Abstract
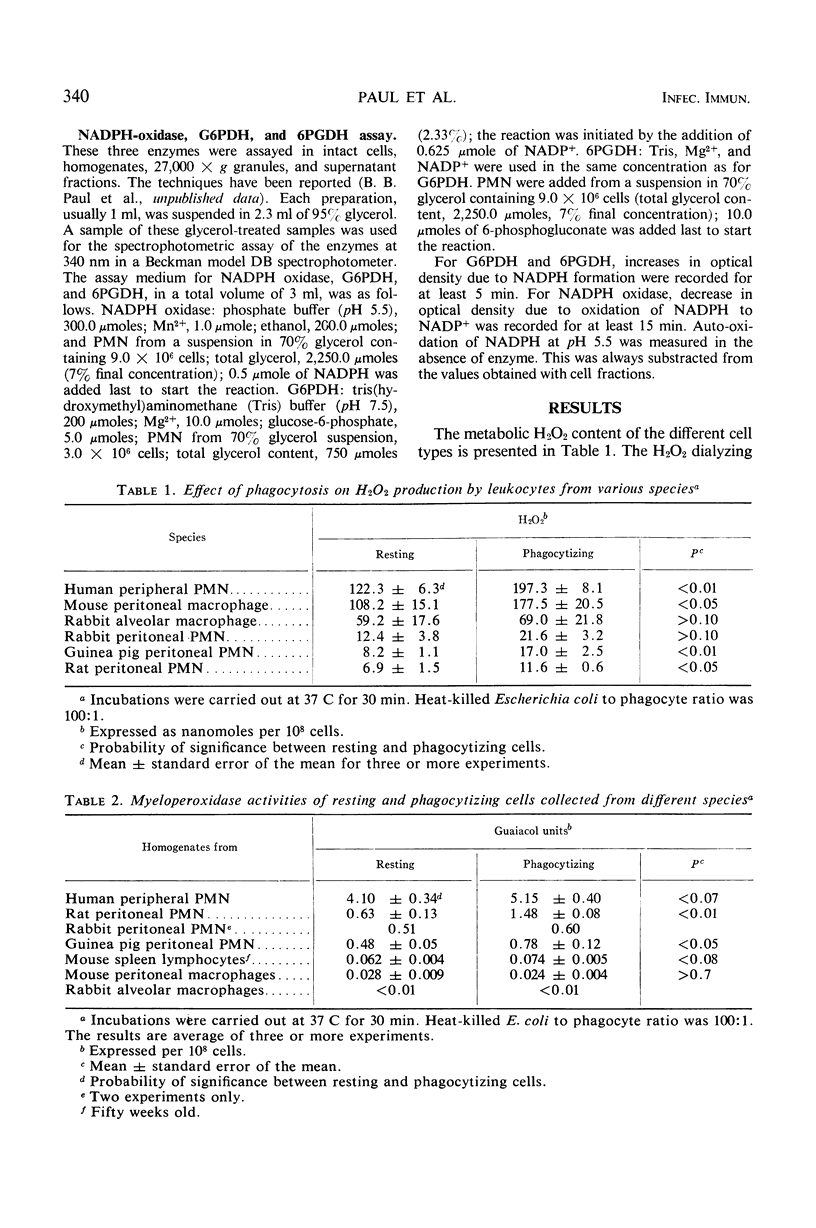
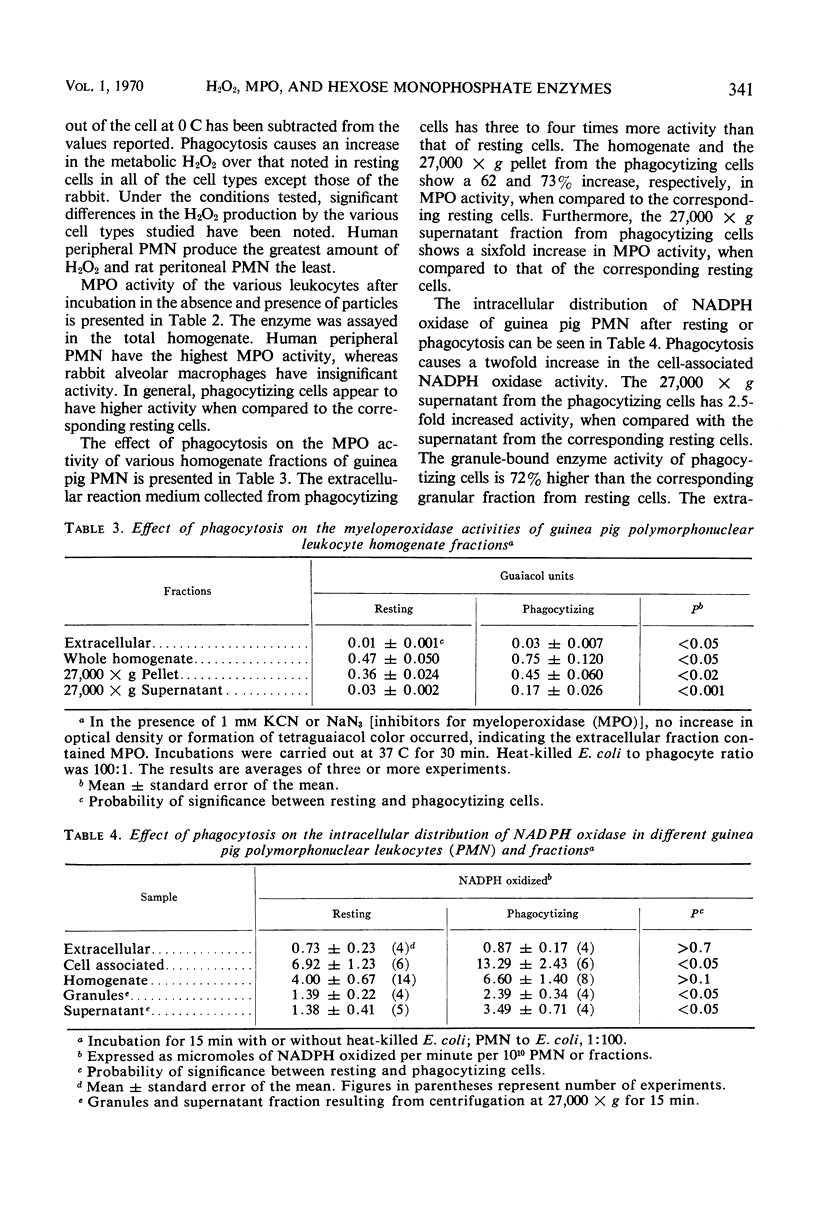
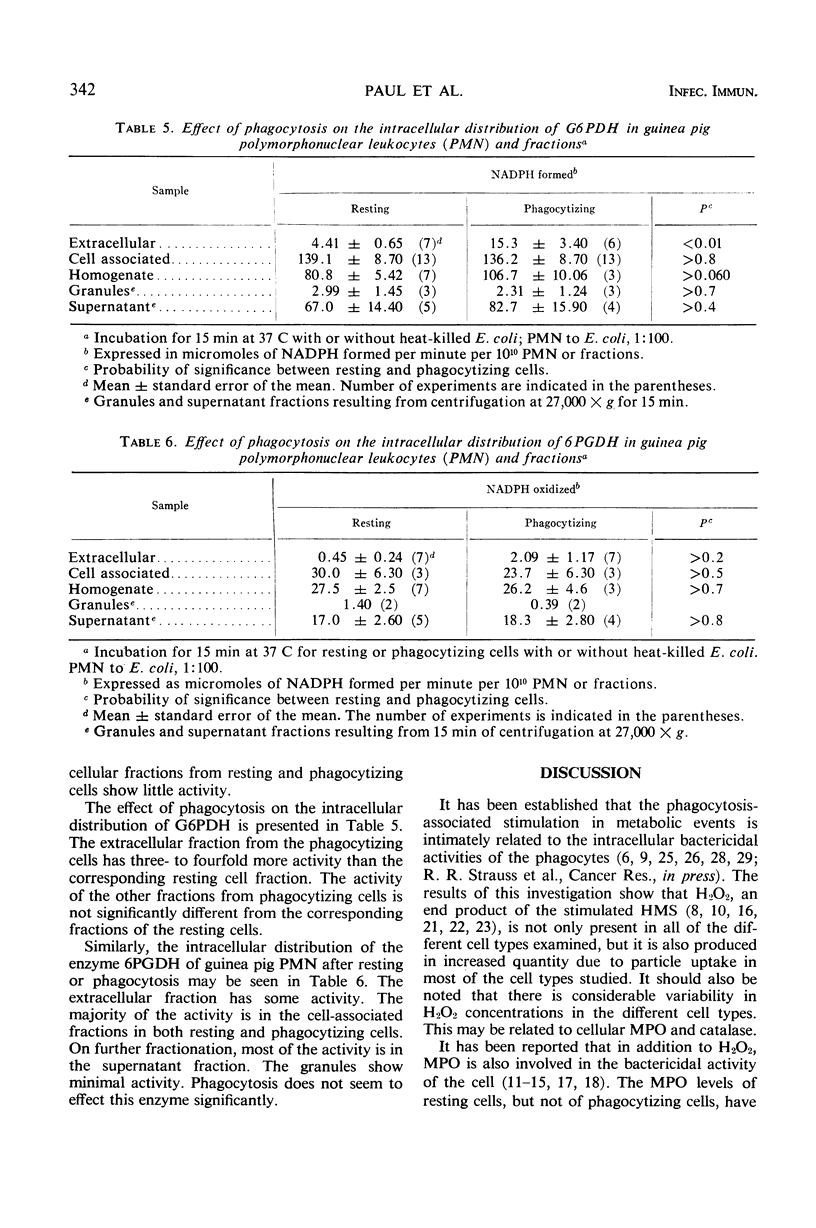
The effect of phagocytosis on the H2O2 production and myeloperoxidase (MPO) activities of leukocytes from various species was investigated. The intracellular distribution of MPO, reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, glucose-6-phosphate dehydrogenase, and 6-phosphogluconate dehydrogenase (6PGDH) of resting and phagocytizing guinea pig polymorphonuclear leukocytes has also been studied. Phagocytizing cells produce more H2O2 than the corresponding resting cells. This has been found to be true for human peripheral polymorphonuclear leukocytes, mouse peritoneal macrophage, and guinea pig and rat peritoneal polymorphonuclear leukocytes. All of these cells, except rabbit alveolar macrophages, have significant MPO activity. Generally an increased activity is noted with phagocytizing cells. Homogenization and differential centrifugation of guinea pig peritoneal polymorphonuclear leukocytes indicate that the whole homogenate and its fractions from phagocytizing cells have significantly higher MPO and NADPH oxidase activities, when compared to the corresponding fractions from the resting cells. The 27,000 × g supernatant fluid from phagocytizing cells has 6-fold more MPO and 2.5-fold more NADPH oxidase activity than similar supernatant fractions from resting cells. The enzyme 6PGDH was unaffected by phagocytosis. The relationship of these stimulated activities to the intracellular bactericidal function of the phagocytes has been discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. C., Feinstein R. N. Relationship of hydrogen peroxide production by Mycoplasma pulmonis to virulence for catalase-deficient mice. J Bacteriol. 1969 Jun;98(3):1036–1040. doi: 10.1128/jb.98.3.1036-1040.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTLIEB A. R., SBARRA A. J., BARDAWIL W. A. Isolation of highly purified, viable leukocytes from blood. Am J Clin Pathol. 1962 Mar;37:257–262. doi: 10.1093/ajcp/37.3.257. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Rechcigl M., Jr Factors influencing myeloperoxidase and catalase activities in polymorphonuclear leukocytes. Biochim Biophys Acta. 1967 Oct 9;148(1):243–250. doi: 10.1016/0304-4165(67)90299-1. [DOI] [PubMed] [Google Scholar]
- Good R. A., Quie P. G., Windhorst D. B., Page A. R., Rodey G. E., White J., Wolfson J. J., Holmes B. H. Fatal (chronic) granulomatous disease of childhood: a hereditary defect of leukocyte function. Semin Hematol. 1968 Jul;5(3):215–254. [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER G. Y., QUESTEL J. H. NADPH and NADH oxidation by guinea pig polymorphonuclear leucocytes. Can J Biochem Physiol. 1963 Feb;41:427–434. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Antifungal effects of peroxidase systems. J Bacteriol. 1969 Aug;99(2):361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Mandell G. L., Hook E. W. Leukocyte bactericidal activity in chronic granulomatous disease: correlation of bacterial hydrogen peroxide production and susceptibility to intracellular killing. J Bacteriol. 1969 Oct;100(1):531–532. doi: 10.1128/jb.100.1.531-532.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XI. Relationship between stimulated oxidative metabolism and hydrogen peroxide formation, and intracellular killing. J Bacteriol. 1967 Nov;94(5):1417–1424. doi: 10.1128/jb.94.5.1417-1424.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E. Killing and lysis of gram-negative bacteria through the synergistic effect of hydrogen peroxide, ascorbic acid, and lysozyme. J Bacteriol. 1969 Jun;98(3):949–955. doi: 10.1128/jb.98.3.949-955.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi E., Selvaraj R. J., Sbarra A. J. The biochemical activities of rabbit alveolar macrophages during phagocytosis. Exp Cell Res. 1965 Dec;40(3):456–468. doi: 10.1016/0014-4827(65)90226-0. [DOI] [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- Paul B., Strauss R., Sbarra A. J. The role of the phagocyte in host-parasite interactions. XVI. Effect of x-irradiation on H2O2 production in guinea pig exudate cells. J Reticuloendothel Soc. 1968 Dec;5(6):538–549. [PubMed] [Google Scholar]
- Roberts J., Camacho Z. Oxidation of NADPH by polymorphonuclear leucocytes during phagocytosis. Nature. 1967 Nov 11;216(5115):606–607. doi: 10.1038/216606a0. [DOI] [PubMed] [Google Scholar]
- Rossi F., Zatti M. The mechanism of the respiratory stimulation during phagocytosis in polymorphouclear leucocytes. Biochim Biophys Acta. 1966 Feb 14;113(2):395–397. doi: 10.1016/s0926-6593(66)80079-6. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Schultz J., Corlin R., Oddi F., Kaminker K., Jones W. Myeloperoxidase of the leucocyte of normal human blood. 3. Isolation of the peroxidase granule. Arch Biochem Biophys. 1965 Jul;111(1):73–79. doi: 10.1016/0003-9861(65)90324-3. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966 Sep 17;211(5055):1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. 8. Effect of whole-body x-irradiation on nicotinamides, lysosomal enzymes and bactericidal activities of leukocytes during phagocytosis. J Bacteriol. 1967 Jul;94(1):149–156. doi: 10.1128/jb.94.1.149-156.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes R. C. Leukin, a bactericidal agent from rabbit polymorphonuclear leucocytes. Nature. 1967 Nov 25;216(5117):806–808. doi: 10.1038/216806a0. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Sbarra A. J. Effect of phenylbutazone on phagocytosis and intracellular killing by guinea pig polymorphonuclear leukocytes. J Bacteriol. 1968 Dec;96(6):1982–1990. doi: 10.1128/jb.96.6.1982-1990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G. The role of lysosomes in inflammation and disease. Annu Rev Med. 1967;18:97–112. doi: 10.1146/annurev.me.18.020167.000525. [DOI] [PubMed] [Google Scholar]
- West J., Morton D. J., Esmann V., Stjernholm R. L. Carbohydrate metabolism in leukocytes. 8. Metabolic activities of the macrophage. Arch Biochem Biophys. 1968 Mar 20;124(1):85–90. doi: 10.1016/0003-9861(68)90306-8. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



