Abstract
Background: Previous studies have suggested that violence victimization is prevalent among women with premenstrual syndrome (PMS). However, it is unclear whether early life abuse contributes directly to PMS or whether associations are explained by the high prevalence of PMS risk factors including smoking and obesity among women reporting childhood abuse.
Methods: We have assessed the relation of early life abuse and the incidence of moderate-to-severe PMS in a study nested within the prospective Nurses' Health Study 2. Participants were aged 27–44 years and free from PMS at baseline, including 1,018 cases developing PMS over 14 years and 2,277 comparison women experiencing minimal menstrual symptoms. History of early life emotional, physical, and sexual abuse was self-reported in 2001.
Results: After adjustment for obesity, smoking, and other factors, emotional abuse was strongly related to PMS (pTrend<0.0001); women reporting the highest level of emotional abuse had 2.6 times the risk of PMS as those reporting no emotional abuse (95% confidence interval, 1.7–3.9). Women reporting severe childhood physical abuse had an odds ratio of 2.1 (95% confidence interval, 1.5–2.9; pTrend<0.001) compared with those reporting no physical abuse. Sexual abuse was less strongly associated with risk. Adjustment for childhood social support minimally affected findings.
Conclusions: Findings from this large prospective study suggest that early life emotional and physical abuse increase the risk of PMS in the middle-to-late reproductive years. The persistence of associations after control for potential confounders and mediators supports the hypothesis that early life abuse is importantly related to PMS.
Introduction
Premenstrual syndrome (PMS), characterized by the cyclic occurrence of physical and emotional symptoms prior to menses that causes substantial impairment, is experienced by 8%–20% of reproductive aged women.1–4 The development of PMS likely involves a complex interplay of hormonal, neural, dietary and behavioral factors.5 Evidence suggests that psychosocial factors including exposure to early life emotional, physical, and sexual abuse also may contribute. Several small studies have reported that abuse history is common among women experiencing PMS or premenstrual dysphoric disorder (PMDD), a more severe form of PMS in which emotional symptoms predominate.6–13 For example, Girdler et al. reported that PMDD cases (n=28) were significantly more likely than controls to report sexual and physical abuse, as well as younger age of first abuse.7 Similarly, Perkonigg et al. found that women with PMDD were 6.7 times more likely to report childhood sexual abuse than controls (95% confidence interval [95%CI], 3.2–14.1).4
Most previous studies of abuse and PMS have been unable to control for potentially important confounders, such as smoking, alcohol use, and obesity. It thus remains unclear whether abuse contributes directly to the pathophysiology of PMS, or whether observed associations are explained largely by behavioral factors associated with risk of PMS14–16 that are also common among women with a history of abuse.17,18 It is also unknown whether abuse in childhood and adolescence have different relations with PMS, and whether emotional, physical, and sexual abuse each independently influence risk. We have assessed these relations in a substudy nested within the Nurses' Health Study 2 (NHS2).
Methods
Study population
The NHS2 is a prospective cohort study of 116,686 U.S. female registered nurses who responded to a mailed questionnaire in 1989. Aged 25–42 years at baseline, participants provided information on medical history and health-related behaviors including smoking and oral contraceptive use. Cohort members have completed questionnaires every two years thereafter to update information on risk factors and report new diagnoses. The response rate for each questionnaire has been ≥89%. The study protocol was approved by the Institutional Review Board at Brigham and Women's Hospital.
Assessment of premenstrual syndrome
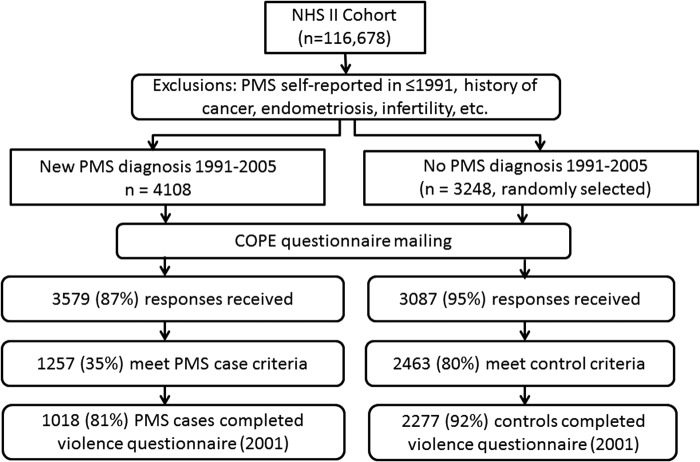
This analysis includes members of the NHS2 PMS Substudy, described previously.19,20 Briefly, we identified all NHS2 members who had not reported a diagnosis of PMS on their main NHS2 questionnaire in either 1989 or 1991, and were thus at risk for being diagnosed with PMS during follow-up. We then identified all premenopausal women who self-reported on a NHS2 questionnaire between 1991 and 2005 that they had been newly diagnosed with PMS by a physician (n=4,108; Fig. 1). As a comparison group, we randomly selected 3,248 premenopausal women who did not report PMS during the follow-up period (1991–2005). To make sure that women with PMS and comparison women provided information about comparable time periods, we assigned each a “reference year.” For PMS cases, reference year was equal to year of PMS diagnosis. Because comparison women did not have a year of diagnosis, we assigned each a randomly chosen reference year between 1991 and 2005. To reduce the likelihood of including women with PMS-type symptoms caused by conditions other than PMS, from both groups we excluded women who reported cancer, endometriosis, highly irregular menstrual cycles, infertility, or hysterectomy prior to their reference year.
FIG. 1.
Selection of participants into the Nurses' Health Study 2 Premenstrual Syndrome Substudy and present analysis.
Participants were sent a questionnaire based on the Calendar of Premenstrual Experiences,21 on which we assessed the occurrence of 26 physical, behavioral and affective symptoms, symptom timing during the menstrual cycle, and the impact of symptoms on multiple domains of daily functioning. Completed questionnaires were received from 3,579 (87%) of the women self-reporting PMS and 3,087 (95%) women in the comparison group. Women responding to the PMS questionnaire did not differ substantially from nonrespondents in terms of age (34.4 vs. 34.5 years), body mass index (BMI) (24.0 vs. 24.8 kg/m2), ever use of oral contraceptives (81.9% vs. 83.7%) and other baseline characteristics.
We used responses to further limit our case group to women who met established criteria for moderate-to-severe PMS.21 Case criteria included: (1) the occurrence of at least one physical/behavioral and one affective menstrual symptom; (2) overall symptom severity of moderate or severe, or symptom impact on one or more life activities and social relationships rated as moderate or severe; (3) symptoms beginning within 14 days before onset of menses; (4) symptoms ending within 4 days after onset of menses; and (5) symptoms absent in the week after menses ends. Overall, 1,257 (35%) self-reported cases met these additional criteria (Fig. 1).
We also used menstrual symptom questionnaires to further limit the comparison group to women who confirmed that they experienced no menstrual symptoms or only mild symptoms of no personal impact. Ultimately, 2,463 (80%) comparison women met these control criteria. Women who did not meet either case or control criteria were excluded from further analysis. This procedure allowed us to compare women at extreme ends of the spectrum of menstrual symptom experience, thereby minimizing the likelihood of misclassification between PMS cases and controls.
The validity of our approach to identifying PMS cases and controls was assessed previously among 135 substudy members first reporting PMS in 2001 and 371 not reporting PMS (1989–2001).20 Cases meeting our criteria for PMS were very similar to cases also reporting clinician-supervised prospective symptom charting, in terms of symptom frequency (e.g., mean number of physical symptoms: 5.5 vs. 6.1, p>0.05), timing of occurrence (e.g., mean number of days symptoms began before onset of menses: 5.7 vs. 6.1, p>0.05), and severity (e.g., symptoms caused moderate-severe social isolation: 10% vs. 17%, p>0.05). Furthermore, odds ratios for the associations of the two risk factors evaluated (age and calcium intake) with PMS risk using both case definitions were nearly identical, suggesting that our method is comparable to prospective charting in its ability to classify PMS cases and controls in large epidemiologic studies.
Assessment of early life abuse
A violence questionnaire was sent to 91,286 NHS2 members in 2001. Completed responses were received by 68,518 (75%) women, including 1,018 PMS Substudy cases (81%) and 2,277 controls (92%). This questionnaire was designed after an extensive literature review, in consultation with experts in the field of violence assessment, and has been described in detail previously.17,18 Questions measuring punitive parenting occurring before age 11 were derived from the Childhood Trauma Questionnaire (CTQ) short form.22,23 Two items measured physical abuse and corporal-type punishment: “People in my family hit me so hard that it left me with bruises and marks”; and “I was punished with a belt, a board, a cord, or some other hard object.” Two items measured emotional abuse: “Someone in my family yelled or screamed at me”; and “People in my family said hurtful or insulting things to me.” A fifth item measured perception of punishments: “The punishments I received seemed cruel to me.” Responses were reported on a Likert scale, with the following scores assigned: never true=1; rarely true=2; sometimes true=3; often true=4; very often true=5. We calculated total CTQ score by summing responses to these five items, with total scores ranging from 5 (no evidence of maltreatment) to 25 (frequent maltreatment). Additionally, one CTQ item measured social support: “Someone in my family made me feel special.” This item was not included in the CTQ score, but was instead evaluated as a mediator of the effects of childhood maltreatment.
Questions on physical abuse in childhood (before age 11) and adolescence (ages 11–17) physical abuse were adapted from the Revised Conflict Tactics Scale.24 Women were asked: Did a parent, stepparent or other adult guardian ever: spank you for discipline; push, grab, or shove you; kick, bite, or punch you; hit you with something that hurt your body; choke or burn you; or physically attack you in some other way. Response options included never, once, a few times, and more than a few times. We derived four categories of abuse based on the most severe level of abuse reported at each time period, following methods established previously by Rich-Edwards et al (2010).25 “None” corresponded with no reported physical abuse. “Mild” included being pushed, grabbed, or shoved one or more times; being kicked, bitten, or punched once; or being hit with something once. “Moderate” included being hit with something more than once or being physically attacked once. “Severe” included being kicked, bitten, or punched, physically attacked more than once, or ever choked or burned. The question on spanking for discipline was not included in the abuse categorization.
Questions on inappropriate sexual touching or forced sex were adapted from the Sexual Experiences Survey.26 Questions were: “Were you ever touched in a sexual way by an adult or an older child, or were you forced to touch an adult or an older child in a sexual way when you did not want to?” and “Did an adult or older child ever force you or attempt to force you into any sexual activity by threatening you, holding you down, or hurting you in some way when you did not want to?” Response options for these questions were: “No, this never happened”; “Yes, this happened once”; or “Yes, this happened more than once.” We categorized sexual abuse during childhood (<11 years) and adolescence (11–17 years) as follows: “none,” “unwanted sexual touching,” “forced sex once,” or “forced sex more than once.” As with physical abuse, we assigned women to categories based on the most severe level of abuse reported at each time period.25
Finally, we created summary variables that captured co-occurrence of exposure to emotional, physical and sexual abuse in childhood, and cumulative violence exposure in childhood and adolescence, following the method described previously by Boynton-Jarrett et al.27
Assessment of other factors
Factors measured at baseline included age, age at menarche, and race/ethnicity. Information on number of full-term pregnancies, age at first birth, oral contraceptive use, and smoking history was collected at baseline and then updated every 2 years. Childhood socioeconomic factors including mother's education, father's education, and home ownership when the participant was an infant were reported in 2005. Current household income was assessed in 2001.
Participation in physical activity was measured in 1991, 1997, and 2001 by asking how much time women spent each week participating in specific recreational activities. Responses were used to calculate metabolic equivalent hours per week of activity. Information on food and nutrient intake was collected in 1991, 1995, 1999, and 2003 by validated semiquantitative foods frequency questionnaire28 and was used to estimate intake of calcium, vitamin D, B vitamins, and other nutrients. All nutrients were adjusted for total energy intake using the residual method.29
Participants reported their height at baseline and reported current weight on each biennial questionnaire, which we used to calculate BMI {weight (kg)/[height (m)]2}. In addition, at baseline participants were shown diagrams of female body figures and asked to identify which best represented their body at age 5 years (range from 1=very thin to 9=extremely obese). Responses were used to derive a childhood somatogram score.30
Physical and sexual abuse occurring in adulthood (i.e., between age 18 and the year 2001) was measured on the 2001 violence questionnaire. Finally, participants reported history of clinically diagnosed depression, use of antidepressant medications and the timing of each, on the questionnaire used to assess menstrual symptoms.
Statistical analysis
All statistical analyses were conducted with SAS (SAS Institute, Inc., Cary, NC). We compared age-standardized baseline characteristics of PMS cases and controls with generalized linear models (PROC GLM) adjusting for age. We then compared characteristics of women reporting different levels of abuse.
We used logistic regression to estimate odds ratios for PMS for women across categories of abuse, and calculated 95% confidence intervals (95%CI). We built three sets of models following methods used previously in studies of early life abuse and risk of conditions including type 2 diabetes,25 hypertension,31 chronic inflammation,32 heart disease, and stroke.33 In Model 1, we adjusted for age and characteristics during early life (i.e., prior to or contemporaneous with abuse), which we considered potential confounders. These included race/ethnicity, somatogram at age 5, maternal education, paternal education, and home ownership in the nurse's infancy. In Model 2, we further adjusted for characteristics that (1) could vary between early life and PMS onset, (2) may be affected by abuse, and/or (3) were associated with risk of PMS in previous studies in our population.14–16,19,34–36 We considered these factors potential mediators. These included BMI, physical activity, age at menarche, household income (in adulthood), alcohol intake, pack-years of cigarette smoking, history of depression, physical abuse occurring in adulthood (between age 18 and 2001), sexual abuse in adulthood, and dietary intake of vitamin D, calcium, vitamin B6, and potassium. Under assumptions including the absence of exposure-mediator interaction and no residual confounding, adjustment for mediators may yield estimates of direct effects.37 In Model 3, we further adjusted for social support in childhood.
We conducted two sensitivity analyses. First, we assessed relations only among women first reporting PMS after completion of the 2001 violence questionnaire (i.e., 2003–2005), to address potential recall bias related to the timing of abuse assessment versus PMS diagnosis. Second, we excluded women reporting any clinical diagnosis of depression or antidepressant use prior to their reference year, to determine whether associations were robust to potential misclassification of depression as PMS. Finally, we assessed whether childhood social support was an effect modifier of the abuse–PMS relation by stratifying by high versus low social support and comparing abuse–PMS associations across strata and by testing for multiplicative interaction.
Results
Age-standardized baseline characteristics of the 1,018 PMS cases and 2,277 controls are presented in Table 1. Cases were slightly younger, had slightly higher BMI, and had slightly lower intake of vitamin D from foods than controls. Cases were more likely to have used oral contraceptives, and to have smoked cigarettes. History of depression and baseline antidepressant use was more common in cases than controls (p<0.0001 for both). Cases and controls did not differ significantly in terms of alcohol intake, parity, or physical activity.
Table 1.
Age-Standardized Baseline (1991) Characteristics of Premenstrual Syndrome Cases and Controls Completing Childhood Violence Questionnaires, Nurses' Health Study 2 Premenstrual Syndrome Substudy
| Cases n=1018 | Controls n=2277 | ||||
|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | p Valuea |
| Age | 33.9 | 4.3 | 34.4 | 3.9 | <0.0001 |
| Body mass index (1991) | 24.6 | 4.9 | 23.8 | 5.4 | <0.0001 |
| Body mass index at age 18 | 21.3 | 3.3 | 21.1 | 3.6 | 0.051 |
| Age at menarche | 12.4 | 1.4 | 12.5 | 1.6 | 0.056 |
| Number of full term pregnancies | 1.6 | 1.2 | 1.6 | 1.3 | 0.26 |
| Age at first birthb | 25.6 | 4.4 | 25.8 | 4.9 | 0.34 |
| Physical activity (METs/week) | 38.5 | 115 | 32.6 | 126 | 0.16 |
| Alcohol intake (grams/day) | 3.1 | 6.3 | 3.0 | 6.9 | 0.80 |
| Vitamin D intake from foods (IU/day) | 257 | 126 | 267 | 134 | 0.03 |
| % | % | ||
|---|---|---|---|
| Parents owned home in nurse's infancy | 45 | 49 | 0.10 |
| Household income ≥$100,000c | 31 | 34 | 0.19 |
| Ever used oral contraceptives | 87 | 79 | <0.0001 |
| Used oral contraceptives >4 years | 43 | 38 | 0.009 |
| Current smoker | 13 | 7 | <0.0001 |
| Former smoker | 26 | 18 | <0.0001 |
| History of clinician-diagnosed depression | 8 | 4 | <0.0001 |
| Ever used antidepressantsd | 12 | 4 | <0.0001 |
All characteristics except age standardized to the age distribution of cases and controls in 1991.
Calculated using the F statistic.
Among parous women.
Assessed in 2001.
Assessed in 1993.
MET, metabolic equivalent; SD, standard deviation.
Childhood Trauma Questionnaire (CTQ) scores of ≥16 were reported by 11% (n=373), and 34% (n=1,120) reported moderate or severe physical abuse in childhood or adolescence (Table 2). Unwanted sexual touching was reported by 22% (n=729), and 9% (n=303) reported forced sexual activity during childhood or adolescence. Current smoking, history of depression, and antidepressant use were more common among women reporting the highest levels of each type of abuse (p<0.001). Mean adult BMI increased by severity for each type of abuse (p<0.01). Other factors including physical activity, alcohol intake, and oral contraceptive use did not differ by abuse history. As expected, experiences of each type of abuse were highly correlated.
Table 2.
Age-Standardized Baseline (1991) Characteristics of Study Participants by Experience of Early Life Abuse, Nurses' Health Study 2 Premenstrual Substudy
| Childhood Trauma Questionnaire score | Physical abuse in childhood or adolescence | Sexual abuse in childhood or adolescence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 (low) n=353 | 6–10 n=1860 | 11–15 n=709 | 16–20 n=265 | 21–25 (high) n=108 | None n=1597 | Mild n=573 | Moderate n=866 | Severe n=254 | None n=2251 | Touching n=729 | Forced sex n=303 | |
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | |
| Age (years) | 34.2 | 34.2 | 34.4 | 34.4 | 34.5 | 34.2 | 33.9 | 34.4 | 34.4 | 34.2 | 34.3 | 34.5 |
| Body mass index at age 18 (kg/m2) | 20.8 | 21.1 | 21.5 | 21.5 | 21.5* | 21.2 | 21.1 | 21.3 | 21.4 | 21.2 | 21.2 | 21.4 |
| Adult body mass index (kg/m2) | 23.4 | 24.0 | 24.7 | 24.8 | 25.4** | 24.0 | 24.2 | 24.3 | 25.2* | 24.0 | 24.4 | 25.1* |
| Age at menarche | 12.5 | 12.5 | 12.4 | 12.5 | 12.6 | 12.4 | 12.6 | 12.5 | 12.5 | 12.5 | 12.4 | 12.4 |
| Number of full term pregnancies | 1.7 | 1.7 | 1.6 | 1.6 | 1.6 | 1.7 | 1.7 | 1.7 | 1.6 | 1.7 | 1.8 | 1.6 |
| Age at first birtha | 25.5 | 25.9 | 25.8 | 25.9 | 24.3* | 25.9 | 25.8 | 25.6 | 25.3 | 26.0 | 25.4 | 25.2* |
| Physical activity (METs/week) | 38.3 | 28.5 | 30.1 | 29.1 | 35.6 | 30.8 | 37.3 | 25.5 | 26.1 | 29.4 | 29.1 | 38.1 |
| Alcohol intake (grams/day) | 3.2 | 3.1 | 3.4 | 2.8 | 2.6 | 3.0 | 3.3 | 3.4 | 2.8 | 3.1 | 3.6 | 2.5 |
| Vitamin D intake from foods (IU/day) | 265 | 260 | 254 | 251 | 253 | 265 | 258 | 250 | 245** | 258 | 261 | 253 |
| % | % | % | % | % | % | % | % | % | % | % | % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parents owned home in nurse's infancy | 59 | 45 | 44 | 49 | 29** | 47 | 49 | 44 | 40 | 46 | 47 | 46 |
| Household income ≥$100,000b | 36 | 32 | 32 | 30 | 34 | 33 | 34 | 32 | 30 | 34 | 29 | 27** |
| Ever used oral contraceptives | 82 | 80 | 80 | 83 | 90 | 81 | 79 | 81 | 86 | 79 | 84 | 84* |
| Used oral contraceptives >4 years | 41 | 40 | 35 | 43 | 44 | 40 | 41 | 38 | 41 | 38 | 40 | 47 |
| Current smoker | 5 | 8 | 11 | 16 | 18** | 6. | 12 | 12 | 15** | 8 | 10 | 14* |
| Former smoker | 20 | 21 | 22 | 30 | 27* | 19 | 25 | 22 | 32** | 21 | 22 | 27 |
| History of clinician-diagnosed depression | 2 | 4 | 7 | 9 | 13** | 4 | 6 | 5 | 11** | 4 | 6 | 10** |
| Ever used antidepressants | 3 | 5 | 9 | 12 | 17** | 5 | 8 | 7 | 14** | 5 | 8 | 14** |
| % | % | % | % | % | % | % | % | % | % | % | % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTQ Score ≥16 | – | – | – | – | – | 0 | 4 | 21 | 65** | 9 | 13 | 28** |
| Early life moderate-severe physical abuse | 1 | 18 | 59 | 91 | 97** | – | – | – | – | 30 | 40 | 49** |
| Early life forced sex | 6 | 6 | 13 | 21 | 29** | 6 | 12 | 10 | 29** | – | – | – |
| Adult physical abuse | 12 | 19 | 21 | 30 | 49** | 15 | 22 | 25 | 39** | 18 | 23 | 29** |
| Adult sexual abuse | 5 | 8 | 11 | 13 | 23** | 7 | 9 | 10 | 18** | 7 | 11 | 16** |
All characteristics except age standardized to the age distribution of cases and controls in 1991.
p Values from F test: *p<0.01; **p<0.001.
Analysis limited to parous women.
Assessed in 2001.
CTQ, Childhood Trauma Questionnaire.
CTQ score was significantly associated with risk of PMS (Table 3). After adjustment for confounders including maternal and paternal education and race/ethnicity, women with the highest CTQ scores had 3.7 times the risk of developing PMS as those with the lowest CTQ score (95%CI=2.3–6.0; pTrend<0.0001). Results were attenuated but remained significant after adjustment for mediating factors including BMI, smoking, and abuse during adulthood; the odds ratio (OR) for women in the highest vs. lowest category of CTQ score was 2.1 (95%CI=1.2–3.5; pTrend<0.0001). Social support in childhood was inversely associated with risk of PMS. In analyses adjusted for confounders and mediators, women reporting the highest social support had an OR of 0.7 (95%CI=0.1–1.1) compared to those reporting low social support (pTrend=0.0002). Adjustment of CTQ score for social support (Model 3) attenuated findings modestly, but associations remained strong and significant (OR for highest vs. lowest score=1.9; 95%CI=1.1–3.3; pTrend<0.0001).
Table 3.
Relative Risks of Premenstrual Syndrome by History of Punitive Parenting, Nurses' Health Study 2 Premenstrual Syndrome Substudy (1991–2005)
| Model 1: Adjusted for age and confoundersa | Model 2: Adjusted for age, confounders and mediatorsb | Model 3: Adjusted for age, confounders, mediators and social supportc | ||||||
|---|---|---|---|---|---|---|---|---|
| Type of abuse | Cases | Controls | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| CTQ score | ||||||||
| 5 (none) | 65 | 288 | 1.0 | 1.0 | 1.0 | |||
| 6–10 | 510 | 1350 | 1.7 | 1.2–2.2 | 1.6 | 1.1–2.1 | 1.5 | 1.1–2.1 |
| 11–15 | 269 | 440 | 2.7 | 2.0–3.7 | 2.3 | 1.6–3.2 | 2.2 | 1.5–3.1 |
| 16–20 | 122 | 143 | 3.7 | 2.5–5.3 | 2.8 | 1.9–4.2 | 2.6 | 1.7–3.9 |
| 21–25 (high) | 52 | 56 | 3.7 | 2.3–6.0 | 2.1 | 1.2–3.5 | 1.9 | 1.1–3.2 |
| pTrend<0.0001 | pTrend<0.0001 | pTrend<0.0001 | ||||||
| CTQ emotional abuse itemsd | ||||||||
| 2 (none) | 82 | 347 | 1.0 | 1.0 | 1.0 | |||
| 3–4 | 308 | 881 | 1.4 | 1.1–1.9 | 1.4 | 1.0–1.8 | 1.4 | 1.0–1.8 |
| 5–6 | 321 | 663 | 1.9 | 1.4–2.5 | 1.7 | 1.2–2.2 | 1.6 | 1.2–2.2 |
| 7–8 | 172 | 245 | 2.7 | 1.9–3.7 | 2.2 | 1.5–3.2 | 2.1 | 1.5–3.0 |
| 9–10 | 135 | 141 | 3.4 | 2.3–5.0 | 2.6 | 1.7–4.0 | 2.5 | 1.6–3.8 |
| pTrend<0.0001 | pTrend<0.0001 | pTrend<0.0001 | ||||||
| CTQ corporal punishment itemsd | ||||||||
| 2 (none) | 439 | 1219 | 1.0 | 1.0 | 1.0 | |||
| 3–4 | 355 | 732 | 1.1 | 1.0–1.4 | 1.1 | 0.9–1.3 | 1.1 | 0.9–1.3 |
| 5–6 | 128 | 218 | 1.1 | 0.9–1.5 | 1.1 | 0.8–1.4 | 1.1 | 0.8–1.4 |
| 7–8 | 71 | 78 | 1.3 | 0.9–1.9 | 1.1 | 0.7–1.7 | 1.1 | 0.7–1.7 |
| 9–10 | 25 | 30 | 1.1 | 0.6–2.0 | 0.8 | 0.4–1.5 | 0.8 | 0.4–1.5 |
| pTrend=0.16 | pTrend=0.68 | pTrend=0.74 | ||||||
| CTQ social support iteme | ||||||||
| Never | 31 | 46 | 1.0 | 1.00 | ||||
| Rarely | 125 | 167 | 1.2 | 0.7–2.0 | 1.1 | 0.6–1.9 | ||
| Sometimes | 213 | 396 | 0.8 | 0.5–1.4 | 0.9 | 0.5–1.4 | ||
| Often | 286 | 635 | 0.7 | 0.4–1.1 | 0.8 | 0.5–1.3 | ||
| Very often | 361 | 1027 | 0.6 | 0.4–0.9 | 0.7 | 0.4–1.1 | ||
| pTrend<0.0001 | pTrend=0.0002 | |||||||
Adjusted for age and confounders: mother's education, father's education, white race, home ownership in infanc,y and childhood somatogram.
Adjusted for age, confounders listed above, and mediators: income, parity, oral contraceptive use and duration, body mass index, and pack-years of cigarette smoking, MET hours per week of physical activity, history of depression, physical abuse during adulthood, sexual abuse during adulthood, alcohol intake, and dietary intake of calcium, vitamin D, vitamin B2, vitamin B6, and potassium.
Adjusted for age, confounders and mediators listed above, and the Childhood Trauma Questionnaire (CTQ) social support item.
Emotional and corporal punishment items adjusted for each other in all models.
“Someone made me feel special.”
95% CI, 95% confidence interval; OR, odds ratio.
The CTQ emotional abuse items were strongly related to PMS risk (Table 3). After full adjustment (Model 3), women reporting the highest level of emotional abuse had an OR of 2.5 (95%CI=1.6–3.8) compared with those reporting no emotional abuse, with evidence of a linear trend (pTrend<0.0001). When we repeated this analysis among the 345 cases and 1,064 controls reporting no other types of abuse on the CTQ, emotional abuse remained significantly and strongly associated with risk of PMS (results not shown); for example, the OR for highest level of emotional abuse versus none was 3.8 (pTrend=0.001). In contrast, the CTQ corporal punishment items were not related to risk.
Physical abuse during childhood and adolescence as assessed by the Revised Conflict Tactics Scale were associated with higher risk of PMS (Table 4). For example, after adjustment for confounders and mediators, women reporting severe physical abuse in childhood had an OR of 2.0 (95%CI=1.5–2.8; pTrend=0.0001) compared to those reporting no physical abuse. Social support modestly attenuated associations, but results remained significant.
Table 4.
Relative Risks of Premenstrual Syndrome by History of Physical Abuse and Sexual Abuse in Childhood and Adolescence in the Nurses' Health Study 2 Premenstrual Syndrome Substudy (1991–2005)
| Model 1: Adjusted for age and confoundersa | Model 2: Adjusted for age, confounders and mediatorsb | Model 3: Adjusted for age, confounders, mediators and social supportc | ||||||
|---|---|---|---|---|---|---|---|---|
| Type of abuse | Cases | Controls | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Physical abuse in childhood | ||||||||
| None | 440 | 1300 | 1.0 | 1.0 | 1.0 | |||
| Mild | 178 | 323 | 1.6 | 1.3–2.1 | 1.5 | 1.2–1.9 | 1.4 | 1.1–1.8 |
| Moderate | 293 | 556 | 1.5 | 1.3–2.0 | 1.4 | 1.7–1.7 | 1.4 | 1.1–1.7 |
| Severe | 107 | 97 | 3.1 | 2.3–4.2 | 2.0 | 1.5–2.8 | 1.8 | 1.3–2.6 |
| pTrend<0.0001 | pTrend<0.0001 | pTrend<0.0001 | ||||||
| Physical abuse in adolescence | ||||||||
| None | 609 | 1644 | 1.0 | 1.0 | 1.0 | |||
| Mild | 171 | 306 | 1.5 | 1.2–1.8 | 1.3 | 1.0–1.6 | 1.3 | 1.0–1.6 |
| Moderate | 144 | 229 | 1.7 | 1.4–2.2 | 1.4 | 1.1–1.8 | 1.3 | 1.0–1.7 |
| Severe | 93 | 95 | 2.5 | 1.8–3.4 | 1.8 | 1.3–2.5 | 1.6 | 1.1–2.3 |
| pTrend<0.0001 | pTrend<0.0001 | pTrend=0.001 | ||||||
| Physical abuse in childhood and adolescence | ||||||||
| None | 389 | 1208 | 1.0 | 1.0 | 1.0 | |||
| Mild in childhood and/or adolescence | 198 | 375 | 1.6 | 1.3–2.0 | 1.4 | 1.2–1.8 | 1.4 | 1.1–1.8 |
| Moderate in childhood and/or adolescence | 297 | 569 | 1.6 | 1.3–1.9 | 1.4 | 1.2–1.8 | 1.4 | 1.1–1.7 |
| Severe in childhood or adolescence but not both | 66 | 51 | 3.9 | 2.6–5.7 | 3.0 | 2.0–4.6 | 2.8 | 1.8–4.2 |
| Severe in both childhood and adolescence | 67 | 70 | 2.7 | 1.9–3.9 | 1.8 | 1.2–2.6 | 1.6 | 1.1–2.4 |
| Sexual abuse in childhood | ||||||||
| None | 792 | 1869 | 1.0 | 1.0 | 1.0 | |||
| Unwanted touching | 163 | 321 | 1.2 | 1.0–1.5 | 1.1 | 0.9–1.3 | 1.0 | 0.8–1.3 |
| Forced sex once | 26 | 37 | 1.5 | 0.9–2.5 | 1.1 | 0.6–1.9 | 1.1 | 0.6–1.9 |
| Forced sex more than once | 35 | 46 | 1.6 | 1.0–2.6 | 1.2 | 0.7–2.0 | 1.1 | 0.7–1.8 |
| pTrend=0.004 | pTrend=0.34 | pTrend=0.63 | ||||||
| Sexual abuse in adolescence | ||||||||
| None | 765 | 1852 | 1.0 | 1.0 | 1.0 | |||
| Unwanted touching | 153 | 290 | 1.3 | 1.0–1.6 | 1.2 | 0.9–1.5 | 1.2 | 0.9–1.5 |
| Forced sex once | 58 | 88 | 1.6 | 1.1–2.2 | 1.3 | 0.9–2.0 | 1.3 | 0.9–1.9 |
| Forced sex more than once | 38 | 43 | 2.1 | 1.3–3.3 | 1.4 | 0.9–2.3 | 1.3 | 0.8–2.1 |
| pTrend<0.0001 | pTrend=0.02 | pTrend=0.07 | ||||||
| Sexual abuse in childhood and adolescence | ||||||||
| None | 637 | 1614 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Unwanted touching in childhood and/or adolescence | 247 | 482 | 1.3 | 1.1–1.6 | 1.2 | 1.0–1.5 | 1.2 | 1.0–1.4 |
| Forced sex in childhood or adolescence but not both | 101 | 136 | 1.9 | 1.4–2.5 | 1.5 | 1.1–2.0 | 1.4 | 1.0–1.9 |
| Forced sex in both childhood and adolescence | 28 | 38 | 1.7 | 1.0–2.8 | 1.2 | 0.7–2.0 | 1.1 | 0.6–1.8 |
History of sexual abuse was not consistently associated with risk of PMS (Table 4). After adjustment for confounders and mediators, women reporting forced sex more than once in adolescence had a non-significant 40% higher risk compared to those not reporting sexual abuse (pTrend=0.02). Findings were attenuated after adjustment for social support.
Table 5 presents results for emotional, physical, and sexual abuse evaluated together. For analyses of childhood abuse only, results suggested that emotional and physical abuse were more strongly related to risk of PMS than sexual abuse. When type, severity, and chronicity of abuse were evaluated simultaneously in our cumulative abuse measure, ORs for PMS were significantly elevated for every level of abuse. Women with the highest level of abuse (forced sex during both childhood and adolescence and severe physical abuse during both time periods) had an OR of 2.8 (95%CI=1.5–5.3) compared to those without any exposure to abuse. Social support attenuated these findings only modestly.
Table 5.
Relative Risks of Premenstrual Syndrome by Combined History of Emotional, Physical and Sexual Abuse in Childhood and Adolescence in the NHS2 Premenstrual Syndrome Substudy (1991–2005)
| Model 1: Adjusted for age and confoundersa | Model 2: Adjusted for age, confounders and mediatorsb | Model 3: Adjusted for age, confounders, mediators and social supportc | ||||||
|---|---|---|---|---|---|---|---|---|
| Type of abuse | Cases | Controls | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Emotional, physical, sexual abuse in childhood | ||||||||
| None | 226 | 817 | 1.0 | 1.0 | 1.0 | |||
| Emotional abuse only | 137 | 298 | 1.6 | 1.3–2.1 | 1.5 | 1.1–1.9 | 1.4 | 1.1–1.9 |
| Physical abuse only | 104 | 233 | 1.6 | 1.2–2.1 | 1.5 | 1.1–2.0 | 1.5 | 1.1–2.0 |
| Sexual abuse only | 41 | 126 | 1.1 | 0.8–1.7 | 1.1 | 0.7–1.6 | 1.0 | 0.7–1.6 |
| Emotional and physical abuse | 325 | 520 | 2.2 | 1.8–2.7 | 1.8 | 1.5–2.3 | 1.7 | 1.4–2.2 |
| Emotional and sexual abuse | 35 | 57 | 2.2 | 1.4–3.4 | 1.6 | 1.0–2.6 | 1.5 | 0.9–2.5 |
| Physical and sexual abuse | 18 | 50 | 1.3 | 0.7–2.3 | 1.2 | 0.6–2.1 | 1.2 | 0.6–2.1 |
| Emotional, physical, and sexual abuse | 130 | 171 | 2.6 | 2.0–3.4 | 1.9 | 1.4–2.6 | 1.8 | 1.3–2.4 |
| Cumulative abuse history in early lifed | ||||||||
| No exposure to violence | 181 | 691 | 1.0 | 1.0 | 1.0 | |||
| Emotional abuse only | 94 | 228 | 1.5 | 1.1–2.0 | 1.4 | 1.0–1.9 | 1.4 | 1.0–1.9 |
| Mild/moderate single type | 390 | 862 | 1.7 | 1.4–2.1 | 1.5 | 1.2–1.9 | 1.5 | 1.2–1.9 |
| Mild-multiple or severe-single type(s) | 182 | 300 | 2.3 | 1.8–2.9 | 1.9 | 1.4–2.4 | 1.8 | 1.3–2.3 |
| Moderate-chronic or multiple types | 95 | 118 | 2.9 | 2.1–3.9 | 2.2 | 1.6–3.2 | 2.1 | 1.4–2.9 |
| Severe-chronic or multiple types | 40 | 45 | 3.3 | 2.1–5.3 | 1.8 | 1.1–3.1 | 1.7 | 1.0–2.9 |
| Severe chronic and multiple types | 30 | 22 | 4.6 | 2.5–8.2 | 2.8 | 1.5–5.3 | 2.5 | 1.3–4.8 |
Adjusted for age and confounders listed in Table 2.
Adjusted for age, confounders listed above, and mediators listed in Table 2.
Adjusted for age, confounders and mediators, and the CTQ social support item.
Cumulative abuse history combines violence severity and chronicity; categories derived from factor analysis and frequency trends within each factor by Boynton-Jarrett et al.27
Results from sensitivity analyses limited to women reporting PMS after completion of the violence questionnaire (138 cases and 390 controls with reference years 2003–2005) were consistent with those from the main analysis (results not shown), though our power for these comparisons was relatively low. For example, after adjusting for mediators, women with the highest CTQ scores had an OR of 4.1 compared to those with the lowest scores (pTrend=0.09). Results limited to women with no report of depression or antidepressant use prior to PMS diagnosis or reference year (811 cases and 2,071 controls) were highly similar to those from the main analysis (results not shown). For example, after adjusting for mediators, women reporting with the highest CTQ scores had an OR of 2.3 compared to those with the lowest (pTrend<0.001). Finally, analyses stratifying by level of childhood social support (high vs. low) did not suggest that this factor modified the associations between emotional, physical and sexual abuse and risk of PMS (all pInteraction>0.10; results not shown).
Discussion
We observed evidence that early life abuse substantially increased risk of premenstrual syndrome in the middle-to-late reproductive years, even after accounting for the effects of age, smoking, obesity, and other PMS risk factors. Associations were stronger for physical abuse and emotional abuse than for sexual abuse. While social support in childhood was inversely related to PMS, adjustment for social support had limited effect on the abuse–PMS relation. Finally, while results did not suggest that abuse in childhood versus in adolescence had markedly different effects, risk was highest overall among women reporting chronic abuse of multiple types.
Early life abuse may impact PMS development by altering hypothalamic-pituitary-adrenal axis function in response to stress.38,39 Girdler and colleagues suggest that persistent physiologic effects of abuse may differ in women with and without PMS or PMDD.5 In laboratory experiments, abuse history was associated with abnormal response to stressors in all subjects, but the direction of effect was modified by PMDD status; among women with PMDD, abuse was associated with heightened stress reactivity, while in women without PMDD, abuse was associated with blunted cortisol and sympathetic nervous system responses to stress. In other experiments, PMDD cases reporting prior abuse demonstrated higher resting and stress-induced blood pressure than both symptom-free controls and PMDD cases without a history of abuse.5 Additional evidence suggests that abuse history may contribute to dysfunction of the hypothalamus-pituitary-thyroid axis in women with PMDD.40
Furthermore, a common polymorphism in the serotonin transporter gene (5-HTTLPR), which appears more common in women with PMDD than controls, may contribute to more severe consequences of early life abuse in women with an underlying genetic vulnerability to PMS/PMDD.41
Early life abuse may also contribute to dysregulation of immune function leading to chronic inflammation, as suggested by laboratory experiments in humans and in animal studies.38,42 In the NHS2, we found that plasma levels of C-reactive protein and interleukin-6 were higher in women reporting sexual abuse in adolescence and childhood compared to those reporting no abuse (p=0.04 and 0.03, respectively), even after adjustment for obesity, smoking and other factors.32 To date, very few studies have assessed whether menstrual symptoms may be associated with chronic inflammation and results have been inconsistent.43,44 This hypothesis warrants further study.
It is unclear why emotional abuse, and perhaps physical abuse, was more strongly related to PMS risk than sexual abuse, but these findings are consistent with studies of other affective disorders.45–47 For example, in the Adverse Childhood Experiences Study (n=9,460), women with a history of childhood emotional abuse had a 2.7-fold higher lifetime risk of depressive disorders (95% CI=2.3–3.2), while risks associated with childhood physical (OR=2.1; 95%CI=1.8–2.4) and sexual abuse (OR=1.8; 95%CI=1.5–2.0) were somewhat lower.45 In 857 psychiatric outpatients, Gibb et al. found scores on the emotional abuse subscale of the 53-item version of the Childhood Trauma Questionnaire more strongly related to prevalent depressive disorder than scores on either the physical or sexual abuse subscales.46 In this study, emotional abuse was significantly related to depression even among patients reporting no physical or sexual abuse. Clinical experiments suggest that hypothalamus-pituitary-adrenal axis function and stress reactivity may be impacted by early-life emotional abuse, independently of other types of childhood maltreatment.48 Given the high prevalence of childhood emotional abuse in the general population,45 additional investigation into the impact of emotional abuse on PMS is highly warranted.
Our study of incident PMS is nested within a large, ongoing prospective cohort of over 116,000 women. Consequently, we were unable to use prospective symptom diaries to classify PMS. However, we used established criteria to identify women meeting strict criteria for moderate to severe PMS,21 and to identify controls experiencing few symptoms of no personal impact. We excluded women meeting neither case nor control criteria from analysis, and excluded women with a history of depression and/or antidepressant use in subanalyses. Some investigators have suggested that retrospective assessment of PMS could potentially be subject to overreport of symptom severity49 and consequently lead to the inclusion of women without true PMS in the case group in studies using retrospective measures. However, it is important to note that this type of misclassification would attenuate associations between PMS and abuse, rather than exaggerate them, and thus cannot explain our findings. Finally, our results are comparable to the few other population-based surveys of abuse and PMS/PMDD, which have also relied on single questionnaire assessments to identify cases.4.10,12
It is possible that the likelihood of completion of the violence questionnaire was influenced by abuse history, but response rates were very high. Self-report of childhood abuse is likely more accurate than objective reports (i.e., police records), as only a small proportion of cases are reported to authorities. The levels of physical and sexual abuse reported by our participants are comparable to other population surveys using similar instruments.50 It is conceivable that experience of PMS may have influenced recall of abuse. However, results from a sensitivity analysis limited to cases diagnosed after abuse was reported in 2001 were very similar to those from the main analysis and suggest that associations are not explained by recall bias.
In addition, it is possible that our analysis is affected by uncontrolled confounding, both with regard to main analyses and assessment of mediation. However, we note that between the large sample size, large number of measured confounders and mediators, and the minimal influence adjustment for confounding had on risk estimates, the impact of such confounding is likely to be minimal and is unlikely to alter our main findings.
Our participants were ≥27 years old at baseline and we followed them prospectively for new PMS diagnoses. We were therefore not able to assess how early life abuse is associated with PMS developing before age 27. We would expect early life abuse to be strongly related to PMS developing in the teens and early 20's, as was reported in another population-based study evaluating abuse history and PMS/PMDD in women aged 14–27 years.4
Conclusions
Ours is one of the first large population-based studies of the impact of early life abuse on PMS developing in the middle-to-late reproductive years. Findings suggest that abuse, especially emotional and physical abuse, is a strong risk factor for moderate-to-severe PMS. The persistence of associations between abuse and PMS, even after control for established risk factors for PMS and for potential mediators of direct effects, supports the hypothesis that early life abuse is importantly related to the etiology of PMS. These findings provide further evidence that early life abuse may have long-term consequences on women's physical and emotional health.
Acknowledgments
This work was supported by Public Health Services grants UM1CA176726 and HL064108 from the U.S. National Institutes of Health, Department of Health and Human Services.
Disclosure Statement
No competing financial interests exist.
References
- 1.Yonkers KA, O'Brien PMS, Eriksson E. Premenstrual syndrome. Lancet 2008;371:1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tschudin S, Bertea PC, Zemp E. Prevalence and predictors of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample. Arch Womens Ment Health 2010;13:485–494 [DOI] [PubMed] [Google Scholar]
- 3.Johnson SR. Premenstrual syndrome, premenstrual dysphoric disorder, and beyond: A clinical primer for practitioners. Obstet Gynecol 2004;104:845–859 [DOI] [PubMed] [Google Scholar]
- 4.Perkonigg A, Yonkers KA, Pfister H, Lieb R, Wittchen HU. Risk factors for premenstrual dysphoric disorder in a community sample of young women: The role of traumatic events and posttraumatic stress disorder. J Clin Psychiatry 2004;65:1314–1322 [DOI] [PubMed] [Google Scholar]
- 5.Halbreich U. The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinol 2003;28:55–99 [DOI] [PubMed] [Google Scholar]
- 6.Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in biological profiles in women with abuse histories: Influence of premenstrual dysphoric disorder. Health Psychol 2007;26:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girdler SS, Sherwood A, Hinderliter AL, et al. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med 2003;65:849–856 [DOI] [PubMed] [Google Scholar]
- 8.Lustyk MK, Widman L, de Laveaga Becker L. Abuse history and premenstrual symptomatology: Assessing the mediating role of perceived stress. Women Health 2007;46:61–80 [DOI] [PubMed] [Google Scholar]
- 9.Koci A, Strickland O. Relationship of adolescent physical and sexual abuse to perimenstrual symptoms (PMS) in adulthood. Issues Ment Health Nurs 2007;28:75–87 [DOI] [PubMed] [Google Scholar]
- 10.Wittchen HU, Perkonigg A, Pfister H. Trauma and PTSD: an overlooked pathogenic pathway for premenstrual dysphoric disorder. Arch Womens Ment Health 2003;6:293–297 [DOI] [PubMed] [Google Scholar]
- 11.Golding JM, Taylor DL, Menard L, King MJ. Prevalence of sexual abuse history in a sample of women seeking treatment for premenstrual syndrome. J Psychosom Obstet Gynaecol 2000;21:69–80 [DOI] [PubMed] [Google Scholar]
- 12.Pilver CE, Levy BR, Libby DJ, Desai RA. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health 2011;14:383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand 2011;90:746–752 [DOI] [PubMed] [Google Scholar]
- 14.Bertone-Johnson ER, Hankinson SE, Johnson SR, Manson JE. Cigarette smoking and the development of premenstrual syndrome. Am J Epidemiol 2008;168:938–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertone-Johnson ER, Hakinson SE, Johnson SR, Manson JE. Alcohol use at different ages and incidence of premenstrual syndrome and probable premenstrual dysphoric disorder. J Women's Health 2009;18:1945–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertone-Johnson ER, Hankinson SE, Johnson SR, Willett WC, Manson JE. Adiposity and the development of premenstrual syndrome. J Women's Health 2010;19:1955–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun HJ, Rich-Edwards JW, Boynton-Jarrett R, Austin SB, Frazier AL, Wright RJ. Child abuse and smoking among young women: The importance of severity, accumulation, and timing. J Adolesc Health 2008;43:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun HJ, Corliss HL, Boynton-Jarrett R, Spiegelman D, Austin SB, Wright RJ. Growing up in a domestic violence environment: Relationship with developmental trajectories of body mass index during adolescence into young adulthood. J Epidemiol Community Health 2012;66:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertone-Johnson ER, Hankinson SE, Bendich A, Johnson SR, Willett WC, Manson JE. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med 2005;165:1246–1252 [DOI] [PubMed] [Google Scholar]
- 20.Bertone-Johnson ER, Hankinson SE, Johnson SR, Manson JE. A simple method of assessing premenstrual syndrome in large prospective studies. J Reprod Med 2007;52:779–786 [PubMed] [Google Scholar]
- 21.Mortola JF, Girton L, Beck L, Yen SS. Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: The calendar of premenstrual experiences. Obstet Gynecol 1990;76:302–307 [PubMed] [Google Scholar]
- 22.Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M. Initial reliability and validity of the childhood trauma interview: A new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry 1995; 152:1329–1335 [DOI] [PubMed] [Google Scholar]
- 23.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994;151:1132–1136 [DOI] [PubMed] [Google Scholar]
- 24.Straus MA. Gelles RH Physical violence in American families: Risk factors and adaptations to violence in 8145 families. New Brunswick, NJ: Transaction Publishing, 1990 [Google Scholar]
- 25.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med 2010;39:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koss MP, Oros CJ. Sexual experiences survey: A research instrument investigating sexual aggression and victimization. J Consult Clin Psychol 1982;50:455–457 [DOI] [PubMed] [Google Scholar]
- 27.Boynton-Jarrett R, Rich-Edwards JW, Jun HJ, Hibert EN, Wright RJ. Abuse in childhood and risk of uterine leiomyoma: The role of emotional support in biologic resilience. Epidemiology 2011;22:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 29.Willett WC. Nutritional epidemiology, 3rd edition. New York: Oxford University Press, 2013 [Google Scholar]
- 30.Field AE, Franko DL, Striegel-Moore RH, Schreiber GB, Crawford PB, Daniels SR. Race differences in accuracy of self-reported childhood body size among white and black women. Obes Res 2004;12:1136–1144 [DOI] [PubMed] [Google Scholar]
- 31.Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses' Health Study II. J Epidemiol Community Health 2010;64:413–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am J Prev Med 2012; 43:611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rich-Edwards JW, Mason S, Rexrode K, et al. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation 2012;126:920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chocano-Bedoya P, Manson JE, Ronnenberg A, et al. B-complex vitamins and incident premenstrual syndrome. Am J Clin Nutr 2011;93:1080–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Intake of selected minerals and risk of premenstrual syndrome. Am J Epidemiol 2013;177:1118–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertone-Johnson E, Hankinson S, Johnson S, Manson J. Physical activity at different ages and incidence of premenstrual syndrome [Abstract]. Am J Epidemiol 2010;171:S146 [Google Scholar]
- 37.VanderWeele TJ, Vansteelandt S. Odds Ratios for Mediation Analysis for a Dichotomous Outcome. Am J Epidemiol 2010;172:1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA 2007;298:1685–1687 [DOI] [PubMed] [Google Scholar]
- 39.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol 2010;52:671–690 [DOI] [PubMed] [Google Scholar]
- 40.Bunevicius A, Leserman J, Girdler SS. Hypothalamic-pituitary-thyroid axis function in women with a menstrually related mood disorder: Association with histories of sexual abuse. Psychosom Med 2012;74:810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gingnell M, Comasco E, Oreland L, Fredrikson M, Sundstrom-Poromaa I. Neuroticism-related personality traits are related to symptom severity in patients with premenstrual dysphoric disorder and to the serotonin transporter gene-linked polymorphism 5-HTTPLPR. Arch Womens Mental Health 2010;13:417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med 2009;71:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertone-Johnson E, Mumford S, Pollack A, et al. Premenstrual syndrome and cytokine levels across the menstrual cycle [abstract]. Am J Epidemiol. 2011;173(suppl11):S603 [Google Scholar]
- 44.Puder JJ, Blum CA, Mueller B, De Geyter C, Dye L, Keller U. Menstrual cycle symptoms are associated with changes in low-grade inflammation. Eur J Clin Invest 2006;36:58–64 [DOI] [PubMed] [Google Scholar]
- 45.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004;82:217–225 [DOI] [PubMed] [Google Scholar]
- 46.Gibb BE, Chelminski I, Zimmerman M. Childhood emotional, physical, and sexual abuse, and diagnoses of depressive and anxiety disorders in adult psychiatric outpatients. Depress Anxiety 2007;24:256–263 [DOI] [PubMed] [Google Scholar]
- 47.Spertus IL, Yehuda R, Wong CM, Halligan S, Seremetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl 2003;27:1247–1258 [DOI] [PubMed] [Google Scholar]
- 48.Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry 2009;66:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knaapen L, Weisz G. The biomedical standardization of premenstrual syndrome. Stud Hist Phil Biol Biomed Sci 2008;29:120–134 [DOI] [PubMed] [Google Scholar]
- 50.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the Adverse Childhood Experiences Study. Am J Psychiatry 2003;160:1453–1460 [DOI] [PubMed] [Google Scholar]