Abstract
Significance: We provide a conceptual framework for the interactions between the cellular redox signaling hub and the phytohormone signaling network that controls plant growth and development to maximize plant productivity under stress-free situations, while limiting growth and altering development on exposure to stress. Recent Advances: Enhanced cellular oxidation plays a key role in the regulation of plant growth and stress responses. Oxidative signals or cycles of oxidation and reduction are crucial for the alleviation of dormancy and quiescence, activating the cell cycle and triggering genetic and epigenetic control that underpin growth and differentiation responses to changing environmental conditions. Critical Issues: The redox signaling hub interfaces directly with the phytohormone network in the synergistic control of growth and its modulation in response to environmental stress, but a few components have been identified. Accumulating evidence points to a complex interplay of phytohormone and redox controls that operate at multiple levels. For simplicity, we focus here on redox-dependent processes that control root growth and development and bud burst. Future Directions: The multiple roles of reactive oxygen species in the control of plant growth and development have been identified, but increasing emphasis should now be placed on the functions of redox-regulated proteins, along with the central roles of reductants such as NAD(P)H, thioredoxins, glutathione, glutaredoxins, peroxiredoxins, ascorbate, and reduced ferredoxin in the regulation of the genetic and epigenetic factors that modulate the growth and vigor of crop plants, particularly within an agricultural context. Antioxid. Redox Signal. 21, 1305–1326.
Introduction
Organ formation in plants consists of an initial phase of cell proliferation and primary morphogenesis, followed by a second phase of cell expansion, secondary morphogenesis, and endoreduplication (91). The orchestration of organ formation is complex, involving a diverse range of genes that promote or inhibit component pathways or processes. While the components that control plant cell cycle machinery have been identified in plants (48), relatively little is known about how they may be influenced by redox controls. Nevertheless, accumulating evidence indicates that cell proliferation and shoot and root meristem activities are influenced by cellular redox state (14, 152). Redox controls play a key role in the regulation of cell cycle progression (102, 148). Oxidation of the cytosol occurs at an early stage in cell cycle progression (67). The G1 and G2 checkpoints that regulate the cell cycle are highly responsive to oxidation (94). However, the mechanisms that underpin these responses and the pathways of oxidative activation of the cell cycle are not as clearly defined in plants as they are in animals (67).
Literature evidence suggests that the redox-dependent control of growth involves a network of interactions between reactive oxygen species (ROS; also called active oxygen species), antioxidants, and phytohormones such as auxin and cytokinin, which are major regulators of the plant cell cycle (50, 54, 182). The pleiotropic developmental changes observed in plants that are deficient in NADPH oxidases (also called respiratory burst oxidase homologs [RBOH]) imply defects in auxin and strigolactone (SL)-responses (152). The Arabidopsis thaliana genome has 10 RBOH genes (AtRBOHA-AtRBOHJ) (173). Although the functions of many of these NADPH oxidase forms remain to be characterized, RBOHC is considered important in the control of root hair tip growth (120). Similarly, the transcriptional mediator subunits, phytochrome and flowering time 1/mediator 25 (PFT1/MED25), have a crucial role in root hair formation by activating a subset of hydrogen peroxide (H2O2)-producing class III peroxidases (165).
Loss of SL-dependent control of shoot branching in the more axillary growth 2 (MAX2) loss-of-function allele ore9-1 correlated with increased ROS tolerance (193). Studies on A. thaliana mutants that are deficient in glutathione and thioredoxin have established that thiol-dependent steps in auxin transport are required for postembryonic root meristem development (14, 101).
Phytohormones such as auxin, abscisic acid (ABA), salicylic acid (SA), and brassinosteroids produce H2O2 via the activation of NADPH oxidases during signal transduction (13). The production of H2O2 as a result of phytohormone action is considered as amplifying oxidative signaling. In this system, enhanced cellular oxidation, as occurs for example in the A. thaliana mutants that are deficient either in the photorespiratory form of catalase or in the cytosolic form of glutathione reductase, triggers SA and jasmonate (JA) signaling (115, 116, 142). SA, in turn, induces a rapid increase in NADPH-oxidase activity (96), resulting in ROS production and leading to further cellular oxidation. This process and the associated changes in Ca2+ signaling and mitogen-activated protein kinase (MAPK) cascades leads to adaptive responses in growth, as well as in biotic and abiotic stress responses (118). Here, we discuss the evidence supporting the view that redox-dependent step(s) are important in the control of the development of plant organs, with specific reference to the root and perennial buds.
Redox Regulation of Cell Division and Cell Expansion
Cellular redox homeostasis plays an important role in every aspect of plant biology, including growth. The spatial regulation of ROS production is an important factor controlling plant form (66). Regulated ROS production is an important control of plant development operating through the regulation of cell division and cell expansion. Moreover, the responses of cells to cellular oxidation, responses to abiotic stress and to defense phytohormones such as ABA, which generate ROS, very much depend on cell identity (49, 86). Redox controls are important in the renewal and differentiation of stem cells, such as those that drive the growth of the root apical meristem. The arrest of cell cycle activity in the cells of root quiescent center (QC) is linked to the auxin maximum and the maintenance of a highly oxidized state in the stem cell niche (49, 86–88). While QC cells appear to be physiologically indistinguishable from the adjacent, actively dividing cells, for example in terms of mitochondrial function, they do not enter programmed cell death (87, 88). Stem cells, thus, avoid oxidative stress and the oxidative activation of genetically programmed cell suicide pathways, even though they are deficient in low-molecular-weight antioxidants (99). The cell cycle in the QC cells is arrested at G1, and this appears to be associated with the very low abundance of ascorbate and the thiol tripeptide, glutathione (GSH; γ-glutamyl-L-cysteinylglycine) in the QC, because the addition of GSH stimulates the cells to progress from the G1 to the S phase of the cell cycle (99). The addition of glutathione disulfide (GSSG; oxidized glutathione) or oxidized ascorbate (dehydroascorbate [DHA]) to proliferating cells causes an arrest in the cell cycle in G1 (137, 138). Moreover, the very low GSH levels observed in the root meristemless 1 (rml1) mutants of A. thaliana lead to arrest of the cell cycle at G1, specifically in the root but not in the shoot (186). There is net movement of GSH from the cytosol to the nucleus in G1, leading to cytosolic oxidation and reduction in the nucleus (67). The sequestration of GSH in the nucleus of proliferating cells may suggest that GSH has important roles in safeguarding the nuclear architecture (67).
In addition to regulating cell cycle progression, redox regulation has been implicated in the control of the transition from cell proliferation to cell differentiation in both plants and animals. While a few components involved in these processes have been identified in plants, the negative regulation of peroxidases by the UPBEAT1 transcription factor appears to influence the balance between H2O2 and superoxide in the complex regulation of the transition from cell proliferation to differentiation in the root (175).
Redox Regulation of Root Growth and Architecture
Redox regulation impinges on nearly every stage of root development, from the breaking of ABA-induced seed dormancy, by nitric oxide (NO), cyanide, or H2O2, to the development of lateral roots (LRs) and root hairs. For example, ROS production is required for oxidative signaling underpinning cellular elongation in root hair expansion (60). Similarly, ROS are positive signals for seed dormancy release, interacting with ABA, gibberellins (GA), and NO in signal transduction pathways that underpin seed germination (20, 154). Seed germination begins with the hydration of the quiescent seed, leading to the elongation of the embryonic axis from a seed, enabling the subsequent emergence of the metabolically active seedling. This transition from quiescent to metabolically active states is associated with the accumulation of superoxide radicals, H2O2, hydroxyl radicals, and NO. It is generally assumed that most of the ROS production originates from mitochondrial respiration, which resumes in imbibed seeds, but NADPH oxidases such as RBOHB are also required for seed ripening and germination (154). The nuclei in seeds contain antioxidant defense systems, including a 1-Cys peroxiredoxin that is almost exclusively expressed in seeds, to protect against the oxidative stress which occurs during seed maturation and germination (140, 161). The enhanced cellular oxidation that is triggered on germination is considered important for the regulated oxidative modification of proteins and of mRNAs that underpin regulatory pathways (56). In some species, ROS are required to overcome the mechanical resistance imposed by the endosperm, enabling the radicle to protrude through the weakened tissues. This process is regulated by ABA and GA, which regulate redox processes that are associated with cell wall loosening; high ABA maintains a high antioxidant capacity and prevents germination, while GA reverses this process (55, 58). GA regulates the stability of the DELLA proteins that control growth and also influences the extent of ROS accumulation through effects on the expression of antioxidative enzyme genes (3). While the levels of GA and ABA in germinating seeds are regulated by transcriptional control of genes involved in the phytohormone biosynthetic pathways, and by deactivating genes, GA and ABA signal transduction mainly depends on post-translational regulation.
The founder cells of the primary root are produced by cell division during the development of the embryo. A single cell in the embryonic root called the hypophysis gives rise to both the QC and the columella root cap. Primary root development after germination strongly depends on the phytohormone auxin. The key role of GSH in the postembryonic development of the primary root was established on characterization of A. thaliana rml mutants (33, 186). Four rml mutants were originally identified due to their inability to maintain cell division after germination; thus, they fail to produce a functional postembryonic root meristem (33). Cell division is unimpaired in the embryos, shoots, and calli of these mutants, but the cell cycle is arrested in the primary root at an early stage. GSH was subsequently identified as the factor in the root that is specifically required to activate and maintain the cell division cycle in the root apical cells (182). The effects of GSH deficiency on root architecture in A. thaliana are illustrated in Figure 1. In contrast to the wild-type (Col-0) seedlings, the roots of the rml1 mutant fail to develop because of arrest of root meristem development (Fig. 1). In addition, arrest of the root, but not the shoot, meristem, can be induced in wild-type seedlings by incubation with the glutathione synthesis inhibitor, L-buthionine sulfoximine (BSO; Fig. 1). However, LR density is not decreased in the presence of BSO (111).
FIG. 1.

Root phenotypes in the Arabidopsis thaliana wild type (Col-0) and the root meristemless 1 (rml1) mutant and also in the wild-type seedlings after treatment with the glutathione synthesis inhibitor, L-buthionine sulfoximine (BSO). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The rml1 mutant is one of a number of A. thaliana mutants that have been characterized and shown to have defects in the GSH1 gene which encodes the enzyme γ-glutamate-cysteine ligase (GCL; also called γ-glutamylcysteine synthase), which catalyzes the first step of GSH synthesis. This pathway involves two ATP-dependent steps, catalyzed by GCL and GSH synthetase (GSHS; also called GSH synthase). The rml1 mutant has less than 5% of wild-type GSH levels and is unable to establish a postembryonic root meristem because of the arrest of the cell cycle at G1 (186). The addition of BSO to wild-type A. thaliana seedlings also leads to an arrest of the root meristem (101). Further genetic analysis demonstrated that there is a redundancy in GSH and thioredoxin functions in the control of shoot apical meristem development (14, 143). Studies involving crosses between the rml1 mutant that is deficient in GSH and mutants which are unable to regenerate reduced thioredoxin in the cytosol because they lack functional cytosolic NADPH-thioredoxin reductases (NTRs) display root and shoot meristemless phenotypes (143). Other mutations in the GSH1 gene result in less severe restrictions in the GSH synthesis pathway. For example, the rax1-1, cad2-1, and pad2-1 mutants exhibit characteristic decreases in LR density relative to the wild type (37, 111).
LR development is initiated by auxin (18, 29, 62). Auxin is required for the initiation process that enables the cells in the pericycle layer to re-enter the cell cycle (29). LR production is central to the architecture of the root and determines the spatial arrangement of the root system in the soil (110). The development of the LR primordia is sensitive to environmental stimuli such as the availability of nitrogen or water, enabling plants to optimize their root architecture according to environmental cues. The initiation of LRs requires the activation of cell division from the pericycle layer in the differentiation zone of the root that is distant from the root apical meristem (46, 57, 110). The developmental process starts with an asymmetric transverse division of the pericycle cells adjacent to the two xylem poles of the main root, followed by a series of periclinal and transverse cell divisions, leading to the formation of a dome-shaped structure called the LR primordium (LRP). The LRP then grows by both cell division and cell expansion through the overlaying cell layers and emerges via cell expansion. After emergence, the LRP undergoes an activation step to form a fully functional meristem (110). The generation of each LRP is regulated by a network of interacting pathways that affect different phases of the developmental process (46, 110). Auxin is required for the correct control of cell division and patterning throughout the entire developmental process (17, 45). The effects of auxin (1-naphthaleneacetic acid; NAA) and the auxin transport inhibitor (1-N-naphthylphthalamic acid; NPA) on the development of LRs in A. thaliana seedlings are illustrated in Figure 2.
FIG. 2.
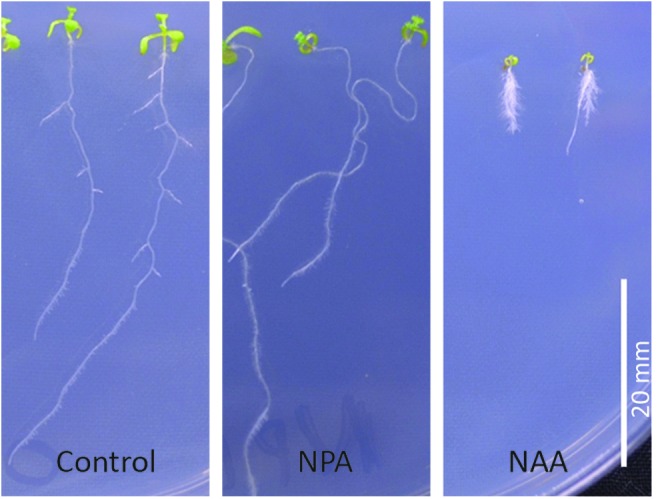
The effects of auxin (NAA) and the auxin transport inhibitor (NPA) on lateral root (LR) development in A. thaliana seedlings. NAA, 1-naphthaleneacetic acid; NPA, 1-N-naphthylphthalamic acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Markers for auxin accumulation and response such as DR5::GUS are observed during LR formation (18, 53). The formation of the LRP is also influenced by other phytohormones such as ethylene. Ethylene stimulates LR formation, while ABA, cytokinins, and SLs act antagonistically to auxin and ethylene (5, 62). However, unlike ethylene, the ABA pathway functions at the later stages of LR development (45). Moreover, the ABA pathway is independent of auxin, and it is important in plant responses to nitrogen deficiency and water deficits (45).
Phytohormones such as auxin, ABA, and brassinosteroids trigger superoxide production and H2O2 accumulation through the activation of NADPH oxidases and cell wall peroxidases, such as pH-dependent cell wall peroxidases, germin-like oxalate oxidases, and polyamine oxidases (136, 158). The regulated production of ROS in the apoplastic face of the plasma membrane underpins the phenomenon called oxidative burst, which is important in the hypersensitive response to pathogens (47). The oxidative burst is activated in response to many types of stimuli, particularly the action of a number of phytohormones. For example, H2O2 accumulation in response to ABA is required for the control of stomatal movements (118, 158).
Unlike the cytosol, which is rich in low-molecular-weight antioxidants, the apoplast/cell compartment of the cell has little glutathione and only relatively low levels of ascorbate. The low antioxidant buffering capacity of the apoplast means that H2O2 is able to accumulate in the apoplast to a much greater extent than would be possible within the cytosol, enabling the formation of the oxidative burst and leading to oxidation specifically on the outside plasma membrane. This strong oxidative signal on the external face of the plasma membrane can cause adjustments in calcium transport and signaling, alter other ion fluxes, and modify plasma membrane-based electron transport systems. Moreover, H2O2 can be transported via the aquaporins from the apoplast into the cytoplasm, where it is metabolized (19).
Genetic evidence has demonstrated links between thioredoxins, GSH, and auxin in the control of shoot and root development (14, 101, 143, 186). For example, the expression of auxin transporters was decreased by GSH depletion (101). Moreover, the triple cad2 ntra ntrb developed in the same way as the wild type up to the rosette stage, but they were unable to sustain floral meristem development, producing a PIN-formed (PIN) structure that is characteristic of impaired auxin transport (14). Auxin transport is regulated by influx (AUX1, LAX1–LAX3) and efflux (PIN) transporters, as well as by B-type ATP-binding cassette subfamily G (ABCG) transporters. The ABCG transporters are largely found on the outer surface of the root epidermis. The direction of auxin transport is regulated to a large degree by the sub-cellular distribution of the PIN proteins (61, 168).
Redox Interactions Involving Auxin and Other Phytohormones Involved in the Control of Plant Growth and Development
Indole-3-acetic acid (IAA) is the principal auxin in plants such as A. thaliana (132). It is synthesized primarily in meristems and nodes and is transported to distal parts of the plant in response to developmental programming or environmental stimuli, in order to activate cell-specific responses (Fig. 3). Tissue-specific auxin gradients and auxin accumulation are highly regulated at a cellular level to control plant growth (32, 41, 171). In addition to its requirement for LR formation, auxin is important in the production of adventitious roots and root hairs, and in the control of apical dominance, stem elongation phototropic, and gravitropic responses (73, 100). Gravitropic stimuli lead to curvature of the roots by triggering asymmetric auxin flow and, hence, localized ROS production at the root tip (92). The localized accumulation of auxin is decreased by conjugation or catabolism when downstream responses have reached their optima (132). Auxin accumulation increases both the 2-oxindole-3-acetic acid (oxIAA) and ROS (largely H2O2) levels in most cell types. Auxin-induced ROS production is mediated, at least in part, by the activation of RBOHD (92, 195, 196).
FIG. 3.
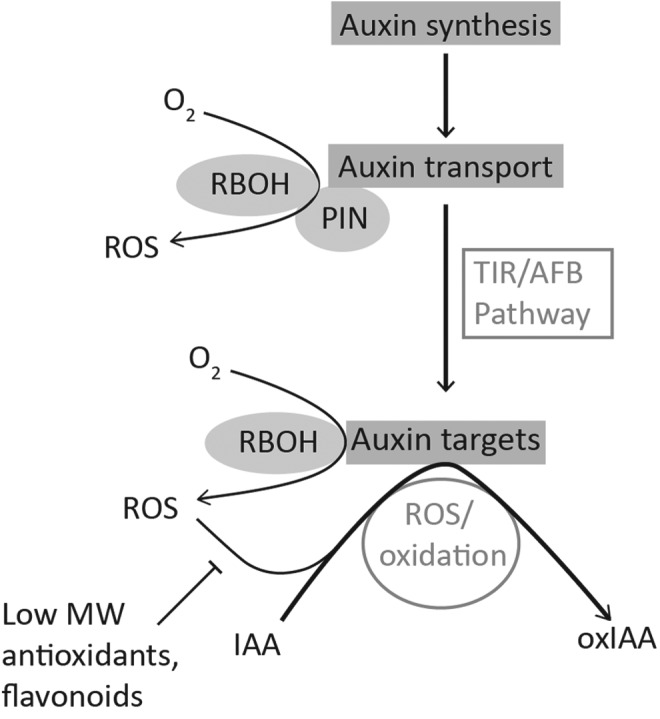
A schematic model of the regulation of auxin (IAA) signaling by redox processes. Auxin is transported from cell to cell in a directional or polar manner that is facilitated by efflux transporters such as the PIN proteins. The spatial distribution of auxin drives plant growth responses and development. The accumulation of auxin in cells is linked to altered cellular redox status, involving the production of reactive oxygen species (ROS) by the NADPH oxidases (also called respiratory burst oxidase homologs [RBOH]) and redox components such as the monothiol glutaredoxin, AtGrxS17 (34). The resultant oxidation of IAA to produce oxIAA attenuates auxin signaling. IAA, indole-3-acetic acid; oxIAA, 2-oxindole-3-acetic acid; PIN, PIN-formed.
Attenuation of the auxin signal is considered as involving oxidation and the irreversible production of the catabolic product, oxIAA. oxIAA is inactive and does not induce the expression of auxin responsive markers such as DR5 (132). IAA signaling is attenuated, at least in part, by IAA catabolism to oxIAA, as illustrated in Figure 3. Unlike IAA, oxIAA is not transported from cell to cell, even though it is a substrate for the ABCG transporters. Mutants that are defective in ABCG transporters accumulate oxIAA, oxIAA-Glc levels, and auxin because of defective cellular export (132).
The amounts of oxIAA measured in the flavonoid-deficient tt4 mutants were higher than those found in the wild-type plants, and they were lower than in tt3 mutants that accumulate excess flavonol-type compounds (132). Antioxidants such as ascorbate and GSH eliminate superoxide and H2O2 and, therefore, serve to prevent ROS accumulation in cell types with localized increases in IAA. However, the abundance of low-molecular-weight antioxidants such as ascorbate and GSH is negligible in the QC cells of the primary root meristem that accumulate auxin, unlike the surrounding cells and those in the elongation zone, which have a high antioxidant capacity. The highly oxidized state of the QC cells will favor rapid attenuation of the auxin signal by catabolism. The interaction between auxin signaling pathways and redox signaling pathways permits flexible regulation that is highly responsive to cell metabolism (130, 170).
The complex interplay between directional cell-to-cell auxin transport that allows auxin gradients and auxin catabolism allows the correct setting of developmental cues. Local auxin maxima, along with the differential distribution of auxin signaling pathways, enables the orchestration of gene expression in a tissue-specific manner at appropriate stages of development (embryogenesis, organogenesis), and facilitates directional controls of growth in response to environmental stimuli. The highly effective buffering of IAA oxidation by the antioxidant network in most tissues could explain why auxin transport and signaling may become localized to cells with a relatively low antioxidant capacity, such as the QC cells in the root apical meristem.
Rapid changes in the protein composition of the plasma membrane are facilitated by a process called “constitutive cycling” that involves an available pool of plasma membrane proteins, including PIN proteins, which are accumulated in early endosomes. The PIN proteins are exchanged between the plasma membrane and the “early” endosomes, with constant cycling in and out of the plasma membranes; the internalization of the PIN proteins is facilitated by a clathrin-dependent endocytosis mechanism (31, 61). Vesicular trafficking of protein requires appropriate control of oxidative folding and reduction of component proteins, a process that is influenced by the availability of GSH and its precursor γ-glutamylcysteine (γ-EC). Moreover, the expression of some of the PIN transporter family members is regulated by cellular reductants, such as GSH (14, 101). In contrast, flavonoids tend to repress polar auxin transporters (133). The redox regulation of genes encoding key auxin transport and signaling proteins, along with oxidative inactivation of IAA by NADPH oxidase-dependent ROS production, may facilitate redox control of polar auxin transport (93). Moreover, antioxidants also influence the interactions of PIN proteins with regulatory interacting components, such as the protein phosphatase 2A, alpha, catalytic subunit (PP2AA), and the PINOID kinase (27, 61, 153). Auxin-induced ROS production by NADPH oxidases in the root cells (93) requires the activation of phosphatidylinositol 3-kinase (PtdIns 3-kinase), which produces PtdIns(3)P, which regulates endocytosis and vesicle trafficking. Phospholipid signaling and redox pathways, therefore, cooperate in the control of PIN-dependent auxin transport (203).
Auxin flow activates the transport inhibitor response 1/auxin-binding f-box protein (TIR1/AFB) pathway that regulates the expression of auxin-induced, primary-response genes and facilitates auxin signaling in roots and other tissues (Fig. 3). Auxin binds to the TIR1 F-box protein subunit of the E3 ubiquitin protein ligase, Skp, Cullin, F-box containing complex (SCFTIR1), which facilitates targeted protein degradation. Auxin binding to TIR1 destabilizes interactions between the TIR1/AFB families of auxin receptors that regulate the expression of auxin-regulated genes. When auxin is low or absent, the transcription factors called auxin response factors (ARFs) bind to negative regulators that keep them in an inactive state, which prevents the expression of target genes. IAA binding to TIR1 liberates the ARFs, enabling target gene expression. The tir1/afb mutants of A. thaliana have more ascorbate and higher leaf ascorbate peroxidase (APX) activities, suggesting that the TIR/AFB signaling pathway can influence the tissue antioxidant capacities (85). Moreover, genes encoding antioxidant enzymes are expressed early in the auxin response (1, 68, 178).
The catalase-deficient (cat2-1) mutants of A. thaliana were reported to have curled leaves (65) when grown at a relatively high light intensity (150 μmol m−2 s−1) compared with a lower light intensity (30 μmol m−2 s−1). Leaf curling in these mutants at the higher light intensity was linked to decreased levels of transcripts encoding auxin synthesis genes and lower auxin levels relative to the wild-type controls (65). Moreover, enhanced cellular oxidation caused by decreased H2O2 metabolism in these mutants was suggested to influence the abundance and availability of auxin (65). However, the enhanced oxidation in cat2-1 leads to oxidation of the leaf glutathione pool and so, although the total glutathione pool is much higher in cat2-1 leaves than in the wild type, the GSH/GSSG ratios are considerably lower. The application of GSH to cat2-1 increased auxin levels and prevented leaf curvature in cat2-1 (65). This observation is consistent with the concept that GSH is an important mediator of the cross-talk between H2O2 and auxin signaling. Accumulating evidence suggests that oxidative signals are partly transmitted by modulation of the redox status of the glutathione pool.
Similar to auxin, SLs fulfill essential roles in the control of plant development (50). The SL synthesis and signaling pathways have now been studied in a wide range of plant species, including A. thaliana, pea, rice, and petunia (197). SL biosynthesis involves the action of β-carotene isomerase (D27) and two carotenoid cleavage dioxygenases (CCDs). In A. thaliana, these CCDs are often referred to as the MAX3 and MAX4 proteins, respectively, as illustrated in Figure 4 (72, 77, 179, 189). A subsequent oxidation by a cytochrome P450 (MAX1) results in the production of a mobile compound that requires an F-box protein (MAX2) and an α/β hydrolase (D14) to elicit its effects on shoot branching (72, 75, 77, 163, 164, 179, 197). Mutants in either SL synthesis or signaling typically exhibit altered shoot and root branching phenotypes, but an analysis of these mutants has also revealed roles for SLs in other processes, such as photomorphogenesis and leaf senescence (72, 151, 174, 179). The responses of root architecture to the addition of the artificial SL (GR24) can be easily illustrated in A. thaliana (Fig. 4). LR development is inhibited by the presence of the artificial SL, GR24 in wild-type A. thaliana (Col-0) seedlings, and in mutants that are deficient in the SL signaling MAX2 protein (max2-1 and max2-3 in Fig. 4). Similar to auxin, SLs interact with the redox signaling network (193). Mutants that are defective in the signaling protein MAX2 (ore9) are defective in SL-dependent control of root branching, and they are more tolerant to oxidative stress than the wild type (164, 192).
FIG. 4.
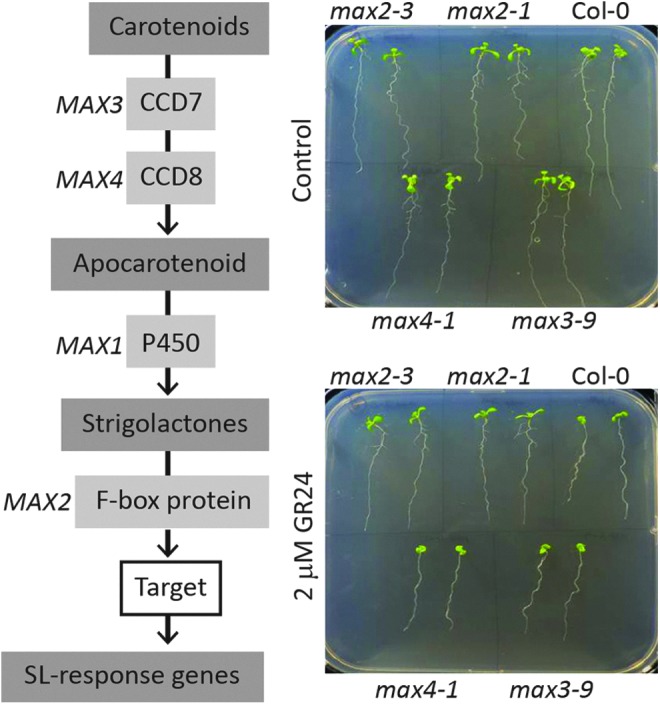
Schematic model of the strigolactone (SL) synthesis pathway, along with a study showing the typical responses of root architecture to the addition of the artificial SL (GR24) in the A. thaliana. The addition of 2 mM GR24 significantly decreased LR density in the wild-type (Col-0) plants and in the max3-9 and max4-1 mutants, but not in max2-1, confirming the role of the MAX2 protein as an important SL signaling component (110). MAX2, more axillary growth 2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
SLs function downstream of auxin for the control of shoot (72, 179) and root branching (97, 98, 151). The auxin and SL signaling pathways influence the total root glutathione pool, but not the GSH/GSSG ratio, as illustrated in Figure 5. The SL signaling pathway, which restricts root branching, favors a high root glutathione pool (Fig. 5). Conversely, the auxin pathway, which controls root architecture and triggers root branching (Fig. 1), restricts root GSH accumulation (Fig. 5). There is, therefore, a link between SLs and the size of the root glutathione (GSH plus GSSG) pool in the control of LR development that occurs in an MAX2-dependent manner (111).
FIG. 5.
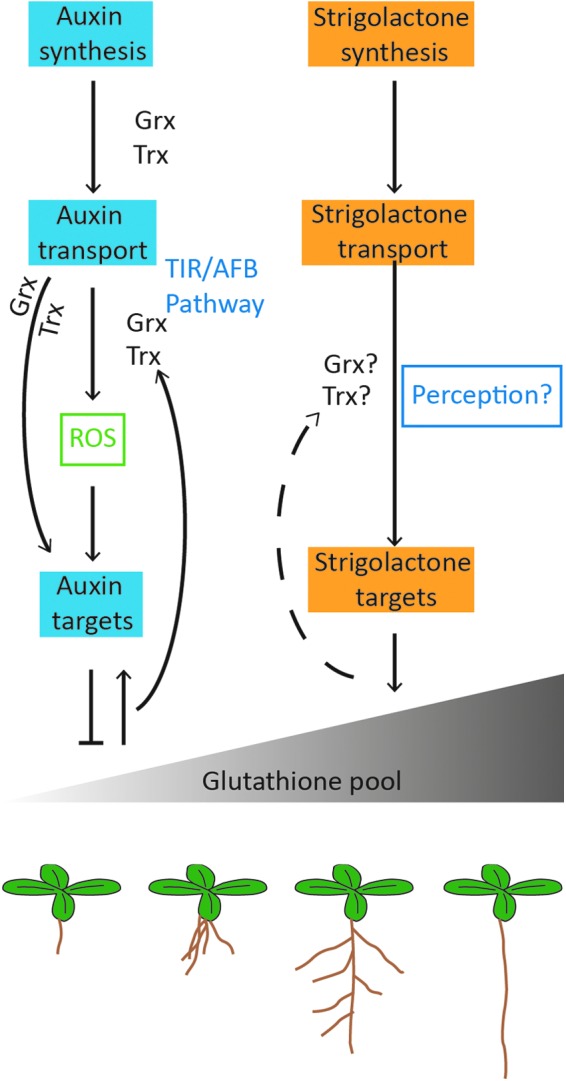
A schematic model linking redox regulation exerted through the glutathione pool to the auxin and SL-mediated control of root architecture. Auxin is transported from cell to cell in a polar manner to control root development. The transport action of auxin is influenced by glutaredoxin (Grx) such as AtGrxS17 (34) and thioredoxins (Trx; 14). The auxin-regulated TIR/AFB pathway elaborates root architecture and stimulates the growth of LRs. However, this process is dependent on the abundance of glutathione. A low abundance of glutathione serves to decrease LR density; for example, all of the GSH synthesis mutants show decreased lateral density compared with the wild type (111). Very low glutathione levels such as those observed in the A. thaliana root meristemless mutant (rml1) arrest cell division in the postembryonic root meristem such that root organogenesis is prevented (186). SLs act antagonistically to auxin in the control of LR proliferation. The addition of SLs to A. thaliana decreases LR density and increases the abundance of glutathione in the roots (111). SLs also enhance tolerance to oxidative stress, and they may, therefore, influence Grx and Trx-mediated processes. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
GA and auxin have many overlapping functions in the control of organ expansion. GA regulates plant growth in response to environmental signals by relieving the constraints on gene expression imposed by a family of growth-repressing regulators, called DELLA proteins (76, 134), which decrease the potential for cell proliferation (159). The GA pathway interacts with auxin, limits the lifespan of these nuclear growth-repressing proteins, and, so, controls plant growth. However, the DELLA proteins also play important roles in stress tolerance (2, 4, 76). For example, A. thaliana mutants that have low GA contents or which have GA-insensitive forms of DELLA proteins exhibit increased salt tolerance (2). Conversely, loss-of-function mutants lacking DELLA proteins exhibit a higher level of sensitivity to salt stress (2). The imposition of salt stress increases the abundance of DELLA proteins, and favors decreased stress-induced ROS accumulation, presumably because of the enhanced expression of genes encoding antioxidant enzymes (3). Increases in the ratio of ABA to GA that occur in response to stresses such as drought and high salt favor DELLA protein accumulation and enhanced antioxidant activities (59).
High ABA/GA ratios tend to induce dormancy in tubers, buds, and seeds. For example, ABA decreased GA accumulation in rice seeds and increased oxidation because of a decrease in ascorbate accumulation (201). Dormancy release was stimulated by GA and ROS, but it was inhibited by antioxidants (127).
Bud Development and Dormancy in Temperate Perennials
Unlike the redox regulation of root development that has received considerable attention over the past decade, the role of redox processes in many other tissues has been less intensively studied. In particular, the role of redox processes in bud dormancy and subsequent bud burst is poorly documented; however, this process is of considerable agronomic interest for the productivity of temperate perennial plants, which cycle between periods of growth and quiescence in synchrony with the annual seasonal climate. The duration of the photoperiod and prevailing temperature are primary cues for both processes (Fig. 6). This is a survival strategy that is common to many perennial horticultural crops, including grapevine, kiwifruit, and most Rosaceae fruits, as well as to temperate and arboreal trees such as poplar, oak, and birch.
FIG. 6.
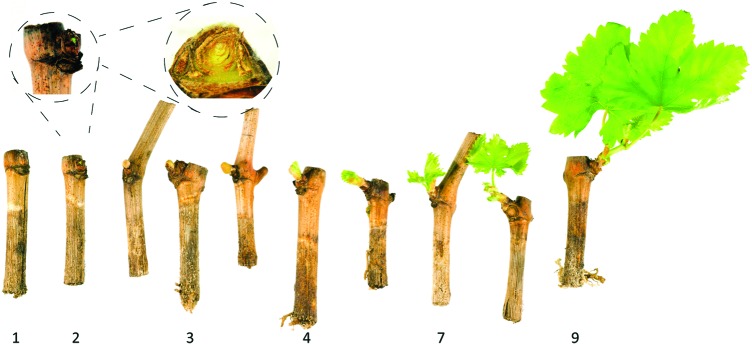
Developmental progression of bud burst in grapevine according to the Modified Eichhorn-Lorenz System (E-L) (40). Once the dormancy requirement has been met and the bud is competent to recommence growth, the bud [stage 1] begins to swell [stage 2] and burst through the bud scales [stages 2–3], showing the compound bud enclosed in protective hairs [stage 3] before the first leaf tips of the primary shoot become visible [stage 4]; this stage defines “bud burst.” Shoot extension and expansion continues through [stage 7], and any inflorescence present in the leaf axils becomes visible after [stage 9]. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
As winter approaches, axillary or apical meristems make the transition to a quiescent state within a hardy bud that protects the vegetative and reproductive initials until favorable growth conditions return. The acquisition of perennial bud dormancy can be divided into three processes, that is, bud formation, the acquisition of cold and dehydration tolerance, and quiescence and dormancy (150). Bud dormancy per se is intrinsically derived from the branching process, which, as discussed earlier, is governed by the relative abundance of phytohormones such as auxin, SLs, and ABA. The formation of the bud is governed by apical dominance (paradormancy), while the transition to “true dormancy” (endodormancy) requires the acquisition of a quiescent or metabolically inhibited state. In this state, the buds are unable to respond to conducive conditions and burst until dormancy is relieved by an appropriate signal that arises within the bud [ecodormancy is not considered here (104, 145, 188)].
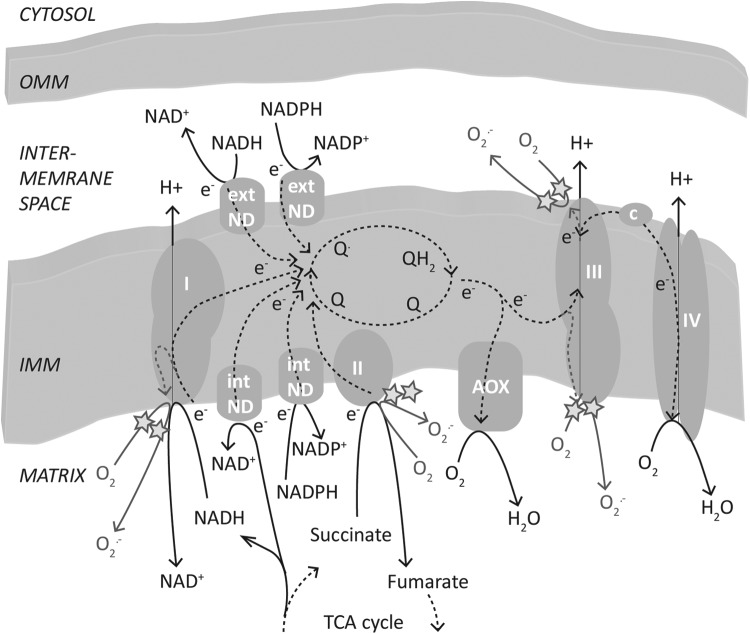
The process of ecodormancy will not be considered here, but it has many similar features to those described here, and ecodormancy succeeds endodormancy. It is the state of developmental “competence,” but this process is repressed by unfavorable environmental conditions. In both cases, the regulation of the hormonal balance in the bud is pivotal in regulating the state of dormancy. The quantitative nature of bud dormancy has similarities to seed dormancy and inflorescence vernalization, and it implies the creation of genetic and epigenetic memories that underpin the regulation of subsequent developmental processes. Moreover, similar to the situation in the mature seed, it is likely that the meristematic tissues in the embryo reside in a hypoxic environment. The availability of oxygen to support respiration, undoubtedly, plays an important role in regulating the state of dormancy and dormancy release to produce the metabolically active state. While relatively few physiological measurements have been performed, our own unpublished data suggest that the variable partial pressure of oxygen (pO2) is regulated within the bud during seed dormancy and bud burst, and this regulation may have important implications for the regulation of respiration and ROS production by the respiratory electron transport chain (Fig. 7).
FIG. 7.
Schematic model of the production of superoxide by the respiratory electron transport chain. Briefly, electrons (e−) involved in either reduction or oxidation of the ubiquinone cycle (Q, Q·, QH2) of the inner mitochondrial membrane may participate in the partial reduction of oxygen (O2) to form superoxide (O2·−). Primary sites for partial reduction in plants are the matrix side of Complexes I and III (CI, CIII) and the intermembrane space side of Complex III, as illustrated by a star symbol. In addition, the partial reduction of oxygen at Complex II (CII) in plants and animals has been demonstrated, although in plants, it is considered a minor contribution to mitochondrial superoxide production. ext ND, external NAD(P)H dehydrogenase of mitochondria; int ND, internal NAD(P)H dehydrogenase of mitochondria; IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane.
Before the availability of genomic data and genetic manipulation in perennials, it was difficult to deduce causal relationships between phytohormone signaling pathways and the control of bud development, dormancy, and burst (188). Unfortunately, A. thaliana meristems do not exhibit dormancy, and this fact alone has probably retarded the development of new concepts underpinning our current understanding of the acquisition and regulation of dormancy in meristems. Gleaning useful information with regard to the influences of various phytohormones on bud development and dormancy by an analysis of database information, particularly transcriptome and information from RNA Sequencing (RNAseq; also called whole transcriptome shotgun sequencing), is also somewhat limited because of the prevalence of post-translational regulation in phytohormone and oxidative signaling processes. Thus, relatively few studies over the past decade have gone beyond inferences derived from our understanding of seed dormancy or our reliance on annual models, where reverse genetic approaches are much more easily performed.
Regulation of Bud Formation by ABA/GA and Ethylene Interactions
Bud formation involves the differentiation of the embryonic leaves and scales that enclose the bud, the acquisition of cold and drought tolerance, and the cessation of metabolic and meristem activities. In the apical buds of poplar (Populus tremula×P. alba>) trees, the transition from long to short photoperiod conditions triggers the coordinated induction of ethylene biosynthesis and related signal transduction, followed by the expression of ethylene-response genes (145). Next, the induction of ABA-modulated pathways is achieved through an increase in ABA biosynthesis and ABA-related signal transduction pathways (150). The accumulation of ethylene-related transcripts precedes bud differentiation, while the presence of enhanced levels of ABA-related transcripts accompanies differentiation and bud formation before the cessation of meristem activity (150). The pivotal role of ethylene signaling early in the bud formation process, induced by the transition to short days, is further illustrated by studies on ethylene-insensitive birch (Betula pendula). Birch trees expressing a dominant negative allele of the A. thaliana ethylene triple response (etr1-1) gene exhibit retarded bud differentiation and incomplete bud formation, compared with wild-type controls (149). Notably, the buds of the etr1-1 lines did not accumulate ABA in the manner observed in the wild type, and they exhibited an impaired response to the application of exogenous ABA. A reciprocal relationship between ABA and ethylene signaling pathways has been demonstrated in poplar lines expressing a dominant negative allele of the A. thaliana abscisic acid-insensitive 1 (abi1-1) gene. ABI1 encodes a type 2C protein phosphatase (PP2C) that functions as a negative regulator of the ABA signaling pathway. H2O2 inactivates PP2Cs, which are often considered targets for ROS in mediating ABA responses. In A. thaliana, ABA-induced ROS production and ABA-dependent activation of Ca2+ channels are impaired in mutants lacking PPC2. The poplar lines expressing the mutated abi1-1 displayed increased ethylene biosynthesis and an ethylene-dependent increase in shoot growth (6). Furthermore, loss of ABI1 function blocked the inhibitory effect of exogenous ABA on the growth of lateral buds. In contrast, transgenic poplar lines with sense or antisense expression of ABI3 constructs had no visible phenotype when grown under long day conditions. However, after the transition to short days, these lines showed a markedly altered differentiation of embryonic leaves and scales, as well as malformed buds (146). Ethylene signaling was not altered by changes in ABI3 function (146). The ABI3 protein is a negative regulator of ABA signaling pathways and is important in the acquisition of cold and drought tolerance in seeds (150 and references therein). It is interesting to note that despite the genetic manipulation of either the ethylene or ABA signaling pathways, the buds examined in these studies still developed an endodormant state (149, 150), indicating the importance of other developmental controls.
The role of GA in bud dormancy is illustrated in studies on the phenological characterization of poplar lines expressing sense or mutant constructs for GA-related transcripts. The altered expression of two DELLA-domain proteins, GA insensitive (GAI) and repressor of GAI-like (RGL), advanced bud formation, although curiously this effect was not observed in lines of either GAI or the dominant negative gai under a constitutive promoter (202). Transgenic poplar lines with constitutive expression of the poplar gene encoding the GA catabolic enzyme GA2 oxidase (GA2ox) did not exhibit an alteration in the timing of bud formation. In contrast, ectopic expression of a bean GA2ox advanced bud formation (202). As described earlier for studies on ABA and ethylene signaling, the altered phenology and phenotypes, observed in the GA-related transformed lines, were not accompanied by the acquisition of endodormancy. Roles for auxin-, SL-, and cytokinin-related regulation during bud formation cannot be excluded, despite the apparent absence of signatures for these regulators in the studies described earlier. This absence may, in part, be due to an under-representation of appropriate transcripts on the array platforms used in these studies (149, 150), along with a difficulty in dissecting the roles of phytohormone signals without consideration of post-translational regulation, for example, through targeted reverse genetic approaches.
The Acquisition of Endodormancy Shares Features of Flowering Time Regulation Pathways and Requires MIKC MADS Box Transcription Factors
As discussed earlier, genetic alterations in the ABA, ethylene, and GA signaling pathways did not prevent the acquisition of endodormancy. The observation that the regulation of bud dormancy may share components with the flowering regulatory pathway was revived in the past decade after the characterization of the long-elusive florigen “flowering phytohormone” (82, 145). The flowering locus T (FT)/constans (CO) regulatory module is a model of photoperiod responses in plants (176). The acquisition of dormancy was retarded in poplar or plum constitutively expressing the flowering promoter FT (23, 160), while poplar RNA interference (RNAi)-FT lines formed buds and acquired dormancy more rapidly than the wild type, even under long photoperiod conditions that do not induce dormancy in the wild type (23). Other photoperiod-related flowering regulators have also been shown to manipulate dormancy and flowering phenology in perennials, including the phytochrome (PHY) A in poplar (23), and FT, centroradialis, and flowering locus D in kiwifruit (183), although CO-overexpressing poplar lines did not display altered phenology (83).
The similarities in the molecular regulation of dormancy and flowering, albeit with some distinction, have been demonstrated further by the genetic characterization of the evergrowing peach (Prunus persica) mutant (evg). The evg peach mutants are unable to form buds or enter dormancy. The evg locus contains a deletion of six tandem short vegetative phase (SVP)-like genes, namely dormancy-associated MADS-box (DAM) (21). Numerous MIKC-type MADS box transcription factors have well-characterized functions in developmental regulation, particularly in response to seasonal signaling underpinning flowering (78). The abundance of DAM transcripts was increased during endodormancy in perennial species but decreased in response to chilling and by the induction of bud burst (90, 107, 194, 199). Transcriptome comparisons have led to the identification of a number of transcripts that are differentially increased in abundance in the evg mutants in wild-type peach meristems, after the transition to short days. These observations demonstrate the importance of ABA signaling pathways (90), along with redox signaling and photoperiod and temperature effects in the acquisition of bud dormancy (90), as summarized in Figure 8.
FIG. 8.
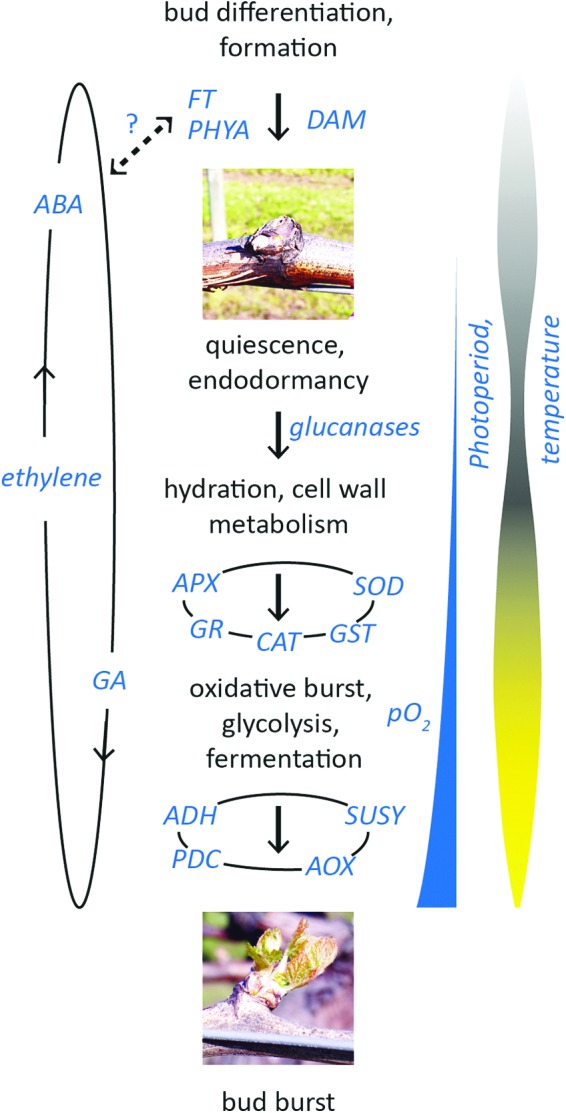
Scheme for primary seasonal and signaling influences on bud endodormancy transitions of temperate perennial plants. Photoperiod and temperature are primary environmental cues; their respective influence is quantitatively and qualitatively determined by genetics and management (in production). The phytohormones abscisic acid (ABA), ethylene, and gibberellic acid (GA) influence spatiotemporal features of bud formation and dormancy acquisition, but not the decision to enter dormancy. These phytohormones are also involved in endodormancy release. Transcription factors flowering locus T (FT), phytochrome A (PHYA), and dormancy-associated MADS-box (DAM), in addition, influence the fate of the meristem in the decision to enter dormancy. Hydration and cell wall metabolism are the first physiological features of endodormancy release, through which cellular signaling and redox perturbation create a hypoxic state (pO2), and subsequent activation of glycolysis and mitochondrial metabolism drive sufficient energy for bud burst. APX, ascorbate peroxidase; SOD, superoxide dismutase; GR, glutathione reductase; CAT, catalase; GST, glutathione S transferase; pO2, partial pressure of oxygen; ADH, alcohol dehydrogenase; PDC, pyruvate decarboxylase; AOX, alternative oxidase; SUSY, sucrose synthase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
While the DAM and FT regulons play a key role in dormancy acquisition in perennial species, little is known about how these factors contribute to the maintenance of dormancy or dormancy release. Studies on the transgenic poplar (P. tremula×P. tremuloides) lines expressing one of two isoforms of the A. thaliana GA2ox have provided some insights into the GA-dependent regulation bud dormancy (113). These data suggest that the abundance of bioactive GAs is important for the acquisition and maintenance of paradormancy (113).
Oxidative Signals and Respiratory Control During Bud Dormancy and Burst
As in seed dormancy, the relative abundance of phytohormones such as ABA and GA determine the dormant state in buds, with accompanying redox controls and oxidative signaling contributing to phytohormone-mediated regulation of dormancy and bud burst. Much of our current understanding of the role of oxidants and reductants in these processes comes from an appreciation of the requirement for stress-induced changes in redox metabolism that perturb phytohormone-mediated control of hypoxia and cellular redox homeostasis in the dormant state. It is generally accepted that exposure to chilling stress is required to overcome endodormancy in temperate perennials. The response to chilling is quantitative, as seen by a gradual increase in the number of differentially expressed transcripts (52, 64, 141, 155, 198, 204). Transcripts associated with cold-hardiness and oxidative stress tolerance accumulate as a result of exposure to chilling, as well as transcripts encoding proteins involved in glycolysis, fermentation, and cellular signaling (52, 80, 126). Transcripts such as alcohol dehydrogenase (ADH), pyruvate decarboxylase (PDC), and sucrose synthase (SUSY) are considered markers of bud endodormancy (74, 114, 126, 128). The transcript signature of bud endodormancy exhibits remodeled oxygen and energy metabolism that strongly resembles the signature generated by tissues in a hypoxic state [refer next to hypoxia (11, 63)].
In an agronomic context, physical and chemical treatments are frequently used to break bud dormancy and to trigger bud burst. This practice is important in horticulture, where seasonal variability and market demands require management intervention to augment bud burst, and particularly, to coordinate the time of bud burst, which, ultimately, affects flowering, fruiting, and harvest time. The allelochemical hydrogen cyanamide is widely used in the perennial fruit industry to break bud dormancy, despite the risks of phytotoxicity. The transcriptional changes in buds triggered by these treatments provide further insights into the requirement for stress-induced changes in metabolism and cellular redox state in regulating the transition to bud burst. Comparisons of transcript profiles generated in response to chilling, exposure to hydrogen cyanamide, heat shock, sodium azide, and H2O2 exhibit a similar induction of a suite of redox-related genes, including ADH, PDC, and SUSY, as well as glutathione-s-transferase, catalase, superoxide dismutase, ascorbate peroxidase, and glutathione reductase (126, 135, 187). Crucially, H2O2 accumulated in buds after treatment with hydrogen cyanamide, or after exposure to hypoxia (5.2–8 kPa pO2), or inhibition of the cytochrome pathway of mitochondrial respiration (126, 185). It is interesting to note in this regard that the altered expression of oxidative stress-induced mitochondrial proteins such as the A. thaliana LEA5 (also called SAG21) has a profound effect on plant growth and stress tolerance (Fig. 9) (119, 121). Another mitochondrial member of this typically unstructured family of late embryogenesis abundant proteins has also been implicated in maintaining integrity and function of the inner mitochondrial membrane (IMM) in pea seed (172).
FIG. 9.
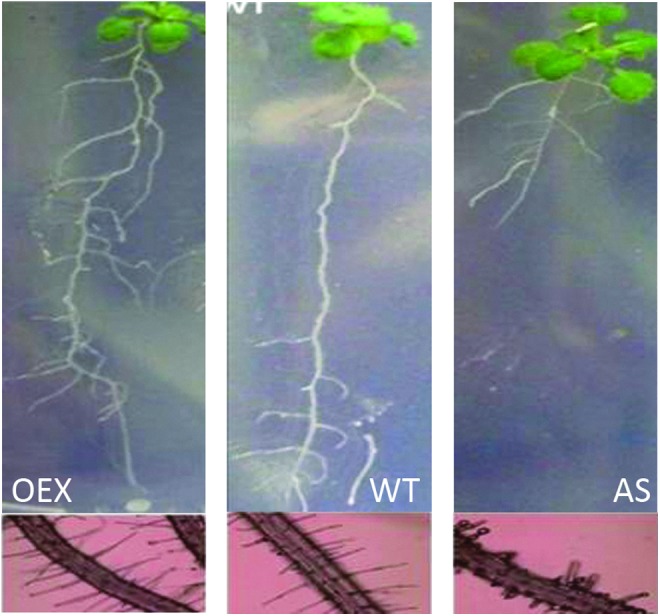
The responses of root architecture to altered abundance of the mitochondrial late embryogenesis-associated protein 5, also called senescence-associated gene 21 (AtLEA5/SAG21) protein in A. thaliana. Root development and the abundance of root hairs were increased in transgenic plants constitutively expressing the AtLEA5/SAG21 protein (OEX; 119). In contrast, antisense expression (AS) inhibited root development and decreased the abundance of root hairs (119). OEX, overexpressed. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The regulation of respiration under hypoxia and the release from hypoxia as a result of bud burst is central to the oxidative regulation of the transition of cells from the quiescent to the metabolically active state. This transition involves changes in the expression of genes encoding proteins involved in the cytochrome pathway of respiration (Fig. 7) and the tricarboxylic acid cycle (TCA) transcripts. Crucially, transcripts encoding the mitochondrial alternative oxidase (AOX) and alternative nicotimamide adenine dinucleotide (reduced state) (NADH) dehydrogenases, as well as glycolysis and fermentation were enhanced under these conditions (126, 185). We consider that the transcript and metabolic signatures observed in bud burst resemble those observed in the release of seed dormancy, in which oxidative signaling plays a key role, along with hormonal signaling which was mediated through changes in the balance between ABA and GA signaling pathways (9, 154).
Exposure to chilling stress induced the expression of a number of GA-related transcripts in buds as well as 1,3-β-glucanases in poplar, during the transition to bud burst (144). The expression of glucanases is also regulated by photoperiod controls and by the addition of exogenous GA (GA3 and GA4). Only bioactive GAs such as GA4 were effective in inducing bud burst (144). The chilling-induced expression of GA biosynthesis and/or signaling pathways is considered central to bud burst, along with the enhanced expression of glucanases, which may serve to augment intercellular signaling, for example, through the floral regulatory pathway, including FT, which has been identified as important in the regulation of seasonality in perennials (23, 144). The central role of GA in bud burst has been corroborated by studies using reverse genetic approaches in almond (12) and rose (36).
Epigenetic Regulation of Bud Dormancy and Flowering
Epigenetic mechanisms are important in the control of bud dormancy and release and in stress tolerance (35). The controlled acceleration of flowering time after exposure to prolonged cold, a process called vernalization, is a paradigm for epigenetic control. The vernalization process triggers environmental stress (low temperature)-mediated epigenetic silencing of gene expression that underpins the timing of the transition to reproductive development. The characterization of the A. thaliana vernalization (vrn) mutants has greatly increased our current understanding not only of the requirements for vernalization, but also of the molecular mechanisms and pathways that contribute to the vernalization process (30). The vrn mutants are defective in components that are involved in the control of the cold-induced repression of flowering locus C (FLC). Vernalization is the acquisition of competence to flower after exposure to prolonged low temperatures. This process quantitatively silences FLC, which is a floral repressor that antagonizes the activation of all the genes required to switch the meristem to a floral fate. Silencing of FLC relieves flowering repression. Studies on the processes that facilitate FLC regulation have greatly enhanced our knowledge of how noncoding RNAs, particularly antisense transcripts, mediate chromatin regulation as well as provide insights into evolutionary mechanisms (156). FLC regulation is an important determinant of plant adaptation to stress. FLC encodes an MADS box transcription factor that represses the expression of the floral integrator genes FT and suppresses the overexpression of constans 1 (SOC1). FLC expression is reduced in response to vernalization, and this establishes a molecular memory of winter that leads to early flowering. The vernalization-induced repression of FLC is stable through subsequent mitotic cell divisions until it is reset in the next sexual generation, properties that are indicative of epigenetic regulation. The most upstream requirement for the repression of FLC is the cold-induced expression of vernalization-insensitive 3 (VIN3) (166). VIN3 encodes a PHD finger protein that initiates FLC chromatin re-modeling (deacetylation of H3 and methylation of H3 at Lys9 and Lys27) via association with a polycomb repressive complex 2-like protein complex (PRC2-like) (43). It is important to note that VIN3 expression during hypoxia in A. thaliana is essential for the survival of low oxygen stress (24, 25). The VIN3 protein appears to function in chromatin remodeling during hypoxia, as it does in vernalization. Chromatin modifications at defined loci may, therefore, help the survival of A. thaliana exposed to prolonged hypoxia (24).
The acquisition of endodormancy shares a number of features in common with the flowering regulatory pathways and requires MIKc MADS box transcription factors, including DAM (78). The role and regulation of DAM during dormancy onset in perennials resembles that of FLC during flowering. Expression of DAM responds quantitatively to chilling, declining before bud burst. DAM are orthologs of the SVP genes, which repress FT in A. thaliana, retarding the onset of flowering independently of FLC (105). These genes are involved in the regulation of flowering in a temperature-dependent manner and whose function may span flowering and dormancy (78). Notably, chilling-exposed DAM1 of leafy spurge shows analogous chromatin modifications to those observed in vernalized A. thaliana; a similar regulation of DAM6 in peach was reported (107). Heterologous expression of DAM6 in A. thaliana leads to a delay in flowering. Within this context, the action of DAM6 resembles that of the SVP genes (81, 194). These observations, taken together with the putative function of VIN3 during hypoxia, suggest that common pathways and signaling systems operate during the chilling and vernalization responses and under hypoxia. While the transcriptome profiles suggest that the dormant bud resides in a hypoxic state, more physiological and biochemical evidence is required to fully understand the metabolic state of the cells in the dormant bud. It would be attractive to suggest that an oxidation event, associated with a hypoxic state, is required to re-initiate the cell cycle before bud burst, but literature evidence in support of this concept is lacking. Nevertheless, it is important to consider the possible roles of hypoxia and the release of cells from the quiescent state by the transition to an oxygen-rich cellular environment.
The Importance of Hypoxia
Hypoxia is a condition in which cellular availability of oxygen is insufficient for oxidative phosphorylation. Glycolytic activity is increased to supply ATP, and fermentation is induced to enable recycling of pyridine nucleotides in a response known as the Pasteur effect. Carbohydrates become mobilized and facilitate the increased glycolytic demand, and the expression of SUSY, ADH, and PDC is triggered by oxygen-limited metabolism. It might be considered that a reduction in oxygen availability (pO2) would be accompanied by a reduction in ROS production by the respiratory electron transport chain (Fig. 7), resulting in a decreased requirement for antioxidants. However, observations of increased or persistent ROS production under oxygen-limiting conditions span kingdoms of aerobic life forms (22, 122). A number of conserved transcriptional and metabolic responses are seen across life forms, notably the re-programming of primary metabolism and response to enhanced oxidation (122). Such datasets demonstrate that ROS play a key signaling role in regulating cellular responses to changes in oxygen availability. The metabolic and hormonal regulation of ROS production and antioxidant defenses are central to ROS/redox signaling and the maintenance of the quiescent state.
Plant tissue oxygen
The cells of higher plants have evolved to experience and accommodate a far greater range and frequency of variation in pO2 than animals; while most mammalian cells are less than two cells away from hemoglobin-rich blood (190 and references therein), plants are sessile, and lack an active oxygen transport system. Despite this, higher plants have evolved complex and highly dense tissues and organs, with spatiotemporally variable metabolic rates and, therefore, requirements for oxidative phosphorylation. Organs such as seeds, storage organs, fruit, and dense stems are developmentally disposed to internal oxygen gradients and prone to hypoxic (21>0 kPa pO2) or potentially anoxic (c. 0 kPa pO2) conditions (see Table 1).
Table 1.
Tissue Oxygen Partial Pressure (pO2) and Concentration ([O2]) in Optimal and Stressed Conditions for a Range of Plant Tissues
| Organ, tissue, species | Stress/development condition | pO2 (kPa) | [O2] (μM) | Reference |
|---|---|---|---|---|
| Broadbean(Fava-bean; Vicia faba) | (147) | |||
| Seed coat | Nonstressed, early-stage development | 14.6 | 180.0 | |
| Seed space | 2.1>0.6 | 26.0>7.5 | ||
| Seed embryo | 0.6<4.2 | 7.5<52.0 | ||
| Seed coat | Nonstressed, mature-stage development | 16.6>4.2 | 206.0>52.0 | |
| Seed space | — | — | ||
| Seed embryo | 4.2<8.4 | 52.0<104.0 | ||
| Garden Pea (Pisum sativum) | ||||
| Seed coat | Ambient light, early-stage development | 18.7>3.1 | 232.0>39.0 | |
| Seed space | 0.1<1.7 | 1.3<21.0 | ||
| Seed embryo | 1.7<2.5 | 21.0<31.0 | ||
| Seed coat | Ambient light, mature-stage development | 18.7>10.4 | 232.0>129.0 | |
| Seed space | — | — | ||
| Seed embryo | 8.3<10.4 | 103.0<129.0 | ||
| Seed coat | Dark, mature-stage development | 18.7>0.2 | 232.0>2.6 | |
| Seed space | — | — | ||
| Seed embryo | 0.1<0.8 | 1.3<10.3 | ||
| Wheat (Triticum aestivum) seed endosperm | Low oxygen atmosphere (8 kPa pO2) | 0.5 | 6.1 | (180) |
| Ambient atmosphere (21 kPa pO2) | 2.1 | 26.0 | ||
| High oxygen atmosphere (40 kPa pO2) | 11.3 | 139.0 | ||
| Castor bean (Ricinus communis) stem | (181) | |||
| Vascular bundle | Ambient atmosphere | 15.0>7.0 | 184.0>86.0 | |
| Parenchyma | 11.0<13.0 | 135.0<160.0 | ||
| Inner cavity | 11.0<15.0 | 135.0<184.0 | ||
| Maize (Zea mays) root | 44.7>37 | (70) | ||
| Cortex | Air-saturated water | 17.5>14.5 | ||
| Stele | 14.5>11.3 | 37>28.9 | ||
| Cortex | Low oxygen water (54 μM) | 2.8>0.6 | 7.2>1.5 | |
| Stele | 0.6>0.0 | 1.5>0.0 | ||
| Halosarcia pergranulata (a succulent halophyte) | (131) | |||
| Succulent stem | Waterlogged, shoot emergent, in light (shoot) | 15.2 | 40.8 | |
| Root | 6.0 | 15.5 | ||
| Succulent stem | Waterlogged, shoot emergent, in dark | 15.7 | 40.5 | |
| Root | 5.1 | 13.2 | ||
| Succulent stem | Completely submerged, in light (shoot) | 17.3 | 44.6 | |
| Root | 2.2 | 5.7 | ||
| Succulent stem | Completely submerged, in dark | 3.6 | 9.3 | |
| Root | 0.7 | 1.8 | ||
| Rice (Oryza sativa) root meristem (10 mm behind apex) | Submerged, in dark 2 h, sans glucose | 0.0 | 0.0 | (39) |
| +light (shoot), initial peak | 3.5 | 9.0 | ||
| +light (shoot), homeostasis | 3.1 | 8.0 | ||
| Submerged, in dark 2 h, 20 mM glucose | 0.0 | 0.0 | ||
| +light (shoot), initial peak | 2.5 | 6.5 | ||
| +light (shoot), homeostasis | 1.7 | 4.4 | ||
| Human endothelial cells | Arterial | 10.7–13.3 | 27.6–34.3 | (190)a |
| Venous | 4.7–5.6 | 12.1–14.4 | ||
| Human (foetal) alveolar epithelium | Birth | 3.1<13.3 | 8.0<34.4 |
See also review in Webster (190).
Where a range is presented, that represents a gradient through the tissue transect from external toward internal. Units were normalized from the cited sources, assuming standard ambient temperature and pressure (25°C, 101.3 kPa, and 0 μS·cm−1 conductivity), atmospheric pO2=20.8 kPa, and water-saturated [O2]=258 μM unless otherwise stated by authors; some data were round approximations from cited data.
pO2, partial pressure of oxygen.
Much of the research to date on the acclimation of plants to low pO2 has been done in the context of flood-prone species and crops (11, 39, 51), where partial or complete submergence limits available oxygen. A considerable overlap was found between the effects of low pO2 and submergence on the transcript response (106), providing credence to this strategy. A recent study of 86 A. thaliana ecotypes from a broad geographic range showed wide variations in tolerance to complete submergence in water (184). A. thaliana is not known to be submergence tolerant, but the abundance of genetic and molecular tools makes it a useful model. Most strikingly, the authors found no correlation between root or shoot tissue oxygen status (pO2) and tolerance to submergence. Further insights to oxygen signaling are discussed in the subsequent sections. Irrespective of submergence, roots are continually exposed to sub-normoxic conditions and a high variance in pO2; the rate of diffusion of O2 in water is 10,000-fold slower than in air, and roots are (typically) nonphotosynthetic, relying on vascular connections to aerial organs for O2 supply if soil O2 is limiting. Further diffusion barriers due to tissue density and composition in roots may create wide spatial variations within the organ, including cores of extreme hypoxia or anoxia, particularly near the apex, where oxygen requirement is high (refer examples in Table 1).
The seed embryo is another well-studied illustration of the range of internal pO2 within which vital plant tissues survive (26, 147); in fact, in an evolutionary sense, no plant organ is more vital. The role of hypoxia in organogenesis has received much attention in mammals; not only suggesting a central role of a regulated hypoxic state in the functional cardiovascular development of the embryo, particularly in the first trimester, but also highlighting the influence of nonoptimal pO2, caused by chemical, physical, or environmental stress, in the development of abnormalities (191 and references therein). Physical diffusion barriers impose a wide range of pO2 values within seeds. The pO2 values decline sharply below the seed coat due to a thick cuticular wax, densely packed epidermal cells, and low stomatal frequency (26, 147). Gradients are not linear, due to the variable cell density and size of gaseous spaces. This is clearly illustrated through the transect of seeds, where transitions in pO2 to near anoxic levels in the seed space are largely influenced by diffusion barriers, but levels in the embryo are elevated [Table 1 (26, 147)]. The influences of development, environment, and stress are also illustrated in Table 1, in seeds, roots, and stems, by a comparison to some well-known examples from human tissues. The embryo of most dicotyledonous species is photosynthetic, which can greatly augment oxygen status (26). Notwithstanding, the external oxygen status has a marked influence on plant tissue oxygen and seed viability [Table 1 (103)].
Respiration under hypoxic conditions
An important preface to this discussion is the acknowledgement of ongoing debate regarding oxygen sensing in plants. This is highlighted in recent discussions with regard to the critical oxygen pressure for respiratory control (7, 8, 124). Plant oxidative phosphorylation displays remarkable plasticity through a number of alternative pathways to cycle carbohydrates and to regenerate reduced pyridine nucleotides (Fig. 7) [for a recent review, see Igamberdiev and Hill (84), Millar et al. (117), and Noctor et al. (125)]. Under normoxic conditions, reduced substrates from glycolysis and the TCA cycle transfer electrons to the ubiquinone pool (UQ) of the inner mitochondrial membrane (IMM) via Complex I and/or Complex II. Reduction of Complex I is coupled to proton transfer across the IMM, against the proton gradient, generating an electrochemical potential; reduction of Complex II is not coupled. Plants also possess additional internal (matrix) and external (intermembrane space) NADH dehydrogenases, which may bypass Complex I or II to reduce the UQ. Reduced UQ may either reduce Complex III, which draws energy to translocate a further proton across the IMM, or directly reduce oxygen to water via the AOX pathway. Regulated engagement of the AOX pathway and the noncoupled reductants of UQ enables full flexibility in electron transfer, at the expense of further proton-coupled electron transfer through Complex III and Complex IV (cytochrome c oxidase [COX]), before reducing oxygen to water. Additional flexibility, although less well understood, may be achieved by the plant uncoupling protein (pUCP; also called plant uncoupling mitochondrial protein), which may function to relieve the transmembrane electrochemical potential, where, for example, phosphorylation is limited by adenylate control.
A comparison of transcriptome data under hypoxia across kingdoms shows a conserved response of reprogrammed primary metabolism, through transcriptional regulation of starch and sugar metabolism, sugar transport, and mitochondrial TCA cycle and electron transfer systems (122). In addition, genes encoding heat shock proteins and enzymes involved in secondary antioxidant metabolism were responsive. The signature was one of declined biosynthesis, increased sugar mobilization, glycolysis, fermentation, and pentose phosphate pathway metabolism, and a parallel decline in TCA metabolism and phosphorylative (coupled) components in the electron transfer system of mitochondria, in addition to the biogenesis of other organelles and cell walls (122). General comparisons of available microarray data reveal a convergence in transcriptional response to hypoxia and ROS-producing conditions (139).
Mitochondria are also active protagonists of ROS; they are a major sub-cellular source of ROS in nonphotosynthetic tissues, such as the root and dormant bud (167). Sources of ROS are concentrated around the UQ in the IMM, principally Complex I and Complex II, where the superoxide anion (O2·−) is formed. The conversion of superoxide to H2O2 is rapid, particularly under highly reducing conditions. While superoxide is predominantly produced in the matrix space, electrons from Complex III may partially reduce oxygen in the intermembrane space, which is more proximal to the cytosol, potentially augmenting intracellular signaling, including mitochondria to nucleus retrograde signaling.
Mammalian oxygen signaling
Hypoxic acclimation integrates mitochondrial and glycolytic metabolism and gene regulation. The regulation of the mammalian cell cycle and cell differentiation by oxygen availability is well established and remains the focus of clinical studies in chronic disease (28, 71, 95, 148, 157). Progression through the cell cycle is inhibited under hypoxic conditions in many cell types, leading to arrest of the cell cycle at G1. Changes in gene expression in response to low pO2 are centrally mediated by the hypoxia-inducible factor 1 (HIF1) transcription factor [for a recent review, see Kaelin and Ratcliffe (95)]. Briefly, HIF1 is a heterodimer comprising α and β subunits. Under normoxic conditions, the HIF1α-subunit is post-translationally hydroxylated by an oxygen-dependent prolyl hydroxylase, and is, thus, targeted for degradation in the proteasome after recognition and hydrolysis by a Von Hippel-Lindau tumor suppressor protein. Hypoxia both promotes transcription of the HIF1α subunits and limits the post-translational hydroxylation and subsequent degradation, leading to the accumulation of the HIF1 heterodimer. The HIF1 heterodimer binds to DNA at specific locations containing the hypoxia response element RCGTG, inducing gene expression that enables an appropriate metabolic response. While oxygen sensors also appear to operate in bacteria and fungi, the dissection of oxygen signaling in plants has proved more elusive (10, 69, 108, 109, 139).
Higher plant oxygen signaling
No HIF1 ortholog has been identified in plant genomes to date, and while several prolyl hydroxylases have been identified, there is no clear evidence for their role in plant oxygen sensing. Reports to date suggest that several interdependent mechanisms, or networks, may operate in response to changes in pO2 in plants. In the previous decade, the dissection of some key players in the early sensing and signaling of plant tissue oxygen has emerged. Two major models have been proposed: These are (i) signaling via the Roh-like GTPases, ROH of plant GTPase (ROP) (15, 63, 139) and (ii) the N-end rule pathway of targeted proteolysis (10, 69, 108, 109). The N-end rule states that the N-terminal residue on a protein determines its half life and, hence, its likelihood of being degraded. The presence of amino-terminal destabilizing residues enables marking of the proteins for destruction by the ubiquitin proteasome system. These N-terminal residues are recognized by E3 ligases and targeted for proteolysis via the 26S proteasome. Several recent reviews have detailed the N-end rule pathway (10, 108), and here, we only elaborate on the ROP.
Oxygen signaling by ROP
Many plant species have an ability to acclimate to hypoxic conditions through tolerance or avoidance mechanisms. The formation of vacuous aerenchyma in roots and stems can enhance tissue porosity two- to four-fold, and limiting radial oxygen loss further serves to maximize the potential availability and conservation of aerial oxygen to hypoxic roots (38). Ethylene and ROS play a key role in this and other acclimatory responses to flooding and hypoxia. GTP-binding proteins (G proteins; heterotrimeric) and small GTPases (monomeric) have emerged as key mediators of ethylene- and ROS-induced acclimation in plants, not least in response to hypoxia. GTPase signaling is upstream of the mammalian HIF1, and is, in turn, regulated by ROS/redox signaling (79, 177). Several sub-classes of GTPases communicate signals from various phytohormones and extracellular signals in eukaryotes, notably in cell proliferation, actin cytoskeletal organization, and cell polarity (89, 123, 169, and references therein). Plants lack orthologs of these families (89), but the characterization of ROP has greatly advanced our understanding of the interdependence of ROS and oxygen signaling (15, 42, 63).
The signaling activity of G proteins and GTPases is modulated by GTP/GDP binding—the GTP-bound form is active. This, in turn, is regulated by several associated proteins [for a review, see Neves et al. (123), Temple and Jones (169), and Yang (200)]. Guanine exchange factors and GTPase activator proteins (GAP) act in an opposite manner. Guanine exchange factors increase the slow intrinsic rate of guanine nucleotide exchange, promoting the activated state; while GAP enhance the slow intrinsic rate of GTP hydrolysis (200). Heterotrimeric G proteins are also regulated by transmembrane G protein-coupled receptors (GPCR) and regulators of G protein signaling (169). The Gα subunit of the heterotrimeric proteins possesses guanine nucleotide binding activity and is related to the small GTPases.
Several lines of evidence have converged to reveal the inter-relation of hypoxia and redox signaling with G protein signaling in plants. Overexpression of a constitutively active small GTPase in soybean suspension cells resulted in increased production of H2O2, which was dependent on a diphenyleneiodonium (DPI)-sensitive NADPH oxidase, similar to mammalian systems (129). A forward-genetic screen of gene-trap transposon mutant lines of A. thaliana identified a GAP (RopGAP4), by which disruption of RopGAP4 resulted in hyperinduction of ADH under low pO2, but reduced tolerance to low pO2, and particularly, low tolerance to re-oxygenation (15). The hyperinduction of ADH was reduced by treatment with DPI, implicating H2O2 signaling intermediates between GTPase and ADH induction (15). The sensitivity of RopGAP4-1 mutants to re-oxygenation resulted from an inability to down-regulate GTPase-mediated induction of H2O2, through induction of RopGAP, and consequent inactivation of GTPases by GTP hydrolysis (15). This defined a so-called rheostat mechanism of GTPase signaling resulting in increased NADPH oxidase activity under hypoxia, and consequently, not only increased ADH and anaerobic metabolism, but also induced GAP activity to provide feedback and regulate the GTPase activation state, enabling moderation of the cascade on re-oxygenation. Further studies confirmed the relationship between GTPase and hypoxic acclimation. For example, the induction of aerenchyma formation and cell death in normoxic maize roots, when treated with GTPγS substrate, effectively binds and activates GTPases but does not hydrolyze, resulting in constitutive activity (162, and references therein). Extensive analyses of mammalian GTPases have identified conserved redox-modulated motifs. Hence, GTPase signaling and redox signaling have a reciprocal relationship. Detailed mechanisms of the regulation and chemical interactions between redox signals and mammalian GTPases were recently reviewed (79).
Conclusions and Perspectives
The earlier discussion highlights the key concept that redox signaling is an important regulator of growth in plants. Oxidative and reductive signaling arising from metabolism and phytohormone action participate in the control of dormancy and the liberation of meristematic cells from a quiescent state. We have discussed the interactions of phytohormone and redox pathways in the control of root architecture and bud dormancy/bud burst, key factors that enable plants to respond appropriately to environmental cues and stress. Given emerging evidence that the oxidative signal could be partly transmitted by modulation of glutathione status, our hypothesis that liberation from the quiescent hypoxic state requires oxidative activation linked to respiratory metabolism is important both for sensitization and for regulation of signaling through glutathione-dependent systems. Redox changes in the glutathione pool and its interacting glutaredoxins and thioredoxins are sufficient to have an impact on growth through phytohormone pathways. While the concept that perturbations in cellular redox homeostasis exert a major influence on cell cycle progression, expansion growth, and the transition from cell proliferation to cell differentiation is widely accepted (16, 44, 112, 137, 138, 143, 186), many uncertainties remain with regard to the interacting pathways that underpin each process. Moreover, many of the mechanisms and components involved in the redox control of growth remain to be identified. Causal links between changes in cellular redox state and shoot and root meristem activities have been established, but the underlying mechanisms are far from clear. A. thaliana mutants that are impaired in both thioredoxin and glutathione demonstrate that the ability to maintain the floral meristem also depends on thiol reduction systems (14).
Innovation
Each of the 300,000 plant species living in the world these days continuously monitors the environment, using environmental cues to fine-tune growth and development. Environmental inputs and stresses activate common and overlapping cell signaling pathways, leading to co-ordination of acclimation, immunity, and growth responses. This is possible, because perturbations in cellular redox homeostasis and signaling enable the synergistic co-activation of environmental response pathways that cross biotic–abiotic stress boundaries to influence the hormone-dependent regulation of growth. While much remains to be discovered with regard to the specific roles of individual redox mediators, in regulating genetic and epigenetic factors that modulate plant growth and development, recent evidence demonstrates extensive cross-talk between cellular redox homeostasis and plant growth regulators such as auxin, SLs, GA, and ABA, with multiple steps of reciprocal control.
Abbreviations Used
- ABA
abscisic acid
- ABCG
ATP binding cassette subfamily G
- ABI
ABA insensitive
- ADH
alcohol dehydrogenase
- AOX
alternative oxidase
- APX
ascorbate peroxidase
- ARFs
auxin response factors
- AS
antisense
- AUX
auxin (e.g., AUX1; also called auxin resistant)
- BSO
L-buthionine sulfoximine
- c.
circa
- CAT
catalase
- CCD
carotenoid cleavage dioxygenase
- CO
constans
- Col-0
Columbia-0 ecotype of Arabidopsis thaliana (L.) Heynh
- COX
cytochrome c oxidase
- D27
β-carotene isomerase
- DAM
dormancy-associated MADS-box
- DHA
dehydroascorbate (oxidized state of ascorbate)
- DPI
diphenyleneiodonium
- DR5::GUS
DR5 is an auxin response element (AuxRE) (used in this instance as a promoter)::β-glucuronidase reporter system
- evg
evergrowing mutant phenotype of peach
- Ext ND
external NAD(P)H dehydrogenase of mitochondria
- FLC
flowering locus C
- FT
flowering locus T
- GA
gibberellic acid
- GA2ox
GA2 oxidase
- GAI
GA insensitive
- GAP
GTPase activator protein
- GCL
γ-glutamate-cysteine ligase; also called γ-glutamylcysteine synthase
- Glc
glucose
- GPCR
G protein-coupled receptors
- GR24
a synthetic strigolactone analog
- Grx
glutaredoxin
- GSH
glutathione (reduced state); γ-glutamyl-L-cysteinylglycine
- GSH1
(gene encoding) γ-glutamate-cysteine ligase (GCL)
- GSHS
glutathione synthetase; also called glutathione synthase
- GSSG
glutathione disulfide (oxidized glutathione)
- HIF
hypoxia-inducible factor
- IAA
indole-3-acetic acid
- IMM
inner mitochondrial membrane
- Int ND
internal NAD(P)H dehydrogenase of mitochondria
- JA
jasmonic acid
- LAX
like AUX1 (e.g., LAX1)
- LEA5/SAG21
late embryogenesis abundant 5; also called senescence-associated gene 21
- LR
lateral root
- LRP
lateral root primordium
- MAPK
mitogen-activated protein kinase
- MAX
more axillary growth (e.g., MAX2)
- MIKC MADS
a subfamily of MADS-box domain-containing transcription factors that also contain three additional domains; the Intervening (I) domain, the Keratin (K) domain, and the C-terminal (C) domain.
- NAA
1-naphthaleneacetic acid (a synthetic auxin)
- NAD(P)H
nicotinamide adenine dinucleotide (phosphate) (reduced state)
- NADH
nicotimamide adenine dinucleotide (reduced state)
- NPA
1-N-naphthylphthalamic acid (an auxin transport inhibitor)
- NTRs
NADPH-thioredoxin reductases
- OEX
overexpressed
- OMM
outer mitochondrial membrane
- oxIAA
2-oxindole-3-acetic acid
- PDC
pyruvate decarboxylase
- PFT1/MED25
phytochrome and flowering time 1/gene that encodes the mediator 25 subunit of mediator
- PHYA
phytochrome A
- PIN
PIN-formed
- pO2
partial pressure of oxygen (in air or solution)
- PP2AA
protein phosphatase 2A, alpha, catalytic subunit
- PP2C
type 2C protein phosphatase
- PRC2-like
polycomb repressive complex 2-like
- PtdIns 3-kinase
phosphatidylinositol 3-kinase
- pUCP
plant uncoupling protein; also called plant uncoupling mitochondrial protein
- QC
quiescent center of root tips
- RBOH
respiratory burst oxidase homolog; also called NADPH oxidases (NOX); ortholog of the neutrophil gp91phox
- Redox
reduction/oxidation
- RGL
repressor of GAI-like
- rml
root meristemless (e.g., rml1)
- RNAi
RNA interference; a form of post-transcriptional gene silencing
- RNAseq
RNA sequencing; whole transcriptome shotgun sequencing
- ROP
Roh of plant GTPase
- ROS
reactive oxygen species; also called active oxygen species
- SA
salicylic acid
- SCFTIR1
Skp, Cullin, F-box containing complex
- SL
strigolactone
- SOC1
suppressor of overexpression of constans 1
- SUSY
sucrose synthase
- SVP
short vegetative phase
- TCA
tricarboxylic acid (cycle)
- TIR1/AFB
transport inhibitor response 1/auxin-binding f-box protein
- Trx
thioredoxin
- tt
transparent testa (e.g., tt4)
- UQ
ubiquinone pool
- VIN3
vernalization insensitive 3
- vrn
vernalization
- γ-EC
γ-glutamylcysteine
Acknowledgments
M.C. and C.F. thank the Royal Society (UK) for a joint project grant to support this work. M.C. thanks the University of Western Australia Research Collaboration Award for a fellowship, and a University of Western Australia sabbatical grant. Parts of the work reported here were funded by an EU Marie Curie ITN (PITN-GA-2008-215174: Chloroplast Signals), by an EU Marie Curie IEF grant (PIEF-GA-2009-252927: ROXNP), and by Subprograma Estancias de Movilidad Posdoctoral en Centros Extranjeros (2009), Ministerio de Educación (Spain). The authors thank Daniel Schnaubelt for Figure 1, Guillaume Queval for Figures 2 and 5, and Belén Márquez García for Figure 4.
References
- 1.Abel S. and Theologis A. Early genes and auxin action. Plant Physiol 111: 9–17, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, and Harberd N. Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Achard P, Renou J-P, Berthomé R, Harberd NP, and Genschik P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18: 656–660, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Achard P, Vriezen WH, Van Der Straeten D, and Harberd NP. Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15: 2816–2825, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aloni R, Aloni E, Langhans M, and Ullrich CI. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot 97: 883–893, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arend M, Schnitzler J-P, Ehlting B, Hänsch R, Lange T, Rennenberg H, Himmelbach A, Grill E, and Fromm J. Expression of the Arabidopsis mutant abi1 gene alters abscisic acid sensitivity, stomatal development, and growth morphology in gray poplars. Plant Physiol 151: 2110–2119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong W. and Beckett PM. Experimental and modelling data contradict the idea of respiratory down-regulation in plant tissues at an internal [O2] substantially above the critical oxygen pressure for cytochrome oxidase. New Phytol 190: 431–441, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Armstrong W. and Beckett PM. The respiratory down-regulation debate. New Phytol 190: 276–278, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Bahin E, Bailly C, Sotta B, Kranner I, Corbineau F, and Leymarie J. Crosstalk between reactive oxygen species and hormonal signalling pathways regulates grain dormancy in barley. Plant Cell Environ 34: 980–993, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LACJ, and van Dongen JT. Making sense of low oxygen sensing. Trends Plant Sci 17: 129–138, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Bailey-Serres J. and Voesenek LACJ. Flooding stress: Acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Barros PM, Gonçalves N, Saibo NJM, and Oliveira MM. Cold acclimation and floral development in almond bud break: insights into the regulatory pathways. J Exp Bot 63: 4585–4596, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Bartoli CG, Casalongué CA, Simontacchi M, Marquez-Garcia B, and Foyer CH. Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ Exper Bot 94: 73–88, 2013 [Google Scholar]
- 14.Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, and Reichheld J-P. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 22: 376–391, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baxter-Burrell A, Yang Z, Springer PS, and Bailey-Serres J. RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296: 2026–2028, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Belmonte MF, Donald G, Reid DM, Yeung EC, and Stasolla C. Alterations of the glutathione redox state improve apical meristem structure and somatic embryo quality in white spruce (Picea glauca). J Exp Bot 56: 2355–2364, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Benková E. and Bielach A. Lateral root organogenesis—from cell to organ. Curr Opin Plant Biol 13: 677–683, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, and Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Bethke PC. and Jones RL. Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J 25: 19–29, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Bethke PC, Libourel IGL, Aoyama N, Chung Y-Y, Still DW, and Jones RL. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143: 1173–1188, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielenberg D, Wang Y, Li Z, Zhebentyayeva T, Fan S, Reighard G, Scorza R, and Abbott A. Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genet Genomes 4: 495–507, 2008 [Google Scholar]
- 22.Blokhina O. and Fagerstedt KV. Oxidative metabolism, ROS and NO under oxygen deprivation. Plant Physiol Biochem 48: 359–373, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Bohlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, and Nilsson O. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Bond DM, Dennis ES, and Finnegan EJ. Hypoxia. A novel function for VIN3. Plant Signal Behav 4: 773–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bond DM, Wilson IW, Dennis ES, Pogson BJ, and Finnegan JE. VERNALIZATION INSENSITIVE 3 (VIN3) is required for the response of Arabidopsis thaliana seedlings exposed to low oxygen conditions. Plant J 59: 576–587, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Borisjuk L. and Rolletschek H. The oxygen status of the developing seed. New Phytol 182: 17–30, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, and Muday GK. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524–535, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmeliet P, Dor Y, Herbert J-M, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, and Keshet E. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394: 485–490, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, and Bennett MJ. Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Chandler J, Wilson A, and Dean C. Arabidopsis mutants showing an altered response to vernalization. Plant J 10: 637–644, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, and De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394: 793–797, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Chen H. and Xiong L. The short-rooted vitamin B6-deficient mutant pdx1 has impaired local auxin biosynthesis. Planta 229: 1303–1310, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Cheng JC, Seeley KA, and Sung ZR. RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiol 107: 365–376, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng N-H, Liu J-Z, Liu X, Wu Q, Thompson SM, Lin J, Chang J, Whitham SA, Park S, Cohen JD, and Hirschi KD. Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J Biol Chem 286: 20398–20406, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinnusamy V. and Zhu J-K. Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12: 133–139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choubane D, Rabot A, Mortreau E, Legourrierec J, Péron T, Foucher F, Ahcène Y, Pelleschi-Travier S, Leduc N, Hamama L, and Sakr S. Photocontrol of bud burst involves gibberellin biosynthesis in Rosa sp. J Plant Phys 169: 1271–1280, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Cobbett CS, May MJ, Howden R, and Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2–1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J 16: 73–78, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26: 17–36, 2003 [Google Scholar]
- 39.Colmer TD. and Pedersen O. Oxygen dynamics in submerged rice (Oryza sativa). New Phytol 178: 326–334, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Coombe BG. Grapevine growth stages—the modified E-L system. In: Viticulture 1—Resources, edited by Dry PR. and Coombe BG. Adelaide, Australia: Winetitles, 150–154, 2004 [Google Scholar]
- 41.Cosio C. and Dunand C. Specific functions of individual class III peroxidase genes. J Exp Bot 60: 391–408, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Craddock C, Lavagi I, and Yang Z. New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol 22: 492–501, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Lucia F, Crevillen P, Jones AME, Greb T, and Dean C. A PHD-Polycomb Repressive Complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci U S A 105: 16831–16836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Pinto MC, Francis D, and De Gara L. The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209: 90–97, 1999 [DOI] [PubMed] [Google Scholar]
- 45.De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, and Zhang H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 33: 543–555, 2003 [DOI] [PubMed] [Google Scholar]
- 46.De Smet I, Vanneste S, Inzé D, and Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Mol Biol 60: 871–887, 2006 [DOI] [PubMed] [Google Scholar]
- 47.De Torres M, Mansfield JW, Grabov N, Brown IR, Ammouneh H, Tsiamis G, Forsyth A, Robatzek S, Grant M, and Boch J. Pseudomonas syringae effector AvrPtoB suppresses basal defence in Arabidopsis. Plant J 47: 368–382, 2006 [DOI] [PubMed] [Google Scholar]
- 48.De Veylder L, Larkin JC, and Schnittger A. Molecular control and function of endoreplication in development and physiology. Trends Plant Sci 16: 624–634, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Dinneny JR. and Benfey PN. Plant stem cell niches: Standing the test of time. Cell 132: 553–557, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Domagalska MA. and Leyser O. Signal integration in the control of shoot branching. Nat Rev Molec Cell Biol 12: 211–221, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Drew MC. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48: 223–250, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Druart N, Johansson A, Baba K, Schrader J, Sjödin A, Bhalerao RR, Resman L, Trygg J, Moritz T, and Bhalerao RP. Environmental and hormonal regulation of the activity–dormancy cycle in the cambial meristem involves stage-specific modulation of transcriptional and metabolic networks. Plant J 50: 557–573, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, and Benková E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci U S A 105: 8790–8794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dudits D, Ábrahám E, Miskolczi P, Ayaydin F, Bilgin M, and Horváth GV. Cell-cycle control as a target for calcium, hormonal and developmental signals: the role of phosphorylation in the retinoblastoma-centred pathway. Ann Bot 107: 1193–1202, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Maarouf-Bouteau H. and Bailly C. Oxidative signaling in seed germination and dormancy. Plant Signal Behav 3: 175–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Maarouf-Bouteau H, Meimoun P, Job C, Job D, and Bailly C. Role of protein and mRNA oxidation in seed dormancy and germination. Front Plant Sci 4: 77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esau K. Plant Anatomy. NY: John Wiley & Sons, 1965 [Google Scholar]
- 58.Fath A, Bethke PC, and Jones RL. Enzymes that scavenge reactive oxygen species are down-regulated prior to gibberellic acid-induced programmed cell death in barley aleurone. Plant Physiol 126: 156–166, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finkelstein R, Reeves W, Ariizumi T, and Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol 59: 387–415, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, and Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Friml J. Subcellular trafficking of PIN auxin efflux carriers in auxin transport. Eur J Cell Biol 89: 231–235, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Fukaki H. and Tasaka M. Hormone interactions during lateral root formation. Plant Mol Biol 69: 437–449, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Fukao T. and Bailey-Serres J. Plant responses to hypoxia–is survival a balancing act? Trends Plant Sci 9: 449–456, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Gai S, Zhang Y, Mu P, Liu C, Liu S, Dong L, and Zheng G. Transcriptome analysis of tree peony during chilling requirement fulfillment: Assembling, annotation and markers discovering. Gene 497: 256–262, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Gao X, Yuan H-M, Hu Y-Q, Li J, and Lu Y-T. Mutation of Arabidopsis CATALASE2 results in hyponastic leaves by changes of auxin levels. Plant Cell Environ 2013[Epub ahead of print]; DOI: 10.1111/pce.12144 [DOI] [PubMed] [Google Scholar]
- 66.Gapper C. and Dolan L. Control of plant development by reactive oxygen species. Plant Physiol 141: 341–345, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.García-Giménez JL, Markovic J, Dasí F, Queval G, Schnaubelt D, Foyer CH, and Pallardó FV. Nuclear glutathione. Biochem Biophys Acta 1830: 3304–3316, 2013 [DOI] [PubMed] [Google Scholar]
- 68.George S, Venkataraman G, and Parida A. A chloroplast-localized and auxin-induced glutathione S-transferase from phreatophyte Prosopis juliflora confer drought tolerance on tobacco. J Plant Phys 167: 311–318, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Gibbs DJ, Lee SC, Md Isa N, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, and Holdsworth MJ. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479: 415–418, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gibbs J, Turner DW, Armstrong W, Darwent MJ, and Greenway H. Response to oxygen deficiency in primary maize roots. I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem. Funct Plant Biol 25: 745–758, 1998 [Google Scholar]
- 71.Goda N, Dozier SJ, and Johnson RS. HIF-1 in cell cycle regulation, apoptosis, and tumor progression. Antioxid Redox Signal 5: 467–473, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, Bouwmeester H, Becard G, Beveridge CA, Rameau C, and Rochange SF. Strigolactone inhibition of shoot branching. Nature 455: 189–194, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Grieneisen VA, Xu J, Maree AFM, Hogeweg P, and Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449: 1008–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Halaly T, Pang X, Batikoff T, Crane O, Keren A, Venkateswari J, Ogrodovitch A, Sadka A, Lavee S, and Or E. Similar mechanisms might be triggered by alternative external stimuli that induce dormancy release in grape buds. Planta 228: 79–88, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Hamiaux C, Drummond Revel SM, Janssen Bart J, Ledger Susan E, Cooney Janine M, Newcomb Richard D, and Snowden Kimberley C. DAD2 Is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol 22: 2032–2036, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Harberd NP, Belfield E, and Yasumura Y. The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21: 1328–1339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayward A, Stirnberg P, Beveridge C, and Leyser O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151: 400–412, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hemming MN. and Trevaskis B. Make hay when the sun shines: The role of MADS-box genes in temperature-dependant seasonal flowering responses. Plant Sci 180: 447–453, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Heo J. Redox control of GTPases: From molecular mechanisms to functional significance in health and disease. Antioxid Redox Signal 14: 689–724, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Horvath D, Chao W, Suttle J, Thimmapuram J, and Anderson J. Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). BMC Genomics 9: 536, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horvath D, Sung S, Kim D, Chao W, and Anderson J. Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant Mol Biol 73: 169–179, 2010 [DOI] [PubMed] [Google Scholar]
- 82.Horvath DP, Anderson JV, Chao WS, and Foley ME. Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci 8: 534–540, 2003 [DOI] [PubMed] [Google Scholar]
- 83.Hsu C-Y, Adams JP, No K, Liang H, Meilan R, Pechanova O, Barakat A, Carlson JE, Page GP, and Yuceer C. Overexpression of CONSTANS homologs CO1 and CO2 fails to alter normal reproductive onset and fall bud set in woody perennial poplar. PLoS One 7: e45448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Igamberdiev AU. and Hill RD. Plant mitochondrial function during anaerobiosis. Ann Bot 103: 259–268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iglesias M, Terrile M, Bartoli C, D'Ippólito S, and Casalongué C. Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Mol Biol 74: 215–222, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Jiang K, Ballinger T, Li D, Zhang S, and Feldman L. A role for mitochondria in the establishment and maintenance of the maize root quiescent center. Plant Physiol 140: 1118–1125, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang K. and Feldman LJ. Regulation of root apical meristem development. Annu Rev Cell Dev Biol 21: 485–509, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Jiang K, Meng YL, and Feldman LJ. Quiescent center formation in maize roots is associated with an auxin-regulated oxidizing environment. Development 130: 1429–1438, 2003 [DOI] [PubMed] [Google Scholar]
- 89.Jiang S-Y. and Ramachandran S. Comparative and evolutionary analysis of genes encoding small GTPases and their activating proteins in eukaryotic genomes. Physiol Genomics 24: 235–251, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Jimenez S, Li Z, Reighard G, and Bielenberg D. Identification of genes associated with growth cessation and bud dormancy entrance using a dormancy-incapable tree mutant. BMC Plant Biol 10: 25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson K. and Lenhard M. Genetic control of plant organ growth. New Phytol 191: 319–333, 2011 [DOI] [PubMed] [Google Scholar]
- 92.Joo JH, Bae YS, and Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126: 1055–1060, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joo JH, Yoo HJ, Hwang I, Lee JS, Nam KH, and Bae YS. Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase. FEBS Lett 579: 1243–1248, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Kadota Y, Furuichi T, Sano T, Kaya H, Gunji W, Murakami Y, Muto S, Hasezawa S, and Kuchitsu K. Cell-cycle-dependent regulation of oxidative stress responses and Ca2+ permeable channels NtTPC1A/B in tobacco BY-2 cells. Biochem Biophys Res Commun 336: 1259–1267, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Kaelin WG. and Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Kalachova T, Iakovenko O, Kretinin S, and Kravets V. Involvement of phospholipase D and NADPH-oxidase in salicylic acid signaling cascade. Plant Physiol Biochem 66: 127–133, 2013 [DOI] [PubMed] [Google Scholar]
- 97.Kapulnik Y, Delaux P-M, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, Séjalon-Delmas N, Combier J-P, Bécard G, Belausov E, Beeckman T, Dor E, Hershenhorn J, and Koltai H. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233: 209–216, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Kapulnik Y, Resnick N, Mayzlish-Gati E, Kaplan Y, Wininger S, Hershenhorn J, and Koltai H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J Exp Bot 62: 2915–2924, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Kerk NM, Jiang K, and Feldman LJ. Auxin metabolism in the root apical meristem. Plant Physiol 122: 925–932, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kleine-Vehn J. and Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol 24: 447–473, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Koprivova A, Mugford ST, and Kopriva S. Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Rep 29: 1157–1167, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Kovtun Y, Chiu W-L, Tena G, and Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci U S A 97: 2940–2945, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuang A, Crispi M, and Musgrave ME. Control of seed development in Arabidopsis thaliana by atmospheric oxygen. Plant Cell Environ 21: 71–78, 1998 [DOI] [PubMed] [Google Scholar]
- 104.Lang GA, Early JD, Martin GC, and Darnell RL. Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. HortScience 22: 371–377, 1987 [Google Scholar]
- 105.Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, and Ahn JH. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev 21: 397–402, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee SC, Mustroph A, Sasidharan R, Vashisht D, Pedersen O, Oosumi T, Voesenek LACJ, and Bailey-Serres J. Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytol 190: 457–471, 2011 [DOI] [PubMed] [Google Scholar]
- 107.Leida C, Conesa A, Llácer G, Badenes ML, and Ríos G. Histone modifications and expression of DAM6 gene in peach are modulated during bud dormancy release in a cultivar-dependent manner. New Phytol 193: 67–80, 2012 [DOI] [PubMed] [Google Scholar]
- 108.Licausi F. Molecular elements of low-oxygen signaling in plants. Physiol Plant 148: 1–8, 2013 [DOI] [PubMed] [Google Scholar]
- 109.Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, and van Dongen JT. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479: 419–422, 2011 [DOI] [PubMed] [Google Scholar]
- 110.Malamy JE. and Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44, 1997 [DOI] [PubMed] [Google Scholar]
- 111.Marquez-Garcia B, Njo M, Beeckman TOM, Goormachtig S, and Foyer CH. A new role for glutathione in the regulation of root architecture linked to strigolactones. Plant Cell Environ 2013[Epub ahead of print]; DOI: 10.1111/pce.12172 [DOI] [PubMed] [Google Scholar]
- 112.Maughan S. and Foyer CH. Engineering and genetic approaches to modulating the glutathione network in plants. Physiol Plant 126: 382–397, 2006 [Google Scholar]
- 113.Mauriat M, Sandberg LG, and Moritz T. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. Plant J 67: 805–816, 2011 [DOI] [PubMed] [Google Scholar]
- 114.Mazzitelli L, Hancock R, Haupt S, Walker P, Pont S, McNicol J, Cardle L, Morris J, Viola R, Brennan R, Hedley P, and Taylor M. Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J Exp Bot 58: 1035–1045, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou J-P, and Noctor G. Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol 153: 1144–1160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mhamdi A, Han Y, and Noctor G. Glutathione-dependent phytohormone responses: Teasing apart signaling and antioxidant functions. Plant Signal Behav 8: e24181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Millar AH, Whelan J, Soole KL, and Day DA. Organization and regulation of mitochondrial respiration in plants. Annu Rev Plant Biol 62: 79–104, 2011 [DOI] [PubMed] [Google Scholar]
- 118.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, and Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci 16: 300–309, 2011 [DOI] [PubMed] [Google Scholar]
- 119.Mohd Salleh F, Evans K, Goodall B, Machin H, Mowla SB, Mur LAJ, Runions J, Theodoulou FL, Foyer CH, and Rogers HJ. A novel function for a redox-related LEA protein (SAG21/AtLEA5) in root development and biotic stress responses. Plant Cell Environ 35: 418–429, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Monshausen GB. and Gilroy S. The exploring root—root growth responses to local environmental conditions. Curr Opin Plant Biol 12: 766–772, 2009 [DOI] [PubMed] [Google Scholar]
- 121.Mowla SB, Cuypers A, Driscoll SP, Kiddle G, Thomson J, Foyer CH, and Theodoulou FL. Yeast complementation reveals a role for an Arabidopsis thaliana late embryogenesis abundant (LEA)-like protein in oxidative stress tolerance. Plant J 48: 743–756, 2006 [DOI] [PubMed] [Google Scholar]
- 122.Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, and Bailey-Serres J. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol 152: 1484–1500, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Neves SR, Ram PT, and Iyengar R. G protein pathways. Science 296: 1636–1639, 2002 [DOI] [PubMed] [Google Scholar]
- 124.Nikoloski Z and van Dongen JT. Modeling alternatives for interpreting the change in oxygen-consumption rates during hypoxic conditions. New Phytol 190: 273–276, 2011 [DOI] [PubMed] [Google Scholar]
- 125.Noctor G, De Paepe R, and Foyer C. Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12: 125–134, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Ophir R, Pang X, Halaly T, Venkateswari J, Lavee S, Galbraith D, and Or E. Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene-ABA interplay and cell enlargement. Plant Mol Biol 71: 403–423, 2009 [DOI] [PubMed] [Google Scholar]
- 127.Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, and Bailly C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol 150: 494–505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pacey-Miller T, Scott K, Ablett E, Tingey S, Ching A, and Henry R. Genes associated with the end of dormancy in grapes. Funct Integr Genomics 3: 144–152, 2003 [DOI] [PubMed] [Google Scholar]
- 129.Park J, Choi H-J, Lee S, Lee T, Yang Z, and Lee Y. Rac-related GTP-binding protein in elicitor-induced reactive oxygen generation by suspension-cultured soybean cells. Plant Physiol 124: 725–732, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pasternak T, Potters G, Caubergs R, and Jansen MAK. Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J Exp Bot 56: 1991–2001, 2005 [DOI] [PubMed] [Google Scholar]
- 131.Pedersen O, Vos H, and Colmer TD. Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant Cell Environ 29: 1388–1399, 2006 [DOI] [PubMed] [Google Scholar]
- 132.Peer WA, Cheng Y, and Murphy AS. Evidence of oxidative attenuation of auxin signalling. J Exp Bot 64: 2629–2639, 2013 [DOI] [PubMed] [Google Scholar]
- 133.Peer WA. and Murphy AS. Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci 12: 556–563, 2007 [DOI] [PubMed] [Google Scholar]
- 134.Peng J, Richards DE, Moritz T, Caño-Delgado A, and Harberd NP. Extragenic suppressors of the Arabidopsis gai mutation alter the dose-response relationship of diverse gibberellin responses. Plant Physiol 119: 1199–1208, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perez FJ, Vergara R, and Rubio S. H2O2 is involved in the dormancy-breaking effect of hydrogen cyanamide in grapevine buds. Plant Growth Reg 55: 149–155, 2008 [Google Scholar]
- 136.Petrov VD. and Van Breusegem F. Hydrogen peroxide—a central hub for information flow in plant cells. AoB Plants 2012: pls014, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Potters G, Horemans N, Bellone S, Caubergs RJ, Trost P, Guisez Y, and Asard H. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiol 134: 1479–1487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Potters G, Horemans N, Caubergs RJ, and Asard H. Ascorbate and dehydroascorbate influence cell cycle progression in a tobacco cell suspension. Plant Physiol 124: 17–20, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pucciariello C, Parlanti S, Banti V, Novi G, and Perata P. Reactive oxygen species-driven transcription in Arabidopsis under oxygen deprivation. Plant Physiol 159: 184–196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pulido P, Cazalis R, and Cejudo FJ. An antioxidant redox system in the nucleus of wheat seed cells suffering oxidative stress. Plant J 57: 132–145, 2009 [DOI] [PubMed] [Google Scholar]
- 141.Qiu Z, Wan L, Chen T, Wan Y, He X, Lu S, Wang Y, and Lin J. The regulation of cambial activity in Chinese fir (Cunninghamia lanceolata) involves extensive transcriptome remodeling. New Phytol 199: 708–719, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Queval G, Neukermans J, Vanderauwera S, Van Breusegem F, and Noctor G. Day length is a key regulator of transcriptomic responses to both CO2 and H2O2 in Arabidopsis. Plant Cell Environ 35: 374–387, 2012 [DOI] [PubMed] [Google Scholar]
- 143.Reichheld J-P, Khafif M, Riondet C, Droux M, Bonnard G, and Meyer Y. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell 19: 1851–1865, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rinne PLH, Welling A, Vahala J, Ripel L, Ruonala R, Kangasjärvi J, and van der Schoot C. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-β-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell 23: 130–146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rohde A. and Bhalerao RP. Plant dormancy in the perennial context. Trends Plant Sci 12: 217–223, 2007 [DOI] [PubMed] [Google Scholar]
- 146.Rohde A, Prinsen E, De Rycke R, Engler G, Van Montagu M, and Boerjan W. PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar. Plant Cell 14: 1885–1901, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, and Weber H. Legume embryos develop in a hypoxic environment. J Exp Bot 53: 1099–1107, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Rothstein EC. and Lucchesi PA. Redox control of the cell cycle: a radical encounter. Antioxid Redox Signal 7: 701–703, 2005 [DOI] [PubMed] [Google Scholar]
- 149.Ruonala R, Rinne PLH, Baghour M, Moritz T, Tuominen H, and Kangasjärvi J. Transitions in the functioning of the shoot apical meristem in birch (Betula pendula) involve ethylene. Plant J 46: 628–640, 2006 [DOI] [PubMed] [Google Scholar]
- 150.Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, and Rohde A. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 19: 2370–2390, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ruyter-Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, Cardoso C, Lopez-Raez JA, Matusova R, Bours R, Verstappen F, and Bouwmeester H. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another belowground role for strigolactones? Plant Physiol 155: 721–734, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, and Fluhr R. Plant Respiratory Burst Oxidase Homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16: 616–628, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, Bailly A, Lee Y, Friml Jí, Geisler M, and Martinoia E. Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J Biol Chem 283: 31218–31226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sarath G, Hou G, Baird L, and Mitchell R. Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C4-grasses. Planta 226: 697–708, 2007 [DOI] [PubMed] [Google Scholar]
- 155.Schrader J, Moyle R, Bhalerao R, Hertzberg M, Lundeberg J, Nilsson P, and Bhalerao R. Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. Plant J 40: 173–187, 2004 [DOI] [PubMed] [Google Scholar]
- 156.Searle I, He Y, Turck F, Vincent C, Fornara F, Krober S, Amasino RA, and Coupland G. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 20: 898–912, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Semenza GL. Hypoxia. Cross talk between oxygen sensing and the cell cycle machinery. Am J Physiol Cell Physiol 301: C55 0–C552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sierla M, Rahikainen M, Salojärvi J, Kangasjärvi J, and Kangasjärvi S. Apoplastic and chloroplastic redox signaling networks in plant stress responses. Antioxid Redox Signal 18: 2220–2239, 2013 [DOI] [PubMed] [Google Scholar]
- 159.Skirycz A, Claeys H, De Bodt S, Oikawa A, Shinoda S, Andriankaja M, Maleux K, Eloy NB, Coppens F, Yoo S-D, Saito K, and Inzé D. Pause-and-stop: The effects of osmotic stress on cell proliferation during early leaf development in Arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell 23: 1876–1888, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Srinivasan C, Dardick C, Callahan A, and Scorza R. Plum (Prunus domestica) trees transformed with poplar FT result in altered architecture, dormancy requirement, and continuous flowering. PLoS One 7: e40715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stacy RAP, Nordeng TW, Culiáñez-Macià Francisco A, and Aalen RB. The dormancy-related peroxiredoxin anti-oxidant, PER1, is localized to the nucleus of barley embryo and aleurone cells. Plant J 19: 1–8, 1999 [DOI] [PubMed] [Google Scholar]
- 162.Steffens B. and Sauter M. G proteins as regulators in ethylene-mediated hypoxia signaling. Plant Signal Behav 5: 375–378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stirnberg P, Furner IJ, and Ottoline Leyser HM. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 50: 80–94, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Stirnberg P, van de Sande K, and Leyser HMO. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141, 2002 [DOI] [PubMed] [Google Scholar]
- 165.Sundaravelpandian K, Chandrika NNP, and Schmidt W. PFT1, a transcriptional Mediator complex subunit, controls root hair differentiation through reactive oxygen species (ROS) distribution in Arabidopsis. New Phytol 197: 151–161, 2013 [DOI] [PubMed] [Google Scholar]
- 166.Sung S. and Amasino RM. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427: 159–164, 2004 [DOI] [PubMed] [Google Scholar]
- 167.Sweetlove LJ. and Foyer CH. Roles for reactive oxygen species and antioxidants in plant mitochondria. In: Plant Mitochondria: From Genome to Function, edited by Day DA, Millar AH, and Whelan J. Dortrecht: Kluwer Academic Publishers, 2004, pp. 307–320 [Google Scholar]
- 168.Teale WD, Ditengou FA, Dovzhenko AD, Li X, Molendijk AM, Ruperti B, Paponov I, and Palme K. Auxin as a model for the integration of hormonal signal processing and transduction. Mol Plant 1: 229–237, 2008 [DOI] [PubMed] [Google Scholar]
- 169.Temple BRS. and Jones AM. The plant heterotrimeric G-protein complex. Annu Rev Plant Biol 58: 249–266, 2007 [DOI] [PubMed] [Google Scholar]
- 170.Tognetti VB, Muhlenbock PER, and Van Breusegem F. Stress homeostasis–the redox and auxin perspective. Plant Cell Environ 35: 321–333, 2012 [DOI] [PubMed] [Google Scholar]
- 171.Tognetti VB, Van Aken O, Morreel K, Vandenbroucke K, van de Cotte B, De Clercq I, Chiwocha S, Fenske R, Prinsen E, Boerjan W, Genty B, Stubbs KA, Inzé D, and Van Breusegem F. Perturbation of indole-3-butyric acid homeostasis by the UDP-Glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 22: 2660–2679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tolleter D, Jaquinod M, Mangavel C, Passirani C, Saulnier P, Manon S, Teyssier E, Payet N, Avelange-Macherel M-H, and Macherel D. Structure and function of a mitochondrial Late Embryogenesis Abundant protein are revealed by desiccation. Plant Cell 19: 1580–1589, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Torres MA. and Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8: 397–403, 2005 [DOI] [PubMed] [Google Scholar]
- 174.Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S, and McCourt P. A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol 6: 741–749, 2010 [DOI] [PubMed] [Google Scholar]
- 175.Tsukagoshi H, Busch W, and Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616, 2010 [DOI] [PubMed] [Google Scholar]
- 176.Turck F, Fornara F, and Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59: 573–594, 2008 [DOI] [PubMed] [Google Scholar]
- 177.Turcotte S, Desrosiers RR, and Béliveau R. HIF-1α mRNA and protein upregulation involves Rho GTPase expression during hypoxia in renal cell carcinoma. J Cell Sci 116: 2247–2260, 2003 [DOI] [PubMed] [Google Scholar]
- 178.Tyburski J, Dunajska K, and Tretyn A. A role for redox factors in shaping root architecture under phosphorus deficiency. Plant Signal Behav 5: 64–66, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, and Yamaguchi S. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200, 2008 [DOI] [PubMed] [Google Scholar]
- 180.van Dongen JT, Roeb GW, Dautzenberg M, Froehlich A, Vigeolas H, Minchin PEH, and Geigenberger P. Phloem import and storage metabolism are highly coordinated by the low oxygen concentrations within developing wheat seeds. Plant Physiol 135: 1809–1821, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.van Dongen JT, Schurr U, Pfister M, and Geigenberger P. Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol 131: 1529–1543, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vanstraelen M. and Benková E. Hormonal interactions in the regulation of plant development. Annu Rev Cell Dev Biol 28: 463–487, 2012 [DOI] [PubMed] [Google Scholar]
- 183.Varkonyi-Gasic E, Moss SMA, Voogd C, Wang T, Putterill J, and Hellens RP. Homologs of FT, CEN and FD respond to developmental and environmental signals affecting growth and flowering in the perennial vine kiwifruit. New Phytol 198: 732–746, 2013 [DOI] [PubMed] [Google Scholar]
- 184.Vashisht D, Hesselink A, Pierik R, Ammerlaan JMH, Bailey-Serres J, Visser EJW, Pedersen O, van Zanten M, Vreugdenhil D, Jamar DCL, Voesenek LACJ, and Sasidharan R. Natural variation of submergence tolerance among Arabidopsis thaliana accessions. New Phytol 190: 299–310, 2011 [DOI] [PubMed] [Google Scholar]
- 185.Vergara R, Parada F, Rubio S, and Pérez FJ. Hypoxia induces H2O2 production and activates antioxidant defence system in grapevine buds through mediation of H2O2 and ethylene. J Exp Bot 63: 4123–4131, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vernoux T, Wilson RC, Seeley KA, Reichheld J-P, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, May MJ, and Sung ZR. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Walton E, Wu R, Richardson A, Davy M, Hellens R, Thodey K, Janssen B, Gleave A, Rae G, Wood M, and Schaffer R. A rapid transcriptional activation is induced by the dormancy-breaking chemical hydrogen cyanamide in kiwifruit (Actinidia deliciosa) buds. J Exp Bot 60: 3835–3848, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wareing PF. and Saunders PF. Hormones and dormancy. Annu Rev Plant Physiol 22: 261–288, 1971 [Google Scholar]
- 189.Waters MT, Brewer PB, Bussell JD, Smith SM, and Beveridge CA. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol 159: 1073–1085, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Webster KA. Hypoxia: life on the edge. Antioxid Redox Signal 9: 1303–1308, 2007 [DOI] [PubMed] [Google Scholar]
- 191.Webster WS. and Abela D. The effect of hypoxia in development. Birth Defects Res Part C 81: 215–228, 2007 [DOI] [PubMed] [Google Scholar]
- 192.Woo HR, Chung KM, Park J-H, Oh SA, Ahn T, Hong SH, Jang SK, and Nam HG. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 13: 1779–1790, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Woo HR, Kim JH, Nam HG, and Lim PO. The delayed leaf senescence mutants of Arabidopsis, ore1, ore3, and i are tolerant to oxidative stress. Plant Cell Physiol 45: 923–932, 2004 [DOI] [PubMed] [Google Scholar]
- 194.Wu R-M, Walton EF, Richardson AC, Wood M, Hellens RP, and Varkonyi-Gasic E. Conservation and divergence of four kiwifruit SVP-like MADS-box genes suggest distinct roles in kiwifruit bud dormancy and flowering. J Exp Bot 63: 797–807, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xia X-J, Wang Y-J, Zhou Y-H, Tao Y, Mao W-H, Shi K, Asami T, Chen Z, and Yu J-Q. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150: 801–814, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xia X-J, Zhou Y-H, Ding J, Shi K, Asami T, Chen Z, and Yu J-Q. Induction of systemic stress tolerance by brassinosteroid in Cucumis sativus. New Phytol 191: 706–720, 2011 [DOI] [PubMed] [Google Scholar]
- 197.Xie X, Yoneyama K, and Yoneyama K. The strigolactone story. Annu Rev Phytopathol 48: 93–117, 2010 [DOI] [PubMed] [Google Scholar]
- 198.Yakovlev IA, Asante DKA, Fossdal CG, Junttila O, and Johnsen Ø. Differential gene expression related to an epigenetic memory affecting climatic adaptation in Norway spruce. Plant Sci 180: 132–139, 2011 [DOI] [PubMed] [Google Scholar]
- 199.Yamane H, Ooka T, Jotatsu H, Hosaka Y, Sasaki R, and Tao R. Expressional regulation of PpDAM5 and PpDAM6, peach (Prunus persica) dormancy-associated MADS-box genes, by low temperature and dormancy-breaking reagent treatment. J Exp Bot 62: 3481–3488, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang Z. Small GTPases. Plant Cell 14: S375–S388, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ye N, Zhu G, Liu Y, Zhang A, Li Y, Liu R, Shi L, Jia L, and Zhang J. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J Exp Bot 63: 1809–1822, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zawaski C, Kadmiel M, Pickens J, Ma C, Strauss S, and Busov V. Repression of gibberellin biosynthesis or signaling produces striking alterations in poplar growth, morphology, and flowering. Planta 234: 1285–1298, 2011 [DOI] [PubMed] [Google Scholar]
- 203.Zegzouti H, Anthony RG, Jahchan N, Bögre L, and Christensen SK. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc Natl Acad Sci U S A 103: 6404–6409, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhong W, Gao Z, Zhuang W, Shi T, Zhang Z, and Ni Z. Genome-wide expression profiles of seasonal bud dormancy at four critical stages in Japanese apricot. Plant Mol Biol 83: 247–264, 2013 [DOI] [PubMed] [Google Scholar]