Abstract
Influenza virus infection causes severe respiratory disease such as that due to avian influenza (H5N1). Influenza A viruses proliferate in human epithelial cells, which produce inflammatory cytokines/chemokines as a “cytokine storm” attenuated with the viral nonstructural protein 1 (NS1). Cytokine/chemokine production in A549 epithelial cells infected with influenza A/H1N1 virus (PR-8) or nonstructural protein 1 (NS1) plasmid was examined in vitro. Because tumor necrosis factor-α (TNF-α) and regulated upon activation normal T-cell expressed and secreted (RANTES) are predominantly produced from cells infected with PR-8 virus, the effects of mRNA knockdown of these cytokines were investigated. Small interfering (si)TNF-α down-regulated RANTES expression and secretion of RANTES, interleukin (IL)-8, and monocyte chemotactic protein-1 (MCP-1). In addition, siRANTES suppressed interferon (IFN)-γ expression and secretion of RANTES, IL-8, and MCP-1, suggesting that TNF-α stimulates production of RANTES, IL-8, MCP-1, and IFN-γ, and RANTES also increased IL-8, MCP-1, and IFN-γ. Furthermore, administration of TNF-α promoted increased secretion of RANTES, IL-8, and MCP-1. Administration of RANTES enhanced IL-6, IL-8, and MCP-1 production without PR-8 infection. These results strongly suggest that, as an initial step, TNF-α regulates RANTES production, followed by increase of IL-6, IL-8, and MCP-1 and IFNs concentrations. At a later stage, cells transfected with viral NS1 plasmid showed production of a large amount of IL-8 and MCP-1 in the presence of the H2O2-myeloperoxidse (MPO) system, suggesting that NS1 of PR-8 may induce a “cytokine storm” from epithelial cells in the presence of an H2O2-MPO system.
Keywords: bronchial epithelial cells, influenza viral NS1, myloperoxidase, regulated upon activation normal T-cell expressed and secreted
Influenza virus infection causes severe respiratory disease such as that caused by avian influenza virus A (H5N1), which produces a wide range of clinical manifestations, including asymptomatic infections, mild to severe respiratory disease, diarrhea, vomiting, abdominal and pleuritic pain in the early stages and severe disease with high morbidity and mortality (1). Since 2004, 10 young patients have died of A/H5N1 infection in Vietnam. Commonly occurring prognostic indicators for ARDS and death are leukocytopenia and thrombocytopenia (2, 3). Most patients with influenza virus infection of the H5N1 subtype manifest ARDS during their clinical course, often followed by deterioration and death from respiratory failure. The histopathology of these cases confirms that ARDS is a disorder of the respiratory system (4). Indeed, the serious prognosis of these patients is due to the respiratory manifestations, because influenza viruses replicate in the epithelial cells of the respiratory tract (5). On the other hand, H5N1 influenza infection also results in systemic tissue damage mediated by immunological responses, inflammatory cytokine levels in the blood increasing dramatically during severe infections (6–8). In addition, other types of influenza virus infection cause reduced production of MCP-1, RANTES, IL-8, and IP-10 in the late stages of infection. Moreover, pretreatment with TNF-α or IFN-α greatly enhances influenza-A-virus-induced chemokine production (9). Thus, TNF-α, IFN-α, MCP-1, RANTES, and IL-8 play key roles in the early stages of influenza virus infection of epithelial cells. Thus, innate immunology responses occur when influenza viruses infect epithelial cells. Many studies using PR-8 have shown that cytokines are produced from human lung tissue models in response to influenza virus PR8 (H1N1) infection. In addition, influenza A/PR/8/34 virus infection results in significant induction of genes involved in the IFN pathway (10). Moreover, PR-8 influenza virus infection in cells rapidly activates mitogen activated protein kinase signaling in vitro. When influenza A virus replicates in epithelial cells, macrophages and leukocytes respond by producing chemokines and proinflammatory cytokines. Uncontrolled viral replication and the associated “cytokine storm” of IL-6, IL-8, IP-10, MIG, and MCP-1 is responsible for this infection's serious clinical manifestations and poor outcomes (6, 11, 12). In fact, our previous study showed that IL-12p40 and TNF-R2 in plasma and IL-6R in NPA increase in ARDS patients infected with influenza virus (13). In addition, H1N1 (PR-8)-virus-infected mice show severe ARDS in pulmonary epithelial cells.
Thus, virus-infected macrophages and dendritic cells can produce significant amounts of TNF-α and type I IFNs in response to influenza A infection. These cytokines may act locally in virus-infected tissues to enhance the expression of proteins involved in virus recognition and signal transduction. The cytokine priming leads to strong virus-induced activation of transcription factors and enhanced secondary cytokine and chemokine responses in later phases of influenza A virus infection (9). Type I IFNs and inflammatory cytokine expression are attenuated with viral NS1, which is a potent virulence factor for influenza A virus (14). The NS1 protein of influenza A virus is a multifunctional protein that contributes significantly to disease pathogenesis by modulating many virus and host-cell processes (15, 16). In addition, NS1 has the ability to limit IFN-β induction by both pre-transcriptional and post-transcriptional nuclear processes (17). Recently, NS1 has been demonstrated to induce apoptosis of epithelial cells (18). Furthermore, MPO activity increases in the plasma of patients with influenza virus infection (13). Neutrophil-derived MPO in the inflammation of lung infected with influenza virus causes pulmonary pathology, in which recruitment and activation of neutrophils are associated with oxidative tissue damage (19).
In the present study, we examined the sequential order of the stream of cytokines and chemokines produced in A549 epithelial cells infected with PR-8 in vitro. In addition, we analyzed the role of neutrophil-derived MPO and NS1 of PR-8 in “cytokine storms” associated with influenza infection.
MATERIALS AND METHODS
Viruses
Human influenza A/(H1N1)PR-8 virus was originally obtained from the strain collection of the National Institute of Infectious Disease (Tokyo, Japan).
Preparation of nonstructural protein 1 plasmid
The full length of cDNA encoding NS1 of PR-8 was amplified by primers combined with EcoRI and XhoI sites. The cDNA fragment was ligated into the pCR II-TOPO vector (Invitrogen, Carlsbad, CA, USA), and DH5α-T1R Escherichia coli (Invitrogen) was transformed with the vector for subcloning. The purified plasmid was treated with EcoRI and XhoI enzymes, and ligated with pCMV-myc vector (Clontech, Palo Alto, CA, USA) treated with same enzyme pair to create the pCMV-myc-NS1 construct. The construct was amplified with DH5α-T1R E. coli, and purified using a QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) after electrophoresis.
Culture of human alveolar epithelial cell line A549
Human alveolar epithelial cell line (A549) cells were maintained in DMEM (Sigma-Aldrich, St Louis, MO, USA) containing 10% FBS (Gibco, Gaithersburg, MD, USA), 100 units/mL penicillin, and 100 units/mL streptomycin in tissue culture flasks (Corning, Cambridge, MA, USA) at 37°C in a 5% CO2 incubator.
Virus infection to A549 cells
Monolayers of A549 cells at a concentration of 1 × 105 cells/ml in 6-well plates incubated for 24 hr (1 × 106) were infected with the viruses at 1000 pfu diluted in Opti-MEM (Invitrogen). This titer as pfu of the virus was employed for optimal minimization of the concentration into the A549 cells. After 1 hr the infective solution was removed; the cells washed twice with DMEM and supplemented with a growth medium of DMEM, 5% FBS, 100 units/mL penicillin, and 100 units/mL streptomycin and incubated at 37°C in a 5% CO2 incubator.
Transfection of nonstructural protein 1 to A549 cells
A549 cells were transfected with pCMV-myc ligated with NS1 (pCMV-myc-NS1) at a concentration of 0.008 μg/mL in 3 mL of DMEM culture medium containing 1% of 100 units/mL penicillin and 100 units/mL streptomycin, and 5% FBS using lipofectamine 2000 reagent (Invitrogen). Transfection followed the procedure provided by the manufacturers: the cells were incubated for 6 hr after being washed twice with DMEM and replacement of the growth medium with DMEM, 5% FBS, 100 units/mL penicillin, and 100 units/mL streptomycin at 37°C in a 5% CO2 incubator. Production of NS1 in the cells transfected with NS1 plasmid was determined by mRNA expression.
Transfection of small interfering RNA to A549 infected with PR-8
siRNA of the following eight cytokines: siIFN-α, siIFN-β, siTNF-α, siIL-1β, siIL-6, siIL-12p40, siRANTES, and siIL-5 (Sigma Genosys siRNA Service, Sapporo, Japan) were transfected into A549 cells using lipofectamine RNAiMAX (Invitrogen). After transfection of siRNA into A549 cells (1 × 106 cells/mL) according to the standard procedure supplied by the manufacturers, they were incubated for 6 hr. The cells were then washed twice with DMEM and infected with PR-8 for 1 hr. Then the cells were washed twice with DMEM, placed in a growth medium of DMEM, 5% FBS, 100 units/mL penicillin, and 100 units/mL streptomycin, and incubated at 37°C in a 5% CO2 incubator. Cells and culture fluid were harvested at 2 and 4 days after infection.
Administration of recombinant tumor necrosis factor-α and recombinant regulated upon activation normal T-cell expressed and secreted to uninfected-A549 cells
R tumor necrosis factor-α or rRANTES at a concentration of 10 ng/mL in Opti-MEM (Invitrogen) was added to the uninfected-A549 cells at a concentration of 1 × 106 cells/mL in 6-well plates. The cells were incubated for 1 hr and washed with DMEM. After further incubation in DMEM containing 5% FBS, 100 units/mL penicillin, and 100 units/mL streptomycin for 2 days at 37°C in a 5% CO2 incubator, the culture fluid was obtained from the wells.
Administration of human myeloperoxidase to A549 cell culture infected with PR-8 or nonstructural protein 1
Human myeloperoxidase was isolated from neutrophils of volunteers as has been described elsewhere (20). After infection with PR-8 or transfection with NS1 plasmid, the A549 cells were cultured for 2 hr at 37°C in a 5% CO2 incubator, then hMPO (1 and 3 units/mL) in PBS containing 0.001% BSA (ICN Biomedicals 81-028, Aurora, OH, USA) was added to the cells with H2O2 (0.01 mM in PBS). The cells and culture fluid were harvested at 2 and 4 days after infection.
Polymerase chain reaction
Total RNA was extracted from the cells with Isogen (Nippon Gene, Toyama, Japan) and 1.0 μg of the total RNA was transcribed to first strand of cDNA using a Rever Tra Ace α-First Strand cDNA Synthesis Kit (Toyobo, Osaka, Japan) in a total of 20 μL. Using a primer set for internal and target genes, the PCR was performed in 1.5 mM MgCl2 and 0.2 pmol of primers and Taq DNA Polymerase Hot Start (Takara, Kyoto, Japan) in a total reaction volume of 20 μL. The PCR used the following protocol: denaturation at 95°C for 3 min, 95°C for 30 sec, 60°C for 1 min, and 72°C for 5 min for 25–35 cycles, and then 4°C.
Quantitative polymerase chain reaction analysis of gene expression by using SYBR Green
The differential expression data were validated by qPCR. One hundred nanograms of total RNA from control and infected A549 cells was used for qPCR analysis. All qPCR assays were performed in the same format and run on the StepOne Real-Time PCR System in a 48-well plate (ABI Life Technologies, Carlsbad, CA, USA). The reaction was performed from cDNA using the SYBR Green PCR Master Mix QuantiTect (ABI, Life Technologies) according to the manufacturer's instructions using primers (Supplemental Table 1). Reaction efficiency was calculated by using serial 10-fold dilutions of the housekeeping gene encoding GAPDH and sampled with identical cycle conditions: 95°C for 5 min, and 45 cycles of PCR at 95°C for 15 sec and 60°C for 60 sec.
Determination of concentration of cytokines and chemokines
Cytokines and chemokines in culture fluid were measured by a multiplex assay (Bio-Plex, Bio-Rad, Hercules, CA, USA) after centrifugation of culture fluid harvested from A549 cell cultures. Human RANTES Immunoassay ELISA (Quantikine, R&D Systems, Minneapolis, MN, USA) and a IL-12p40 minikit (BD OptEIA) was used.
Cell proliferation assay for survival rate
A MTT assay kit (Roche, Mannheim, Germany) was used for survival rate calculation. MTT labeling reagent (10 μL of 0.5 mg/mL) provided in the kit was added to the cultured cells in a 96-well microplate with flat-bottom wells in a total volume of 100 μL, and then incubated for 24 hr at 37°C in a 5% CO2 incubator. Solubilization solution (100 μL) was added to each well and the spectrophoto-metric absorbance of the samples was measured with a microplate reader at a wavelength of 550 nm.
Statistical analysis
Analysis of variance was used to evaluate the statistical significance of the data in the quantitative analysis of cytokines and chemokines, using Student's t-test. Differences with P values > 0.05 were considered significant.
RESULTS
Survival of A549 cells during influenza virus infection
When human A549 cells were infected with PR-8 influenza virus at 1000 pfu viral NS1 gene was expressed at 2 days post-infection, and its degree of expression was reduced at 4 days post-infection (Fig. 1a). The survival rate of the infected cells was not significantly different from that of uninfected cells (Fig. 1b). No morphological differences between infected and uninfected cells were observed at 2 and 4 days post-infection (Supplemental Fig. 1).
Fig. 1. The survival rate of A549 cells during infection with PR-8.
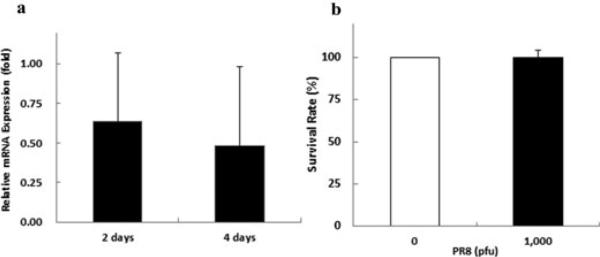
(a) Expression of viral NS1 gene in A549 cells infected with PR-8.
(b) Survival rates of infected (black bar) and uninfected (white bar) cells.
Amounts of cytokine in A549 cells after influenza virus infection
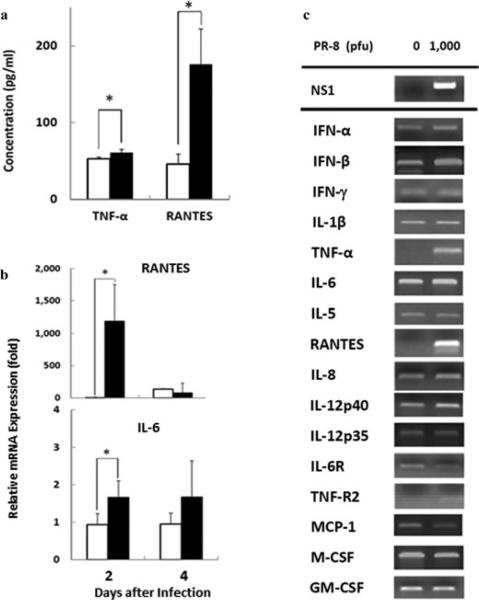
Inflammatory cytokines such as IL-12p40, TNF-R2, TNF-α, IL-6, IL-12p70, and IL-6 increase in the plasma and/or NPA in patients infected by avian (A/H5N1) influenza virus (13), and IFN-α, IFN-γ and IL-10 in patients infected with other influenza viruses (6, 21, 22). We, therefore, measured these cytokines/chemokines in the culture fluid of cells infected with PR-8 virus in vitro. Production of TNF-α and RANTES was remarkably enhanced at 2 days after infection (P = 0.01 and 0.044, respectively, Fig. 2a). Expression of RANTES and IL-6 in the A549 cells was significantly promoted by the infection at 2 days (P = 0.046 and 0.021, respectively), but not increased at 4 days (Fig. 2b). These results indicate that mRNA expression of TNF-α, RANTES, and IL-6 was predominantly produced from the A549 cells as a result of influenza virus infection. Other cytokines and chemokines, such as type I and II IFNs and IL-12p40, were slightly promoted, whereas mRNA expression of IL-1β, IL-5, IL-8, IL-12p35, IL-6R, TNF-R2, MCP-1, M-CSF, and GM-CSF was not up-regulated by the infection (Fig. 2c). We also measured amounts of cytokine and chemokine in the culture fluid of the infected cells. IL-6, IL-8, MCP-1, and MIP-1β were slightly induced, but there was no difference in the amounts of IL-1β, IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-13, IL-17, G-CSF, GM-CSF, and IFN-γ (Supplemental Fig. 2).
Fig. 2. Protein production and gene expression of cytokines and chemokines in A549 cells after infection.
(a) Concentrations of TNF-α and RANTES secreted by infected (black bars) and uninfected (white bars) cells.
(b) Quantitative analyses of expression of IL-6 and RANTES genes in infected (black bars) and uninfected (white bars) cells with PR-8 at 2 days post-infection. (c) mRNA expression of cytokine and chemokine genes at 2 days post-infection. Data are shown as mean ± SD of results from three individuals. *P < 0.05 (Student's t-test).
Knockdown of transcripts of interferon-α and -β, tumor necrosis factor-α, interleukin-1β, -6, and -5, regulated upon activation normal T-cell expressed and secreted and interleukin12p40 with small interfering RNA
To investigate further the system by which release of inflammatory mediators such as cytokines and chemokines is amplified in associated with infection, knockdown procedures using siRNA were applied. siTNF-α significantly down-regulated the expression of RANTES (P = 0.012) and slightly blocked IFN-γ expression in the infected-cells (Supplemental Fig. 3), but did not suppress expression of the other target genes, IFN-β, TNF-α, IL-6, and IL-8. siTNF-α also slightly suppressed expression of TNF-α, IFN-γ and MIP-1β in the infected cells at 4 days. Furthermore, siRANTES suppressed expression of target gene IFN-γ in the infected cells at 4 days post-infection (P = 0.033) (Supplemental Fig. 3). However, siIFN-α, siIFN-β, siIL-1β, siIL-6, and siIL-12p40 did not suppress the target genes IFN-α, IFN-β, IFN-γ, TNF-α, RANTES, IL-6, IL-8, and GM-CSF (data not shown).
As shown in Figure 3, siTNF-α suppressed secretion of RANTES, IL-8, and MCP-1 into the culture fluid of the infected cells at 2 days post-infection (P = 0.01, 0.002, and 0.00001, respectively). In addition, the siTNF-α significantly reduced the amount of RANTES, IL-8, and MCP-1 at 4 days (P = 0.03, 0.003, and 0.03, respectively). More over, siRANTES reduced secretion of RANTES, IL-8, and MCP-1 into the culture fluid of the infected cells at 2 days (P = 0.0005, 0.006, and 0.004, respectively), and then suppressed the amount of RANTES (P = 0.015) in the culture fluid at 4 days (Fig. 3). It also slightly reduced the amounts of IL-8, MCP-1, MIP-1β, and IFN-γ, but not of TNF-α (Fig. 3). We did not find any differences in amounts of other cytokines and chemokines, such as IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12p70, IL-13, IL-17, G-CSF, and GM-CSF, in the culture fluid of the cells at 2 and 4 days post-infection. These results suggest that TNF-α stimulates production of RANTES, IL-8, MCP-1, and IFN-γ, and RANTES enhances production of IL-8, MCP-1, and IFN-γ, but not of TNF-α, in A549 cells during influenza virus infection.
Fig. 3. Knockdown of expression of TNF-α and RANTES genes.
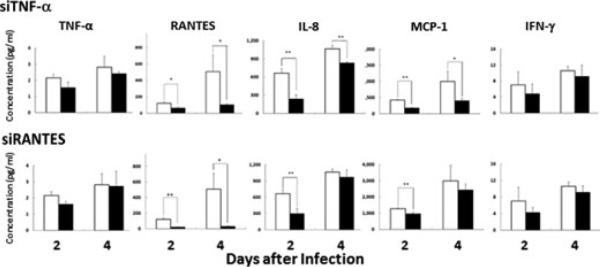
Blockade by siTNF-α and siRANTES of cytokine secretion by A549 cells transfected with si-TNF-α or siRANTES (black bars) or transfected with siGAPDH as controls (white bars). Data are shown as mean ± SD of results from three individuals. *P < 0.05; **P < 0.01 (Student's t-test).
Effect of r tumor necrosis factor-α and r regulated upon activation normal T-cell expressed and secreted on cytokine production of uninfected A549
To examine the relationship between TNF-α and RANTES in infection, we mimicked an inflammatory process in A549 cells using rTNF-α and rRANTES. Addition of rTNF-α to the culture fluid markedly increased the secretion of RANTES, IL-8, and MCP-1 from uninfected A549 cells (P = 0.000007, 0.00005, and 0.0000003, respectively), and slightly increased TNF-α and IL-6 production in the cells (Fig. 4). Moreover, administration of rRANTES also markedly enhanced IL-6, IL-8 and MCP-1 production (P = 0.02, 0.000006, and 0.000003, respectively) in the culture fluid of the A549 cells (Fig. 4). These results provide evidence that in the inflammatory process soluble TNF-α protein first stimulates RANTES production, and then the released RANTES induces production of large amounts of IL-8 and MCP-1.
Fig. 4. Stimulation of uninfected-A549 cells by rTNF-α or rRANTES protein.
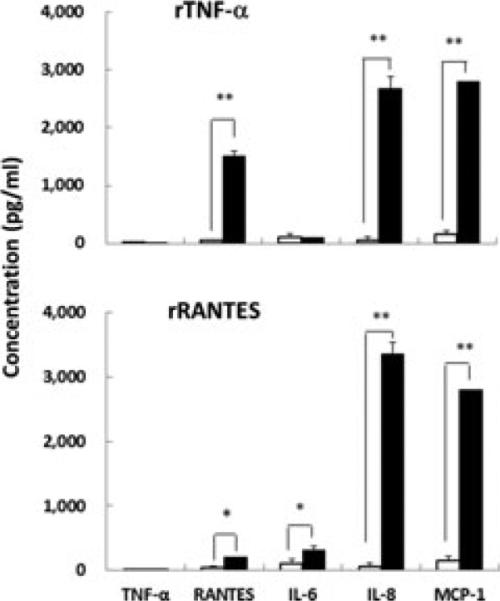
Addition of rTNF-α or rRANTES protein (black bars), and medium (white bars). Data are shown as mean ± SD of results from three individuals. *P < 0.05; **P < 0.01 (Student's t-test).
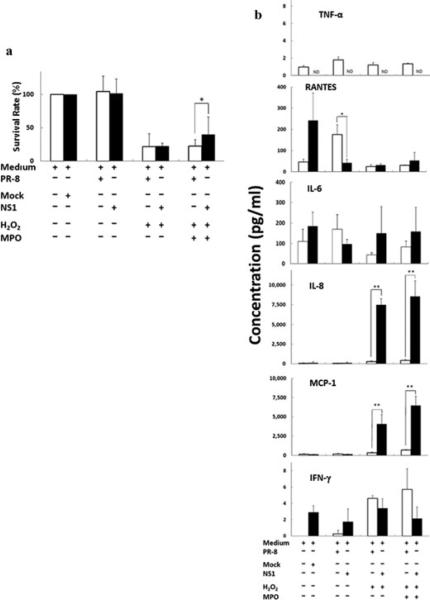
Inhibitory role of viral nonstructural protein 1 in cells damaged by H2O2-myeloperoxidase
Myeloperoxidase is a multifunctional enzyme with unique abilities that is involved in both host defense and tissue damage at inflammatory sites. MPO activity is increased in the plasma of patients infected with avian influenza viruses (13). A recent report of ARDS induced by avian influenza virus infection A suggested that there is a relationship between MPO and IL-12 (13). We therefore analyzed the role of MPO in cell damage during influenza virus infection. In addition, because it is known to be a key factor influencing the severity of inflammation and epithelial damage, we also examined the role of NS1 of influenza virus in an in vitro system. First, microscopic observations showed that almost all A549 cells were damaged at 2 days after infection (Supplemental Fig. 4). In contrast, cells transfected with NS1 plasmid showed survival advantages compared to PR-8-infected cells (Fig. 5a). In addition, administration of H2O2 and MPO under uninfected or infected conditions did not result in significant differences in damage and survival rate (Fig. 5a). However, cell damage caused by H2O2 and MPO administration (3 units/mL) was significantly diminished by transfection with NS1 plasmid (P = 0.049, Fig. 5a), indicating that NS1 significantly protects against this type of cell damage compared with that caused by PR-8 infection. We next quantitated the con centrations of cytokines and chemokines in the culture fluid of cells that had been subjected to PR-8-infection or NS1 plasmid transfection in the presence or absence of H2O2 and MPO (3 units/mL). The increased release of RANTES from cells infected with PR-8 was suppressed by NS1 transfection (Fig. 5b). In addition, production of RANTES was decreased by the addition of MPO to the PR-8-infected cells (Fig. 5b white bar), but there was no change in concentrations of IL-8 and MCP-1. Surprisingly, addition of MPO to cells transfected with NS1 markedly enhanced concentrations of IL-8 and MCP-1 compared with PR-8 infection, but did not change the amounts of RANTES and IL-6 produced (Fig. 5b). These results indicate that NS1 produces a large amount of the chemokines IL-8 and MCP-1 in association with the H2O2-MPO system.
Fig. 5. Explosion of IL-8 and MCP-1 secretion by NS1 of influenza virus collaborating with the H2O2-MPO axis.
(a) The influence of H2O2-MPO on the survival rate of A549 cells infected with PR-8 (white bars) or NS1 (black bars). (b) Cytokine production of A549 cells infected with NS1 plasmid (black bars) or PR-8 (white bars) in the presence of H2O2 and MPO. Data are shown as mean ± SD of results from three individuals. *P < 0.05; **P < 0.01 (Student's t-test).
DISCUSSION
H5N1 viruses mainly infect type II alveolar epithelial cells, causing damage to lung tissue. Histopathology of samples from patients shows diffuse alveolar damage, reflecting how severely this infection damages the respiratory system (23). H5N1 infection can rapidly proceed to serious respiratory failure with a poor lower survival rate (fulminant ARDS) (4). In addition, the concentrations of cytokines and chemokines, such as IL-12p40, TNF-R2, TNF-α, IL-6, IL-12p70, and IL-6R, are greatly increased in the plasma and NPA of ARDS patients with H5N1 infection (13). Other types of influenza viruses besides H5N1 can induce INF-α, INF-γ, and IL-10 (21, 22). H1N1 (PR-8) virus infection targets epithelial cells in mice, causing severe ARDS.
In the present study, concentrations of TNF-α and RANTES had increased significantly by 2 days after PR-8 infection. On the other hand, mRNA of TNF-α and RANTES was strongly increased in PR-8-infected cells and that of IFN-α, IFN-β, IFN-γ, and IL-12p40 slightly increased. These results confirm that influenza virus infection can induce expression of RANTES by normal human bronchial and nasal epithelial cells (24) and that H5N1 is more potent in inducing IP-10, TNF-α, and RANTES (25). IFN-α and TNF- α have a significant role in priming epithelial cells to produce more cytokines and chemokines in influenza A virus infection (9). In addition, influenza A viruses induce IL-6, IL-8, and RANTES secretion from transformed bronchial epithelial cell lines in vitro (26), and the amounts of cytokines IL-6, TNF-α, IFN-γ, IL-10, MCP-1, and MIP-1α and MIP-1β increase in nasal lavage fluid in influenza A infection (27). On the other hand, in experiments in vitro, large amounts of IL-6 and IL-8 are released by normal human bronchial epithelial cells and by a human alveolar epithelial cell line treated with swine dust, which is a strong stimulus for IL-8 production by both bronchial epithelial cells and human alveolar macrophages (28, 29). These results strongly suggest that primary influenza A virus infection results in cytokine and chemokine production in human epithelial cells and that production of these factors may have a serious impact on the establishment of inflammation and virus-specific immune responses. Cytokines TNF-α and RANTES were being secreted from human epithelial cells A549 by 2 days after infection in the present in vitro study, suggesting that, in addition to the abundant cytokine secretion by infected macrophages in the alveolar region, TNF-α and RANTES from epithelial cells play a key role in creating “cytokine storm”. Furthermore, virus-infected A549 cells express mRNA for the cytokines and chemokines TNF-α, RANTES, IFN-α, IFN-β, IFN-γ, and IL-12p40. In response to influenza virus infection, airway epithelial cells may express TNF-α, RANTES, and IFNs.
When influenza A virus infects epithelial cells, it replicates in them. Macrophages and leukocytes respond to this infection by producing chemokines and proinflammatory and other immunoregulatory cytokines, leading to inflammation and alveolar damage. siRNA-induced knockdown of strong expression of inflammatory cytokines (30) clarifies the potential for induction of inflammatory cytokines and interferon responses to influenza infection (31). siRNA provides specific and robust gene silencing that can improve our understanding of gene expression and production of cytokines and chemokines. In the present study, siTNF-α suppressed increased expression of RANTES and IFN-γ in cells and secretion of RANTES, IL-8, and MCP-1 by cells infected with PR-8. Moreover, siRANTES suppressed increased expression of IFN-γ in these cells and secretion of RANTES, IL-8, and MCP-1 by them. Furthermore, in the present study administration of rTNF-α enhanced release of RANTES, IL-8, MCP-1, and IL-6 from uninfected A549 cells. Administration of rRANTES also enhanced the secretion of IL-6, IL-8, and MCP-1 from the cells, but not of TNF-α. These results strongly suggest that TNF-α protein may be initially released from epithelial cells, then stimulate RANTES production downstream, following which released RANTES may sequentially induce the production of IL-6, IL-8, and MCP-1. Taken together, these results of studies on siRNA and administration of rTNF-α and rRANTES strongly suggest that production of RANTES may enhance stimulation of production of IL-6, IL-8, and MCP-1 in A549 cells infected with PR-8 virus. Therefore, TNF-α, MCP-1, IL-8, and RANTES are primary cytokines and chemokines of cells that are associated with the pathogenesis of ARDS and may amplify the inflammatory response.
Finally, MPO, a multifunctional enzyme that is mainly found at sites of neutrophil accumulation, is involved in tissue damage at inflammatory sites in addition to its primary role in host defense. MPO activity is increased in the plasma of patients infected with influenza virus H5N1 (13). MPO not only plays an important role in the development of lung neutrophilia but also indirectly contributes to chemokine and cytokine production that may govern inflammatory processes (13, 32). In the present study, MPO provided evidence of damage to A549 cells infected with PR-8 virus. On the other hand, NS1 of type A influenza plays a key role in the regulation of production of cytokines by epithelial cells (33). However, we observed an improvement in the survival rate of A549 cells transfected with NS1 plasmid which we attributed to recovery from damage caused by the H2O2-MPO system. Surprisingly, cells transfected with NS1 showed greatly enhanced concentrations of IL-8 and MCP-1 in the culture fluid. These results suggest that NS1 of PR-8 may cause production of the chemokines IL-8 by neutrophils and MCP-1 by macrophages transfected with NS1, strongly suggesting collaboration with the H2O2-MPO system in epithelial cells. On the other hand, NS1 protein is considered to be an IFN antagonist (33) and a multifunctional protein with three domains that have a number of regulatory functions during influenza virus infection (34). Therefore, explosion of IL-8 and MCP-1 induced by the NS1-H2O2-MPO system seems to be one of the roles of NS1 in epithelial cells.
TheH2O2-MPOsystemoccursinactivatedneutrophils, one of the first cells to immigrate to sites of inflammation. Influenza A virus infection causes acute inflammatory disease of the lung, recruiting neutrophils and macrophages in response to chemokines IL-8 and MCP-1. When activated, these cells produce degranulation in the lung tissue due to agents such as MPO. MPO enhances mRNA expression of IL-1α, IL-1β, and TNF-α (19) and may play key roles in acute lung injury, resulting in respiratory dys-function (13). In addition, reactive oxygen intermediates produced from these cells have been shown to stimulate the production of inflammatory cytokines (19). Our study shows that NS1 and H2O2-MPO stimulate chemokine production associated with inflammatory responses. In addition, in the present study we found greatly increased concentrations of IL-8 and MCP-1 in NS1-transfected cells. This finding is similar to the observation that MPO promotes the development of lung neutrophilia and indirectly influences subsequent chemokine and cytokine production by other cell types in the lung (35). Therefore, for influenza A virus the biological activities of NS1 are likely to be potent virulence factors that implicate viral presence and inhibition of immunity. Indeed, in human virus strains and primary human cells the respective truncated NS1 polypeptides of the mutant viruses are poorly expressed and only barely detectable in overexposed blots compared to the clearly visible amounts observed in TX WT-infected A549 cells (14). In addition, Schultz-Cherry et al. reported that the multimerization domain of the NS1 protein, but not the effector domain, is required for apoptosis (36). However, this mutation is not sufficient to inhibit apoptosis using whole virus. In fact, the NS1 gene shows reduced pathogenesis and protection from wild-type influenza virus infection (18, 37) if the H2O2-MPO system is not present at the influenza infection site. Taken together, these observations strongly suggest that TNF-α regulates RANTES production, which leads to increases in IL-6, IL-8, MCP-1, and IFN concentrations. Furthermore, in the presence of the H2O2-MPO system viral NS1 protein produced in the cells is associated with enhanced production of large amounts of the chemokines IL-8 by neutrophils and MCP-1 by macrophages, suggesting that NS1 of H1N1 (PR-8) influenza virus may play a key role in “cytokine storm” when the H2O2-MPO system is active. In the initial stages of influenza viral infection, RANTES secretion, which is stimulated by TNF-α, enhances induction of IFN-γ in the bronchial epithelial cells. The subsequent synthesis of NS1 protein may enhance IL-8 and MCP-1 in the presence of the H2O2-MPO system produced by activated neutrophils (Fig. 6).
Fig. 6. A scheme for sequential secretion and explosion of “cytokine storm” in bronchial epithelial cells infected with in fluenza viruses.
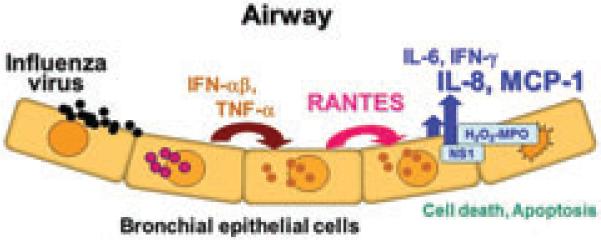
The first step is TNF-α released from bronchial epithelial cells infected with influenza virus enhances induction of RANTES secretion. In a later phase the epithelial cells associated with viral NS1 protein may enhance production of large amounts of chemokines IL-8 and MCP-1 in the presence of the H2O2-MPO system, suggesting that NS1 of PR-8 may play a key role in “cytokine storm” when the H2O2-MPO system is active.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Noriko Nakajima, National Institute of Infectious Diseases, Tokyo, Japan for her valuable discussions. This study was supported by a Research-in-Aid Grant from the Ministry of Health, Labor and Welfare of Japan (H22-S-I-014) and the RONPAKU program of the Japan Society for the Promotion of Science (VAST-10937).
List of Abbreviations
- ARDS
acute respiratory distress syndrome
- DMEM
Dulbecco's modified Eagle's minimal essential medium
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GM-CSF
granulocyte macrophage colony-stimulating factor
- IFN
interferon
- IL
interleukin
- IL-6R
IL-6-recepter
- IP-10
interferon-γ-induced protein 10
- MCP-1
monocyte chemotactic protein 1
- M-CSF
macrophage colony-stimulating factor
- MIG
monokine induced by interferon γ
- MPO
myeloperoxidase
- MTT
thiazolyl blue tetrazolium bromide
- NHP
National Hospital of Pediatrics
- NPA
nasopharyngeal aspirate
- NS1
nonstructural protein 1
- pfu
plaque-forming units
- qPCR
quantitative polymerase chain reaction
- RANTES
regulated upon activation normal T-cell expressed and secreted
- rRANTES
recombinant RANTES
- rTNF-α
recombinant TNF-α
- sIL-6R
soluble interleukin 6 receptor
- siRNA
small interfering RNA
- TNF-α
tumor necrosis factor α
- TNFR2
tumor necrosis factor 2 receptor
Footnotes
DISCLOSURE
The authors have no financial conflicts of interest.
REFERENCES
- 1.Wong SSY, Yuen K. Avian influenza virus infections in humans. Chest. 2006;129:156–68. doi: 10.1378/chest.129.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–82. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 3.Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VVC, Pham TS, Vo CD, Le TQM, Ngo TT, Dao BK, Le PP, Nguyen TT, Hoang TL, Cao VT, Le TG, Nguyen DT, Le HN, Nguyen TKT, Le HS, Le VT, Dolecek C, Tran TT, de Menno J, Schultsz C, Cheng P, Lim W, Horby P, Farrar J. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–88. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 4.Kawachi S, Luong TS, Shigematsu M, Furuya H, Phung TBT, Phan HP, Nunoi H, Nguyen TL, Suzuki K. Risk parameters of fulminant acute respiratory distress syndrome and avian influenza (H5N1) infection in Vietnamese children. J Infect Dis. 2009;200:510–15. doi: 10.1086/605034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suarez DL, Schultz-Cherry S. Immunology of avian influenza virus: a review. Dev Comp Immunol. 2000;24:269–83. doi: 10.1016/s0145-305x(99)00078-6. [DOI] [PubMed] [Google Scholar]
- 6.Jong de MD, Simmons PC, Tran TT, Vo MH, Smith JDG, Tran NBC, Dang MH, Nguyen VVC, Truong HK, Vo CD, Phan TQ, Bach VC, Do QH, Guan Y, Peiris JSM, Nguyen TC, Tran TH, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–7. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–37. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 8.Chan CWM, Chan WYR, Yu CLW, Ho CCC, Chui WH, Lo CK, Yuen MK, Guan Y, Nicholls JM, Peiris JSM. Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells. Respir Res. 2009;10:102–12. doi: 10.1186/1465-9921-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veckman V, Osterlund P, Fagerlund R, Melen K, Matikainen S, Julkunen I. TNF-α and IFN-α/β enhance influenza-A-virus-induced chemokine gene expression in human A549 lung epithelial cells. Virology. 2006;345:96–104. doi: 10.1016/j.virol.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Geiss GK, Salvatore M, Tumpey TM, Carter VS, Wang X, Basler CF, Taubenberger JK, Bumgarner RE, Palese P, Katze MG, García-Sastre A. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc Natl Acad Sci USA. 2002;99:10736–41. doi: 10.1073/pnas.112338099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beigel JH, Farrar J, Han AM, Hayden FG, Hyer CR, de Jong MD, Lochindarat S, Nguyen TKT, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen K. Avian influenza A (H5N1) infection in humans. New Engl J Med. 2005;353:1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 12.Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF, Nicholls JM, Ng TK, Chan KH, Lai ST, Lim WL, Yuen KY, Guan Y. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–19. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phung TBT, Luong TS, Kawachi S, Nunoi H, Nguyen TL, Nakayama T, Suzuki K. Interleukin 12 and myeloperoxidase (MPO) in Vietnamese children with acute respiratory distress syndrome due to Avian influenza (H5N1) infection. J Infect. 2011;62:104–08. doi: 10.1016/j.jinf.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Kester H, Svetlana B, Thomas M, Adolfo G, Ana F. The NS1 protein of a human influenza virus inhibits type I interferon production and the induction of antiviral responses in primary human dendritic and respiratory epithelial cells. J Virol. 2009;83:6849–62. doi: 10.1128/JVI.02323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krug RM, Yuan W, Noah DL, Latham AG. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology. 2003;309:181–89. doi: 10.1016/s0042-6822(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 16.Hale GB, Jackson D, Chen YH, Lamb AR, Randall ER. Influenza A virus NS1 protein binds p85 and activates phosphatidylinositol-3-kinase signaling. Proc Natl Acad Sci USA. 2006;103:14194–99. doi: 10.1073/pnas.0606109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hale GB, Randall ER, Ortin J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J Gen Virol. 2008;89:2359–76. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 18.Chuanfu Z, Yutao Y, Xiaowei Z, Xuelin L, Hongbin S, Yuxian H, Peitang H. Highly pathogenic avian influenza A virus H5N1 NS1 protein induces caspase-dependent apoptosis in human alveolar basal epithelial cells. J Virol. 2010;7:51–6. doi: 10.1186/1743-422X-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grattendick K, Stuart R, Roberts E, Lincoln J, Lefkowitz SS, Bollen A, Moguilevsky N, Friedman H, Lefkowitz LD. Alveolar macrophage activation by myeloperoxidase: a model for exacerbation of lung inflammation. Am J Respir Cell Mol Biol. 2002;26:716–22. doi: 10.1165/ajrcmb.26.6.4723. [DOI] [PubMed] [Google Scholar]
- 20.Ishida-Okawara A, Ito-Ihara T, Muso E, Ono T, Saiga K, Nemoto K, Suzuki K. Neutrophil contribution to the crescentic glomerulonephritis in SCG/Kj mice. Nephrol Dial Transplant. 2004;19:1708–15. doi: 10.1093/ndt/gfh275. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser L, Fritz SR, Straus ES, Gubareva L, Hayden GF. Symptom pathogenesis during acute influenza: Interleukin-6 and other cytokine responses. J Med Virol. 2001;64:262–8. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 22.de Maeyer E, de Maeyer-Guinard J. Interferons. In: Thomson A, editor. The Cytokine Handbook. 3rd edn. Academic Press; San Diego: 1998. pp. 491–516. [Google Scholar]
- 23.Nguyen TL, Nakajima N, Le PP, Sato Y, Hoang NT, Pham VH, Luong TS, Katano H, Kumasaka T, Oka T, Kawachi S, Matsushita T, Sata T, Kudo K, Suzuki K. H5N1-infected cells in lung with diffuse alveolar damage in exudative phase from a fatal case in Vietnam. Jpn J Infect Dis. 2008;61:157–60. [PubMed] [Google Scholar]
- 24.Matsukura S, Kokubu F, Kubo H, Tomita T, Tokunaga H, Kadokura M, Yamamoto T, Kuroiwa Y, Ohno T, Suzaki H, Adachi M. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am J Respir Cell Mol Biol. 1998;18:255–64. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- 25.Lam WY, Apple CMY, Ida MTC, Paul KSC. Profiles of cytokine and chemokine gene expression in human pulmonary epithelial cells induced by human and avian influenza viruses. J Virol. 2010;7:344–53. doi: 10.1186/1743-422X-7-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adachi M, Matsukura S, Tokunaga H, Kokubu F. Expression of cytokines on human bronchial epithelial cells induced by influenza virus A. Int Arch Allergy Immunol. 1997;113:307–11. doi: 10.1159/000237584. [DOI] [PubMed] [Google Scholar]
- 27.Fritz RS, Hayden FG, Calfee DP, Cass LMR, Peng AW, Alvord WG, Strober W, Straus SE. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. J Infect Dis. 1999;180:586–93. doi: 10.1086/314938. [DOI] [PubMed] [Google Scholar]
- 28.Palmberg L, Larsson BM, Malmberg P, Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells. Thorax. 1998;53:260–64. doi: 10.1136/thx.53.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Malmberg P, Ek A, Larsson K, Palmberg L. Swine dust induces cytokine secretion from human epithelial cells and alveolar macrophages. Clin Exp Immunol. 1999;115:6–12. doi: 10.1046/j.1365-2249.1999.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins M, Judge A, MacLachlan I. siRNA and innate immunity. Oligonucleotides. 2009;19:89–101. doi: 10.1089/oli.2009.0180. [DOI] [PubMed] [Google Scholar]
- 31.Barik S. siRNA for influenza therapy. Viruses. 2010;2:1448–57. doi: 10.3390/v2071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haegens A, Heeringa P, van Suylen RJ, Steele C, Aratani Y, O'Donoghue RJJ, Mutsaers SE, Mossman BT, Wouters EFM, Vernooy JHJ. Myeloperoxidase deficiency attenuates lipopolysaccharide-induced acute lung inflammation and subsequent cytokine and chemokine production. J Immunol. 2009;182:7990–6. doi: 10.4049/jimmunol.0800377. [DOI] [PubMed] [Google Scholar]
- 33.Newby CM, Sabin L, Pekosz A. The RNA binding domain of influenza A virus NS1 protein affects secretion of tumor necrosis factor alpha, interleukin-6, and interferon in primary murine tracheal epithelial cells. J Virol. 2007;81:9469–80. doi: 10.1128/JVI.00989-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noah DL, Twu KY, Krug RM. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 39 end processing of cellular pre-mRNAS. Virology. 2003;307:386–95. doi: 10.1016/s0042-6822(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 35.Krug RM, Yuan W, Noah DL, Latham AG. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology. 2003;309:181–9. doi: 10.1016/s0042-6822(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 36.Schultz-Cherry S, Dybdahl-Sissoko N, Neumann G, Kawaoka Y, Hinshaw VS. Influenza virus ns1 protein induces apoptosis in cultured cells. J Virol. 2001;75:7875–81. doi: 10.1128/JVI.75.17.7875-7881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyland L, Webby R, Sandbulte MR, Clarke B, Hou S. Influenza virus NS1 protein protects against lymphohematopoietic pathogenesis in an in vivo mouse model. Virology. 2006;349:156–63. doi: 10.1016/j.virol.2006.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.