Abstract
We monitored microbiological properties in two forest sites over a period of 17 years (1993–2010) within the International Cooperative Programme on Integrated Monitoring of Air Pollution Effects on Ecosystems (ICP IM). The two study sites were located in South Tyrol in the Italian Alps at altitudes of 1,737 m a.s.l. (subalpine site IT01) and 570 m a.s.l. (submontane site IT02). Soil samples were collected in the late spring and autumn of 1993, 2000, and 2010, and were characterized by measuring respiration, key enzyme activities involved in the C, N, P, and S cycles and litter degradation, and the abundance of viable bacterial and fungal populations. Over the study period, an increase in mean annual air temperatures at both sites (+0.6°C and +0.8°C at IT01 and IT02, respectively) was calculated from trendlines. Significantly lower mean annual air temperatures, higher temperature fluctuations, and higher annual precipitation rates were observed at site IT01 than at site IT02. Subalpine site IT01 was characterized by significantly lower microbial activity (respiration, enzymes) and abundance than those at submontane site IT02. The year of sampling had a significant effect on all the parameters investigated, except for nitrification. Fungal abundance decreased consistently over the study period, while no consistent trend was noted among the other parameters investigated. Season only affected a few of the measured microbiological parameters: respiration and bacterial numbers were significantly higher in the spring than in the autumn, while the opposite was noted for xylanase and phosphatase activities. Soil fungi contributed essentially to xylanase and protease activities, while soil bacteria were mainly involved in degradation processes that required the activity of sulfatase.
Keywords: forest soils, subalpine, submontane, soil microbiology, enzymes
Climate change is increasing surface temperatures worldwide. The European Alps already experienced an increase in the annual minimum temperature of approximately 2°C during the 20th century (5). Mountain regions are especially vulnerable to climate change (12). In Alpine environments, climate change is expected to cause an upward migration of vegetation zones. This may alter the composition of vegetation as well as the quantity and quality of plant litter, which in turn affects the composition and functioning of microbial communities (14). Climate has one of the greatest effects on soil microbial activities (56).
Forests are expected to face significant pressures in the future from climate change. Data from long-term monitoring programs can be used to answer questions on the impacts of climate change and air pollution on forest ecosystems as well as the feedback of forests to the climate (9). Such a long-term monitoring program has been undertaken by the multi-disciplinary International Cooperative Programme on Integrated Monitoring of Air Pollution Effects on Ecosystems (ICP IM), which is part of the Effects Monitoring Strategy under the UN ECE (United Nations Economic Commission for Europe) Convention on Long-Range Transboundary Air Pollution (LRTAP) and became a permanent monitoring program from the beginning of 1993 (26). In this framework, integrated monitoring is carried out at 57 sites, mostly in Europe, in 23 countries.
ICP IM includes the monitoring of bioindicators that describe the state of the ecosystem (1). Since microbiological processes are central to forest growth, their monitoring constitutes an important tool for the characterization of forest soils (48). The ICP IM subprogram “Microbial Decomposition” is focused on monitoring microbiological parameters, with special consideration of soil respiration, microbial biomass, soil enzymatic activity, and N turnover dynamics (22, 53).
Soil biology is a significant component of soil quality, and microorganisms play vital roles in soil fertility and primary production through the decomposition of organic matter and nutrient cycling (2). Microbiological activity determines the net-mineralization of nutrients in an ecosystem; thus, any disturbance in ordinary activity will result in changes in both decomposition and nutrient uptake. Since the microbiological composition of soil is extremely difficult to monitor directly, indications and indirect biochemical measurements have to be applied (40). Soil biological properties, including soil respiration, soil biomass, microbial numbers, and enzyme activities, have been used successfully as biological indicators to evaluate soil metabolic activity and the effects of changing environmental conditions on soil ecosystems (17, 34, 38, 43, 45, 52). Soil (basal) respiration is considered to represent overall microbial activity that reflects the mineralization of organic matter in soil and is the most commonly used biological variable in soil studies, including forest ecosystems (53, 54). Soil enzyme activities are the direct expression of the soil community to metabolic requirements and available nutrients; the capacity of soil microorganisms to maintain soil enzyme functional diversity could ultimately be more important to the stability of an ecosystem than taxonomic diversity (10).
This study was performed within the ICP IM subprogram “Microbial Decomposition” and presented the microbiological characterization of two permanent monitoring sites (IT01 Ritten, IT02 Montiggl) located in South Tyrol in the Italian Alps that were established in 1992 as B class sites as classified by the ICP IM. The objective of this study was to monitor soil microbiological properties in the late spring and autumn of 1993, 2000, and 2010. Soil samples were characterized with regard to respiration, the key enzyme activities involved in the C, N, P, and S cycles, and litter degradation. Culture-dependent methods were used to obtain information on the abundance of bacterial and fungal populations. We evaluated the effects of the sampling year, season, and altitude (by comparing submontane and subalpine sites) on soil microbial activity at these sites. So far, the impact of global warming on microbial communities and activities in forest soils has been evaluated in short-term (2–3 years) soil warming experiments; however, no monitoring studies have been conducted for a period comparable to our 17-year study.
Materials and Methods
Description of the study sites
The two investigated sampling sites IT01 (Ritten) and IT02 (Montiggl) are two long-term monitoring sites that were installed in the Italian Alps in 1992 within the framework of ICP IM. These sites represent two widely distributed and forestally significant forest types in South Tyrol; their characteristics are shown in Table 1.
Table 1.
Characteristics of the sites sampled in the late spring (May) and autumn (October) of 1993, 2000, and 2010.
| Site | IT01 (Ritten) | IT02 (Montiggl) |
|---|---|---|
| Location | below Rittner Horn | above Small Lake Montiggl |
| Size | 0.9 ha (confined) | 0.9 ha (confined) |
| Altitude | 1,737 m a.s.l. | 570 m a.s.l. |
| Altitudinal vegetation belt | subalpine | submontane |
| Phytoclimate | Piceetum, subzone warm | Castanetum, subzone cold |
| Vegetation | Coniferous forests and shrubs | Mixed deciduous forests |
| Piceetum subalpinum | Quercetum pubescentis | |
| Bedrock | Silicate (porphyry) | Silicate (porphyry) |
| Soil type* | Haplic podsol | Dystric cambisol |
| Soil properties | ||
| Sand (%; <2,000–63 μm) | 48 | 36 |
| Silt (%; <63–2 μm) | 36 | 51 |
| Clay (%; <2 μm) | 16 | 13 |
| CaCO3 (%) | <1% | <1% |
| pH (CaCl2) | 3.3 | 4.1 |
| SOM (%) | 33 | 12 |
| Corg (%) | 19 | 7 |
| C/N | 24 | 21 |
| N (mg 100 g−1) | 826 | 327 |
| P2O5 (mg 100 g−1) | 4.1 | 1.6 |
| K2O (mg 100 g−1) | 21 | 11 |
| Mg (mg 100 g−1) | 16 | 14 |
According to the soil classification of the Federal Republic of Germany (60)
The subalpine site IT01 is located 7 km north of Bozen/Bolzano below the Rittner Horn at an altitude of 1,737 m above sea level (a.s.l.). The pedogenetic substratum consists of a mixed morainic and detritus cover of porphyritic pebbles and stones on quattziferous porphyry of the Permian period (Paleozoic). The soil was classified (FAO) as haplic podsol. The site consists of coniferous forests close to the timber line and is dominated by Picea abies, Pinus cembra, and Larix decidua. The climate is subalpine-continental with a mean annual temperature of 4°C and mean annual rainfall of 1,000 mm. Before confining the study site, high stress factors arose from grazing and touring (7).
The submontane site IT02 is located 8 km south of Bozen/Bolzano above the Small Lake Montiggl at an altitude of 570 m a.s.l. The pedogenetic substratum consists of colluvial material on quartziferous porphyry, which often comes to the surface in outcrops. The soil was classified (FAO) as dystric cambisol. The site consists of deciduous forests and is dominated by Quercus pubenscens, Q. robur, Fraxinus ornus, and Ostrya carpinifolia. The climate is mild continental with submediterranean influences, with a mean annual temperature of 11°C and mean annual rainfall of 900 mm. Before confining the study site, high stress factors resulted from grazing, touring, clear-cutting, and reforestation with allochthonous species (Picea abies) (7).
Climatic conditions
Air temperature at the two sites was constantly monitored according to WMO standards at open field stations using the sensor t026 TTEPRH (Siap+Micros, Italy). Bulk precipitation was monitored by open field pluviometers using the sensor t027 TP500 (Siap+Micros, Italy).
Soil sampling and sample preparation
In each site, 10 sampling spots that were distributed uniformly over the site were chosen in 1992 when the sites were established. Soil samples (ca. 5 kg) were collected from each of these sampling spots from the Ah horizon (top 10 cm). Sampling occurred in 1993, 2000, and 2010. To determine the effects of season, soil samples were collected in each sampling year from each site both in the late spring (May) and autumn (October). Immediately after sampling, soil samples were transported in cooled boxes to the laboratory, sieved (<2 mm), and immediately analyzed or stored at 2°C. Each soil sample was analyzed with three replicates.
Physical and chemical soil properties
Soil samples were analyzed after sampling in the spring and autumn for dry mass (24 h at 105°C), maximum water-holding capacity, and soil organic matter (SOM; loss on ignition after 3 h at 430°C) as described (45, 46).
Soil microbial activities
Soil respiration (basal respiration) was determined according to the ICP IM Manual (20). Soil samples were pre-incubated for 12 d at 50% of the maximum water-holding capacity to stabilize basal respiration. Soil samples were then incubated in air-tight 1-L vessels that contained NaOH solution to absorb the CO2 produced during incubation. After 18 h of incubation at a temperature of 22°C, BaCl2 was added and excess NaOH was titrated with HCl to the endpoint of phenolphthalein. Substrate-induced respiration (SIR) was determined using the same method in soil samples that were amended with glucose and incubated at 22°C in bottles containing NaOH.
Soil dehydrogenase activity was examined using trinitrophenyltetrazolium chloride (TTC, 1.5%, w/v) as a substrate and 0.1 M Tris-HCl, pH 7.8, as a buffer. After 16 h of incubation at 25°C, the produced triphenyl formazan (TPF) was extracted and measured spectrophotometrically (51, modified according to reference no. 45).
Soil xylanase activity was assessed using xylane (1.2%, w/v) as a substrate and 2 M sodium acetate-acetic acid buffer, pH 5.5. After 24 h of incubation at 50°C, the reduced sugars released during the incubation period were determined colorimetrically (45).
Protease activity was determined using casein (2%, w/v) as a substrate and 0.05 M Tris-HCl, pH 8.1, as buffer. After 2 h of incubation at 50°C, the amino acids released during incubation were extracted and quantified colorimetrically (27, 45).
Phosphomonoesterase activity (acidic phosphatase) was measured as described in the ICP IM Manual (20) by using 0.115 M p-nitrophenyl phosphate as a substrate and 0.5 M sodium acetate-acetic acid buffer, pH 5.0. After 1 h of incubation at 37°C, the p-nitrophenol released during the incubation was extracted and colorimetrically determined.
Arylsulfatase activity was determined using 0.02 M p-nitrophenyl sulfate as a substrate and 0.5 M sodium acetate-acetic acid buffer, pH 5.8. After 1 h of incubation at 37°C, the p-nitrophenol released during the incubation was extracted and colorimetrically determined (50, modified according to reference no. 45).
Nitrification was determined after the incubation of soil samples amended with ammonium chloride solution for 2 weeks (samples from site IT02) or 3 weeks (samples from site IT01 were incubated for longer due to a higher SOM content) at 25°C and the released amounts of ammonium and nitrate were determined colorimetrically. Oxidation of the substrate (ammonium chloride) was equivalent to the nitrification dynamics and was expressed as a percentage of the added nitrogen (3, 4, 45).
Enumeration of culturable aerobic soil bacteria and soil fungi
Culturable heterotrophic soil bacteria and soil fungi were quantified by the plate-count method for viable cells. Appropriate dilutions of soil suspensions were spread on the surfaces of agar plates. The numbers of heterotrophic bacteria were determined on Standard I agar, supplemented with cycloheximide (400 μg mL−1) to exclude fungal growth. Soil fungi were enumerated on malt extract agar (5% [w/v] malt extract) supplemented with 0.02% (w/v) dl-lactic acid and chloramphenicol (100 μg mL−1) to exclude bacterial growth. Plates were incubated at 25°C for 48–72 h.
The microbial abundance of heterotrophic microorganisms was determined using culture-dependent methods. Since culturable cells may only represent ≤0.1–1% of the total microbial community in an environment (2, 41), culture-independent, molecular assays, such as profiling soil DNA, rRNA, or phospholipid fatty acids, are increasingly used (19, 33, 37, 43, 61). However, these methods were not well established at the beginning of the long-term monitoring in 1993 and were, thus, not included in this study. In order to allow for a comparison of the results obtained in 1993, 2000, and 2010, we consistently used the culture-dependent method as applied in 1993.
Calculations and statistical treatment of data
Soil microbiological data are usually calculated on a dry mass basis. However, soil microbiological activity is highly dependent on the soil organic matter (SOM) content, which constitutes the basis of nutrition for heterotrophic soil microorganisms (45), while soil dry mass also contains material without relevance for microbial activity. Thus, the calculation of soil microbiological properties on a SOM basis is more appropriate for comparative studies of soils with highly differing SOM contents, as was the case in the present study. All measured parameters were calculated on a SOM basis.
Properties were determined for each soil sample (10 soil samples per site) in the spring and autumn of each sampling year, and the mean values of replicate determinations were used for statistical calculations (Statistica, version 8.0). Normal distributions were evaluated by the Kolmogorov-Smirnov-test. ANOVA was applied to determine whether the sampling year, sampling season, and/or the sampling site had a significant (P<0.05) effect on the soil microbiological parameters investigated. Kruskal-Wallis ANOVA and Mood’s median test were additionally applied for non-parametric data; however, these tests confirmed the results obtained by ANOVA. Multivariate statistics (principal component analysis) was applied to evaluate the relationship between the measured soil microbiological parameters and determining factors. Correlations between the determined properties in each site were tested by Spearman’s rank correlation coefficient since most of the data were non-parametric.
Results and Discussion
Climate conditions
The climate for the two studied sites is subalpine-continental with a solstitial pluvial curve (maximum values in summer and minimum values in winter). Site IT02 exhibited submediterranean influences with equinoctial pluvial regimes. This atmospheric condition, along with factors such as poor soil formation and low water retention capacity denoted occasional periods of summer aridity (1).
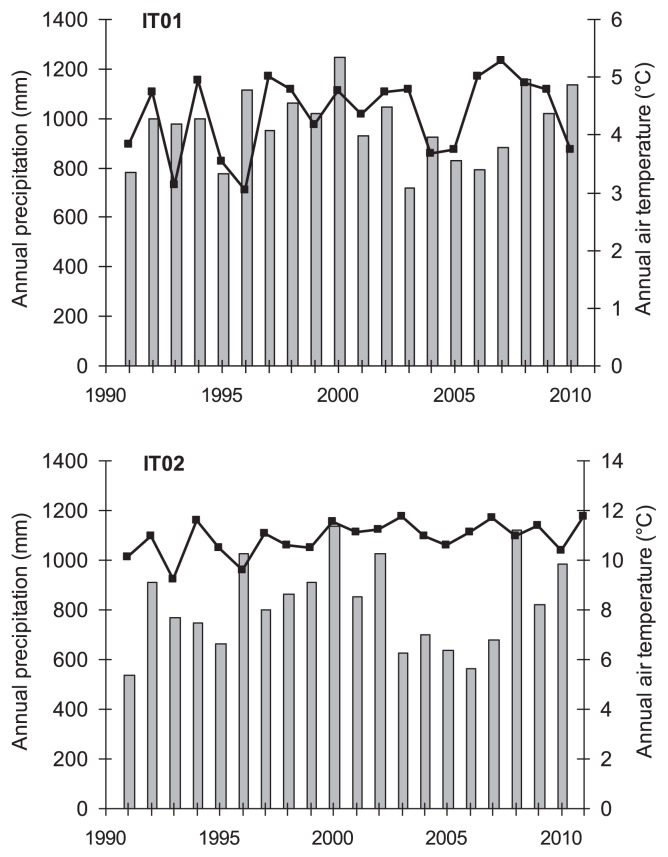
Mean annual air temperatures and precipitation at the two sites during the study period are shown in Fig. 1. Site IT01 was characterized by significantly (P<0.05) colder climatic conditions and larger temperature fluctuations than those at site IT02. Mean annual air temperatures were 4.3±0.7°C at site IT01 and 10.9±0.7°C at site IT02. Minimum and maximum air temperatures were 1.9±0.7°C and 7.1±0.9°C at site IT01, and were markedly higher at IT02 (5.8±0.5°C and 15.9±1.0°C). Site IT01 was also characterized by significantly higher annual precipitation (977±142 mm) than that at IT02 (830±174 mm).
Fig. 1.
Climatograms showing mean annual air temperature (lines) and precipitation (columns) for the two study sites.
Over the study period (1993–2010), an increase in the mean annual air temperature (+0.6°C and +0.8°C at IT01 and IT02, respectively) at both sites was calculated from trendlines. A 34-year-climatic series (1977–2011) at site IT02 even showed an increase in mean annual air temperatures over time from 10°C to 11.4°C (data not shown). An almost identical increase in temperature was reported at a site in northern Italy (6). An analysis of the climate regime in the period 2005–2009 over the Alpine area and northern Italy demonstrated the repeated occurrence of seasonal deviations (such as very cold winters, warm winters, rainy winters) over the last decade (6).
Physical and chemical soil properties
Marked differences were observed in the nutrient contents of soil at the two studied sites. Soil from site IT01 contained significantly higher amounts of SOM (33%), Corg (19%), and nitrogen (0.8%) than that from site IT02 (12% SOM; 7% Corg, 0.3% nitrogen); however, the C/N ratios were similar (Table 1). This is typical for subalpine forest soils, which contain high amounts of SOM in the Ah horizon due to slow litter degradation processes (especially at cold and humid sites). Soil from site IT01 was further characterized by its higher acidity (pH 3.3) than that from site IT02 (pH 4.1) (Table 1).
Soil microbiological properties
Effect of the sampling year
The year of sampling had a significant effect on all investigated parameters, except nitrification (Tables 2 and 3). However, no consistent trend was observed among the investigated parameters. Phosphatase, sulfatase, respiration, SIR, and bacterial numbers were significantly higher in the second sampling period (2000) than in the first sampling period (1993), while the opposite was observed for dehydrogenase, xylanase, and protease as well as fungal numbers. Phosphatase, sulfatase and respiration were significantly lower in the third sampling period (2010) than in 2000, whereas no significant differences were observed in dehydrogenase, protease, and xylanase activities, SIR, and bacterial numbers between 2000 and 2010. These data can be explained by the climate conditions prevailing in each of the three sampling years: at both sites, air temperatures and precipitation were lower in 1993 than in 2000, while values recorded in 2010 were lower than those in 2000, but still higher than those in 1993 (Fig. 1). The constant decrease in fungal abundance over the study period could be attributed to a reduction in the C/N ratio because of accelerated litter degradation (due to increasing temperatures), which favored bacteria over fungi. C/N ratios decreased in spring from 24 (1993) to 22 (2000) and 21 (2010) at site IT01 and from 21 (1993) to 18 (2000) and 17 (2010) at site IT02. These decreases may have been due to a constant decrease in soil organic C with time, whereas N contents remained almost unchanged. Decreases in the relative abundance of fungi as a response to global warming have already been described (49, 58); however, these findings were obtained from experimental soil warming studies performed over short periods of up to 2 years, which makes it difficult to compare them with the results obtained in our study.
Table 2.
Mean values of the soil microbiological properties determined in this study. Different letters indicate significant (P<0.05) differences.
| VARIATION | RESP | SIR | DHG | XYL | PROT | PME | SULF | NITRIF | BACT | FUNGI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | IT01 | 0.040 a | 2157 a | 77 a | 3776 a | 427 a | 452 a | 720 a | 0.168 a | 6.36 a | 4.33 a |
| IT02 | 0.057 b | 3910 b | 464 b | 8238 b | 817 b | 391 a | 1240 b | 0.311 a | 7.36 b | 6.38 b | |
| Year | 1993 | 0.048 b | 2047 a | 548 b | 521 b | 130 b | 99 a | 352 a | 0.033 a | 6.68 a | 6.23 a |
| 2000 | 0.059 c | 3749 b | 62 a | 265 a | 60 a | 1097 c | 1523 c | 0.596 a | 7.02 b | 5.19 b | |
| 2010 | 0.040 a | 3305 b | 202 a | 130 a | 75 a | 167 b | 1063 b | 0.089 a | 6.88 b | 4.65 c | |
| Season | Spring | 0.052 b | 2976 a | 284 a | 5065 a | 1268 a | 362 a | 1094 a | 0.111 a | 6.96 b | 5.33 a |
| Autumn | 0.046 a | 3092 a | 257 a | 9950 b | 776 a | 481 b | 865 a | 0.368 a | 6.77 a | 5.38 a | |
Abbreviations: RESP, respiration (mg CO2 [g SOM * h]−1); SIR, substrate-induced respiration (mg CO2 [g SOM * h]−1); DHG, dehydrogenase (μg TPF [g SOM * 16 h]−1); XYL, xylanase (μg glucose [g SOM * 24 h]−1); PROT, protease (μg tyrosine [g SOM * 2 h]−1); PME, acidic phosphatase (μmol pNP [g SOM * h]−1); SULF, sulfatase (μmol pNP [g SOM * h]−1); NITRIF, nitrification (% N d−1); BACT, bacterial numbers (log cfu); FUNG, fungal numbers (log cfu).
Table 3.
Effects of the study site, sampling season, and sampling year as well as their interactions on soil microbiological properties, as determined by ANOVA (Kruskal-Wallis ANOVA and Mood’s median test were additionally applied for non-parametric data and confirmed the results obtained by ANOVA).
| Parameter | Effect of | Significant effect? | Result | |
|---|---|---|---|---|
|
| ||||
| P-value | yes/no | |||
| RESP | Year | 0.000 | yes | 2010 < 1993 < 2000 |
| Season | 0.000 | yes | Spring > Autumn | |
| Site | 0.000 | yes | IT01 < IT02 | |
| Site * year | 0.504 | no | ||
| Site * season | 0.681 | no | ||
| Year * season | 0.006 | yes | ||
| Site * year * season | 0.148 | no | ||
| SIR | Year | 0.000 | yes | 1993 < 2000 = 2010 |
| Season | 0.588 | no | Spring = Autumn | |
| Site | 0.000 | yes | IT01 < IT02 | |
| Site * year | 0.019 | yes | ||
| Site * season | 0.64 | no | ||
| Year * season | 0.001 | yes | ||
| Site * year * season | 0.064 | yes | ||
| DHG | Year | 0.000 | yes | 1993 > 2000 = 2010 |
| Season | 0.670 | no | Spring = Autumn | |
| Site | 0.000 | yes | IT01 < IT02 | |
| Site * year | 0.000 | yes | ||
| Site * season | 0.761 | no | ||
| Year * season | 0.755 | no | ||
| Site * year * season | 0.224 | no | ||
| XYL | Year | 0.000 | yes | 1993 > 2000 = 2010 |
| Season | 0.000 | yes | Spring < Autumn | |
| Site | 0.000 | yes | IT01 < IT02 | |
| Site * year | 0.000 | yes | ||
| Site * season | 0.256 | no | ||
| Year * season | 0.000 | yes | ||
| Site * year * season | 0.286 | no | ||
| PROT | Year | 0.000 | yes | 1993 > 2000 = 2010 |
| Season | 0.119 | no | Spring = Autumn | |
| Site | 0.000 | yes | IT01 < IT02 | |
| Site * year | 0.000 | yes | ||
| Site * season | 0.303 | no | ||
| Year * season | 0.096 | no | ||
| Site * year * season | 0.356 | no | ||
| PME | Year | 0.000 | yes | 1993 < 2010 < 2000 |
| Season | 0.000 | yes | Spring < Autumn | |
| Site | 0.069 | no | IT01 = IT02 | |
| Site * year | 0.159 | no | ||
| Site * season | 0.151 | no | ||
| Year * season | .011 | yes | ||
| Site * year * season | 0.167 | no | ||
| SULF | Year | 0.000 | yes | 1993 < 2010 < 2000 |
| Season | 0.096 | no | Spring = Autumn | |
| Site | 0.000 | yes | IT01 < IT02 | |
| Site * year | 0.135 | no | ||
| Site * season | 0.972 | no | ||
| Year * season | 0.135 | no | ||
| Site * year * season | 0.799 | no | ||
| NITRIF | Year | 0.205 | no | 1993 = 2000 = 2010 |
| Season | 0.365 | no | Spring = Autumn | |
| Site | 0.614 | no | IT01 = IT02 | |
| Site * year | 0.761 | no | ||
| Site * season | 0.992 | no | ||
| Year * season | 0.210 | no | ||
| Site * year * season | 0.733 | no | ||
| BACTERIA | Year | 0.001 | yes | 1993 < 2000 = 2010 |
| Season | 0.012 | yes | Spring > Autumn | |
| Site | 0.000 | yes | IT01 < IT02 | |
| Site * year | 0.245 | no | ||
| Site * season | 0.040 | yes | ||
| Year * season | 0.006 | yes | ||
| Site * year * season | 0.656 | no | ||
| FUNGI | Year | 0.000 | yes | 1993 > 2000 > 2010 |
| Season | 0.651 | no | Spring = Autumn | |
| Site | 0.000 | yes | IT01 < IT02 | |
| Site * year | 0.000 | yes | ||
| Site * season | 0.055 | no | ||
| Year * season | 0.055 | no | ||
| Site * year * season | 0.082 | no | ||
The significance level was P<0.05. Abbreviations: RESP, respiration; SIR, substrate-induced respiration; DHG, dehydrogenase; XYL, xylanase; PROT, protease; PME, acidic phosphatase; SULF, sulfatase; NITRIF, nitrification; BACTERIA, bacterial numbers; FUNGI, fungal numbers.
Overall, an increase in air temperatures of 0.6°C (IT01) and 0.8°C (IT02) over the study period of 17 years was noted at the two sites. A 35-year monitoring period (1977–2011) also revealed an increase in temperature of 1.4°C at site IT02. As already described above, the impact of increased temperatures on microbial communities and activities in forest soils has been evaluated in soil warming experiments conducted over a period of up to 2 years (15, 43, 49, 58); however, no studies have conducted experiments over a period of time that is similar to our long-term study. In addition, artificially warmed soils are characterized by a lower moisture content than that in controls without experimental warming, which is a serious drawback because soil moisture is the primary factor controlling decomposition rates in alpine forest soils (59).
Effect of seasonality
Season affected only a few of the measured microbiological parameters. Respiration and bacterial numbers were significantly higher in the spring than in the autumn, while the opposite was noted for the activities of xylanase and phosphatase. No significant differences were observed in any of the other parameters examined in this study between samples measured in the spring and autumn (Tables 2 and 3).
Significantly higher bacterial numbers in the spring than in the autumn, and the opposite trend for xylanase activity (higher activity in autumn than in spring), can be explained by the microorganisms involved in litter degradation. The highest amount of litter accumulates in autumn. This was clearly shown by comparing SOM contents in the spring and autumn: For example, in 2000, SOM contents at site IT01 were 36.5% and 80.3% in the spring and autumn, respectively; values recorded at site IT02 were 44.1% and 81.9%, respectively. Although litter contains components that are not easy to degrade, the degradation of these compounds by fungal cellulases and xylanases starts in autumn, and, consequently these activities are higher in the autumn than in the spring. After the snow melts in spring, easily degradable compounds are available and mainly converted by bacteria. Consequently, bacterial numbers are higher in the spring than in the autumn, which is consistent with the results of our study. Seasonal variations in microbial communities in forest soil were attributed to an altered resource supply, i.e., winter communities had a higher capacity for the degradation of complex C substrates (plant cell walls), while summer communities were better adapted to utilize labile C sources (24). Seasonal variations in community structures and the function of alpine and subalpine soil microbial communities play a central role in biogeochemical cycling (16, 28). Forest soil microorganisms are characterized by high metabolic versatility and a capability to adapt to climatic changes associated with seasonality (8). At higher altitudes the decomposition of soil organic matter was shown to be more responsive to global warming because it is affected in a more sensitive (colder) temperature range than sites at lower altitudes (42). This has also been shown for proteolytic enzymes in forest soils (8).
Comparison of sites
All measured parameters, except for nitrification and phosphatase activity, were significantly different between the two investigated sites; the subalpine site IT01 was generally characterized by significantly lower microbial activity and abundance than those observed at the submontane site IT02 (Tables 2 and 3).
This can be explained by the climatic conditions prevailing at the two sites. Site IT01, located at 1,737 m a.s.l., is exposed to significantly lower temperatures (lower by approximately 7°C on average over the study period), higher temperature fluctuations and higher precipitation (higher by 147 mm on average) than site IT02, which is located at 570 m a.s.l. High altitude ecosystems experience extreme temperature fluctuations with changing seasons and climates, higher precipitation, and a shorter vegetation period (25, 31). Due to these climatic conditions, microbial metabolism is slowed down and accumulating litter cannot be easily degraded, which results in the accumulation of raw humus.
Effect of combinations of the sampling year, seasonality, and site
An evaluation of the effects of the combinations of the three independent factors (year, season, site) clearly indicated that a number of activities were influenced by the combination of sampling year and season (respiration, SIR, xylanase and phosphatase activities, and bacterial numbers) as well as by the combination of sampling year and site (SIR, dehydrogenase, xylanase, and protease activities, and fungal numbers). The combination of season and site only influenced bacterial numbers, while the combination of all three independent factors (year, season, and site) only had a significant effect on SIR (Table 3).
Microbiological activities and the role of soil bacteria and soil fungi
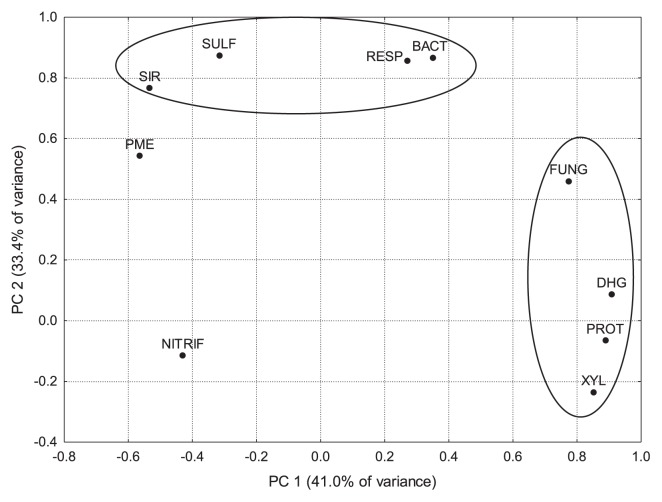
Principal components (PC) were extracted for soil microbiological parameters measured over the whole study period at both sites in the spring and autumn. The first principal component (PC1) explained 41.0% of the variance in data while the second (PC2) accounted for 33.4%. Two clusters could be observed. PC1 was significantly (>0.7) determined by the activities of dehydrogenase, xylanase, and protease as well as fungal numbers (Fig. 2), and this could be explained by the important role of fungi in forest soils for primary litter degradation. Since litter mainly consists of xylanes and also contains a large amount of proteins, the activities of xylanases and proteases are required. In the course of fungal litter degradation, the C/N ratio is lowered, which in turn favors and allows for the activities of soil bacteria. PC2 was significantly determined by respiration, SIR, sulfatase activity, and bacterial numbers (Fig. 2). Bacteria in forest soils utilize the products released by fungal degradation and mineralize these products to CO2 and H2O, as demonstrated by the strong relationship between bacteria, respiration and SIR. Bacterial sulfatases degrade S-containing peptides and amino acids.
Fig. 2.
Loadings for the two first principal components (PC1 and PC2) of soil microbiological parameters determined in this study. Ellipses show parameters significantly (<0.7) contributing to PC1 or PC2. Abbreviations: RESP, respiration; SIR, substrate-induced respiration; DHG, dehydrogenase; XYL, xylanase; PROT, protease; PME, acidic phosphatase; SULF, sulfatase; NITRIF, nitrification; BACT, bacterial numbers; FUNG, fungal numbers.
Nitrification and phosphatase activity did not contribute significantly to any of the clusters (to PC1 or PC2). Nitrification is mainly carried out by autotrophic bacteria (rarely and to a weak extent by heterotrophic bacteria) that were not detected by the culturable method for heterotrophic microorganisms used in the present study. Nitrification has mainly been attributed to acid-sensitive chemoautotrophic nitrifiers; however, this process has also been documented in acid forest soil (18, 29). Seasonal changes in ammonia-oxidizing microorganisms have also been observed in temperate forest soils (36).
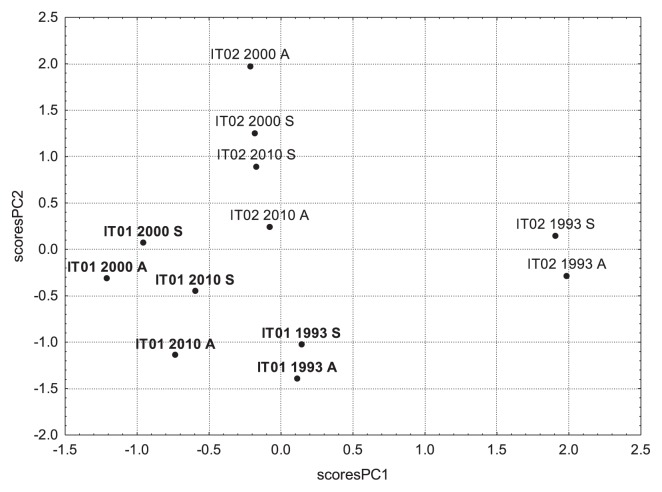
When plotting the PC1 and PC2 scores for all soil samples analyzed, we obtained a clear separation between the two studied sites (Fig. 3). Within each site, PC2 factor scores were higher for values obtained in the spring than for those obtained in the autumn (except for site IT02 in the spring of 2000), which resembles the significantly higher microbial activities and bacterial abundance (significantly contributing to PC2) in the spring than in the autumn. Regarding the sampling years, factor scores were separated along PC1 and PC2. Variability over the study period was markedly higher in site IT02 than in site IT01. Especially, values obtained in 1993 were clearly separated from those obtained in 2000 and 2010, and this could be attributed to the higher temperature and precipitation recorded in 1993 than in 2000 at site IT02 (2.3°C, 372 mm) than at site IT01 (1.6°C, 266 mm).
Fig. 3.
Score plot of PCA showing the separation of the two studied sites sampled in 1993, 2000, and 2010 in the spring (S) and in autumn (A).
Data obtained by PCA were confirmed by correlation analysis (Table 4) in which all data obtained at the two sites in the spring and autumn of 1993, 2000, and 2010 were included. The weak interaction of nitrification was confirmed by the absence of a correlation with most soil microbiological parameters. The positive correlation between xylanase and protease activities and fungal numbers, but the absence of a correlation between these activities and bacterial numbers confirmed the essential role of soil fungi with regards to these enzymes. On the other hand, sulfatase activity was positively correlated with bacterial numbers, but not with fungal numbers, which showed the dominating role of soil bacteria in soil metabolic processes such as the degradation of sulfur-containing compounds. The correlations observed between respiration, SIR, and dehydrogenase activity with bacterial as well as fungal numbers revealed that these microbiological parameters cannot be attributed exclusively to bacteria or fungi.
Table 4.
Correlation matrix (Spearman rank order coefficients and significance level) between the soil microbiological parameters determined in this study
| Parameter | RESP | SIR | DHG | XYL | PROT | NITRIF | PME | SULF | BACT |
|---|---|---|---|---|---|---|---|---|---|
| SIR | 0.657 *** | ||||||||
| DHG | 0.410 *** | 0.415 *** | |||||||
| XYL | 0.295 ** | −0.175 NS | 0.279 | ||||||
| PROT | 0.276 ** | −0.052 NS | 0.562 *** | 0.754 *** | |||||
| NITRIF | 0.034 NS | 0.114 NS | 0.009 NS | −0.186 | −0.140 *** | ||||
| PME | 0.259 ** | 0.504 *** | −0.250 ** | −0.528 *** | −0.656 *** | 0.052 NS | |||
| SULF | 0.451 *** | 0.647 *** | 0.292 ** | −0.263 ** | −0.094 NS | −0.042 NS | 0.565 *** | ||
| BACT | 0.562 *** | 0.651 *** | 0.627 *** | 0.061 NS | 0.170 NS | −0.077 NS | 0.214 | 0.523 *** | |
| FUNG | 0.423 *** | 0.249 | 0.633 *** | 0.573 *** | 0.538 *** | −0.177 NS | −0.263 | −0.027 NS | 0.614 *** |
NS, not significant;*P<0.05;
P<0.01;
P<0.001
Abbreviations: RESP, respiration; SIR, substrate-induced respiration; DHG, dehydrogenase; XYL, xylanase; PROT, protease; PME, acidic phosphatase; SULF, sulfatase; NITRIF, nitrification; BACT, bacterial numbers; FUNG, fungal numbers.
Conclusions
At the two studied forest sites in the Italian Alps, increases in the mean annual air temperature of +0.6°C (IT01) and +0.8°C (IT02) over the monitoring period (1993–2010) were calculated from trendlines. A significant effect of the sampling year was detected on all microbiological properties studied (except nitrification), but with no consistent trend, whereas the effect of seasonality resulted in a significant response in a few parameters only. Soil microbial activities may be weakly correlated with climatic factors because they are typically estimated under optimized rather than in situ conditions (13). Enzyme activity measurements in soil are limited by the use of artificial reaction conditions and substrates and represent potential activities and may be insensitive to small temperature increases (21). However, they are a useful tool to compare the intensity, kind, and duration of specific effects (e.g., environmental conditions) on the metabolic activity of soil (38, 39, 45, 57). In addition, the effects of climate change are likely to be driven by site-specific characteristics; therefore, it is important to consider the location-specific effects of climate change (11). The role of the microclimate in soil biological processes is often overlooked (47). The soil microclimate (temperature, moisture) is highly variable with time, which has been shown to cause temporal variations in microbial structures and activities (21, 23).
Biomonitoring sites also have the objective to quantify the variations between sites (26). We found significant differences in soil microbiological properties between the two investigated sites, i.e. microbial activity and abundance were lower in the subalpine site IT01 than in the submontane site IT02. These differences were mainly attributed to significant (altitude-dependent) differences in the climate conditions prevailing at these sites. However, other factors such as physical and chemical soil properties also play a role. Altitude, which strongly influences climate, was found to affect a number of soil biochemical properties (38). The relationship between altitude (increasing environmental harshness, i.e. lower annual temperature and soil nutrient contents) and a decrease in the microbial population size and diversity (28, 30, 32) as well as a decrease in a number of microbial activities (35, 44, 55) has been reported previously. However, other studies reported no consistent altitudinal trends regarding bacterial activity and community (14).
Therefore, further studies are warranted in order to obtain an insight into changing climate conditions on soil microorganisms. The combination of studies on soil microbial activity and diversity with special consideration of the soil microclimate should allow for a better understanding of the functional role of soil microorganisms in forest soils and will be the focus of future investigations.
Acknowledgements
This study was financed within the ICP IM Programme by the Autonomous Province of Bozen/Bolzano, Section Forestry. We thank P. Illmer for the useful advice regarding statistical data and P. Thurnbichler and J. Mair for their technical assistance. Data on air temperature and precipitation were kindly provided by Umweltagentur-Chemisches Landeslabor (site IT01) and Hydrographisches Amt (site IT02) of the Autonomous Province of Bozen/Bolzano, Section Forestry.
References
- 1.Ambrosi P, Bertolini F, George E, Minerbi S, Salvadori C. Integrated monitoring in alpine forest ecosystems in Trentino and South Tyrol, Italy. Chemosphere. 1998;36:1043–1048. [Google Scholar]
- 2.Alexander M. Introduction to Soil Microbiology. 2nd ed. John Wiley Sons Inc; New York: 1977. [Google Scholar]
- 3.Beck T. Verlauf und Steuerung der Nitrifikation in Bodenmodellversuchen. Landw Forschung. 1976;30:85–94. [Google Scholar]
- 4.Beck T. Die Nitrifikation in Böden (Sammelreferat) J Soil Sci Plant Nutr. 1979;142:344–364. [Google Scholar]
- 5.Beniston M, Diaz HF, Bradley RS. Climate change at high elevation sites: an overview. Clim Change. 1997;36:233–251. [Google Scholar]
- 6.Bertini G, Amoriello T, Fabbio G, Piovosi M. Forest growth and climate change: evidences from the ICP-Forests intensive monitoring in Italy. iForest—Biogeosci Forestry. 2011;4:262–267. [Google Scholar]
- 7.Bonavita P, Chemini C, Ambrosi P, Minerbi S, Salvadori C, Furlanello C. Biodiversity and stress level in four forests of the Italian Alps. Chemosphere. 1998;36:1055–1060. [Google Scholar]
- 8.Brzostek ER, Finzi AC. Seasonal variation in the temperature sensitivity of proteolytic enzyme activity in temperate forest soils. J Geophys Res. 2010;117:G01018. doi: 10.1890/10-1803.1. [DOI] [PubMed] [Google Scholar]
- 9.Clarke N, Fischer R, de Vries W, et al. Availability, accessibility, quality and comparability of monitoring data for European forests for use in air pollution and climate change science. iForest. 2011;4:62–166. [Google Scholar]
- 10.Caldwell BA. Enzyme activities as a component of soil biodiversity: a review. Pedobiologia. 2005;49:637–644. [Google Scholar]
- 11.Dale VH, Tharp ML, Lannom KO, Hodges DG. Modeling transient response of forests to climate change. Sci Total Environ. 2010;408:1888–1901. doi: 10.1016/j.scitotenv.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 12.Diaz HF, Grosjean M, Graumlich L. Climate variability and change in high elevation regions: past, present and future. Clim Change. 2003;59:1–4. [Google Scholar]
- 13.Dilly O, Blume HP, Munch JC. Soil microbial activities in Luvisols and Anthrosols during 9 years of region-typical tillage and fertilisation practices in northern Germany. Biogeochemistry. 2003;65:319–339. [Google Scholar]
- 14.Djukic I, Zehetner F, Mentler A, Gerzabek MH. Microbial community composition and activity in different alpine vegetation zones. Soil Biol Biochem. 2010;42:155–161. [Google Scholar]
- 15.Djukic I, Zehetner F, Watzinger A, Horacek M, Gerzabek MH. In situ carbon turnover dynamics and the role of soil microorganisms therein: a climate warming study in an Alpine ecosystem. FEMS Microbiol Ecol. 2013;83:112–124. doi: 10.1111/j.1574-6941.2012.01449.x. [DOI] [PubMed] [Google Scholar]
- 16.Edwards KA, Jefferies RL. Nitrogen uptake by Carex aquatilis during the winter-spring transition in a low Arctic wet meadow. J Ecol. 2010;98:737–744. [Google Scholar]
- 17.Gömöryová E, Strelcová K, Fleischer P, Gömöry D. Soil microbial characteristics at the monitoring plots on windthrow areas of the Tatra National Park (Slovakia): their assessment as environmental indicators. Environ Monit Assess. 2011;174:31–45. doi: 10.1007/s10661-010-1755-2. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson TR, Schmidt EL. An acidophilic and a neutrophilic Nitrobacter strain isolated from the numerically predominant nitrite-oxidizing population of an acid forest soil. Appl Environ Microbiol. 1988;54:1536–1540. doi: 10.1128/aem.54.6.1536-1540.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedrick DB, Peacock A, White DC. Interpretation of fatty acid profiles of soil microorganisms. In: Margesin R, Schinner F, editors. Manual of Soil Analysis. Springer; Berlin: 2005. pp. 252–259. [Google Scholar]
- 20.ICP-IM Manual. International Cooperative Programme on Integrated Monitoring of Air Pollution Effects on Ecosystems. ICP-IM Programme Centre, Finnish Environment Institute; Helsinki: 1998. Manual for Integrated Monitoring. UN ECE Convention on Long-Range Transboundary Air Pollution. [Google Scholar]
- 21.Jing X, Wang Y, Chung H, Mi Z, Wang S, Zeng H, He J-S. No temperature acclimation of soil extracellular enzymes to experimental warming in an alpine grassland ecosystem on the Tibetan plateau. Biogeochemistry. 2014;117:39–54. [Google Scholar]
- 22.Kandeler E, Margesin R, Ohlinger R, Schinner F. Bodenmikrobiologisches Monitoring—Vorschläge für eine Bodenzustandsinventur. Die Bodenkultur. 1993;44:357–377. [Google Scholar]
- 23.Kennedy NM, Gleeson DE, Connolly J, Clipson NJW. Seasonal and management influences on bacterial community structure in an upland grassland soil. FEMS Microbiol Ecol. 2005;53:329–337. doi: 10.1016/j.femsec.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Koranda M, Kaiser C, Fuchslueger L, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S, Richter A. Seasonal variation in functional properties of microbial communities in beech forest soil. Soil Biol Biochem. 2013;60:95–104. doi: 10.1016/j.soilbio.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn M. The climate of snow and ice as boundary condition for microbial life. In: Margesin R, Schinner F, Marx JC, Gerday C, editors. Psychrophiles: from Biodiversity to Biotechnology. Springer Verlag; Berlin Heidelberg: 2008. pp. 3–15. [Google Scholar]
- 26.Kleemola S, Forsius M. The Finnish Environment. Helsinki: 2002. 11th Annual Report 2002. UN ECE ICP Integrated Monitoring; p. 567. [Google Scholar]
- 27.Ladd JN, Butler JHA. Short-term assay of soil proteolytic enzyme activites using proteins and dipeptide derivates as substrates. Soil Biol Biochem. 1972;4:19–30. [Google Scholar]
- 28.Lipson DA. Relationships between temperature responses and bacterial community structure along seasonal and altitudinal gradients. FEMS Microbiol Ecol. 2007;59:418–427. doi: 10.1111/j.1574-6941.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 29.Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci USA. 2011;108:15892–15897. doi: 10.1073/pnas.1107196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma X, Chen T, Zhang G, Wang R. Microbial community structure along an altitude gradient in three different localities. Folia Microbiol. 2004;49:105–111. doi: 10.1007/BF02931382. [DOI] [PubMed] [Google Scholar]
- 31.Margesin R. Alpine microorganisms: useful tools for low-temperature bioremediation. J Microbiol. 2007;45:281–285. [PubMed] [Google Scholar]
- 32.Margesin R, Jud M, Tscherko D, Schinner F. Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol Ecol. 2009;67:208–218. doi: 10.1111/j.1574-6941.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- 33.Margesin R, Plaza GA, Kasenbacher S. Characterization of bacterial communities at heavy-metal-contaminated sites. Chemosphere. 2011;82:1583–1588. doi: 10.1016/j.chemosphere.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 34.Margesin R, Zimmerbauer A, Schinner F. Monitoring of bioremediation by soil biological activities. Chemosphere. 2000;40:339–346. doi: 10.1016/s0045-6535(99)00218-0. [DOI] [PubMed] [Google Scholar]
- 35.Niklinska M, Klimek B. Effect of temperature on the respiration rate of forest soil organic layer along an elevation gradient in the Polish Carpathians. Biol. Fertil Soils. 2007;43:511–518. [Google Scholar]
- 36.Onodera Y, Nakagawa T, Takahashi R, Tokoyama T. Seasonal change in vertical distribution of ammonia-oxidizing archaea and bacteria and their nitrification in temperate forest soil. Microbes Environ. 2010;25:28–35. doi: 10.1264/jsme2.me09179. [DOI] [PubMed] [Google Scholar]
- 37.Ottow JCG. Mikrobiologie von Böden Biodiversität, Ökophysiologie und Metagenomik. Springer; Berlin: 2011. [Google Scholar]
- 38.Paz-Ferreiro J, Trasar-Cepeda C, Leiros MC, Seoane S, Gil-Sotres F. Effect of management and climate on biochemical properties of grassland soils from Galicia (NW Spain) Eur J Soil Biol. 2010;46:136–143. [Google Scholar]
- 39.Płaza GA, Nałęcz-Jawecki G, Pinyakong O, Illmer P, Margesin R. Ecotoxicological and microbiological characterization of soils from heavy metal and hydrocarbon contaminated sites. Environ Monit Assess. 2010;163:477–488. doi: 10.1007/s10661-009-0851-7. [DOI] [PubMed] [Google Scholar]
- 40.Pylvänäinen M. International Cooperative Programme on Integrated Monitoring of Air Pollution Effects on Ecosystems. ICP-IM Environment Data Centre, National Board of Waters and the Environment; Helsinki: 1993. Manual for Integrated Monitoring. Programme phase 1993–1996. UN ECE Convention on Long-Range Transboundary Air Pollution. [Google Scholar]
- 41.Rappé M, Giovannoni SJ. The uncultured microbial majority. Annu Rev Microbiol. 2003;57:369–394. doi: 10.1146/annurev.micro.57.030502.090759. [DOI] [PubMed] [Google Scholar]
- 42.Schindlbacher A, de Gonzalo C, Diaz-Pines E, Gorria P, Matthews B, Inclan R, Zechnmesiter-Boltenstern S, Rubio A, Jandl R. Temperature sensitivity of forest soil organic matter decomposition along two elevation gradients. J Geophys Res. 2010;115:G03018. [Google Scholar]
- 43.Schindlbacher A, Rodler A, Kuffner M, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S. Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biol Biochem. 2011;43:1417–1425. doi: 10.1016/j.soilbio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schinner F, Gstraunthaler G. Adaptation of microbial communities to the environmental conditions in alpine soils. Oecologia. 1981;50:113–116. doi: 10.1007/BF00378803. [DOI] [PubMed] [Google Scholar]
- 45.Schinner F, Öhlinger R, Kandeler E, Margesin R. Methods in Soil Biology. Springer; Berlin: 1996. [Google Scholar]
- 46.Schlichting E, Blume HP, Stahr K. Auflage. Vol. 2. Blackwell Wissenschafts-Verlag; Berlin: 1995. Bodenkundliches Praktikum. [Google Scholar]
- 47.Seyfried MS, Flerchinger GN, Murdock MD, Van Vactor SS. Long-Term Soil Microclimate Database. USDA-Agricultural Research Service; Boise, Idaho: 2000. (ARS Technical Bulletin NWRC-2000-7). [Google Scholar]
- 48.Staddon WJ, Duchesne LC, Trevors JT. The role of microbial indicators of soil quality in ecological forest management. Forest Chron. 1999;75:81–86. [Google Scholar]
- 49.Sun XF, Su YY, Zhang Y, Wu MY, Zhang Z, Pei KQ, Sun LF, Wan SQ, Liang Y. Diversity of arbuscular mycorrhizal fungal spore communities and its relations to plants under increased temperature and precipitation in a natural grassland. Chin Sci Bull. 2013;58:4109–4119. [Google Scholar]
- 50.Tabatabai MA, Bremner JM. Arylsulfatase activity of soils. Soil Sci Soc Am Proc. 1970;34:225–229. [Google Scholar]
- 51.Thalmann A. Zur Methodik der Bestimmung der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC) Landw Forschung. 1968;21:249–258. [Google Scholar]
- 52.Valentini R, Matteucci G, Dolman AJ, et al. Respiration as the main determinant of carbon balance in European forests. Nature. 2000;404:861–865. doi: 10.1038/35009084. [DOI] [PubMed] [Google Scholar]
- 53.Vanhala P, Kiikkillä O, Fritze H. Microbial responses of forest soil to moderate anthropogenic air pollution. Water Air Soil Poll. 1996;86:173–186. [Google Scholar]
- 54.Vanhala P, Tamminen P, Fritze H. Relationship between basal soil respiration rate, tree stand and soil characteristics in boreal forests. Environ Monit Assess. 2005;101:85–92. doi: 10.1007/s10661-005-9134-0. [DOI] [PubMed] [Google Scholar]
- 55.Väre H, Vestberg M, Ohtonen R. Shifts in mycorrhiza and microbial activity along an oroarctic altitudinal gradient in Northern Fennoscandia. Arct Alp Res. 1997;29:93–104. [Google Scholar]
- 56.Wardle DA. Controls of temporal variability of the soil microbial biomass: a global-scale synthesis. Soil Biol Biochem. 1998;30:1627–1637. [Google Scholar]
- 57.Weedon JT, Aerts R, Kowalchuk GA, van Bodengom PM. Enzymology under global change: organic nitrogen turnover in alpine and sub-Arctic soils. Biochem Soc Trans. 2011;39:309–314. doi: 10.1042/BST0390309. [DOI] [PubMed] [Google Scholar]
- 58.Wei H, Guenet B, Vicca S, Nunan N, AbdElgawad H, Pouteau V, Shen WJ, Janssens IA. Thermal acclimation of organic matter decomposition in an artificial forest soil is related to shifts in microbial community structure. Soil Biol Biochem. 2014;71:1–12. [Google Scholar]
- 59.Withington CL, Sanford RL. Decomposition rates of buried substrates increase with altitude in the forest-alpine tundra ecotone. Soil Biol Biochem. 2007;39:68–75. [Google Scholar]
- 60.Wittmann O. Soil classification of the Federal Republic of Germany. Mitt Dt Bodenkundl Ges. 1997;84:253–275. [Google Scholar]
- 61.Zelles L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol. Fertil Soils. 1999;29:111–129. [Google Scholar]