this context, p-values< 0.05 would suggest the model was not well calibrated and there was a significant difference between expected and observed event rates (19). The Harrell’s c-statistic was calculated for each model and predicted risks were categorized into <5%, 5-<10%, 10-<20%, and ≥20%.(20). The predicted risk categories were used to compute the net reclassification improvement (NRI) statistic for those experiencing events, those without events and overall. The event NRI was calculated as the proportion of individuals who were reclassified to a higher risk category minus the proportion reclassified to a lower risk category. The non-event NRI was calculated as the proportion of individuals who were reclassified to a lower risk category minus the proportion reclassified to a higher risk category. The overall NRI was calculated as the sum of the event NRI and the non-event NRI. The NRI data and difference in c-statistic were used to compare the CIMT and full models to the base model and the full model to the CIMT model. Further analyses examined the NRI statistic comparing the CIMT model to the base model restricted to those with plaque and to those with high-risk plaque.
It has been suggested that it is in those individuals with intermediate FRS risk, the addition of the carotid ultrasound measures (e.g., of CIMT and plaque) to the base model is likely to be most useful clinically. Consequently, clinical NRIs (cNRI) were calculated for the primary analysis by restricting the calculation of the NRI to those who were in the intermediate FRS-risk groups (5-20%) for the initial model. Other than this restriction, cNRI was calculated similarly to the NRI except that reclassification was only upward or downward if movement occurred to the ≥20% category or the 0-5% category, respectively; movements within the intermediate categories were not considered to be reclassifications. As a sensitivity analysis, we also examined NRI statistics comparing the addition of plaque categories to the base model and comparing the full model to the base plus plaque categories model. In additional sensitivity analyses, the NRI statistics were calculated comparing the CIMT model to the FRS-type model and comparing the full model to the CIMT model with three different restrictions. First, we excluded individuals who were taking lipid medications; second, we excluded individuals with prevalent peripheral arterial disease (PAD); and third, we included only ischemic stroke as the stroke outcome and censored those with hemorrhagic or unclassified stroke type. PAD was defined as the presence of either claudication (adjudicated) or an ankle-arm index <0.9. The NRI statistics for the first two were calculated for both stroke and CVD, while those for ischemic stroke were not calculated for CVD.
Results
Of the 5,888 CHS participants, 1,406 were excluded from analysis because of the presence of CHD, heart failure, or stroke at baseline. In addition, 25 were excluded because of missing carotid ultrasound data and 73 were excluded because of missing data for the clinical covariates. Consequently, the analyses presented included 4,384 CHS participants—61% women, 14% black, baseline age 72 ± 5 years. There were 482 strokes included in this analysis. Of these, 450 were classified as ischemic or non-ischemic: 397 (88.2%) were ischemic. Of the 1510 cases of CVD, considering only time at first event, 9 had CHD, CHF, and stroke at the same time; 9 had stroke and HF; 19 had stroke and CHD; 247 had HF and CHD; 248 had only HF; 641 had only CHD; and 337 had only stroke. With regard to the stroke outcome, 6 were lost to follow-up and 1,009 died prior to a stroke or 10 years of follow-up; for the CVD outcome, 5 were lost to follow-up and 584 died prior to a CVD event or 10 years of follow-up.
Demographic and risk factor characteristics in the cohort are presented in Table 1 as a function of CIMT percentile category. As can be seen from Table 1, higher age, systolic blood pressure, total cholesterol, percent of participants taking hypertension medication, percent with diabetes, and percent who are current smokers are associated with higher CIMT percentile category. The mean (SD) of maximal CCA IMT was 1.066 (0.217) and the mean (SD) of maximal ICA IMT was 1.440 (0.567); these values were used to standardize these measures. After taking the mean of the standardized maximal CCA IMT and maximal ICA IMT, the 25th and 75th percentiles of the summary CIMT measure were −0.895 and 0.176 for women and −0.583 and 0.721 for men, respectively; these cutpoints were used to categorize CIMT.
Table 1. Demographic and risk factor characteristics by CIMT percentile category.
| CIMT range | <25th %tile (1095) | 25th-75th %tile (2194) | >75th %tile (1095) |
|---|---|---|---|
| Age, mean ± SD | 70.78±4.63 | 72.25±5.17 | 74.46±6.00 |
| Systolic BP (mm Hg), mean ± SD | 129.35±18.70 | 136.33±21.10 | 142.94±22.26 |
| Cholesterol (mg/dl), mean ± SD | 208.82±37.95 | 211.32±37.57 | 217.47±41.84 |
| HDL (mg/dl), mean ± SD | 57.70±16.71 | 55.46±15.86 | 53.39±14.23 |
| African American, n (%) | 112 (10.2%) | 339 (15.5%) | 184 (16.8%) |
| Male, n (%) | 428 (39.1%) | 857 (39.1%) | 428 (39.1%) |
| Hypertension medication, n (%) | 329 (30.0%) | 876 (39.9%) | 512 (46.8%) |
| Diabetes, n (%) | 100 (9.1%) | 287 (13.1%) | 221 (20.2%) |
| Smoking category, n (%) | |||
| Never smoked | 585 (53.4%) | 1063 (48.5%) | 432 (39.5%) |
| Former smoker | 415 (37.9%) | 889 (40.5%) | 460 (42.0%) |
| Current smoker | 95 (8.7%) | 242 (11.0%) | 203 (18.5%) |
| Plaque risk category, n (%) | |||
| None | 675 (61.6%) | 363 (16.5%) | 18 (1.6%) |
| Intermediate-risk | 169 (15.4%) | 586 (26.7%) | 183 (16.7%) |
| High-risk | 251 (22.9%) | 1245 (56.7%) | 894 (81.6%) |
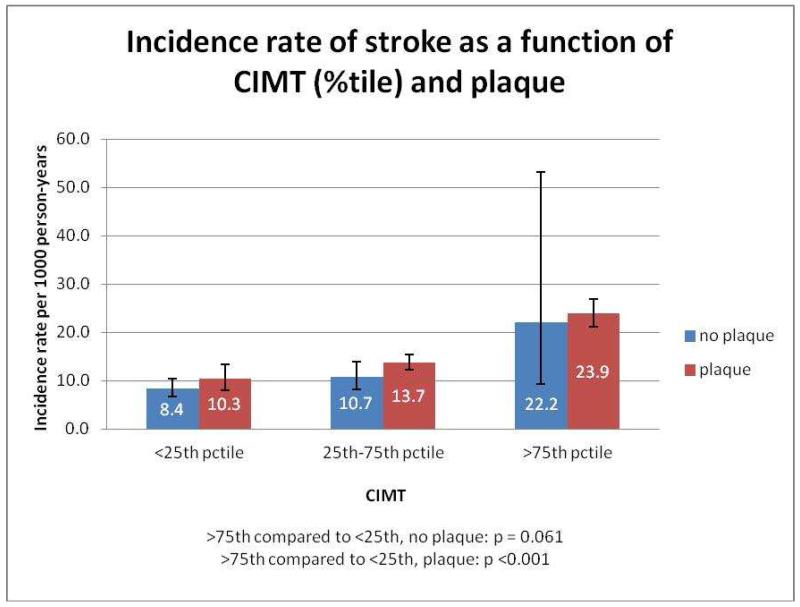
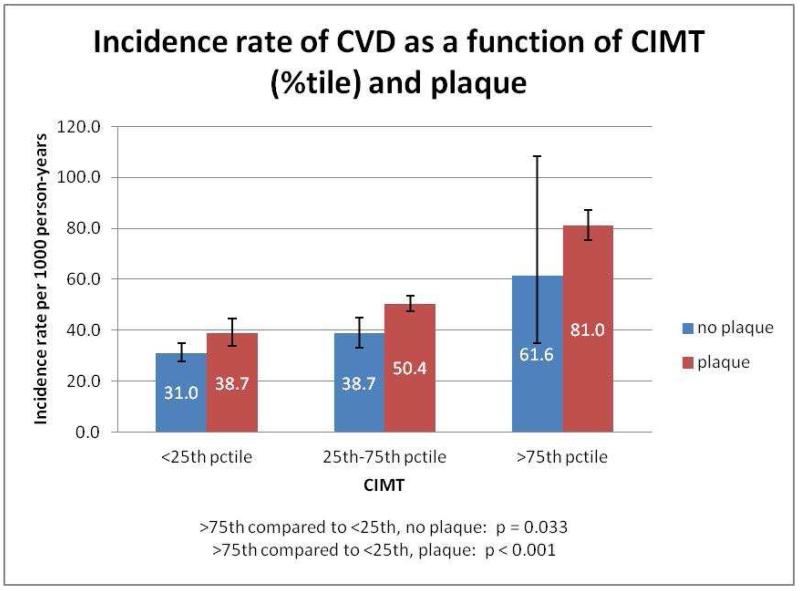
Figure 1 presents the incidence rates of stroke per 1,000 person-years as a function of CIMT percentile in those with or without plaque. Note that there is a graded relationship between CIMT percentile and the incidence rate of stroke in both those with and without plaque (p-values comparing >75th percentile of CIMT category to <25th percentile of CIMT are <0.001 and 0.025 respectively). Figure 2 displays the incidence rate for overall CVD as a function of CIMT percentile in those with or without plaque. Similarly, in individuals both with (p<0.001) and without (p=0.022) plaque, there was a significantly higher incidence rate for CVD among participants in the >75th percentile for CIMT versus those in the <25th percentile for CIMT.
Figure 1.
Incidence rate of stroke as a function of CIMT (%tile) and plaque
Figure 2.
Incidence rate of CVD as a function of CIMT (%tile) and plaque
CIMT categories and plaque categories were both associated with incident CVD and incident stroke within 10 years when added individually to the baseline model (all p<0.001, data not shown). Hazards ratios for all variables in our prediction models for stroke and CVD outcomes are presented in Supplemental Table 1. Analysis of the c-statistics revealed that the addition of CIMT improved the ability of the base FRS model to discriminate cases from non-cases of incident stroke (Harrell’s c: 0.711 versus 0.699, p=0.01), as well as overall CVD (Harrell’s c: 0.679 versus 0.669, p<0.001). There was no significant improvement by adding plaque category to the CIMT model (p =0.464 and p = 0.609, respectively).
Table 2 presents results for reclassification of 10-year stroke risk related to adding CIMT to the base model. The upper section of the table presents data in participants without incident stroke (non-cases), whereas the lower section presents data on participants with incident stroke. In those without incident stroke, there were 381 reclassified into a higher risk category and 541 reclassified into a lower risk category by adding CIMT to the base model. Subtracting 381 from 541 and dividing by the overall number of participants in this table, resulted in a calculated NRI of 0.041 for non-events (p <0.001). Similarly calculated, the NRI in the incident stroke group was 0.021 (p = 0.391) and the overall NRI for stroke was 0.062 (p=0.015). Table 3 shows an analysis for reclassification of 10-year overall CVD risk related to adding CIMT to the base model. Note that a similar NRI analysis for overall CVD revealed an overall NRI of 0.027 (p<0.001) also driven by reclassifying those without incident CVD into a lower risk category.
Table 2. Reclassification of 10-year risk by adding CIMT to FRS-type model.
| 10-year risk in model with standardized CIMT in participants without incident stroke (non-cases) |
||||
|---|---|---|---|---|
|
| ||||
|
10-year risk in model
without standardized CIMT |
0-5% | 5-10% | 10-20% | ≥20% |
| 0 - <5% | 307 | 19 | 0 | 0 |
| 5 - <10% | 129 | 1370 | 195 | 0 |
| 10 - <20% | 0 | 278 | 874 | 167 |
| ≥20% | 0 | 0 | 134 | 429 |
| NRI non-events=0.041 | 541 = reclassified into lower risk | 381 = reclassified into higher risk | ||
|
10-year risk in model with standardized CIMT in participants with
incident stroke (cases) |
||||
|---|---|---|---|---|
|
| ||||
|
10-year risk in model
without standardized CIMT |
0-5% | 5-10% | 10-20% | ≥20% |
| 0 - <5% | 8 | 1 | 0 | 0 |
| 5 - <10% | 4 | 84 | 23 | 0 |
| 10 - <20% | 0 | 23 | 142 | 49 |
| ≥20% | 0 | 0 | 36 | 112 |
| NRI events=0.021 | 63 = reclassified into lower risk | 73 = reclassified into higher risk | ||
Overall NRI=0.062, p=0.015
Table 3. Reclassification of 10-year CVD risk by adding CIMT to FRS-type model.
| 10-year risk in model with standardized CIMT in participants without CVD (non-cases) |
||||
|---|---|---|---|---|
|
| ||||
|
10-year risk in model
without standardized CIMT |
0-5% | 5-10% | 10-20% | ≥20% |
| 0 - <5% | 0 | 0 | 0 | 0 |
| 5 - <10% | 0 | 1 | 0 | 0 |
| 10 - <20% | 0 | 2 | 369 | 67 |
| ≥20% | 0 | 0 | 180 | 2255 |
| NRI non-events=0.040 | 182 = reclassified into lower risk | 67 = reclassified into higher risk | ||
|
10-year risk in model with standardized CIMT in participants with
CVD (cases) |
||||
|---|---|---|---|---|
|
| ||||
|
10-year risk in model
without standardized CIMT |
0-5% | 5-10% | 10-20% | ≥20% |
| 0 - <5% | 0 | 0 | 0 | 0 |
| 5 - <10% | 0 | 0 | 0 | 0 |
| 10 - <20% | 0 | 1 | 52 | 13 |
| ≥20% | 0 | 0 | 31 | 1413 |
| NRI events=−0.013 | 32 = reclassified into lower risk | 13 = reclassified into higher risk | ||
Overall NRI=0.027, p<0.001
Additional NRI analyses revealed that addition of plaque category to the CIMT model did not significantly improve prediction of incident stroke or CVD (p = 0.184 and 0.307, respectively, data not shown). cNRI calculations revealed that adding CIMT to the base model was significant for stroke (cNRI=0.126, p< 0.001) and adding plaque category to the CIMT model also resulted in a significant cNRI (0.086, p<0.001) for stroke (See Supplemental Table 2). Calculations of the cNRI for overall CVD revealed that neither the addition of CIMT to the base model (cNRI=0.044, p=0.44), nor the addition of plaque category to the CIMT model (cNRI=0.013, p=0.73) resulted in a statistically significant cNRI.
In a secondary analysis, in participants with any plaque, addition of CIMT to the base model improved prediction for both stroke (NRI=0.065, p=0.013) and overall CVD events (Table 4, NRI=0.019, p<0.001). Even in participants with high-risk plaque, there was a modest incremental benefit of adding CIMT to the base model for CVD (NRI=0.014, p=0.016) and for stroke (NRI=0.058, p=0.039).
Table 4. Reclassification of 10-year CVD risk by adding CIMT in those with any plaque.
| 10-year risk in model with standardized CIMT in participants with plaque and without disease (non-cases) |
||||
|---|---|---|---|---|
|
| ||||
| 10-year risk in model with no CIMT |
0-5% | 5-10% | 10-20% | ≥20% |
| 0 - <5% | 0 | 0 | 0 | 0 |
| 5 - <10% | 0 | 0 | 0 | 0 |
| 10 - <20% | 0 | 1 | 116 | 12 |
| ≥20% | 0 | 0 | 64 | 1869 |
| NRI non-events=0.026 | 65 = reclassified into lower risk | 12 = reclassified into higher risk | ||
|
10-year risk in model with standardized CIMT in participants with
plaque and disease (cases) |
||||
|---|---|---|---|---|
|
| ||||
|
10-year risk in model with
no CIMT |
0-5% | 5-10% | 10-20% | ≥20% |
| 0 - <5% | 0 | 0 | 0 | 0 |
| 5 - <10% | 0 | 0 | 0 | 0 |
| 10 - <20% | 0 | 0 | 19 | 3 |
| ≥20% | 0 | 0 | 11 | 1233 |
| NRI events =−0.006 | 11 = reclassified into lower risk | 3 = reclassified into higher risk | ||
Overall NRI=0.019, p≤0.001
Of note, among participants in the 25th-75th percentile of CIMT, 83.4% also had plaque (26.7% characterized as intermediate-risk and 56.7% characterized as high-risk), whereas in the group with >75th percentile of CIMT, 98.3% had plaque (16.7% characterized as intermediate-risk and 81.6% as high-risk). In a sensitivity analysis, the addition of plaque categories to the base model added modest incremental benefit for classification of overall CVD events (NRI=0.022, p=0.005) but not stroke (NRI=0.022, p=0.25). Furthermore, adding CIMT to a plaque categories plus base model did not significantly improve the prediction of overall CVD (NRI=0.011, p=0.088), but did provide incremental value for the prediction of stroke (NRI=0.061, p=0.005). Of note, the addition of CIMT to a plaque categories plus base model improved the risk reclassification of those without incident stroke (NRI=0.021, p=0.002) or incident CVD (NRI=0.018, p< 0.001) by reclassifying them into a lower risk category.
In additional sensitivity analyses (Supplemental Table 3), the NRI statistics were similar to our main findings when those with prevalent PAD were excluded, when those taking lipid medications were excluded, or when the stroke outcome was limited to ischemic stroke. Specifically, after excluding those with PAD at baseline (n=456), the NRI for stroke when CIMT was added to the base model was 0.070, p=0.015 and the NRI comparing the full model to the CIMT model was 0.037, p = 0.039. For CVD, the NRI when CIMT was added to the base model was 0.026, p<0.001 and the NRI comparing the full model to the CIMT model was 0.003, p =0.591. After excluding those taking lipid medications (n=197) at baseline, the NRI when CIMT was added to the base model was significant (NRI = 0.076, p = 0.005) and the NRI comparing the full model to the CIMT model was non-significant (0.031, p = 0.057). The findings for the CVD outcome were similar. Finally, when restricting the stroke outcome to ischemic stroke (nevent=397), the NRI for stroke when CIMT was added to the base model was significant (NRI = 0.077, p = 0.007) and the NRI comparing the full model to the CIMT model was non-significant (0.028, p = 0.097).
The Hosmer-Lemeshow test was significant for the base model (p=0.003 ), the CIMT model (p=0.002 ), and the full model (p<0.001 ) for the prediction of stroke. In contrast, for the prediction of overall CVD, the Hosmer-Lemeshow test was only significant (p=0.026 ) for the base model.
Discussion
Our study has shown that in older adults, addition of ultrasound measurements of CIMT modestly improves 10-year risk prediction for stroke and CVD beyond the predictive ability of a traditional FRS-type risk model. This improvement is mainly the result of net improvement in down-classifying risk in participants who did not experience incident stroke or CVD. In our elderly cohort, addition of plaque category to CIMT provided no incremental benefit in risk prediction or reclassification in the overall cohort. In participants with any plaque, addition of CIMT modestly improved risk prediction and reclassification. This was true despite the high prevalence of intermediate- and high-risk plaque in our participants in the 25th-75th percentile and >75th percentile CIMT groups. The results remained substantially unchanged when those with prevalent PAD, taking lipid medication or with non-ischemic stroke at baseline were excluded. In the clinically important intermediate FRS risk subgroup, our cNRI findings suggest that the addition of CIMT to the base model, as well as the addition of plaque (categories) to the CIMT model, adds predictive value for stroke but not overall CVD. The assessment of plaque in our study was qualitative and therefore similar to the general approach in use clinically. Our findings suggest that even in the clinical setting, it is worth measuring CIMT with or without plaque assessment—mostly to prevent over-classification (estimation) of risk. A recently-published ACC/AHA guideline on the assessment of cardiovascular risk classified the use of CIMT risk assessment with a Level III—i.e., no benefit—and concluded that CIMT was not recommended for routine measurement in clinical practice for risk assessment for first CVD event (21). Consequently, we felt that it was important in the CHS cohort to evaluate whether the addition of carotid plaque categories to a model that included the FRS-type risk factors plus CIMT added predictive value.
The current findings differ in magnitude from those previously reported in the middle-aged participants of the Atherosclerosis Risk in Communities (ARIC) Study. Nambi, et al. reported that the addition of CIMT and plaque was associated with an overall reclassification of 23% of eligible subjects (NRI=0.127 or 12.7% and cNRI=0.189 or 18.9% in men; NRI=0.077 or 7.7% and cNRI=0.212 or 21.2% in women) over and above a traditional risk factor model. The CHS cohort was, on average, 18 years older than the ARIC cohort (mean age 54.0 ± 5.8) (5). One might conjecture that this older CHS cohort underwent a certain selection relative to the younger ARIC cohort in that CHS participants had lived, on average, 18 years longer without experiencing stroke or other incident CVD events. In addition, although we know that aging is associated with progressive accumulation of atherosclerotic lesions in the carotid arteries (22), it is unclear how the difference in age might have affected the relative contribution of traditional risk factors versus CIMT or plaque in terms of predicting a future stroke or CVD event. Of interest, in the Multi-Ethnic Study of Atherosclerosis (MESA), in which the mean participant age was 61.1 years, plaque, using a definition similar to the present manuscript, was associated with an increased NRI for CHD and CVD, but not stroke—as was plaque measured along the far wall of the ICA (6).
A second potentially important difference between CHS and ARIC relates to their carotid imaging protocols. In ARIC, ICA IMT was measured in one selected projection, separately for the carotid bulb and ICA. In CHS, IMT measurements were performed from one longitudinal image of the CCA and 3 longitudinal images of the ICA. The IMT values in ARIC were always measured from the far wall, whereas IMT was measured from both the near and far walls in CHS. In ARIC, the success rate for obtaining IMT measurements in either the bulb or ICA was low–whenever full models were used, more than half of the bulb/ICA measurements needed to be imputed–whereas the completeness rate for the CHS protocol was over 98% (8). Both ARIC and CHS protocols evaluated for the presence of plaque, but ARIC defined this as an IMT >1.5 mm or the presence of a protrusion even if the IMT was not measured, whereas CHS defined plaque qualitatively based on the presence of the greatest perceived protrusion of the carotid wall (specifically the IMT) in either the carotid bulb or proximal ICA.
In the Rotterdam Study, comprised of 3,580 subjects (mean age = 65 years), the addition of CIMT to Framingham risk factors resulted in an NRI of 8.2% (0.082) in older women for hard CHD and 8.0% (0.08) for stroke, but did not improve prediction of hard CHD or stroke in older men over a 10-year follow-up period (11). In the more recently-reported IMPROVE Study, comprised of 3,703 subjects (median age = 64.4 years), a number of CIMT measurements, as well as the interadventitia common carotid artery diameter (ICCAD), were associated with an NRI percent ranging from 3-12% (0.03-0.12) over and above FRS. The addition of plaque to this combination added only 1% (0.01) to the NRI—similar to the findings in the current study (12). In the Framingham Offspring Study cohort, 2,965 participants (mean age 58 years, 55% women) without a history of CVD were followed for an average of 7.2 years. NRI increased significantly (7.6% or 0.076, p<0.001) after adding ICA CIMT to the baseline FRS model, but not after addition of CCA CIMT thickness. When the presence in the ICA of plaque, defined as a CIMT >1.5 mm, was added to the FRS model, the NRI was 7.3% (0.073, p=0.01) with a c-statistic (0.762) slightly improved from the c-statistic (0.758) for the model including the FRS plus ICA CIMT (13).
In a recent individual level meta-analysis of 14 population-based cohorts, including data for 45,828 individuals who were followed for a median of 11 years, 4,007 first-time myocardial infarctions or strokes were reported (9). Absolute 10-year risks to develop a first-time myocardial infarction or stroke were estimated in a model with FRS-type risk factors and a second model in which the CCA CIMT was added. The authors found that the NRI with the addition of CCA CIMT was not significant (0.8%, or 0.008; 95% CI: 0.1%-1.6%). In those at intermediate-risk, the NRI was 3.6%, or 0.036 (95% CI: 2.7%-4.6%), with no differences between men and women. Despite the fact that in the studies included in the meta-analysis, the cardiovascular endpoint (first-time myocardial infarction or stroke) and carotid site (common carotid only, with no information on plaque) differed from those in the current study, the order of magnitude of NRI was not substantially different from the findings in the current study.
A previous report from CHS has documented the association of increased CIMT percentile with 7-year event risk and CVD survival (3). In another report from CHS, participants in the lowest quintile for CIMT demonstrated a 95% cumulative survival free of myocardial infarction or stroke events compared with a 74% event-free survival for those in the highest quintile of CIMT (14). In addition, in a previous analysis of 5,711 participants free of baseline stroke who were followed for a median of 6.3 years, CIMT was statistically significant when added to a stroke-prediction model that included age, systolic blood pressure, diabetes, ECG diagnosis of atrial fibrillation or left ventricular hypertrophy, confirmed history of CVD, time to walk 15 feet, and serum creatinine. However, CIMT did not significantly improve this model for predicting stroke, so it was not included in the final 5-year risk prediction model (23). This previous CHS report differs from the currently presented analysis in that it: (1) included baseline participants free of baseline stroke, but not myocardial infarction or HF; (2) established a risk prediction model for 5-year risk rather than 10-year risk; and (3) included different non-carotid variables in the prediction models (23).
Previous cross-sectional analyses from CHS have also demonstrated that a history of TIA was more likely when hyperechoic, heterogeneous, and irregular plaques were seen in the carotid arteries. Furthermore, the presence and prevalence of hyperechoic, heterogeneous, and irregular lesions were also associated with an increased degree of ICA stenosis based on analyses of carotid Doppler flow velocity recordings (2). Of interest, in the current longitudinal analyses reported from the same cohort, hypoechoicity (i.e., echolucency)—rather than hyperechoicity—appeared more characteristic of high-risk plaques. Although the reason for this difference is unknown, it may well be due to differences in the way that the high-risk plaque category was defined in these analyses. In the current paper, the definition of “high-risk” plaque included—in addition to echodensity/echolucency (echogenicity)—surface characteristics and texture as opposed to the earlier publication in which only echodensity was evaluated. In the current study, only 57% who were characterized as having high-risk plaque exhibited echolucent (i.e., hypoechoic) plaques. As such, the interaction between plaque size, plaque echodensity, texture, and plaque surface characteristics likely contributed to the difference in relationship of events (e.g., TIA and stroke) to echodensity in these two publications.
Strengths of the current study include the large well-characterized elderly cohort and the 10-year follow-up. Furthermore, there were few missing data for either the carotid ultrasound or other variables used in these analyses. Limitations to be considered include the fact that there is no currently standardized methodology for recording carotid ultrasound measurements, as evidenced by the different approaches reported in the various published center population studies and clinical trials. For example, the Rotterdam Study and Kuopio Ischemic Heart Disease Study recorded measurements from the CCA only, whereas ARIC, IMPROVE, and CHS included both CCA and ICA measurements (5,9,11,12,14-16,23,24). Furthermore, we did not gate our carotid measurements to the electrocardiogram. It is unknown what effect, if any, differences in recording and reading protocols may have had on the published results. Moreover, most of our subjects (being of older age) with significant CIMT also had plaque; this may have limited our ability to detect additional predictive value from plaque over CIMT in our study. However, we found CIMT significantly added to the plaque model for predicting stroke; CIMT also added to the base model among participants with (high-risk) plaque. The older age of our cohort was also accompanied by few people being classified into the lower risk categories—e.g., none were classified into the 0-5% risk category for CVD—and a greater number of people not surviving to 10 years than would be the case in younger cohorts. Also, while our results are relevant to classification by 10-year (shorter-term) risk, results could differ if our data were stratified based on longer-term or life-time risk where more persons would be classified as high risk, so fewer would have the opportunity to have their risk upgraded and more would likely have their risk downgraded. Marma, et al. have suggested that 56% of United States adults at low-risk in 10 years would get reclassified to high lifetime risk (25). However, in our CHS cohort, with a mean (± SD) age of 72 years (±5 years) at baseline, it is likely that the 10-year risk would approach the lifetime risk. Another limitation of our analysis was that our models lacked goodness-of-fit for predicting stroke in our population. In contrast, adding CIMT and plaque to the base FRS-type risk model improved the fit for predicting CVD. Potential reasons for the lack of goodness-of-fit for the models for stroke include the fact that the base FRS-type model was developed in a younger population, which we tried to overcome by using CHS-specific coefficients and the fact that many participants died before they experienced another adverse CVD event due to common risk factors for death and CVD.
Finally, it is important to note that the recent publication of the American College of Cardiology/American Heart Association Guidelines on the Assessment of Cardiovascular Risk and on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults encourages the use of new Pooled Cohort risk equations to estimate 10-year CVD risk for nonfatal myocardial infarction, CHD death, and nonfatal and fatal stroke and help guide the clinical decision regarding initiating statins—without the necessity of adding carotid ultrasound measurements to traditional FRS-type risk factors (21,26). Nonetheless, our study has shown that in an elderly cohort, addition of ultrasound CIMT modestly improves 10-year risk prediction for stroke and CVD beyond the capability of a FRS-type risk factor model—mainly by down-classifying risk estimates for those not ultimately experiencing incident stroke or CVD. Addition of plaque category information to CIMT appears to provide no incremental benefit in the overall cohort, but does add predictive value for stroke in intermediate FRS-risk elderly individuals.
Supplementary Material
Acknowledgement
Funding: This research was supported by contracts HHSN268201200036C, N01HC85239, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
We would like to thank Danielle Rivas for her expert assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
These data were presented in part at the American Heart Association Annual Scientific Sessions, Los Angeles, CA, November 2012.
References
- 1.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 2.Polak JF, O’Leary DH, Kronmal RA, Wolfson SK, Bond MG, Tracy RP, et al. Sonographic evaluation of carotid artery atherosclerosis in the elderly: relationship of disease severity to stroke and transient ischemic attack. Radiology. 1993;188:363–370. doi: 10.1148/radiology.188.2.8327679. [DOI] [PubMed] [Google Scholar]
- 3.O’Leary DH, Polak JF, Wolfson SK, Jr, Bond MG, Bommer W, Sheth S, et al. CHS Collaborative Research Group Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 4.O’Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, et al. Cardiovascular Health Study Collaborative Research Group Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Stroke. 1996;27:224–231. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 5.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nambi V, Chambless L, He M, Folsom AR, Mosley T, Boerwinkle E, et al. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J. 2012;33:183–90. doi: 10.1093/eurheartj/ehr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, et al. The CHS Collaborative Research Group Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. Stroke. 1992;23:1752–60. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 9.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379:2053–62. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elias-Smale SE, Kavousi M, Verwoert GC, Koller MT, Steyerberg EW, Mattace-Raso FU, et al. Common carotid intima-media thickness in cardiovascular risk stratification of older people: the Rotterdam Study. Eur J Prev Cardiol. 2011;19:698–705. doi: 10.1177/1741826711414623. [DOI] [PubMed] [Google Scholar]
- 12.Baldassarre D, Hamsten A, Veglia F, de Faire U, Humphries SE, Smit AJ, et al. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population) study. J Am Coll Cardiol. 2012;60:1489–99. doi: 10.1016/j.jacc.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Polak JF, Pencina MD, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB., Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–21. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr., Cardiovascular Health Study Collaborative Research Group Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 15.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 16.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Duller LH, Crowley PM, et al. The Cardiovascular Health Study Surveillance and ascertainment of cardiovascular events. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 19.May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998;4:109–120. doi: 10.1023/a:1009612305785. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 21.Goff DC, Jr, Lloyd-Jones DM, Bennett G, O’Donnell CJ, Coady S, Robinson J, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solberg LA, Eggen DA. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation. 1971;43:711–724. doi: 10.1161/01.cir.43.5.711. [DOI] [PubMed] [Google Scholar]
- 23.Lumley T, Kronmal RA, Cushman M, Manolio TA, Goldstein S. A stroke prediction score in the elderly: validation and Web-based application. J Clin Epidemiol. 2002;55:129–136. doi: 10.1016/s0895-4356(01)00434-6. [DOI] [PubMed] [Google Scholar]
- 24.Salonen JT, Salonen R. Arterial wall thickness, carotid atherosclerosis and the risk of myocardial infarction and cerebrovascular stroke. In: Touboul PJ, Crouse JR III, editors. Intima-media thickness and atherosclerosis: predicting the risk? Parthenon Publishing Group; New York: 1997. pp. 97–104. [Google Scholar]
- 25.Marma AK, Berry JD, Ning H, Persell SD, Lloyd-Jones DM. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes. 2010;3:8–14. doi: 10.1161/CIRCOUTCOMES.109.869727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Lloyd-Jones DM, Blum CB, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.