Abstract
Fragile X syndrome is the world’s most common hereditary cause of developmental delay in males and is now well characterized at the biological, brain and cognitive levels. The disorder is caused by the silencing of a single gene on the X chromosome, the FMR1 gene. The premutation (carrier) status, however, is less well documented but has an emerging literature that highlights a more subtle profile of executive cognitive deficiencies that mirror those reported in fully affected males. Rarely, however, has the issue of age-related declines in cognitive performance in premutation males been addressed. In the present study we focus specifically on the cognitive domain of working memory and its sub-components (verbal, spatial and central executive memory) and explore performance across a broad sample of premutation males aged 18–69 years matched on age and IQ to unaffected comparison males. We further tease apart the premutation status into those males with symptoms of the newly identified neurodegenerative disorder, the fragile X-associated tremor/ataxia syndrome (FXTAS) and those males currently symptom-free. Our findings indicate a specific vulnerability in premutation males on tasks that require simultaneous manipulation and storage of new information, so-called executive control of memory. Furthermore, this vulnerability appears to exist regardless of the presence of FXTAS symptoms. Males with FXTAS symptoms demonstrated a more general impairment encompassing phonological working memory in addition to central executive working memory. Among asymptomatic premutation males, we observed the novel finding of a relationship between increased CGG repeat size and impairment to central executive working memory.
Keywords: Fragile X syndrome, Fragile X tremor and ataxia syndrome, premutation status, working memory, central executive, phonological loop, visual-spatial sketchpad, development, aging
Introduction
Fragile X Syndrome (FXS) has been a well-recognized common genetic cause of developmental disabilities for over twenty-five years. Previously known as the Martin-Bell syndrome, FXS represents the world’s most common form of inherited intellectual disability, with most recent estimates suggesting that 1 in 2,500 are affected (Crawford et al. 2002, Hagerman, 2008, Kooy et al. 2000, Turner et al. 1996). By virtue of its single gene etiology, fragile X represents an important model for understanding the impact of early gene expression on the development and normal functioning of the central nervous system. The syndrome is caused by a defect in the fragile X mental retardation 1 gene (FMR1), located near the end of the long arm of the X chromosome. The FMR1 gene carries a CGG trinucleotide repeat in the 5′ untranslated region and abnormal expansion above a threshold of 200 CGG repeats in males is almost always associated with intellectual impairment. In unaffected individuals there are between 7 and 54 repeats, with 30 repeats found on the most common allele. In fully affected individuals the CGG regions expands to over 200 repeats resulting in the loss of the encoded protein, the Fragile X Mental Retardation Protein - FMRP. When the CGG repeats expand to between 55 and 200 an individual is referred to as carrying a “premutation”. Both males and females can be carriers of the premutation. At the molecular level, individuals with premutation alleles actually produce increased levels of FMR1 mRNA (Tassone et al. 2000a,b). These FMR1 mRNA levels range from 2 to 10 times normal levels and increase with increasing CGG repeat size over the premutation range (Tassone et al. 2000b). In the majority of individuals with the premutation, FMRP levels are within normal limits (Tassone et al. 2000a). At the cognitive level, it was initially assumed Fragile X carriers were indistinguishable from those who were unaffected. However, there is now converging evidence to suggest that there are identifiable cognitive strengths and weaknesses in this population that mirror the ‘signature’ of individuals with the full-mutation who are cognitively impaired (see Cornish et al 2008b for a review). What is especially significant about these new findings is that impairments occur in the absence of intellectual disability (Cornish et al. 2005, Cornish et al. 2008a, Grigsby et al. 2006a, Loesch et al. 2003, Moore et al. 2004). Furthermore, the recent discovery of an additional premutation ‘signature’, known as, fragile X tremor and ataxia syndrome (FXTAS) (Hagerman and Hagerman 2007, Hagerman et al. 2001, Jacquemont et al. 2003), suggests that the premutation, at least in males, may well diverge into two dissociable pathways: one that leads to FXTAS and one that results in a milder phenotype (Cornish et al. 2008a, Hay 2008). It is possible that the former effect can be related to the toxicity of increased levels of mRNA that can cause cell death and brain atrophy over time, and eventually the full clinical picture of FXTAS. The latter effect may be primarily related to the mild reduction in FMRP level associated with the premutation condition, which results in a subtle yet measurable Fragile X phenotype including inhibition and attentional problems. The specific timing of when the impact of these molecular events becomes measurable at a behavioral level is hitherto unknown.
Because of the variability in CGG repeat length in this population, it is also possible to examine correlations between repeat length and cognitive performance to infer gene-behaviour relationships. Several studies have begun to explore potential relationships between subthreshold repeat length and degree of cognitive impairment. An initial study examining cognitive performance in 14 female premutation carriers revealed that these participants had Full Scale IQs within the average range (Allingham-Hawkins et al. 1996). Further analysis of the potential correlation between CGG repeat length and measures of cognitive ability similarly indicated no significant impairment. Johnston, Eliez, Dyer-Friedman et al. (2001) also examined the relationship between IQ and expansion size in 85 female premutation carriers (Johnston et al. 2001). Consistent with the earlier study, there were no significant correlations between expansion size and IQ. In contrast, results of the proportion of fibroblasts expressing the unaffected FMR1 gene as the active form were positively correlated with Full Scale IQ. A more recent study examining both male and female premutation carriers examined IQ scores in 66 males and 217 females with a range of CGG repeat expansions (Allen et al. 2005). Only a nominal amount of the variance (4%) in Verbal IQ scores could be explained by the CGG repeat length and only in female carriers.
Rather than focusing on general measures of cognition, which may mask domain-specific impairments, other studies have examined specific cognitive functions that are known to be impaired in individuals with the full mutation. These include: cognitive inattention, visual-spatial processing, social cognition and executive cognitive functioning (e.g. Cornish et al. 2005, Cornish et al. 2008a, Grigsby et al 2008). For example, Cornish et al., (2005) investigated aspects of social cognition in premutation male carriers and found the performance of premutation males compared to comparison males matched on chronological age and IQ was significantly poorer on measures that required recognition of complex emotions. This relative impairment was found to be over and above that which might be anticipated on the basis of IQ scores. However, the authors found no correlation between CGG repeat length and social cognitive measures. The one cognitive domain that does appear to show sensitivity to CGG repeat length is the domain of inhibition where larger repeat sizes correlate with greater impairment (Cornish et al. 2008a). The authors speculated that increasingly large mRNA transcripts of the FMR1 gene may damage highly susceptible neural networks, in particular, those associated with the right inferior frontal cortex. Intriguingly, this study was also the first to demonstrate an important trajectory of cognitive deficit in premutation carrier males that appears to begin early in adulthood and become progressively more severe across the lifespan. Furthermore, when one differentiates premutation males with and without FXTAS symptoms the inhibition deficit is more pronounced in those males with FXTAS symptoms suggesting that the disruption of inhibitory control may serve as a useful neurological soft sign preceding more generalized and profound effects associated with FXTAS (i.e., brain atrophy, ataxia, peripheral neuropathy, progressive intention tremor, dementia) reported in older patients (>50 years) (Hagerman and Hagerman 2004, Jacquemont et al. 2007, Jacquemont et al 2004).
In sum, whereas there is little evidence to suggest that measures of genetic severity correlate with general IQ scores, when specific domains of cognition are evaluated, emerging evidence demonstrates a relationship between severity of performance and CGG repeat length that is domain specific. These findings highlight the importance of selecting cognitive measures that tap known weaknesses in fragile X syndrome when studying genetic-neurocognitive relationships. The relatively high prevalence of the fragile X premutation in the general population, estimated to be 1 in 259 females (Rousseau et al. 1995) and 1 in 813 males (Dombrowski et al. 2002), highlights the necessity of investigating the effect of premutation involvement on cognitive development and functioning.
Present study
In the present study, we focus on the cognitive domain of working memory as described by Baddeley (1986). Working memory involves the temporary storage of information in mind or ‘online’, while processing the same or other information. Baddeley’s model of working memory comprises three main components: the central executive, the phonological loop, and the visual-spatial sketchpad. More recently an episodic buffer has been added to the model (Baddeley 2000). The central executive controls information performing complex mental operations such as planning, manipulation, and organization. It is a limited capacity system involved in regulatory control of working memory. Two ‘slave systems’, the phonological loop and the visuo-spatial sketchpad are limited-capacity, material specific stores that are involved in the maintenance of verbal and visuo-spatial materials, respectively. Both stores are subject to rapid decay. At the brain level, an increasing body of neuroimaging findings indicates dissociations between working memory tasks that employ maintenance (corresponding to the phonological and visuo-spatial components) and those that employ manipulation (corresponding to the central executive component). In the former, a network of parietal, dorsal premotor and the ventral lateral frontal regions, lateralized to the left for verbal tasks and to the right for spatial tasks, have consistently been implicated (Wager and Smith, 2003; Henson, Burgess and Frith 2000). In the latter, the dorsal and ventral lateral prefrontal cortex, anterior prefrontal cortex, the bilateral premotor, and the lateral and medial superior parietal cortices have been implicated (Wager and Smith, 2003; D’Esposito, Postle et al 1999).
Disruptions to one or more of these working memory components has been extensively documented across a wide range of neurodevelopmental disorders including Down syndrome (Brock and Jarrold 2005, Jarrold et al. 1999, Vicari et al. 2006), William syndrome (Devenny et al. 2004, Vicari et al. 2003), and fragile X syndrome (Cornish et al. 2001, Hooper et al. 2008, Jakala et al. 1997, Lanfranchi et al. 2008, Munir et al. 2000). Together, the findings highlight the critical importance of teasing apart subcomponents of the working memory domain to identify syndrome-specific signatures and trajectories. In the case of the FMR1 full mutation, at first glance all components of working memory appear to be significantly impaired especially in males (Munir et al. 2000). However, finer-tuned assessment reveals a specific deficit on measures that require executive capacity rather than a specific sub-domain such as phonological or visual-spatial memory. This range of the impairment also appears to be dependent on whether the information to be retrieved is abstract or meaningful (Munir et al 2000a) and the degree of working memory control required (Lanfranchi et al. 2008). Furthermore, working memory deficits in FXS remain constant throughout the lifespan (Cornish et al. 2001, Hooper et al. 2008, Jakala et al. 1997). In the present study, we therefore attempt to investigate the nature and severity, if any, of working memory impairments in premutation adult carriers of the fragile X syndrome. In the context of our previous finding of an inhibitory deficit associated with the premutation status (Cornish et al. 2008a), we hypothesize that premutation males will exhibit a similar albeit more subtle phenotype than full mutation males who demonstrate severe central executive dysfunction in addition to mental retardation. Importantly, we expect premutation males to be disproportionably more impaired than comparison participants on tasks that tap the central executive capacity but not on other components of working memory. We also predict that this impairment will become progressively more severe with age. Finally, we predict that there will be a disproportionate deterioration in central executive functioning in those carrier males who display FXTAS compared to those who are asymptomatic.
Methods
Study Population
The study enrolled a total of 107 participants, of whom 40 were premutation males (age range 18 – 69 years, mean age = 46.88 years, SD = 14.50 years). See Table 1 for a summary of the CGG repeat length distribution and descriptive statistics. The comparison group comprised 67 adult males with normal FMR1 alleles and were matched individually on the basis of age to the premutation group (age range 20 – 69 years, mean age = 45.33 years, SD = 14.87 years). Recruitment was conducted through the UK Clinical Genetics Services and the UK Fragile X Society. The groups did not differ on measures of socio-economic or occupational status. The entire sample was Caucasian (indigenous white British). Ethics approval to conduct the study was obtained from regional and local ethics committees across the United Kingdom. All participants were tested individually on the Wechsler Abbreviated Scale of Intelligence (Wechsler 1999). This test provides a composite IQ score based on four subtests that tap both verbal and performance domains. Although the comparison group attained higher mean scores on the IQ measures, these did not reach statistical significance (p = 0.12, premutation group: mean full scale IQ 103.8, verbal IQ 101.0, performance IQ 105.6; comparison group: mean full scale IQ 110.5, verbal IQ 107.5, performance IQ 109.9).
Table 1.
CGG repeat distribution and descriptive statistics for the premutation (PM) sample
| A) | CGG repeat length range | # of participants |
|---|---|---|
| 55 – 70 | 9 | |
| 71 – 100 | 10 | |
| 101 – 130 | 15 | |
| 131+ | 6 |
| B) | All PM men (n = 40) |
PM without FXTAS (n = 34)
|
PM with probable FXTAS (n = 6)
|
|
|---|---|---|---|---|
| Mean (SD) | 100.33 (29.49) | 99.53 (28.56) | 104.83 (37.02) | |
| Range | 55 – 161 | 55 – 161 | 63 – 160 |
FXTAS – Fragile X Tremor and Ataxia Syndrome
Fragile X DNA testing
Direct PCR was carried out using the following primer set: forward: 5′CACGACGTTGTAAAACGACACGGAGGCGCCGCTGCCAGG3′, reverse: 5′GAGAGGTGGGCTGCGGGCGCT3′, modified from Wang, Green, Bobrow, and Mathew (1995) at 0.5 pmol final concentration. Conditions were as follows: Final concentration 1mM MgCl2, dATP, dCTP & dTTP at 0.2 mM, 7-deazaGTP (AmershamPhamaciaBiotech Piscataway, NJ) at 0.4 mM supplemented with 5% DMSO in a total volume of 20 μl (Wang et al. 1995). Cycling conditions were 32 cycles at 67°C annealing. Products were separated on PAGE gels and visualized by silver staining according to standard protocols. Where a premutation expansion was visible on the PAGE gel, the repeat size was calculated according to size markers and by electropheresing the products in size order and aligning the stutter bands. Southern blotting was carried out according to standard protocols on genomic DNA using a double digest of EcoR1 (NEB) and the methylation sensitive enzyme Eag1 (NEB) and probed with Ox1.9 (Knight et al. 1993).
Sizing was determined relative to a female control of known repeat size. Where possible, repeat sizes derived from single bands (SB) were compared to those obtained from direct PCR. Repeat sizes of those individuals who gave a result on direct PCR and on SB were congruent. Blots were over-exposed to detect any evidence of mosaicism against a known mosaic control. A premutation is defined here as an allele ranging in size from 55 CGG repeats up to approximately 200 repeats without any evidence of abnormal methylation. Mosaicism was considered present when there was evidence of a methylated cell line as well as an unmethylated premutation cell line.
Working Memory measures
Several cognitive measures were chosen to provide a comprehensive description of working memory, including its constituent components of central executive, phonological loop, and visuospatial sketchpad (Baddeley, 1986). Thus, the tasks were divided into three categories. The first category consisted of the Adult Complete Nonsense Repetition task (Gathercole, personal communication) and the Forward Digit Span task (Wechsler 1997), which is considered to tap the phonological loop; the second category consisted of the Dot Test of Visuospatial Working Memory (Bollini, Arnold et al., 2000) and the Spatial Span Forward task (Wechsler, 1997) which is considered to tap the visual-spatial sketchpad; and the third category consisted of the Backward Digit Span task (Wechsler 1997) and the Letter Number Sequencing test (Wechsler 1997), which are considered to tap central executive resources.
Phonological Loop
-
1
Complete Nonsense Repetition task (CNREP). This task requires participants to repeat single, unfamiliar nonsense words heard from an audiotape recording. These words are made up of either, 2, 3, 4 or 5 syllables. The response is the correct number of syllables recited. The total number of correct word repetitions comprised the total raw score.
-
2
Digit Span Forward task. This task required participants to recall increasingly longer sequences of digits that were orally presented at the rate of one per second. The task began with a two-digit sequence and, dependent on participant performance, continued until a nine-digit sequence was presented. Two trials of each sequence length were administered and the task was discontinued when participants made two consecutive failures on both trials of a given digit length. The highest number of correctly recalled digit sequences was calculated as the total forward digit span score.
Visual-Spatial Sketchpad
-
3
Spatial Span Forward task. This task utilized a spatial span board, where 10 blue blocks are situated on a 275mm × 210mm board. Participants are required to repeat spatial-temporal sequences performed by the examiner. The sequences are a series of taps on 10 identical blocks laid out in two dimensions. The number of blocks tapped increases by one until the participant fails two consecutive trials with the same number of block sequences. The highest number of correctly recalled block sequences was calculated as the total forward spatial span score.
-
4
The Dot Test. Participants were presented with a card in which a dot was present at a specific location. Following a 10-second interval, the stimulus card was removed, and the participant was asked to reproduce the dot at the same location on a blank card. The total score as calculated as the number of correct trials.
Central executive
-
5
Spatial Span Forward task. Utilizing the same spatial span board sequences of blocks, the participant was required to repeat the sequence of blocks tapped by the examiner in reverse order. As above, the task was discontinued when the participant made two incorrect responses on both items of an identical sequence of blocks. The highest number of correctly recalled block sequences was calculated as the total backward spatial span score.
-
6
Digit Span Backward Task. This task was identical to the digit span forward task but the participant was required to recall the digits in reverse order from the order presented. The highest number of correctly recalled digit sequences was calculated as the total backward digit score.
-
7
Letter number sequencing. Letter-number sequences of increasing length were presented orally at a rate of one per second. Several tasks were required of the participant. First, they were asked to recall the letters and numbers as in the Digit Span Task. In addition, the participant was required to recall the numbers in ascending order followed by the letters in alphabetical order. The task began with a two-item sequence and, dependent on participant performance, continued until a nine-item sequence was presented. Two trials of each sequence length were administered and the task was discontinued when participants made two consecutive failures on both trials of a given length. The overall score for the task was the calculated by adding points awarded for repeating the letter-number sequence with additional points given for the correct ordering of the letters and numbers presented.
Statistical Analysis
Of the 107 participants recruited to our study, 40 were premutation males and 67 were comparison males. In order to obtain a single combinatory measure for each of the three working memory sub-domains: phonological, visual-spatial and executive capacity, a principle component analysis (PCA) was conducted, which produced a single summary measure for each sub-domain. Within each sub-domain all measures were standardized across all participants. Subsequently, for each sub-domain summary measure, we carried out two regression analyses. First, a standard linear regression analysis was conducted, with the sub-domain score as the dependent variable, and the premutation status (asymptomatic PM, PM with probable FXTAS, and NC), the participant’s age (Age), and the age and status interaction (Age × Status) as explanatory variables. After a full model was fitted to the data, we then employed a backward stepwise variable elimination procedure. A parsimonious model was then obtained for each working memory domain score. In order to examine the potential effect of CGG repeat length on sub-domain score, a second regression analysis was conducted. Thus, we substituted premutation status, which had been included in the first model as a binary indicator of a participant’s CGG repeat length, with the direct measure of CGG repeat length (CGG). As such, CGG and Age were included in the second analysis as explanatory variables.
Neurology Questionnaire
Participants self-reported neurological symptoms on a questionnaire derived from Jacquemont and colleagues (Jacquemont et al. 2004). The neurology questionnaire comprised 2 domains: Tremor: Questions were asked regarding the presence, characteristics, and time-of-onset of tremors; Gait and lower extremities: Questions were asked related to the onset of balance problems, recent falls, and walking distance. The questionnaire was completed over the phone or in person. For the purpose of the survey, symptoms were scored as present if noticed by the respondent, with clarification of the questions or characterization of the symptoms being provided by the interviewing physician as necessary. The participant gave the final answers. The reliability of this questionnaire was previously evaluated by comparison with blind videotape scoring of matched clinical neurological evaluations and found to be highly congruent (Jacquemont et al. 2004).
Results
Cognitive performance
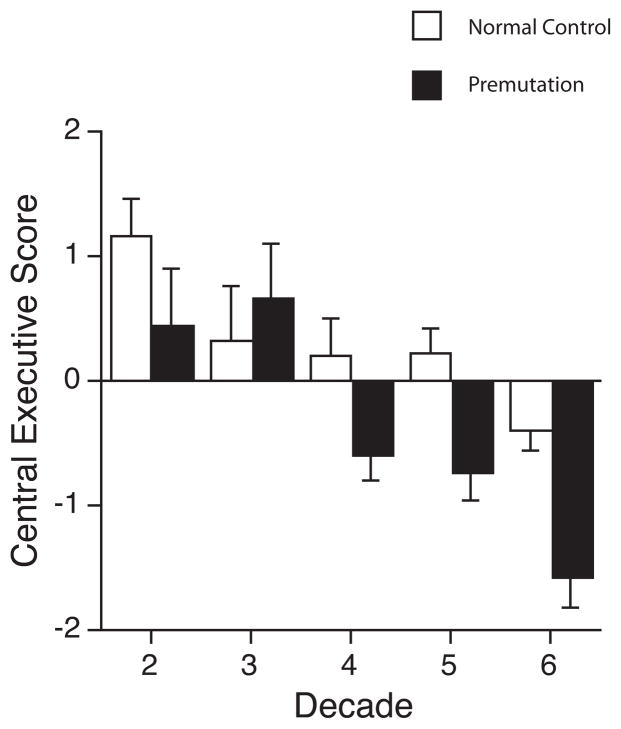
For the central executive sub-domain of working memory, there was no significant main effect of group, instead, differences in performance between the premutation and the comparison groups varied with increasing Age (significant Age X Status interaction, p = 0.0025). This pattern is clearly seen in Figure 1. Younger male premutation participants differ little from the comparison males on their performance of central executive tasks, but with increasing age the two groups’ trajectories diverge. Beginning in their 40s, male carriers follow a significantly different trajectory from comparison individuals, developing progressively more severe problems in performing tasks that tap the central executive of working memory. In order to explore the possibility that FXTAS status may have contributed significantly to the observed pattern of results, we conducted an additional analysis that separated asymptomatic PM from individuals with probable FXTAS and compared performance of these groups to the normal comparison males. Results of this analysis revealed main effects of Age and Status but with no significant interaction effect. Thus, Age and Status served as significant explanatory variables (p = 0.001 and p = 0.033 between asymptomatic PM males and NC, and p = 0.005 and p = 0.002 between probable FXTAS and NC, respectively).
Figure 1.
Age trajectories by decade across the central executive subcomponent of working memory in premutation males versus comparison normal control males. Premutation males exhibit significant impairment in cognitive control beginning in the 4th decade of life, which worsens with each subsequent decade.
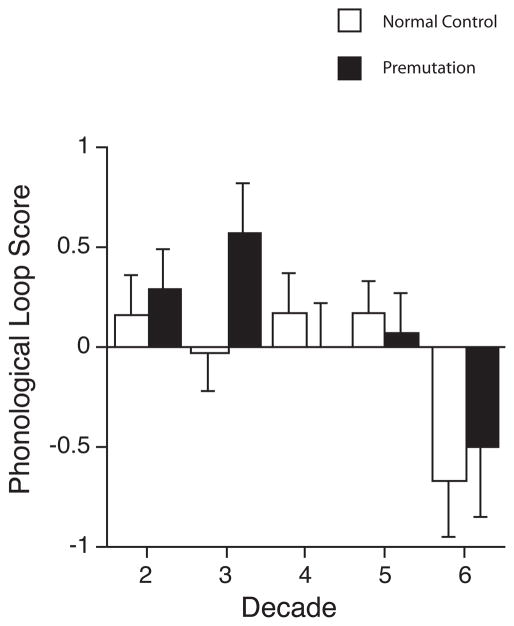
For the sub-domain scores of phonological memory and visual-spatial memory, only Age was the only significant explanatory variable in the final model (p = .044 and p < .001, respectively). In both cases, performance deteriorated significantly with increasing age for the groups (Figures 2 and 3). In order to explore the possibility that FXTAS status may have contributed significantly to the observed pattern of results, we conducted an additional analysis that separated asymptomatic PM from individuals with probable FXTAS and compared performance of these groups to the normal comparison males. For the sub-domain score of visual-spatial memory, Age was the only significant explanatory variable in the final model (p = 0.002 between asymptomatic carriers and NC and p = 0.001 between probable FXTAS and NC, respectively). For phonological memory, no variable was found significant when comparing between asymptomatic carriers and normal comparison males, while Status was found significant when comparing between probable FXTAS and normal comparison males (p = 0.012).
Figure 2.
Age trajectories by decade across the phonological loop subcomponent of working memory in premutation males versus comparison normal control males. Both groups of participants exhibit a significant decline in functioning with age.
Figure 3.
Age trajectories by decade across the visual-spatial sketchpad subcomponent of working memory in premutation males versus comparison normal control males. Both groups of participants exhibit a significant decline in functioning with age.
FXTAS symptoms and working memory performance
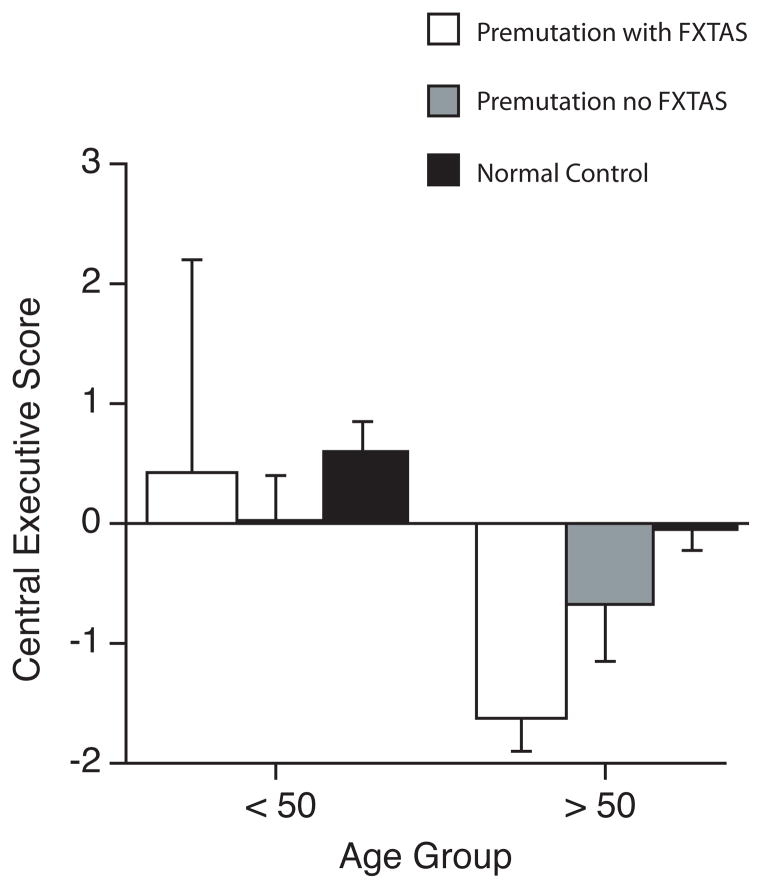
Previous studies have reported differences in cognitive performance among asymptomatic carriers and those with FXTAS (Grigsby et al. 2008, Hagerman and Hagerman 2002, Hagerman et al. 1996, Loesch et al. 2003, Mazzocco et al. 1993, Moore et al. 2004). Therefore, we conducted a secondary analysis to determine the impact of FXTAS related symptoms on performance for the central executive component of working memory. The analysis was restricted to the central executive because it was the only domain with a significant effect of Age x Status interaction. To conduct the analysis, we selected the earliest reported age of onset of diagnosable FXTAS (~ 50 years) (Greco et al. 2002, Greco et al. 2006) as a cut-off age and then compared premutation males with and without possible FXTAS to comparison males across the two age ranges (< 50 and > 50). Given the relatively small sample of premutation males under 50 years who present with FXTAS related symptoms, pair wise comparisons did not include this subgroup. For asymptomatic premutation males under 50 years of age, the central executive of working memory score was not significantly different from that of the comparison males (p = .206). An analysis of premutation males with FXTAS related symptoms over 50 years of age demonstrated that performance for tasks tapping the central executive was significantly impaired relative to comparison males (p < 0.001). In contrast, there was no significant difference between asymptomatic premutation males and individuals in the comparison group (p = .262).
Relation between CGG repeat length and working memory performance
Regression analysis of each working memory sub-domain score given CGG repeat length revealed that CGG length is significantly correlated with both the central executive (p < .001) and the visual-spatial memory (p < .001). However, when we restricted the analysis to the premutation group of participants only, CGG length only remained significantly correlated with the central executive (p = .012), but not with either phonological memory or visual-spatial memory. We explored the effects of FXTAS symptoms on the relationship between CGG repeat length and performance measures. These analyses revealed that CGG length is significantly correlated with the central executive among asymptomatic carriers (p = 0.009), but not among probable FXTAS carriers. Finally, for both PM groups, CGG repeat length is not significantly correlated with either phonological memory or visual-spatial memory.
Discussion
Our primary aim was to establish whether individuals who are carriers of the Fragile X premutation show subtle impairments in working memory as defined by a well-validated theoretical model of the construct (Baddeley, 1986). As such, we administered neuropsychological measures that the tap three core subcomponents of working memory: verbal memory (phonological loop), visual-spatial memory (visual-spatial sketchpad), and the central executive to individuals with molecular confirmation of a Fragile X premutation allele. In addition, we sought to identify the trajectory and specificity of any such working memory impairment as well as to address the extent to which a newly identified neurological disorder associated with the premutation, FXTAS, may impact on performance across the subcomponents of working memory. Four essential findings emerged from the present study. First, when individuals affected by the premutation are examined as a group, they exhibit a weakness only for tasks that reflect functioning of the central executive sub-component of working memory. However, when this group is divided into asymptomatic and FXTAS probable individuals, a slightly different pattern emerges. Ideally, a more comprehensive neurological examination beyond self-report will be needed in future studies to ensure the validity of subgroup membership. Despite this limitation, both sub-groups differ from the normal comparison males, only the individuals with probable FXTAS exhibit an additional weakness in the slave process phonological working memory. Second, when both premutation sub-groups are examined together, the extent of the central executive deficit is significantly correlated with larger CGG repeat expansions. However, when the sub-groups are analyzed separately different profiles emerge. That is, CGG repeat length is correlated with central executive performance only in asymptomatic carriers but not in those also affected by FXTAS. This finding is likely due to the small sample size of the latter group. Finally, consistent with previous reports (Brega et al. 2008, Grigsby et al. 2008, Grigsby et al. 2006a, Grigsby et al. 2007, Grigsby et al. 2006b), older individuals with FXTAS symptomatology exhibit the most severe deficits in central executive processing. Each of these novel findings is discussed in turn below.
Working memory as defined by Baddeley (1986) provides an empirically supported means of conceptualizing this cognitive psychological construct. Thus, working memory involves the temporary storage of information ‘in mind’ or ‘online’, while processing the same or other information. We administered tasks that tap the three main components articulated in Baddeley’s model and observed deficits for the premutation group in central executive functioning only. Measures reflecting the two ‘slave systems’, the phonological loop and the visual-spatial sketchpad, indicate that the material-specific subcomponents remain intact in asymptomatic premutation carriers. In those individuals with probable FXTAS an additional deficit in phonological working memory is observed. These findings highlight the necessity of the importance of teasing apart different processing demands of working memory. Furthermore, they support the idea that findings in the premutation with FXTAS are not limited to executive functioning but also involve other disturbances in cognitive function. Interestingly, Grigsby and colleagues (2006a) reported verbal fluency impairments in men with FXTAS, which is consistent with our findings of a similar impairment in the functioning of the phonological loop. Finally, these findings suggests that in order to reveal the subtle cognitive impairment in asymptomatic carrier males, one must increase the load on working memory.
Previous studies have examined a broad range of executive and working memory functions in premutation males with and without FXTAS (Bacalman et al. 2006, Bourgeois et al. 2006, Brega et al. 2008, Cornish et al. 2008a, Grigsby et al. 2008, Grigsby et al. 2006a, Grigsby et al. 2007, Grigsby et al. 2006b). Taken together, the literature suggests a pattern of impairment for individuals with FXTAS that includes deficits in self-regulation and inhibition, attentional control, working memory, and verbal fluency. For premutation males who are asymptomatic, mild executive dysregulation, inhibitory deficits that worsen with age, and declarative verbal learning and memory are notable. Relevant to the present study, closer examination of findings on working memory specifically, suggests that only premutation males with FXTAS exhibit clear deficits for tasks that tap this cognitive function. For example, Grigsby et al (2008a,b) compared individuals with and without FXTAS on measures of working memory capacity and found that those with FXTAS performed significantly worse than IQ-matched comparison males. In the same study, asymptomatic carriers did no better or worse than those with FXTAS or the IQ-matched comparison group. Our results are not consistent with this profile but rather suggest that asymptomatic carriers are at risk from developing working memory difficulties. In our recently published study of the psychiatric features associated with premutation status with and without FXTAS, the only significant difference between premutation and their unaffected male relatives was on the working memory subscale of the psychometrically sound Brown ADHD self-report measure (Kogan et al. 2008). Thus, impairment to working memory both affects performance on neuropsychological tasks and has a clinically significant impact on the daily functioning of affected individuals.
We speculate that the inconsistency between our findings of significant working memory impairment among asymptomatic carriers and previous studies that suggest that this domain is only impaired in premutation males with FXTAS can be reconciled by taking in to account the tasks employed to assess working memory. Previous reports have included composite measures of working memory that do not differentiate between the subcomponents as conceptualized by Baddeley (1986). In fact, previously administered tasks exclusively tap the auditory system (i.e., phonological loop) and do not differentiate according to the working memory load required for successful performance. In contrast, for the present study we have teased apart the different subcomponents of working memory. Indeed, great demands to working memory lead to greater discrepancies in performance between the groups tested. This confirms the importance of explicitly considering load to working memory as a key variable in future studies. This same approach has been fruitful in delineating the working memory ‘signature’ in individuals with the full mutation (Lanfranchi et al. 2008).
The detection of a deficit in the central executive component of working memory in the 4th decade of life that progressively deteriorates with age suggests a cumulative process that may be a cognitive correlate to the underlying degenerative process identified in premutation males with FXTAS. Prefrontal and parietal neural networks that subserve working memory abilities may be particularly susceptible to the degenerative process observed among some premutation males, which occurs concomitantly with accumulation of eosinophilic intranuclear inclusion bodies in neurons and astroglial cells broadly distributed throughout the CNS (Greco et al. 2002, Hagerman et al. 2003).
We report here for the first time a correlation between CGG repeats length and central executive working memory in asymptomatic premutation males. Analysis of the relationship between CGG repeat length and central executive performance in the FXTAS probable subgroup was not significant. However, our sample of six participants may not have been substantially large enough to capture a significant correlation. Future studies should explicitly examine performance on the separate components of working memory with a larger sample of participants affected by FXTAS.
The significant correlation between CGG repeat length and central executive working memory in asymptomatic carrier males suggests greater neuropathology in carrier males with larger expansions in FMR1 mRNA transcripts. It is therefore possible that expression of FMR1 mRNAs containing repeat expansions approaching the full mutation range (i.e., 200 repeats) produces exceptionally neurotoxic effects to neural circuits critical to central executive functioning, which include dorsal and ventral lateral prefrontal cortex, anterior prefrontal cortex, bilateral premotor, and the lateral and medial superior parietal cortices (Burgess et al. 2000, D’Esposito et al. 1999, Wager and Smith 2003). This result is consistent with previous research of a relationship between CGG repeat size and impairment to inhibitory control (Cornish et al. 2008a). We therefore argue for a model that recognizes two distinct yet overlapping pathways in the fragile X premutation phenotype. The first pathway is attributable to reductions in FMRP levels, which is comparable to what is observed in the full condition, but subtler in nature. These individuals may be represented by the sub-group in our sample who are asymptomatic yet continue to exhibit central executive impairments. Use of age-sensitive measures of central executive functioning will allow future research to examine younger premutation carriers in adolescence and adulthood. A second pathway is attributable to the combined effects of reduced FMRP and RNA toxicity leading ultimately to the full symptomatology of FXTAS.
Lanfranchi et al (2008) tested working memory function across the two ‘slave’ systems with tasks of demanding increasingly greater levels of cognitive control in boys with full mutation Fragile X syndrome and mental age matched typically developing children. Two results are notable. First, visual-spatial working memory deficits were not replicated as in previous research (Cornish et al. 2001, Munir et al. 2000). Rather, individuals with Fragile X Syndrome maintained deficits when compared to typically developing children on both visual-spatial and verbal working tasks that demanded the highest but not low and intermediate levels of cognitive control. The authors interpret these data as consistent with more recent studies of a central executive impairment in Fragile X (Cornish et al 2007; Scerif et al, 2004, 2007; Wilding et al, 2002). Therefore, the central executive and its associated neural networks are particularly vulnerable with alterations to FMR1 mRNA and FMRP expression levels, which appears to be consistent with the data presented here for increasingly larger CGG repeat lengths.
In conclusion, the present study adds a further piece to the puzzle of the Fragile X premutation phenotype by identifying an age-related decline in working memory functioning that is specifically vulnerable on tasks that require both manipulation and storage of new information. This decline appears to begin the early 40’s becoming more pronounced in premutation males with symptoms of FXTAS. Given the nature of these cognitive trajectories in males with the premutation status, it is becoming increasingly urgent to study the premutation trajectory in childhood through adolescence well before the documented onset of FXTAS. The latter will allow a more thorough examination for subtle indicators for cognitive decline that may mirror recently documented impairments that emerge by mid-to late adulthood.
Figure 4.
Central executive functioning is plotted for participants divided according to a cut score of age 50, which represents the mean age of onset for the fragile X tremor and ataxia syndrome (FXTAS). Data for premutation males with a diagnosis of FXTAS, premutation males without FXTAS, and comparison normal control males are illustrated. Data for those premutation individuals with a diagnosis of FXTAS who were younger than 50 at the time of testing were not included in the statistical analysis because of small sample size (n = 2). Premutation males with FXTAS demonstrate significantly worse performance than comparison normal control males. This is not the case for asymptomatic males without FXTAS, who perform as well as normal control males.
Acknowledgments
This research was supported by grants from the Wellcome Trust, Canada Research Chairs Program, NICHD, NIEHS, and NINDS. We express our thanks to the all the regional genetics centers that took part in the study and to the UK Fragile X Society for their support in recruitment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen EG, Sherman S, Abramowitz A, Leslie M, Novak G, Rusin M, Scott E, Letz R. Examination of the effect of the polymorphic CGG repeat in the FMR1 gene on cognitive performance. Behavioral Genetics. 2005;35:435–45. doi: 10.1007/s10519-005-2792-4. [DOI] [PubMed] [Google Scholar]
- Allingham-Hawkins DJ, Brown CA, Babul R, Chitayat D, Krekewich K, Humphries T, Ray PN, Teshima IE. Tissue-specific methylation differences and cognitive function in fragile X premutation females. American Journal of Medical Genetics. 1996;64:329–33. doi: 10.1002/(SICI)1096-8628(19960809)64:2<329::AID-AJMG19>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones BL, Gane LW, Grigsby J, Leehey MA, Tassone F, Hagerman RJ. Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: newly described fronto-subcortical dementia. Journal of Clininical Psychiatry. 2006;67:87–94. doi: 10.4088/jcp.v67n0112. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Science. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Bollini AM, Arnold MC, Keefe RS. Test-retest reliability of the dot test of visuospatial working memory in patients with schizophrenia and controls. Schizophrenia Research. 2000;45:169–73. doi: 10.1016/s0920-9964(99)00216-9. [DOI] [PubMed] [Google Scholar]
- Bourgeois JA, Farzin F, Brunberg JA, Tassone F, Hagerman P, Zhang L, Hessl D, Hagerman R. Dementia with mood symptoms in a fragile X premutation carrier with the fragile X-associated tremor/ataxia syndrome: clinical intervention with donepezil and venlafaxine. Journal of Neuropsychiatry and Clinical Neuroscience. 2006;18:171–7. doi: 10.1176/jnp.2006.18.2.171. [DOI] [PubMed] [Google Scholar]
- Brega AG, Goodrich G, Bennett RE, Hessl D, Engle K, Leehey MA, Bounds LS, Paulich MJ, Hagerman RJ, Hagerman PJ, Cogswell JB, Tassone F, Reynolds A, Kooken R, Kenny M, Grigsby J. The primary cognitive deficit among males with fragile X-associated tremor/ataxia syndrome (FXTAS) is a dysexecutive syndrome. Journal of Clinical and Experimental Neuropsychology. 2008:1–17. doi: 10.1080/13803390701819044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J, Jarrold C. Serial order reconstruction in Down syndrome: evidence for a selective deficit in verbal short-term memory. Journal of Child Psychology and Psychiatry. 2005;46:304–16. doi: 10.1111/j.1469-7610.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–63. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Cornish K, Kogan C, Turk J, Manly T, James N, Mills A, Dalton A. The emerging fragile X premutation phenotype: evidence from the domain of social cognition. Brain and Cognition. 2005;57:53–60. doi: 10.1016/j.bandc.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, Hagerman RJ, Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008a;44:628–36. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Hagerman R, Turk J. The Fragile X continuum: New advances and perspectives. Journal of Intellectual Disability Research. 2008b;52 (6):469–82. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Differential impact of the FMR-1 full mutation on memory and attention functioning: a neuropsychological perspective. Journal of Cognitive Neuroscience. 2001;13:144–50. doi: 10.1162/089892901564126. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, Shubek L, Holmgreen P, Yeargin-Allsopp M, Boyle C, Sherman SL. Prevalence of the fragile X syndrome in African-Americans. American Journal of Medical Genetics. 2002;110:226–33. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proceeding of the National Academy of Science U S A. 1999;96:7514–9. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenny DA, Krinsky-McHale SJ, Kittler PM, Flory M, Jenkins E, Brown WT. Age-associated memory changes in adults with williams syndrome. Developmental Neuropsychology. 2004;26:691–706. doi: 10.1207/s15326942dn2603_3. [DOI] [PubMed] [Google Scholar]
- Dombrowski C, Levesque S, Morel ML, Rouillard P, Morgan K, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Human Molecular Genetics. 2002;11:371–8. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–55. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–71. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, Cook K, Hall DA, Bounds LS, Paulich MJ, Reynolds A. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Jacquemont S, Loesch DZ, Leehey MA, Goodrich GK, Hagerman RJ, Epstein J, Wilson R, Cogswell JB, Jardini T, Tassone F, Hagerman PJ. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) Journal of the Neurological Sciences. 2006a;248:227–33. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Leehey MA, Goodrich GK, Jacquemont S, Loesch DZ, Cogswell JB, Epstein J, Wilson R, Jardini T, Gould E, Bennett RE, Hessl D, Cohen S, Cook K, Tassone F, Hagerman PJ, Hagerman RJ. Impairment of executive cognitive functioning in males with fragile X-associated tremor/ataxia syndrome. Movement Disorders. 2007;22:645–50. doi: 10.1002/mds.21359. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Leehey MA, Jacquemont S, Brunberg JA, Hagerman RJ, Wilson R, Epstein JH, Greco CM, Tassone F, Hagerman PJ. Cognitive impairment in a 65-year-old male with the fragile X-associated tremor-ataxia syndrome (FXTAS) Cognitive and Behavioral Neurology. 2006b;19:165–71. doi: 10.1097/01.wnn.0000213906.57148.01. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Greco CM, Hagerman RJ. A cerebellar tremor/ataxia syndrome among fragile X premutation carriers. Cytogenetic and Genome Research. 2003;100:206–12. doi: 10.1159/000072856. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome (FXTAS) Mental Retardation and Developmental Disabilities Res Rev. 2004;10:25–30. doi: 10.1002/mrdd.20005. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome--an older face of the fragile X gene. Nature Clinical Practice Neurology. 2007;3:107–12. doi: 10.1038/ncpneuro0373. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. Journal of Medical Genetics. 2008;45:498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ. The fragile X premutation: into the phenotypic fold. Current Opinions in Genetics and Development. 2002;12:278–83. doi: 10.1016/s0959-437x(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–30. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Staley LW, O’Conner R, Lugenbeel K, Nelson D, McLean SD, Taylor A. Learning-disabled males with a fragile X CGG expansion in the upper premutation size range. Pediatrics. 1996;97:122–6. [PubMed] [Google Scholar]
- Hay DA. Fragile X - A challenge to models of the mind and to best clinical practice. Cortex. 2008;44:626–7. doi: 10.1016/j.cortex.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hooper SR, Hatton D, Sideris J, Sullivan K, Hammer J, Schaaf J, Mirrett P, Ornstein PA, Bailey DP., Jr Executive functions in young males with fragile X syndrome in comparison to mental age-matched controls: baseline findings from a longitudinal study. Neuropsychology. 2008;22:36–47. doi: 10.1037/0894-4105.22.1.36. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Farzin F, Hall D, Leehey M, Tassone F, Gane L, Zhang L, Grigsby J, Jardini T, Lewin F, Berry-Kravis E, Hagerman PJ, Hagerman RJ. Aging in individuals with the FMR1 mutation. American Journal of Mental Retardation. 2004;109:154–64. doi: 10.1352/0895-8017(2004)109<154:AIIWTF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurology. 2007;6:45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. American Journal of Human Genetics. 2003;72:869–78. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakala P, Hanninen T, Ryynanen M, Laakso M, Partanen K, Mannermaa A, Soininen H. Fragile-X: neuropsychological test performance, CGG triplet repeat lengths, and hippocampal volumes. Journal of Clinical Investigation. 1997;100:331–8. doi: 10.1172/JCI119538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrold C, Baddeley AD, Hewes AK. Genetically dissociated components of working memory: evidence from Down’s and Williams syndrome. Neuropsychologia. 1999;37:637–51. doi: 10.1016/s0028-3932(98)00128-6. [DOI] [PubMed] [Google Scholar]
- Johnston C, Eliez S, Dyer-Friedman J, Hessl D, Glaser B, Blasey C, Taylor A, Reiss A. Neurobehavioral phenotype in carriers of the fragile X premutation. American Journal of Medical Genetics. 2001;103:314–9. [PubMed] [Google Scholar]
- Knight SJ, Flannery AV, Hirst MC, Campbell L, Christodoulou Z, Phelps SR, Pointon J, Middleton-Price HR, Barnicoat A, Pembrey ME, et al. Trinucleotide repeat amplification and hypermethylation of a CpG island in FRAXE mental retardation. Cell. 1993;74:127–34. doi: 10.1016/0092-8674(93)90300-f. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Turk J, Hagerman RJ, Cornish KM. Impact of the Fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated Tremor/Ataxia syndrome: A controlled study. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(6):859–72. doi: 10.1002/ajmg.b.30685. [DOI] [PubMed] [Google Scholar]
- Kooy RF, Willemsen R, Oostra BA. Fragile X syndrome at the turn of the century. Molecular Medicine Today. 2000;6:193–8. doi: 10.1016/s1357-4310(00)01674-9. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Cornoldi C, Drigo S, Vianello R. Working Memory In Individuals With Fragile X Syndrome. Child Neuropsychology. 2008:1–15. doi: 10.1080/09297040802112564. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Grigsby J, Butler E, Epstein J, Huggins RM, Taylor AK, Hagerman RJ. Effect of the fragile X status categories and the fragile X mental retardation protein levels on executive functioning in males and females with fragile X. Neuropsychology. 2003;17:646–57. doi: 10.1037/0894-4105.17.4.646. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Pennington BF, Hagerman RJ. The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. Journal of Developmental and Behavioral Pediatrics. 1993;14:328–35. [PubMed] [Google Scholar]
- Moore CJ, Daly EM, Schmitz N, Tassone F, Tysoe C, Hagerman RJ, Hagerman PJ, Morris RG, Murphy KC, Murphy DG. A neuropsychological investigation of male premutation carriers of fragile X syndrome. Neuropsychologia. 2004;42:1934–47. doi: 10.1016/j.neuropsychologia.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Munir F, Cornish KM, Wilding J. Nature of the working memory deficit in fragile-X syndrome. Brain and Cognition. 2000;44:387–401. doi: 10.1006/brcg.1999.1200. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K. Prevalence of carriers of premutation-size alleles of the FMRI gene--and implications for the population genetics of the fragile X syndrome. American Journal of Human Genetics. 1995;57:1006–18. [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Cornish KM, Wilding J, Driver J, Karmiloff-Smith A. Delineation of early attentional control difficulties in fragile X syndrome: Focus on neurocomputational mechanisms. Neuropsychologia. 2007;45(8):1889–98. doi: 10.1016/j.neuropsychologia.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Cornish KM, Wilding J, Driver J, Karmiloff-Smith A. Visual search in typically developing toddlers and toddlers with Fragile X or Williams syndrome. Developmental Science. 2004;7:118–130. doi: 10.1111/j.1467-7687.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMR1 gene in individuals with fragile X syndrome. American Journal of Medical Genetics. 2000a;97:195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. American Journal of Human Genetics. 2000b;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. American Journal of Medical Genetics. 1996;64:196–7. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vicari S, Bellucci S, Carlesimo GA. Visual and spatial working memory dissociation: evidence from Williams syndrome. Developmental Medicine and Child Neurology. 2003;45:269–73. doi: 10.1017/s0012162203000513. [DOI] [PubMed] [Google Scholar]
- Vicari S, Bellucci S, Carlesimo GA. Evidence from two genetic syndromes for the independence of spatial and visual working memory. Developmental Medicine and Child Neurology. 2006;48:126–31. doi: 10.1017/S0012162206000272. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affectice, and Behavioral Neuroscience. 2003;3:255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wang Q, Green E, Bobrow M, Mathew CG. A rapid, non-radioactive screening test for fragile X mutations at the FRAXA and FRAXE loci. Journal of Medical Genetics. 1995;32:170–3. doi: 10.1136/jmg.32.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale memory. 3. Psychological Press; USA: 1997. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wilding J, Cornish KM, Munir F. Further delineation of the executive deficit in males with fragile-X syndrome. Neuropsychologia. 2002;40(8):1343–1349. doi: 10.1016/s0028-3932(01)00212-3. [DOI] [PubMed] [Google Scholar]